Preparation and Characterization of Salsalate-Loaded Chitosan Nanoparticles: In Vitro Release and Antibacterial and Antibiofilm Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Encapsulation Efficiency
2.2. Mean Particle Size, Polydispersity Index (PDI), and Zeta Potential
2.3. Optical Properties of SSL-Loaded CSNPs
2.4. Surface Properties of SSL-Loaded CSNPs
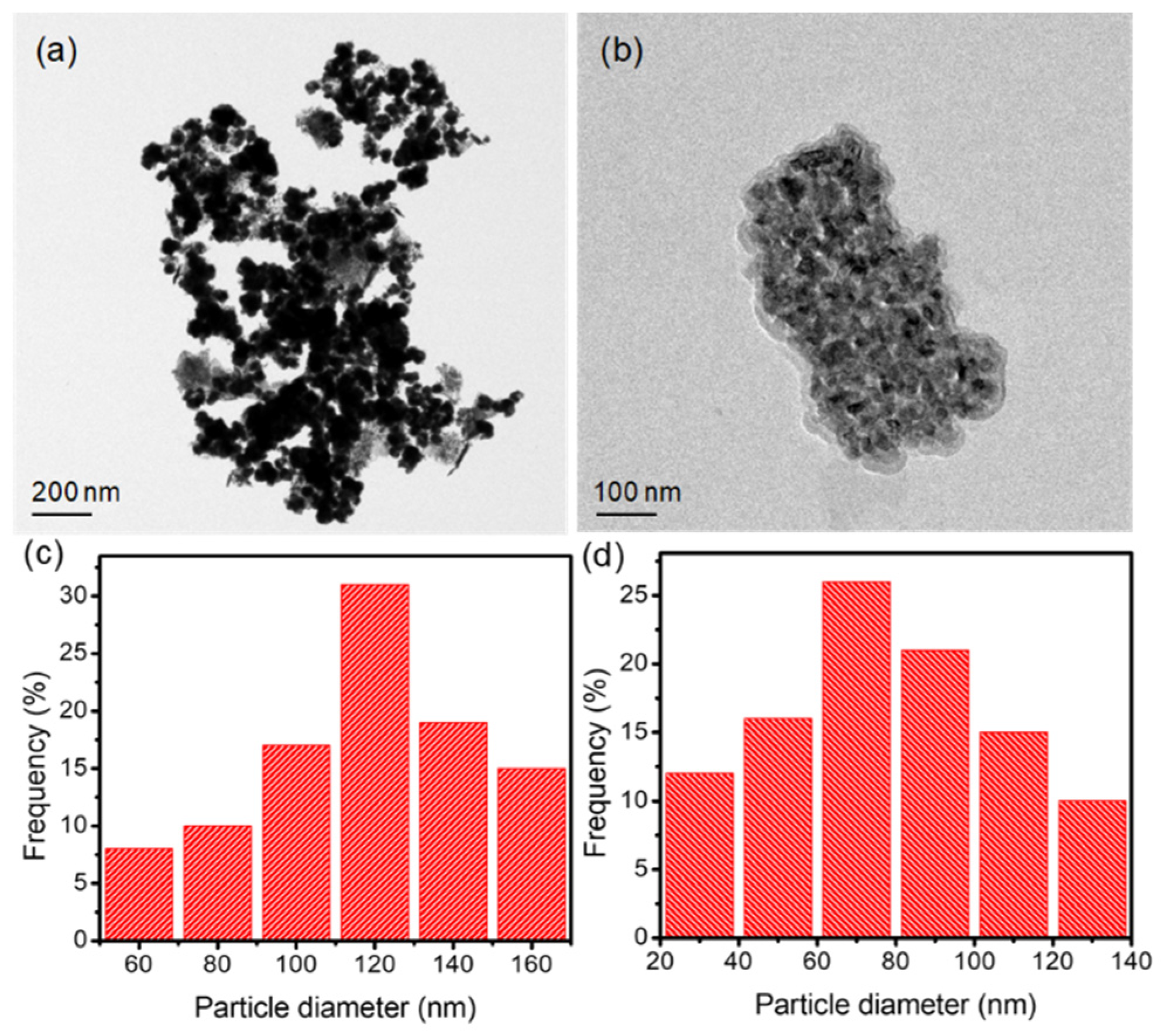
2.5. Morphology of SSL-Loaded CSNPs
2.6. Drug Release and Kinetics
2.7. Antibacterial Activity
2.8. Antibiofilm Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of SSL-Loaded CSNPs
3.3. Characterization
3.4. Encapsulation Efficiency
3.5. In Vitro Drug Release Study
3.6. Evaluation of Antimicrobial Activity
3.7. Evaluation of Antibiofilm Activity
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Zahid Butt, M.T.; Khalid, S.; Shafiq, M.; Islam, A.; Asim, S.; Hafeez, S.; Khan, R.U. In Vitro Study of Chitosan-Based Multi-Responsive Hydrogels as Drug Release Vehicles: A Preclinical Study. RSC Adv. 2019, 9, 31078–31091. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Atay, H.Y. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Springer: Singapore, 2020; ISBN 9789811502637. [Google Scholar]
- de Lima, C.S.A.; Varca, J.P.R.O.; Alves, V.M.; Nogueira, K.M.; Cruz, C.P.C.; Rial-Hermida, M.I.; Kadłubowski, S.S.; Varca, G.H.C.; Lugão, A.B. Mucoadhesive Polymers and Their Applications in Drug Delivery Systems for the Treatment of Bladder Cancer. Gels 2022, 8, 587. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug Release Study of the Chitosan-Based Nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, C.; Bilge, S.S. Effects of Nonsteroidal Anti-Inflammatory Drugs at the Molecular Level. Eurasian J. Med. 2018, 50, 116–121. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Trifilio, S.; Gordon, L.; Rubin, H.; Grosshans, N.; Mehta, J. The Non-Steroidal Anti-Inflammatory Drug Salsalate Provides Safe and Effective Control of Mucositis-Unrelated Pain during Autologous and Allogeneic Hematopoietic Stem Cell Transplantation. Support. Care Cancer 2021, 29, 3643–3648. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Fonseca, V.; Jablonski, K.A.; Pyle, L.; Staten, M.A.; Shoelson, S.E. The Effects of Salsalate on Glycemic Control in Patients with type 2 diabetes: A randomized trial. Ann. Intern. Med. 2010, 152, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Wherle, L.; Park, M.; Nelson, K.; Nguyen, L.D. Salsalate, an Old, Inexpensive Drug with Potential New Indications: A Review of the Evidence from 3 Recent Studies. Am. Health Drug Benefits 2014, 7, 231–235. [Google Scholar] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Choi, K.M.; Kim, M.H.; Cai, H.; Lee, Y.J.; Hong, Y.; Ryu, P.Y. Salicylic Acid Reduces OmpF Expression, Rendering Salmonella Enterica Serovar Typhimurium More Resistant to Cephalosporin Antibiotics. Chonnam Med. J. 2018, 54, 17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poór, P. Effects of Salicylic Acid on the Metabolism of Mitochondrial Reactive Oxygen Species in Plants. Biomolecules 2020, 10, 341. [Google Scholar] [CrossRef]
