Abstract
Chitin is the second most abundant biopolymer consisting of N-acetylglucosamine units and is primarily derived from the shells of marine crustaceans and the cell walls of organisms (such as bacteria, fungi, and algae). Being a biopolymer, its materialistic properties, such as biodegradability, and biocompatibility, make it a suitable choice for biomedical applications. Similarly, its deacetylated derivative, chitosan, exhibits similar biocompatibility and biodegradability properties, making it a suitable support material for biomedical applications. Furthermore, it has intrinsic material properties such as antioxidant, antibacterial, and antitumor. Population studies have projected nearly 12 million cancer patients across the globe, where most will be suffering from solid tumors. One of the shortcomings of potent anticancer drugs is finding a suitable cellular delivery material or system. Therefore, identifying new drug carriers to achieve effective anticancer therapy is becoming essential. This paper focuses on the strategies implemented using chitin and chitosan biopolymers in drug delivery for cancer treatment.
1. Introduction
Chitin is the second most biopolymer composed of N-acetylglucosamine units. It is commonly found in higher quantities in arthropods’ exoskeletons, radula of mollusks, and cell walls of fungi [1]. Commercially, it is marketed as one of the components of natural medicinal products, nutraceutical foods, and 3D scaffolds for biomedical and technological applications [2,3]. It is typically produced using a high-temperature method and is reported to exhibit thermostability [4]. Additionally, because chitin shows high tolerance for high chemical concentrations, some metals, such as copper, can be deposited through an electrochemical process at room temperature [5]. Different forms of chitin are present in nature; the side chain’s backbone arrangement determines the difference between the forms of chitins. The α-chitin, β-chitin, and γ-chitin are the three isoforms of chitin [6].
The parallel chain positioning is found in the α-chitin structure and is usually used for tissue engineering. In contrast, an antiparallel chain positioning is found in the β-chitin structure and is used for wound healing [7]. In comparison, γ-chitin is derived from the cocoon of Orgyia dubia (Moth), shares structural similarities with α-chitin, and is composed of microfibers. The external morphology of α-chitin and β-chitin consists of nanofibers [7]. Moreover, the optimization process is responsible for chitin’s multifunctionality and structural diversity. The internal structure of chitin is an arrangement of building blocks into higher-order fiber structures, which are stabilized by the no-covalent interactions [8].
The chitin arrangement can also be seen as a crystalline structure. Chitin crystallites contain fibrillar rods generated by the supramolecular assembly comprising 19 molecular chains. The chitin rods and protein matrix form a natural composite along with the mineral content. There is considerable evidence that chitin molecules create covalent interactions with their protein matrix, forming in their original form, which includes the involvement of acetyl, amino, and hydroxyl groups in the polymer chain. Additionally, the chitin proteoglycans exhibit higher H-bond networking (intermolecular and intramolecular), resulting in densely linked chitin-derived structures. Because of such high bonding involvement, chitin has poor solubility indexing with most solvents, including water, organic solvents, and to an extent, slightly acidic or basic solutions [8]. Therefore, it serves as a robust support material for various biomedical applications. For example, chitin is used along with carbon nanotubes for neural treatment and growth [9]. It has been seen that by preventing chondrocytes from undergoing apoptosis and stimulating immunomodulation of chondrogenesis and macrophage, chitin hydrogel restored damaged cartilage [10]. Additionally, human bone marrow-derived stromal cells were utilized as a 3D-scaffold for chitin generated from demosponge Aplysina aerophoba, which increased the proliferation of the cell, cell bridging generation, and metabolic functions without producing much toxicity [11]. It is interesting to note that chitin is used as a possible treatment and a biological marker for neurological disorders. Chitin is increased and builds up in the brain of patients with Alzheimer’s disease (AD), providing scaffolding for the deposition of amyloid-β [12,13,14]. Brain tissues from Alzheimer’s patients also contained fungal chitin [15]. AD is one of the most recurrent neurodegenerative disorders that affect a person’s language acquisition, perception, memory, and thoughts [16,17,18,19,20,21,22,23,24,25], which leads to overall impaired cognition [26,27]. Additionally, those with multiple sclerosis had chitin deposition. A condition referred to as multiple sclerosis develops when the body’s immune system attacks the protective covering surrounding the brain’s nerve cells [28].
Chitosan (CS), a polysaccharide, is derived from chitin by deacetylation [29,30,31]. Chitosan has been reported for use in various applications, extending from biomaterials and tissue engineering to antibacterial, antifungal, anticancer, and antioxidant agents due to its strong biocompatibility [32]. Chitosan has undergone several chemical changes that have been suggested to give polysaccharides particular qualities. Chitosan samples that have been altered through phosphorylation, quaternarization, carboxylation, sulfonation, N-alkylation, and acylation can function as stimuli-sensitive materials (pH-, thermo-, or light-sensitive) [33]. Chitin and chitosan have been the focus of numerous investigations to determine their efficacy as agents for drug delivery [34]. For instance, chitosan is commonly used for preparing hydrogels for drug delivery due to its essential characteristics, such as bio-adhesion, having a polycationic surface that makes it easier to form hydrogenic and ionic bonds, and biocompatibility, which means it does not generate any toxins or trigger an immune response when in contact with the body fluids or living tissue [35]. Likewise, several types of research have successfully implemented chitin as one of the support materials for drug delivery. However, many ways are reported to transport the drugs, but the implementation of polymeric carriers received a high interest since they increase the effectiveness of drug targeting and extend the time that drugs stay in circulation by reducing urine elimination [36]. Herein we have emphasized the innovations and advancements in understanding the possible function of chitin and chitosan biopolymers in delivering cancer-treating drugs.