- Ocampo, P.S.; Lázár, V.; Papp, B.; Arnoldini, M.; Zur Wiesch, P.A.; Busa-Fekete, R.; Fekete, G.; Pál, C.; Ackermann, M.; Bonhoeffer, S. Antagonism between Bacteriostatic and Bactericidal Antibiotics Is Prevalent. Antimicrob. Agents Chemother. 2014, 58, 4573–4582. [Google Scholar] [CrossRef]
- Yang, J.; Barra, J.T.; Fung, D.K.; Wang, J.D. Bacillus Subtilis Produces (p)PpGpp in Response to the Bacteriostatic Antibiotic Chloramphenicol to Prevent Its Potential Bactericidal Effect. mLife 2022, 1, 101–113. [Google Scholar] [CrossRef]
- Melander, R.J.; Zurawski, D.V.; Melander, C. Narrow-Spectrum Antibacterial Agents. Medchemcomm 2018, 9, 12–21. [Google Scholar] [CrossRef]
- Kollef, M.H. Broad-Spectrum Antimicrobials and the Treatment of Serious Bacterial Infections: Getting It Right up Front. Clin. Infect. Dis. 2008, 47, 3–13. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Karakasyan, C.; Jouenne, T.; Cerf, D.L.; Dé, E. Application of Polymeric Nanocarriers for Enhancing the Bioavailability of Antibiotics at the Target Site and Overcoming Antimicrobial Resistance. Appl. Sci. 2021, 11, 10695. [Google Scholar] [CrossRef]
- Lim, Y.H.; Tiemann, K.M.; Hunstad, D.A.; Elsabahy, M.; Wooley, K.L. Polymeric Nanoparticles in Development for Treatment of Pulmonary Infectious Diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 842–871. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Mohandoss, S.; Pandimurugan, R.; Lee, Y.R.; Palanisamy, S.; Senthilkumar, M. In Situ Synthesis and Characterization of Colloidal AuNPs Capped Nano-Chitosan Containing Poly(2,5-Dimethoxyaniline) Nanocomposites for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 1083–1101. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Pandimurugan, R.; Palanisamy, S.; Mohandoss, S. Facile Synthesis of Metal Nanoparticle-Loaded Polymer Nanocomposite with Highly Efficient an Optically Enhanced Biocidal and Anticancer Agents. J. Biomater. Sci. Polym. Ed. 2021, 32, 2210–2226. [Google Scholar] [CrossRef]
- Lu, B.; Lv, X.; Le, Y. Chitosan-Modified PLGA Nanoparticles for Control-Released Drug Delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef]
- Yan, J.; Guan, Z.Y.; Zhu, W.F.; Zhong, L.Y.; Qiu, Z.Q.; Yue, P.F.; Wu, W.T.; Liu, J.; Huang, X. Preparation of Puerarin Chitosan Oral Nanoparticles by Ionic Gelation Method and Its Related Kinetics. Pharmaceutics 2020, 12, 216. [Google Scholar] [CrossRef]
- Nunes, R.; Serra, A.S.; Simaite, A.; Sousa, Â. Modulation of Chitosan-TPP Nanoparticle Properties for Plasmid DNA Vaccines Delivery. Polymers 2022, 14, 1443. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, H.; Kjøniksen, A.L.; Hiorth, M. Stability of Chitosan Nanoparticles Cross-Linked with Tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Baek, Y.; Yoo, H.J.; Lee, J.S.; Lee, H.G. Chitosan-Tripolyphosphate Nanoparticles Prepared by Ionic Gelation Improve the Antioxidant Activities of Astaxanthin in the In Vitro and In Vivo Model. Antioxidants 2022, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yu, D.; Ying, Z.; Pan, C.; Wang, N.; Huang, F.; Ling, J.; Ouyang, X.K. Fabrication of Ion-Crosslinking Aminochitosan Nanoparticles for Encapsulation and Slow Release of Curcumin. Pharmaceutics 2019, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, Y.; Zhou, Y.; Guo, D.; Fan, Y.; Guo, F.; Zheng, Y.; Chen, W. Preparation of 5-Fluorouracil-Loaded Chitosan Nanoparticles and Study of the Sustained Release in Vitro and in Vivo. Asian J. Pharm. Sci. 2017, 12, 418–423. [Google Scholar] [CrossRef]
- Porras-Gómez, M.; Vega-Baudrit, J.; Núñez-Corrales, S. Ampicillin-Loaded Chitosan Nanoparticles for In Vitro Antimicrobial Screening on Escherichia coli. In Chitin-Chitosan: Myriad Functionalities in Science and Technology; BoD–Books on Demand: Norderstedt, Germany, 2018. [Google Scholar] [CrossRef]
- Ashvini, H.M.; Balla, A.; Mutta, S.K. Clarithromycin-Loaded Chitosan Nanoparticles: Preparation, Characterisation and Antibacterial Activity on Streptococcus Pneumonia. Indian J. Pharm. Sci. 2019, 81, 302–308. [Google Scholar] [CrossRef]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Gazzarri, M.; Bartoli, C.; Grassi, L.; Esin, S.; Chiellini, F.; Batoni, G. Chitosan Nanoparticles Loaded with the Antimicrobial Peptide Temporin B Exert a Long-Term Antibacterial Activity in Vitro against Clinical Isolates of Staphylococcus Epidermidis. Front. Microbiol. 2015, 6, 372. [Google Scholar] [CrossRef]
- Rashki, S.; Safardoust-Hojaghan, H.; Mirzaei, H.; Abdulsahib, W.K.; Mahdi, M.A.; Salavati-Niasari, M.; Khaledi, A.; Khorshidi, A.; Mousavi, S.G.A. Delivery LL37 by Chitosan Nanoparticles for Enhanced Antibacterial and Antibiofilm Efficacy. Carbohydr. Polym. 2022, 291, 119634. [Google Scholar] [CrossRef]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Cent. Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef]
- Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M.; Hashim, H.S.; Nasrullah, M. Nanostructured Chitosan/Maghemite Composites Thin Film for Potential Optical Detection of Mercury Ion by Surface Plasmon Resonance Investigation. Polymers 2020, 12, 1497. [Google Scholar] [CrossRef]
- Mohandoss, S.; Edison, T.N.J.I.; Atchudan, R.; Palanisamy, S.; Prabhu, N.M.; Napoleon, A.A.; You, S.G.; Lee, Y.R. Ultrasonic-Assisted Efficient Synthesis of Inclusion Complexes of Salsalate Drug and β-Cyclodextrin Derivatives for Potent Biomedical Applications. J. Mol. Liq. 2020, 319, 114358. [Google Scholar] [CrossRef]
- Song, J.; Zhao, L.; Wang, Y.; Xue, Y.; Deng, Y.; Zhao, X.; Li, Q. Carbon Quantum Dots Prepared with Chitosan for Synthesis of CQDS/AuNPs for Iodine Ions Detection. Nanomaterials 2018, 8, 1043. [Google Scholar] [CrossRef] [PubMed]
- Alishahi, A. Antibacterial Effect of Chitosan Nanoparticle Loaded with Nisin for the Prolonged Effect. J. Food Saf. 2014, 34, 111–118. [Google Scholar] [CrossRef]