2. Extraction of Chitin: Chemical and Biological Process
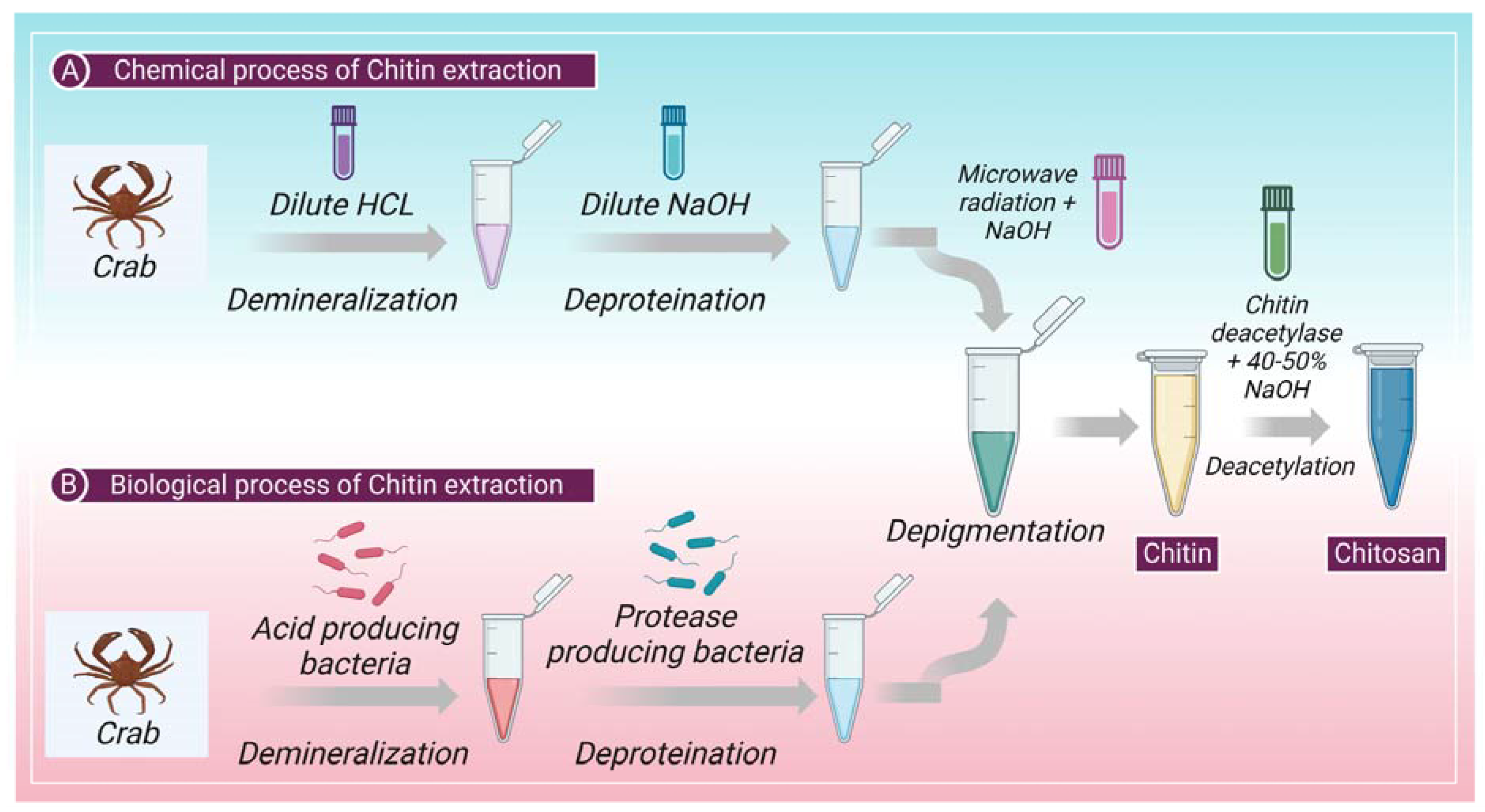
The cuticles of different crustaceans, primarily shrimp and crabs, are the prime producers of raw material for the formation of chitin. Chitin is a complex protein network component mainly found in crustaceans or shellfish, on which the calcium carbonate accumulates to produce a firm shell [37]. Chitin and protein interact very closely; a minor amount of protein is also contained in a polysaccharide-protein complex. The extraction of chitin from shelf fish primarily requires two-step chemical processing (a) removal of inorganic calcium carbonate by demineralization and (b) removal of proteins by deproteinization. Sometimes, a different decolorization phase is also used to eliminate any remaining pigments [38]. Heat and high alkaline or acidic conditions (>1 M NaOH, >3 M HCl) can cause chitin to change itself into the deacetylated state, chitosan, or hydrolyze into C5 and C6 hydrocarbons [39] on prolonged chemical treatments [1]. Figure 1 shows chitin extraction and chitosan production chemical and biological processes. However, several methods of producing pure chitin have been devised, but no method is accepted as standard procedure to this date. Deproteinization and demineralization could also be accomplished by enzymatic (biological) or chemical processes. Additionally, microbial fermentation is used to simultaneously carry out the demineralization and deproteinization processes [40]. Biological processes, an alternative approach, may produce more satisfactory results because they are inexpensive and environmentally friendly, have low energy usage, and are reproducible. In addition, they can extract or manufacture chitin with greater molecular weight and a more robust crystal structure [41].
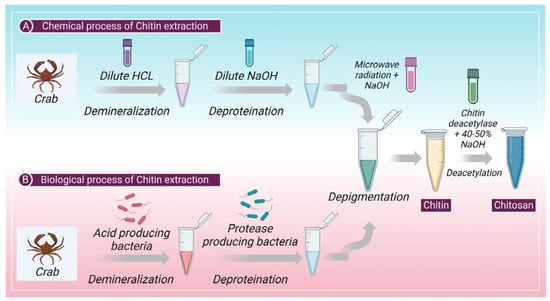
Figure 1.
Shows the (A) chemical and (B) biological process involved in the extraction of chitin and production of chitosan from chitin.
2.1. Chemical Process
2.1.1. Chemical Demineralization
Demineralization is a process of mineral removal, typically calcium carbonate. Demineralization is usually accomplished through acid treatment with H2SO4, HNO3, CH3COOH, HCOOH, and HCl. Dilute hydrochloric acid is the preferred reagent among these acids [42]. Demineralization is simple as it involves breaking down calcium carbonate into calcium salts (water-soluble) with carbon dioxide emission [43]. Treatments involving demineralization are usually empirical and rely on the specificity of samples, including the degree of shell’s mineralization, particle size, temperature, extraction time, solute or solvent ratio, and acid content [44,45].
2.1.2. Chemical Deproteinization
The chemical interactions between chitin and proteins must be broken during the deproteinization process, which is challenging. The biopolymer is depolymerized during this heterogeneous process employing chemicals. For biomedical applications, the total elimination of protein is crucial since a portion of the population suffers from shellfish allergies, with the protein component being the only problem. The first strategy for deproteinization was using chemical reagents. Chemicals, such as K2CO3, KOH, Ca(OH)2, NaOH, Na2CO3, NaHCO3, NaHSO3, Ca(HSO3)2, Na3PO4, and Na2S, have been examined as deproteinization reagents. Each study’s reaction conditions are very different. The preferred reagent, NaOH, is used with different concentrations (0.125 M to 5.0 M), temperatures (up to 160 °C), and treatment times (from a few minutes up to a few days). The NaOH treatment causes deproteinization, and partial deacetylation of chitin, resulting in a decrease in molecular weight [46,47].
2.1.3. Depigmentation
In arachnoids, there are mineral-free cuticles; therefore, the process can start without the demineralization step, and isolation of chitin can begin with deproteinization and depigmentation [48]. Firstly, the lipids and waxes must be removed from the cuticles, which is achieved through microwave irradiation (MWI) [48]. In MWI, cuticles are introduced to microwave radiation while they are treated with ethanol and chloroform. In the second step, microwave radiation is used with NaOH to remove the pigments and other proteins from the cuticle [48]. Figure 1 shows the chemical process of chitin extraction.
2.2. Biological Process
The application of proteolytic enzymes for the digestion of proteins or the fermentation process allows the digestion of proteins and minerals are the two most typical biological methods for the extraction of chitin. This method includes enzymatic deproteinization in which proteases (pepsin, papain, alcalase, trypsin, etc.) are used to remove protein [49]. This process of deproteinization can be performed either before or after demineralization. The method of deproteinization can be conducted through the process of fermentation as well. Further, the depigmentation process takes place to extract pure chitin [46]. Figure 1 shows the biological process of chitin extraction.
3. Drug Delivery System
Combining an appropriate carrier with one or more drugs is the basic building block of drug delivery systems [50]. The two main criteria for perfect delivery systems are concentrating the active substance on the body’s site of action and supplying the correct quantity for a steady, suitable, and predetermined amount of time [51]. The first fundamental element of drug delivery systems is keeping plasma drug concentrations under the therapeutic window, accomplished through drug carriers, typically polymers [51]. This feature helped to promote the notion of controlled delivery systems. The second characteristic results in the creation of specialized delivery systems. The active substance is captured within a delivery system that may deliver the medicine to the particular site, necessitating careful selection of carrier, route of administration, and target of release [52]. The qualities of the medication and polymers, the disease to be treated, the variety of dosage forms, and the method of administration are just among the variables that affect whether delivery systems succeed in achieving their therapeutic goal [51]. As carriers, active compounds are frequently delivered using polymers (such as chitin or chitosan). They can be utilized to create various delivery systems, including micelles, hydrogels, tablets, capsules, and particulate systems (beads, micro-, and nanoparticles). The optimized pharmacokinetics usually get considered before choosing an appropriate polymer as it dictates the kinetics of drug release and the removal of carriers following drug delivery [53]. In a study by Machalowski et al. [42], chitin was utilized to deliver the aceclofenac drug through the topic and transdermal routes. Similarly, Sabitha et al. [54] and Panonnummal et al. [55] utilized chitin as nanogels to deliver 5-fluorouracil and clobetasol through the topical and transdermal route. Chitosan can take numerous ways the delivery of drugs. The routes of chitosan administration for drug delivery have been elaborated in detail further in the below sections.