- Sobhani, Z.; Samani, S.M.; Montaseri, H.; Khezri, E. Nanoparticles of Chitosan Loaded Ciprofloxacin: Fabrication and Antimicrobial Activity. Adv. Pharm. Bull. 2017, 7, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Hao, S.; Wu, D.; Huang, R.; Xu, Y. Preparation, Characterization and in Vitro Release of Chitosan Nanoparticles Loaded with Gentamicin and Salicylic Acid. Carbohydr. Polym. 2011, 85, 803–808. [Google Scholar] [CrossRef]
- Al-Qubaisi, M.S.; Al-Abboodi, A.S.; Alhassan, F.H.; Hussein-Al-Ali, S.; Flaifel, M.H.; Eid, E.E.M.; Alshwyeh, H.A.; Hussein, M.Z.; Alnasser, S.M.; Saeed, M.I.; et al. Preparation, Characterization, in Vitro Drug Release and Anti-Inflammatory of Thymoquinone-Loaded Chitosan Nanocomposite. Saudi Pharm. J. 2022, 30, 347–358. [Google Scholar] [CrossRef]
- Covarrubias, C.; Trepiana, D.; Corral, C. Synthesis of Hybrid Copper-Chitosan Nanoparticles with Antibacterial Activity against Cariogenic Streptococcus Mutans. Dent. Mater. J. 2018, 37, 379–384. [Google Scholar] [CrossRef]
- Duceac, L.D.; Calin, G.; Eva, L.; Marcu, C.; Goroftei, E.R.B.; Dabija, M.G.; Mitrea, G.; Luca, A.C.; Hanganu, E.; Gutu, C.; et al. Third-Generation Cephalosporin-Loaded Chitosan Used to Limit Microorganisms Resistance. Materials 2020, 13, 4792. [Google Scholar] [CrossRef]
- Cover, N.F.; Lai-Yuen, S.; Parsons, A.K.; Kumar, A. Synergetic Effects of Doxycycline-Loaded Chitosan Nanoparticles for Improving Drug Delivery and Efficacy. Int. J. Nanomed. 2012, 7, 2411–2419. [Google Scholar] [CrossRef]
- Zhou, J.; Li, N.; Liu, P.; Liu, Z.; Gao, L.; Jiao, T. Preparation of Fluorescently Labeled Chitosan-Quercetin Drug-Loaded Nanoparticles with Excellent Antibacterial Properties. J. Funct. Biomater. 2022, 13, 141. [Google Scholar] [CrossRef]
- Arafa, M.G.; Mousa, H.A.; Afifi, N.N. Preparation of PLGA-Chitosan Based Nanocarriers for Enhancing Antibacterial Effect of Ciprofloxacin in Root Canal Infection. Drug Deliv. 2020, 27, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Elassal, M.; El-Manofy, N. Chitosan Nanoparticles as Drug Delivery System for Cephalexin and Its Antimicrobial Activity Against Multiidrug Resistent Bacteria. Int. J. Pharm. Pharm. Sci. 2019, 11, 14–27. [Google Scholar] [CrossRef]
- Lu, T.; ten Hagen, T.L.M. A Novel Kinetic Model to Describe the Ultra-Fast Triggered Release of Thermosensitive Liposomal Drug Delivery Systems. J. Control. Release 2020, 324, 669–678. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Qian, J.; Pan, C.; Liang, C. Antimicrobial Activity of Fe-Loaded Chitosan Nanoparticles. Eng. Life Sci. 2017, 17, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Maestu, A.; Ma, Z.; Paik, S.Y.R.; Chen, N.; Ko, S.; Tong, Z.; Jeong, K.C.C. Engineering of Chitosan-Derived Nanoparticles to Enhance Antimicrobial Activity against Foodborne Pathogen Escherichia Coli O157:H7. Carbohydr. Polym. 2018, 197, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial Properties of Chitosan from Different Developmental Stages of the Bioconverter Insect Hermetia Illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Gomes, L.C.; Mergulhão, F.J. SEM Analysis of Surface Impact on Biofilm Antibiotic Treatment. Scanning 2017, 2017, 2960194. [Google Scholar] [CrossRef]