3.1. Rotes of Chitosan Administration
3.1.1. Ocular Drug Delivery of CS
As mentioned above, chitosan’s biodegradability and biocompatibility are important features, making it useful for ocular drug delivery. Chitosan can increase the ocular surface duration of several drugs due to its mucoadhesive nature [56]. It can also transform to gel if smeared on the ocular surface in liquid form as it possesses favorable situ gelling properties. This has led to the therapeutic improvement of ocular drugs. The ocular drugs which are poorly soluble, chitosan nanoparticles (NPs) are a potential alternative, as they can increase the bioavailability of drugs (e.g., naringenin) in the aqueous humor. Moreover, a study by Ping Zhang et al. on rabbit eyes found that chitosan has no irritating effect on the eye [57]. Fluconazole-loaded chitosan NPs were created by Santhi et al., utilizing a cross-linking approach and spontaneous emulsification. They compared the antifungal abilities of these NPs with the traditional eye drops using the cup-plate method. These particles had an average size of 152.85 ± 13.7 nm. All drug-loaded NPs were determined to have an optimal (50%) drug-loading capacity. After completing their research, they deduced that the fluconazole-formulated chitosan NPs were an effective delivery system for fluconazole in drug loading, antifungal efficacy, and prolonged release characteristics [58].
3.1.2. Pulmonary Drug Delivery of CS
The benefits of delivery of drugs to the lungs include immediate and prolonged drug delivery, high effectiveness, and the ability to accomplish both local and systemic effects. Large lung surface area, high vascularity, and a thin absorption barrier are the parameters that improve medication transport via the lungs [59]. Chitosan has been used to enhance the effects of many medications. Rifampicin, an antitubercular medicine, was created as a dry nanoparticle powder inhalation using chitosan as the polymer. This formation demonstrated continuous drug release for up to twenty-four hours without causing any adverse effects on cells or organs [60]. Prothionamide, an antitubercular medication, was given by Debnath et al. as chitosan-coated NPs through the lungs. This change lengthened the drug’s inhalation half-life in the lungs [61]. Itraconazole is an antifungal drug, and due to itraconazole’s poor oral solubility, Jafarinejad et al. produced chitosan NPs for the pulmonary delivery of the antifungal medication as a dry powder formulation. They increased the drug’s aerosolization capabilities by adding chitosan NPs, mannitol, lactose, and leucine to the formulation. As a result, there was an increase in itraconazole pulmonary deposition [62].
3.1.3. Mucosal Drug Delivery of CS
Chitosan and its derivatives encourage mucosal delivery by increasing the absorption of hydrophilic molecules such as protein and peptide medicines. The eminently hydrated glycoproteins (lysozymes, salts, and mucins) that make mucus give it its viscoelastic characteristics [63]. To facilitate the paracellular trafficking of macromolecular medicines, chitosan function by opening the compact intercellular junctions. The positively charged, cell-bound chitosan NPs reduce the transepithelial electrical resistance of living cell monolayers and boost paracellular permeability. Depending on the chitosan’s molecular weight and level of deacetylation, the chitosan solution enhances trans and paracellular permeability [64]. Chitosan’s positive charge interacts with the compact junction proteins ZO-1 and occludin, redistributes F-actin, and somewhat destabilizes the plasma membrane as part of its mechanism of action. The environment has also been demonstrated to affect chitosan’s capacity to increase penetration [65].
3.1.4. Nasal Drug Delivery of CS
Using a non-invasive method like nasal delivery, medications can be administered systemically and locally without experiencing the normal gastrointestinal problems associated with oral management or the effects of hepatic metabolism [66]. Although nasal management can cross the blood–brain barrier (BBB), which has been shown to guide drug delivery from the nose to the brain (NTB) efficiently, NTB is an alternate way of topical management for antibacterial and anti-inflammatory nasal congestion [29]. One of three pathways (three methods of nasal absorption) allows nasally administered drugs to instantly cross the BBB: first, olfactory nerves, which are the foremost effective pathway for NTB delivery of drugs; second, trigeminal nerves, which have the presence of nerve endings in the respiratory epithelia; and third, respiratory epithelium. Some limitations of NTB delivery include the minor volume of the nasal cavity, enzymatic degradation, mucociliary clearance, short drug retention duration, potential nasomucosal toxicity, drug management and deposition technique, and the need for an appropriate delivery device [67]. Due to their limited permeability, the nasal epithelium is challenging to penetrate with hydrophilic medicines, nucleic acids, proteins, and peptides. CS enhances their permeability. The drug’s weight, lipophilicity, and charge affect how well it is absorbed through the nose. The mucociliary system clears medications that cannot pass the nasal membrane. Due to its use in nasal delivery, CS has mucoadhesion qualities combined with low toxicity, biodegradability, and biocompatibility, which can assist in resolving this concern [66].
Moreover, high molecular weight molecules cannot pass through the BBB and blood-cerebrospinal fluid barrier (BCB) into the brain. However, NTB delivery has been a vital strategy in the last few years to overcome these challenges and deliver drugs to the targeted brain regions. Human serum albumin NPs (HSA NPs) coated with chitosan were created by Piazzini et al. functioning as a nose-to-brain carrier for the anti-Alzheimer medicines tacrine and R-flurbiprofen. On ex vivo rabbit nasal mucosa, CS-coated NPs demonstrated improved mucoadhesion and a higher penetrating capacity than uncoated NPs. Additionally, by lowering the levels of ZO-1 expression, they have the benefit of loosening the compact junctions between hCMEC/D3 cells, allowing molecules to pass across the barrier [67].
To increase drug concentration in the active site, direct therapeutic material delivery to the brain is necessary for neurologic illnesses such as Parkinson’s disease (PD). In the central nervous system, PD is characterized by neurodegeneration and dopaminergic neuron loss in the CNS. The current standard of care for managing PD motor symptoms relies on the dopamine (DOPA) replacement strategy, which tries to compensate for the death of dopaminergic neurons and restore adequate neurotransmitter levels. Due to elevated hydrogen-bonding potential, complete ionization in physiological pH, and significant metabolism when administered orally, it is challenging for DOPA to penetrate the BBB. The development of DOPA-loaded nanocarriers as a novel mechanism for treating Parkinson’s disease has received the most significant attention [68]. These nanocarriers should have the capability to traverse BBB and permit persistent transport of the neurotransmitters to the brain. A non-ergoline agonist which works on the brain’s D2- and D3 receptors is ropinirole hydrochloride (RH). Chatzitaki et al. created PLGA (poly(lactic-co-glycolic acid) and PLGA/CS NPs with mucoadhesive characteristics to increase RH transport through the nasal mucosa. RH-loaded PLGA/CS NPs demonstrated full drug release over 24 h in the simulated nasal electrolyte solution (SNES) [68].
3.1.5. Transdermal Drug Delivery of CS
A transdermal drug delivery system is being evolved to overcome the shortcomings of traditional administration methods. The limited skin permeability is the fundamental obstacle to be addressed when creating transdermal dosage forms. Several techniques have been devised to get around the barrier qualities and improve the transportation of medication molecules over the skin [69,70,71]. Numerous transdermal patches made of polysaccharides have been discovered in recent years. Transdermal preparations containing CS are becoming more widespread [71]. NPs have been promoted as one of the prospective delivery systems that can significantly overcome the constraint of the drug’s ability to penetrate the skin. As a transdermal drug delivery technique, polysaccharide NPs are becoming increasingly well-liked. The mucoadhesive, biocompatible, mucodegradable, and mucosal penetration enhancing properties of the CS NPs. They are interrelated with the skin mucosa and fluidize the lipid and protein domains of the epidermis to aid transdermal drug diffusion. Additionally, they might be beneficial therapeutically for local illnesses such as skin infections and malignant melanoma and systemic conditions such as hyperlipidemia and diabetes [72].
3.1.6. Dermal Delivery of CS
The systemic unpropitious effects of traditional oral and injectable delivery could be avoided with topical treatment. Additionally, this can swiftly and directly penetrate the skin and mucous membranes at the illness site [29]. Since they enable the regulated release of drugs and address the issue of their low skin bioavailability, NPs are seen favorably for treating acne. Nicotinamide is one of the potential cosmeceuticals/nutraceuticals lately utilized to treat acne. This medication has anti-inflammatory effects and is said to reduce sebum production. Abd-Allah et al. produced CS NPs as well as added nicotinamide-containing supplements. On individuals with acne vulgaris, the NPs were refined, described, and tested in a clinical setting. Cogent skin adherence ex vivo and elevated nicotinamide deposition in the various skin layers totaling 68%, were used to demonstrate the topical benefits of CS NPs [73]. Furthermore, the nicotinamide CS NPs showed 73% depletion in inflammatory acne lesions when clinically evaluated on patients in contrast to untreated areas, demonstrating that the delivery system could be a therapeutically viable alternative for treating skin diseases [73].
3.1.7. CS Administration for Wound Healing
Various bacteria can infect and colonize injured skin, making it easier for them to get to the underlying tissues [74]. One crucial element that is thought to slow the healing of wounds is infection. In addition to providing a moist surrounding to prevent wound dryness, reducing wound surface necrosis, being oxygen penetrable without dehydrating the wound, and being congenial, wound dressings should also prevent mechanical damage [75]. Less toxicity, biocompatibility, and biodegradability are further important requirements for a material used to make wound dressing [76]. It has been demonstrated that the N-acetyl glucosamine, which is a monomer unit of CS, promotes cell growth, promotes hemostasis, as well as speeds up the healing of wounds.
Regarding biocompatibility, CS has no adverse effects on touch with human cells [77]. Additionally, CS speeds up blood coagulation by attaching to red blood cells. Finally, the constancy of the medications by creating CS NPs increases drug aggregation [77].
4. Cancer: Symptoms, Causes, Treatment Strategies
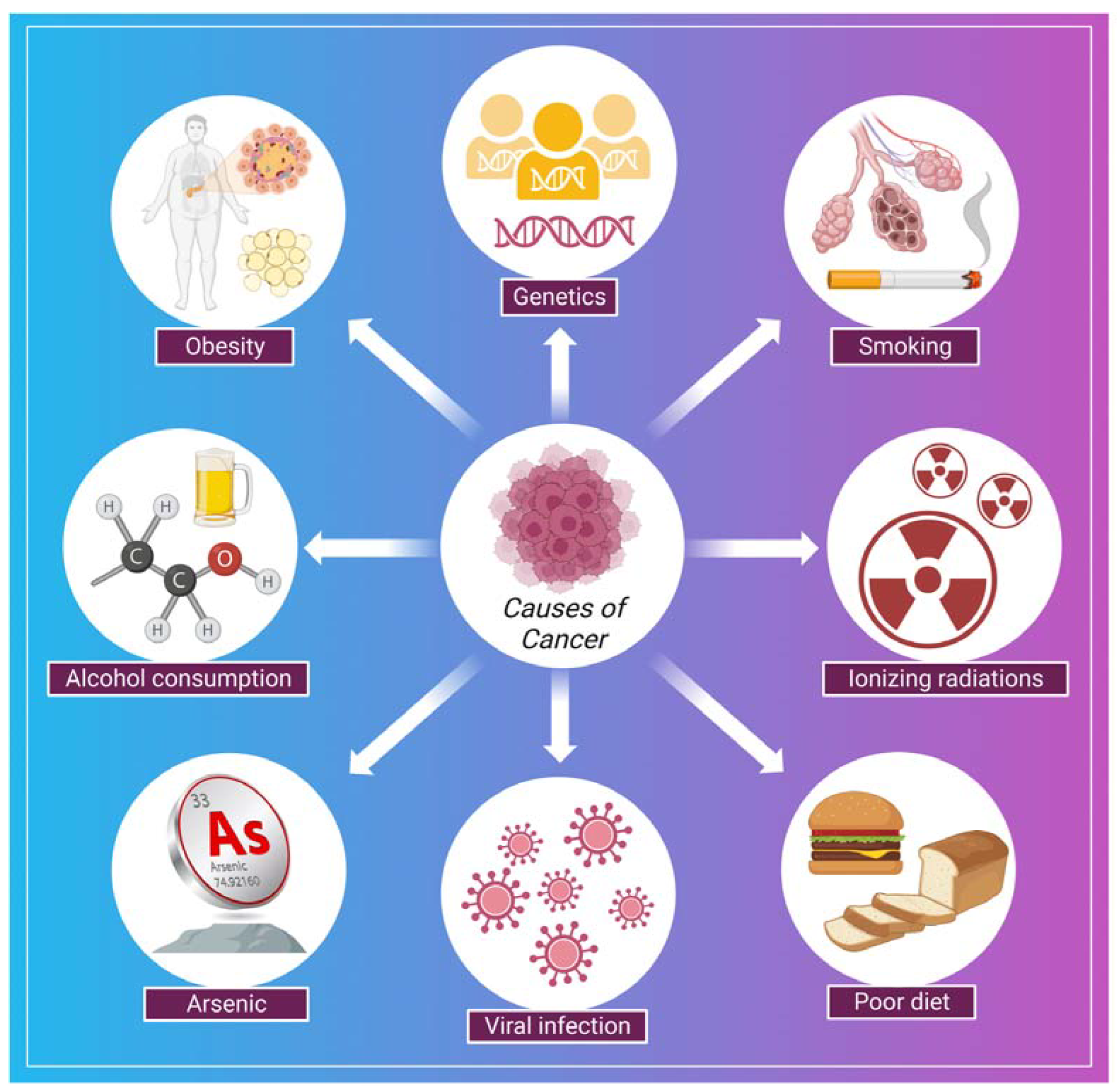
Cancer is a disease in which a group of the cells of the body starts growing and spreading abnormally [78]. The normal and healthy human cells multiply and grow through a process known as cell division, which allows the formation of new cells for the body. When cells are damaged or become old, they die and are replaced by new cells in the body. However, sometimes this process disrupts, leading to the growth and proliferation of abnormal cells (cancerous cells) [79]. In cancer, continuous clonal expansion of cells (somatic cells) is destroyed by destabilizing, eroding, and invading the healthy tissues [80]. The involvement of prior diagnosis assessments in worldwide cancer regulation programs, including symptom recognition campaigns, is expanding. However, if the symptoms specify the early stage of the disease, these strategies will have a nominal effect on refining cancer outcomes [81]. In a study by Koo et al., 7997 patients having cancer (stage IV cancer) were analyzed to examine the symptoms. Some typical symptoms were post-menopausal bleeding, breast lump, abnormal mole, fatigue, weight loss, abdominal pain, rectal bleeding, hoarseness, bowel habit change, haematuria, and symptoms associated with the lower urinary tract [81]. Some of the common causes related to the development of cancer are shown in (Figure 2). There are several strategies for the treatment and early detection of cancer. Some of these are listed in Table 1.
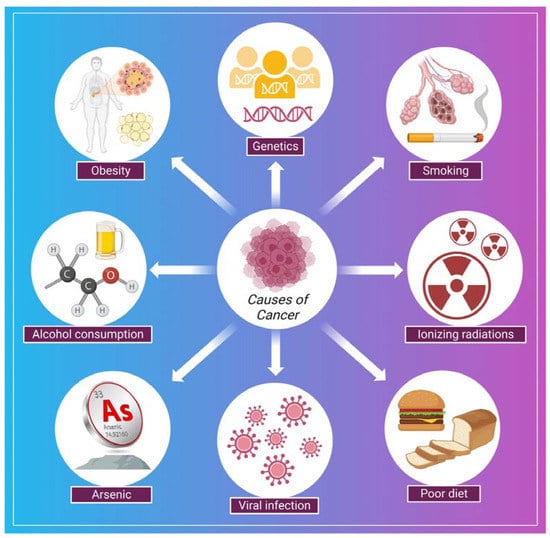
Figure 2.