- Saberpour, M.; Bakhshi, B.; Najar-Peerayeh, S. Evaluation of the Antimicrobial and Antibiofilm Effect of Chitosan Nanoparticles as Carrier for Supernatant of Mesenchymal Stem Cells on Multidrug-Resistant Vibrio Cholerae. Infect. Drug Resist. 2020, 13, 2251–2260. [Google Scholar] [CrossRef]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Biofilm Formation and Persistence on Abiotic Surfaces in the Context of Food and Medical Environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Wang, T.Y.; Webster, T.J.; Duan, H.J.; Qiu, J.Y.; Zhao, Z.M.; Yin, X.X.; Zheng, C.L. Intracellular Disposition of Chitosan Nanoparticles in Macrophages: Intracellular Uptake, Exocytosis, and Intercellular Transport. Int. J. Nanomed. 2017, 12, 6383–6398. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, N.; Kolodkin-Gal, I. The Matrix Reloaded: How Sensing the Extracellular Matrix Synchronizes Bacterial Communities. J. Bacteriol. 2015, 197, 2092–2103. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.Y.; Aleanizy, F.S.; Tahir, E.E.; Alquadeib, B.T.; Alsarra, I.A.; Alanazi, J.S.; Abdelhady, H.G. Preparation, Characterization, and Antibacterial Activity of Diclofenac-Loaded Chitosan Nanoparticles. Saudi Pharm. J. 2019, 27, 82–87. [Google Scholar] [CrossRef]








| CS Concentration | Encapsulation Efficiency (%) |
|---|---|
| 0.2 | 56.7 ± 1.21 |
| 0.4 | 59.2 ± 0.09 |
| 0.6 | 61.4 ± 1.12 |
| 0.8 | 64.7 ± 0.19 |
| 1.0 | 68.9 ± 2.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganesan, S.; Alagarasan, J.K.; Sonaimuthu, M.; Aruchamy, K.; Alkallas, F.H.; Ben Gouider Trabelsi, A.; Kusmartsev, F.V.; Polisetti, V.; Lee, M.; Lo, H.-M. Preparation and Characterization of Salsalate-Loaded Chitosan Nanoparticles: In Vitro Release and Antibacterial and Antibiofilm Activity. Mar. Drugs 2022, 20, 733. https://doi.org/10.3390/md20120733
Ganesan S, Alagarasan JK, Sonaimuthu M, Aruchamy K, Alkallas FH, Ben Gouider Trabelsi A, Kusmartsev FV, Polisetti V, Lee M, Lo H-M. Preparation and Characterization of Salsalate-Loaded Chitosan Nanoparticles: In Vitro Release and Antibacterial and Antibiofilm Activity. Marine Drugs. 2022; 20(12):733. https://doi.org/10.3390/md20120733
Chicago/Turabian StyleGanesan, Sivarasan, Jagadeesh Kumar Alagarasan, Mohandoss Sonaimuthu, Kanakaraj Aruchamy, Fatemah Homoud Alkallas, Amira Ben Gouider Trabelsi, Fedor Vasilievich Kusmartsev, Veerababu Polisetti, Moonyong Lee, and Huang-Mu Lo. 2022. "Preparation and Characterization of Salsalate-Loaded Chitosan Nanoparticles: In Vitro Release and Antibacterial and Antibiofilm Activity" Marine Drugs 20, no. 12: 733. https://doi.org/10.3390/md20120733
APA StyleGanesan, S., Alagarasan, J. K., Sonaimuthu, M., Aruchamy, K., Alkallas, F. H., Ben Gouider Trabelsi, A., Kusmartsev, F. V., Polisetti, V., Lee, M., & Lo, H.-M. (2022). Preparation and Characterization of Salsalate-Loaded Chitosan Nanoparticles: In Vitro Release and Antibacterial and Antibiofilm Activity. Marine Drugs, 20(12), 733. https://doi.org/10.3390/md20120733