Shows some of the common causes of cancer.

Table 1.
Shows some of the standard methods used to treat cancer.
5. Chitin and Chitosan for Drug Delivery and Cancer Treatment
Drug development and delivery have seen significant breakthroughs due to nanotechnology. For instance, the utility of NPs in the treatment and diagnosis of cancer has advanced to the point where it can now detect and target a single cancer cell with the delivery of a carrier to treat it. Traditional cancer therapeutic techniques include side effects, and diagnostic procedures are expensive and time-consuming. Due to their large size, surface charge, and morphology, NPs such as carbon nanotubes (CNTs), calcium NPs (CaNPs), graphene, and polymeric NPs (including chitin and chitosan) have improved cancer diagnostics and treatments. These NPs functionalization with various biological molecules, such as antibodies, aids in the transportation of drugs and the detection of cancerous cells [88]. Chitin holds the ability to generate as a drug delivery system and anticancer agent. It has been demonstrated that chitin can suppress chitinase-3-like protein-1 (CHI3L1), which is overexpressed and stimulates proinflammatory mediators in breast cancer cells [89]. Moreover, the synthesis of vascular endothelial growth factor C (VEGF-C), associated with tumor angiogenesis, can be downregulated with chitin [90]. Chitin has been formed in several kinds that can counteract cancer. For example, cytotoxicity was promoted in human breast cancer cells (MCF-7 Cells) with chitin nanocomposites embedded with silver [91]. Curcumin is an active turmeric substance with anticancer, antibacterial, and antifungal properties [92]. Curcumin-loaded chitin nanogels (CCNGs) is an anticancer drug with chitin and curcumin and are insoluble in water. It has been seen that CCNGs-prepared materals, when treated on porcine skin samples, showed easy penetration in the epidermis od the skin with no signs of inflammation. This shows that the formulation of CCNGs can treat melanoma, which is one of the most serious and common types of skin cancer [93]. Cancer vaccine has evolved as a unique cancer treatment method with the emergence of cancer immunotherapy, and the significance of adjuvants has lately been recognized. Adjuvants are chemical compounds that boost immunity and promote a vaccine’s potency without exhibiting any direct antigenic consequences of their own [94]. In addition to the previously listed applications, chitin and chitosan are essential adjuvants for immunotherapy. Many studies have investigated the adjuvant characteristics of chitin and chitosan due to their immunostimulant capability and structural resemblances to glucans, a subsidiary type of natural polysaccharides [95]. Chitin and chitosan’s antiviral and anticancer properties were first described decades ago. Suzuki et al. initially showed the adjuvant action of chitin and chitosan in the 1980s [96]. Chitin and chitosan are frequently used for non-invasive mucosal management routes, such as oral, intranasal, and ocular mucosa, due to their mucoadhesive characteristics [97]. Specific antigens have been demonstrated to boost adaptive immune responses [97]. Recent studies have shown that chitosan is a potential adjuvant for intranasal vaccination [98].
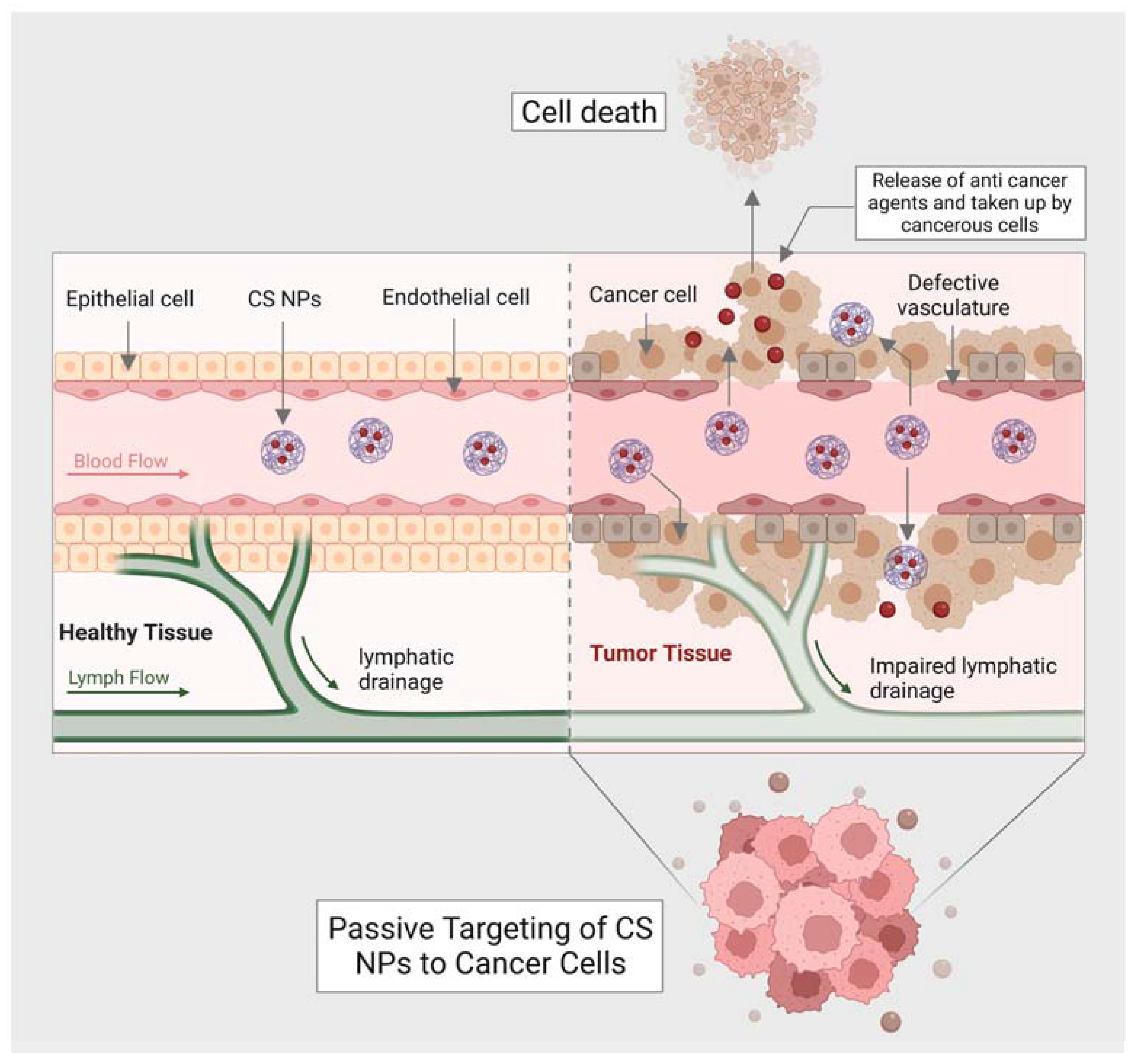
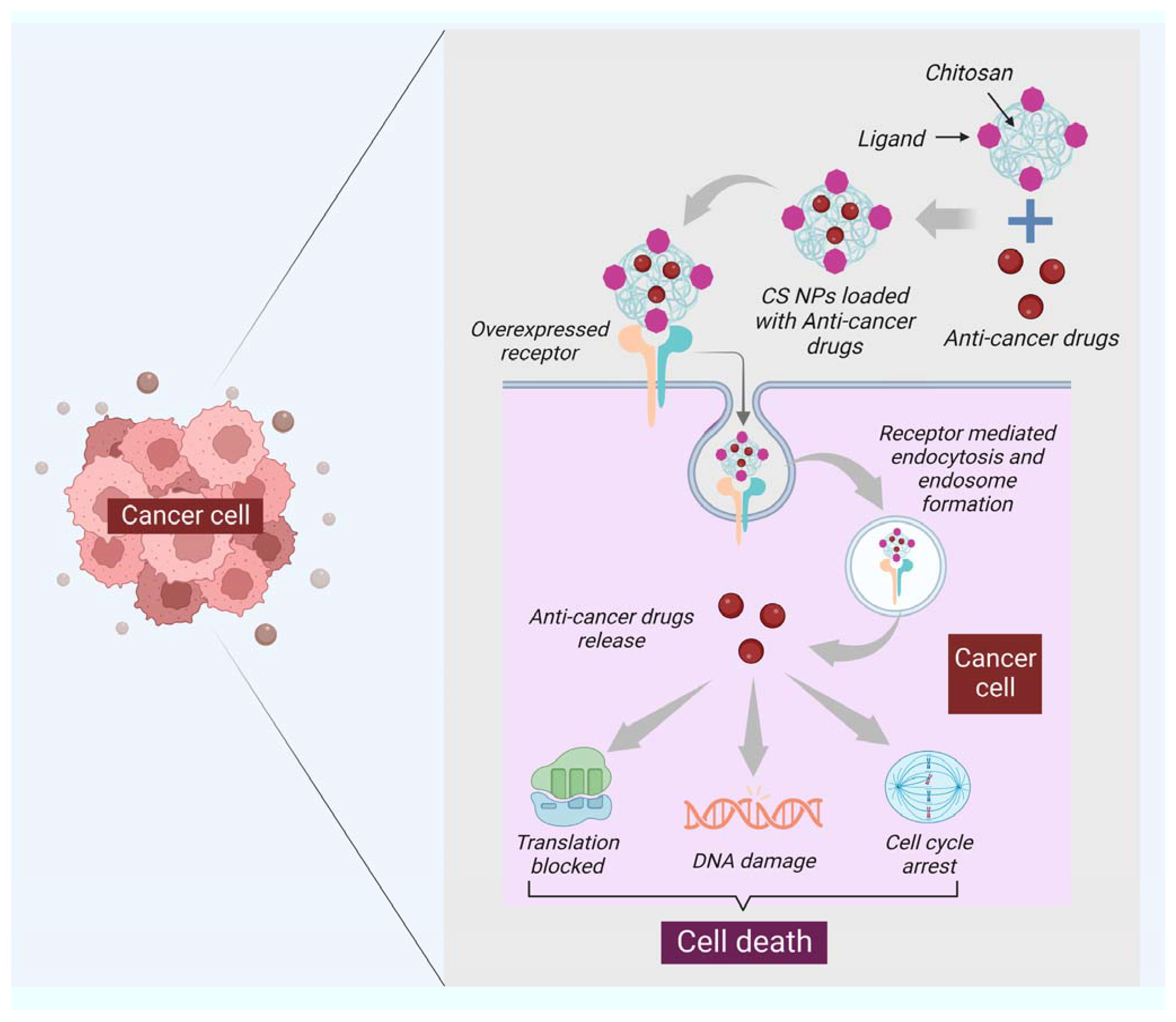
Moreover, chitin has a size-dependent and complex effect on adaptative and innate immune response, including the capability to activate and recruit innate immune cells, which stimulates chemokine and cytokine production [99]. It has been seen that IL-12 is an antitumor cytokine that induces toxicity upon systematic administration. IL-12 can be formulated with chitosan (chitosan/IL-12) and administrated (intratumorally) in tumor mice model, could help in limiting the systematic toxicity by enhancing the local retention in the tumor microenvironment of the IL-12 [100]. Some of the chitin and chitosan-based nanocomposites for drug delivery and treatment of various types of cancer have been mentioned in (Table 2). Moreover, the delivery of most of the NPs or nanocarriers, including chitin and chitosan, is of two types: the passive targeting of NPs for drug delivery and the active targeting by NPs for drug delivery. The passive drug delivery of chitosan for cancer treatment is explained in (Figure 3) and the active drug delivery of chitosan for the treatment of cancer is explained in (Figure 4).

Table 2.
Shows results of experiments performed using chitosan and chitin biopolymers and conjugating or loading them with different types of chemical compounds. The result shows that several conjugated/loaded or encapsulated chemical compounds with chitosan or chitin can be a potential drug for treating various types of cancer and tumor.
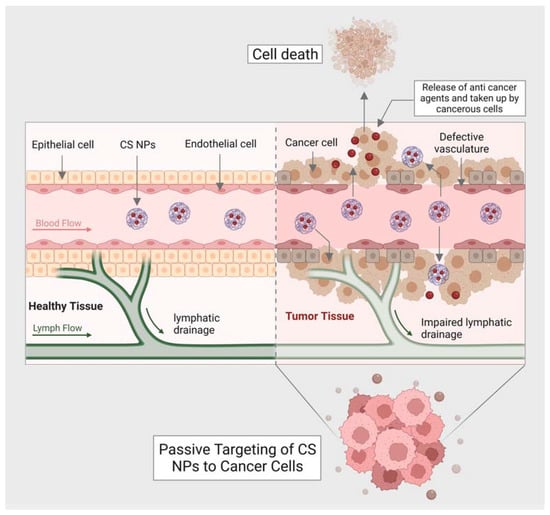
Figure 3.
Shows passive targeting of CS nanocarriers for drug delivery against cancer cells.
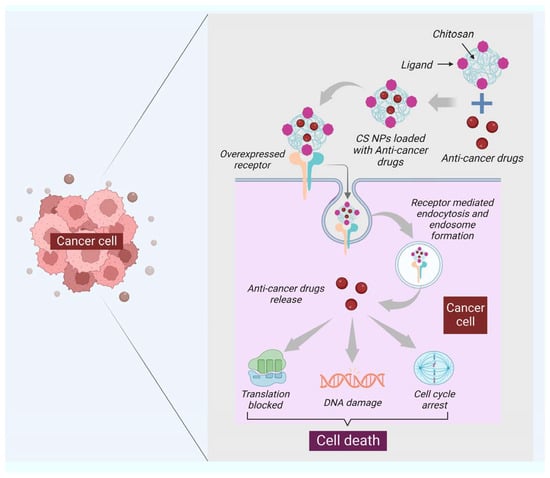
Figure 4.
The functionalization of the surface of NPs specific to the ligand is one of the critical features of active targeting. The ligand selected should be specific to the overexpressed receptor at the surface of cancer cells. The figure shows that the ligand of chitosan nanocarriers binds to the overexpressed receptor of cancer cells; after binding, the receptor-mediated endocytosis takes place, leading to the formation of the endosome, and then the dependent release of drugs will happen. Once the pill is released, the cell will proceed under apoptosis through DNA damage, translation block, and cell cycle arrest.
One of the critical findings of passive targeting is the leaky vasculature or lymphatic drainage of the tumors. This finding has led to a significant conception of EPR (enhanced permeability retention) effect. A neovasculature system is formed by metabolic cancer cells in which the blood vessels are irregular and leaky (exhibit inter-endothelial gaps) compared to healthy vessels. The inter-endothelial gaps help transport nanocarriers and NPs through the formation of paracellular pathways. The tumor cell endothelium has a concentration gradient that further facilitates the accumulation of NPs or, in this case, anticancer agents released by CS nanocarriers into the tumor. Once the anticancer agents are taken by cancer cells, the cell will go under apoptosis. The concentration, size of NPs (less than the size of inter-endothelial gaps of vessels), and blood circulation are the most crucial features responsible for passive targeting.
6. Advantages of Using Chitin and Chitosan in Nanomedicine
6.1. Biocompatibility
Chitin and chitosan can be utilized effectively in the human body without having any negative effects because they are biodegradable and biocompatible. They are, therefore, suitable for a range of biomedical applications, such as wound healing, tissue engineering and medication delivery. Chitin and chitosan have been utilized to create polymer scaffolds. Furthermore, there is growing interest in using chitosan to create nanocarriers and facilitate microencapsulation techniques for the transport of medications, biologics, and vaccines [32,130]. The chitin and chitosan are useful as they can be created as chitin or chitosan-based nano- and micro-particles with certain sizes and cargo-release properties [130].
6.2. Antimicrobial Characteristics
Research has revealed that chitin and chitosan exhibit antimicrobial action against a variety of pathogens, including fungi, bacteria, and viruses. Due to this characteristic, they can be used to create antimicrobial coats for medical equipment and to treat infectious conditions [131,132,133]. Moreover, chitosan films have also been employed as a packaging material to maintain the quality of a wide range of food items [134]. Chitosan exhibits strong antibacterial properties against both Gram-negative and Gram-positive bacteria, fungus, and other pathogenic and spoilage micro-organisms [135,136].
6.3. Mucoadhesive Characteristics
Chitosan and chitin contain mucoadhesive capabilities, which means they could cling to mucosal membranes that are found in the nose, mouth, and gastrointestinal system. Chitosan and chitin can be developed into medication delivery systems for oral and nasal administration because of this characteristic. The use of chitin and chitosan as the vehicles for mucoadhesive system drug delivery has a significant impact that further emphasizes the potential advantages of increased therapeutic agent bioavailability, prolonged drug residence time at the site of administration, and comparatively quicker drug absorption into the systemic circulation [137].
6.4. Biodegradability
Chitin and chitosan can be converted into non-toxic chemicals by the body’s natural mechanisms since they are biodegradable. They are the perfect choice for use in drug delivery systems that call for a sustained drug release over a long timeframe because of this attribute [9]. According to current research, lysozyme and bacterial enzymes in the colon are the main degraders of chitosan in vertebrates [138]. Many different microbes produce and/or breakdown chitin [139].
7. Problematics of Chitin and Chitosan in Nanomedicine
7.1. Allergenicity
It has been demonstrated that chitin and chitosan can cause allergic reactions in some people by inducing an immunological response. Therefore, its usage in some biological applications may be constrained by this fact. Unfortunately, chitin-chitinase-stimulated hypersensitivity is a common cause of occupational allergy. Moreover, current research has studied the immunologic effects of chitin both in vivo and in vitro, and these investigations have shown new facets of how chitin regulates innate and adaptive immune responses. It has been demonstrated that exogenous chitin controls adaptive type 2 allergic inflammation in addition to activating macrophages and other innate immune cells. These results further show that chitin interacts with many cell surface receptors, including the macrophage mannose receptor, to activate macrophages [140].
7.2. Limited Solubility
Chitin and chitosan’s utility in various applications may be restricted by their inability to dissolve in neutral pH water. Yet, by altering their chemical makeup or using the right solvents, solubility can be increased. The degree of acetylation, pH, temperature, and polymer crystallinity are some of the variables that affect how soluble chitosan is [141]. The lower solubility of chitosan was attributed to the polymer’s increased crystallinity following deacetylation, which counterbalances the effect of the polymer’s increased glucosamine moieties. On the other hand, the half-acetylated sample showed a decrease in crystallinity. The solubility window of chitosan is also changed by the application of hydrogen bond disruptors such as urea or guanidine hydrochloride. In actuality, wide solubility is accomplished by combining chemical and physical disruption of the hydrogen bonds [142].
7.3. Variability from Batch to Batch
Depending on the source and preparation techniques used to create chitin and chitosan, its characteristics can change. This may result in batch-to-batch variability, which may have an impact on some applications’ ability to reproduce and maintain consistency in their results [143].
7.4. Limited Stability
Recently, a lot of studies have been put into creating reliable and safe chitosan products. Unfortunately, the issue of chitosan-based systems’ weak stability limits their practical applicability; as a result, it has become extremely difficult to produce chitosan formulations’ adequate shelf-life [141,143]. The degree of chitosan purity has a significant impact on the substance’s solubility and stability in addition to its biological characteristics such as immunogenicity or biodegradability. Moreover, chitosan’s stability is affected by a number of variables, including the degree of deacetylation, moisture content, and molecular weight. Similarly, the stability of chitin is also limited; however, cross-linking chitin with enzymes or other chemical compounds can help in the upgradation of the stability of chitin [141].
8. Challenges and Future Perspectives
The use of chitosan-based medication carriers for cancer treatment is becoming more popular. The chitosan-based nanocarriers appear to hold promise for clinical translation. Chitosan nanoparticles are drug carriers with good biocompatibility and can be easily modified during synthesis [31]. One of the most significant obstacles to chitosan nanoparticle clinical application is its insufficient hemocompatibility. Several studies have shown that chitosan has hemostatic action. Although animal studies have indicated that chitosan-based nanoparticles are tolerable in intravenous injections, deadly emboli formation is dangerous. Off-target distribution of chemotherapeutic drug-loaded chitosan nanocarriers may also represent a clinical translational issue. The reticuloendothelial system, which circulates throughout the body, easily phagocytoses these carriers [104]. Its pharmacological cargo may be delivered to multiple organs during apoptosis. Although the FDA has approved chitosan for oral medicine delivery and wound healing, drug compositions with chemical changes of chitosan may cause in vivo toxicity [144]. Various other pharmaceutical applications have also been cited with chitosan nanoparticles which improve the physicochemical properties of active medicinal agents [39]. As a result, safety assessments for formulating chitosan-based drug carriers and evaluation of their nanotheranostic platforms are critical [145]. Despite these obstacles, chitosan has significant potential in cancer treatment. Current restrictions will be addressed when new technologies emerge and an understanding of the mechanism of action of chitosan-based drug delivery carriers evolves.
9. Conclusions
Cancer is a group of diseases characterized by abnormal cell proliferation and the ability to spread to other body parts, in contrast to benign tumors, which remain stationary. Possible warning signs of cancer include a lump, unusual bleeding, a persistent cough, weight loss, and a change in bowel habits. While these symptoms may indicate the presence of cancer, there could also be other causes. Over 100 different types of cancer can affect humans, and various therapeutic strategies are available for their management and treatment, including the use of nanoparticles. Chitin and chitosan are polymeric nanocarriers that can assist in the delivery of anticancer agents and inhibit the growth of cancer cells. These biopolymers have shown promise in drug delivery due to their biodegradability, biocompatibility, and low toxicity. Chitosan, in particular, has attracted attention as a potential carrier for anticancer drugs due to its ability to target cancer cells and enhance drug efficacy. However, further research is needed to explore the full potential of chitin and chitosan in cancer treatment.
In conclusion, chitin and chitosan biopolymers have emerged as promising materials for drug delivery in cancer treatment. These polysaccharides have demonstrated favorable properties, including biodegradability, biocompatibility, and low toxicity, making them ideal candidates for use as drug carriers. Moreover, chitosan, in particular, has been shown to have the ability to target cancer cells and enhance drug efficacy, indicating its potential in the development of anticancer therapeutics. However, several challenges must be overcome before these biopolymers can be successfully used in clinical settings. For instance, optimization of the drug loading and release properties of chitin and chitosan carriers is crucial to ensure their effectiveness in delivering therapeutic agents to cancer cells. Further studies are also needed to elucidate the mechanisms underlying their antitumor effects and identify any potential adverse effects. Despite these challenges, chitin and chitosan biopolymers hold significant promise in the development of more effective and safer anticancer drugs. With continued research and innovation, these biopolymers may become an essential tool in the fight against cancer.
Author Contributions
Conceptualization, B.S., P.S., J.K.S. and N.K.J.; methodology, B.S.; validation, S.G., S.H. and S.D.; formal analysis, S.K.J. and A.N.; investigation, A.N. and R.B.; resources, J.K.S. and K.K.K.; writing—original draft preparation, P.S., R.B. and J.K.S.; artwork, N.K.J. and P.S.; writing—review and editing, B.S., P.S., A.N., S.G., J.K.S., A.C.P.-S., N.K.J. and K.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This is a review and the majority of the article’s references are cited appropriately in the manuscript.
Acknowledgments
The authors would like to thank their respective institutes for their support. We would like to thank BioRender (BioRender.com) for designing figures incorporated in the manuscript. J.K.S. and S.G. acknowledge support from International Brain Research Organization (IBRO) and Indian Council of Medical Research (ICMR).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| NPs | Nanoparticles |
| CS | Chitosan |
| MWI | Microwave irradiation |
| BBB | Blood–brain barrier |
| BCB | Blood–cerebrospinal fluid barrier |
| NTB | Nose to the brain |
| HAS | Human serum albumin |
| RH | Ropinirole hydrochloride |
| DOPA | Dopamine |
| PD | Parkinson’s disease |
| AD | Alzheimer’s disease |
| PLGA | poly(lactic-co-glycolic acid |
| SNES | Simulated nasal electrolyte solution |
| CNTs | Carbon nanotubes |
| CaNPs | Calcium Nanoparticles |
| CHI3L 1 | Chitinase-3-like protein-1 |
| VEGF-C | Vascular endothelial growth factor |
| CCNGs | Curcumin loaded chitin nanogels |
| EPR | Enhanced permeability retention |
| PDMSCs | Placental-derived mesenchymal stem cells |
| TNBC | Triple-negative breast cancer |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| HA | Hyaluronic acid |
| Cy3 | Cyanine 3 |
| BCL2 | B-cell lymphoma 2 |
| DOX | Doxorubicin |
| AMP | 2-acrylamide-2-methylpropane sulphonic acid |
| SiRNA | Small interfering RNA |
| OQC | Octadecyl quaternized carboxymethyl chitosan |
| FPNs | Folate-targeted chitosan polymeric nanoparticles |
| CMC | Carboxymethyl chitosan |
| sGNPs | Small gold nanoparticles |
| FITC | Fluorescein isothiocyanate |
| CHC | Chitosan hydrochloride |
| OXPt | Oxaliplatin |
| PEG | Poly(ethylene glycol) |
| Ad | Adenoviral |
| FA | Folic acid |
| TDPA | Thiodipropionic acid |
| PBAP | Phenyl boronic acid pinacol ester |
| PDT | Photodynamic therapy |
| LMW | Low molecular weight |
| siRNA | Small interfering RNA |
| OVCAR | Ovarian adenocarcinoma |
References
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in Chitin Analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Machałowski, T.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Galli, R.; Ziętek, J.; Pantović, S.; Voronkina, A.; Kovalchuk, V.; et al. 3D Chitin Scaffolds of Marine Demosponge Origin for Biomimetic Mollusk Hemolymph-Associated Biomineralization Ex-Vivo. Mar. Drugs 2020, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel Chitin Scaffolds Derived from Marine Sponge Ianthella Basta for Tissue Engineering Approaches Based on Human Mesenchymal Stromal Cells: Biocompatibility and Cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Petrenko, I.; Bazhenov, V.V.; Galli, R.; Wysokowski, M.; Fromont, J.; Schupp, P.J.; Stelling, A.L.; Niederschlag, E.; Stöker, H.; Kutsova, V.Z.; et al. Chitin of Poriferan Origin and the Bioelectrometallurgy of Copper/Copper Oxide. Int. J. Biol. Macromol. 2017, 104, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Moussian, B. Chitin: Structure, Chemistry and Biology. In Targeting Chitin-Containing Organisms; Yang, Q., Fukamizo, T., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1142, pp. 5–18. ISBN 9789811373176. [Google Scholar]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On Chemistry of γ-Chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Hou, J.; Aydemir, B.E.; Dumanli, A.G. Understanding the Structural Diversity of Chitins as a Versatile Biomaterial. Philos. Trans. A Math. Phys. Eng. Sci. 2021, 379, 20200331. [Google Scholar] [CrossRef]
- Singh, N.; Chen, J.; Koziol, K.K.; Hallam, K.R.; Janas, D.; Patil, A.J.; Strachan, A.; Hanley, J.G.; Rahatekar, S.S. Chitin and Carbon Nanotube Composites as Biocompatible Scaffolds for Neuron Growth. Nanoscale 2016, 8, 8288–8299. [Google Scholar] [CrossRef]
- Ji, X.; Lei, Z.; Yuan, M.; Zhu, H.; Yuan, X.; Liu, W.; Pu, H.; Jiang, J.; Zhang, Y.; Jiang, X.; et al. Cartilage Repair Mediated by Thermosensitive Photocrosslinkable TGFβ1-Loaded GM-HPCH via Immunomodulating Macrophages, Recruiting MSCs and Promoting Chondrogenesis. Theranostics 2020, 10, 2872–2887. [Google Scholar] [CrossRef]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meißner, H.; Lode, A.; et al. 3D Chitinous Scaffolds Derived from Cultivated Marine Demosponge Aplysina Aerophoba for Tissue Engineering Approaches Based on Human Mesenchymal Stromal Cells. Int. J. Biol. Macromol. 2017, 104, 1966–1974. [Google Scholar] [CrossRef]
- Lomiguen, C.; Vidal, L.; Kozlowski, P.; Prancan, A.; Stern, R. Possible Role of Chitin-Like Proteins in the Etiology of Alzheimer’s Disease. JAD 2018, 66, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Perry, G.; Smith, M.A. The Role of Novel Chitin-like Polysaccharides in Alzheimer Disease. Neurotox. Res. 2007, 12, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.; Siedlak, S.; Fortino, A.; Perry, G.; Ghetti, B.; Smith, M. Chitin-like Polysaccharides in Alzheimers Disease Brains. CAR 2005, 2, 419–423. [Google Scholar] [CrossRef]
- Pisa, D.; Alonso, R.; Rábano, A.; Horst, M.N.; Carrasco, L. Fungal Enolase, β-Tubulin, and Chitin Are Detected in Brain Tissue from Alzheimer’s Disease Patients. Front. Microbiol. 2016, 7, 1772. [Google Scholar] [CrossRef]
- Sachdeva, B.; Sachdeva, P. MXenes for Neurodegenerative Disorders. Mater. Today Proc. 2022, 73, 294–296. [Google Scholar] [CrossRef]
- Ahmad, F.; Sachdeva, P. A Consolidated Review on Stem Cell Therapy for Treatment and Management of Alzheimer’s Disease. Aging Med. 2022, 5, 182–190. [Google Scholar] [CrossRef]
- Ahmad, F.; Sachdeva, P. Critical Appraisal on Mitochondrial Dysfunction in Alzheimer’s Disease. Aging Med. 2022, 5, 272–280. [Google Scholar] [CrossRef]
- Ghosh, S.; Sachdeva, B.; Sachdeva, P.; Chaudhary, V.; Rani, G.M.; Sinha, J.K. Graphene Quantum Dots as a Potential Diagnostic and Therapeutic Tool for the Management of Alzheimer’s Disease. Carbon. Lett. 2022, 32, 1381–1394. [Google Scholar] [CrossRef]
- Ahmad, F.; Sachdeva, P.; Sarkar, J.; Izhaar, R. Circadian Dysfunction and Alzheimer’s Disease—An Updated Review. Aging Med. 2022, 6, 71–81. [Google Scholar] [CrossRef]
- Ahmad, F.; Sachdeva, P.; Sachdeva, B.; Singh, G.; Soni, H.; Tandon, S.; Rafeeq, M.M.; Alam, M.Z.; Baeissa, H.M.; Khalid, M. Dioxinodehydroeckol: A Potential Neuroprotective Marine Compound Identified by In Silico Screening for the Treatment and Management of Multiple Brain Disorders. Mol. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Mukerjee, N.; Al-Khafaji, K.; Maitra, S.; Suhail Wadi, J.; Sachdeva, P.; Ghosh, A.; Buchade, R.S.; Chaudhari, S.Y.; Jadhav, S.B.; Das, P.; et al. Recognizing Novel Drugs against Keap1 in Alzheimer’s Disease Using Machine Learning Grounded Computational Studies. Front. Mol. Neurosci. 2022, 15, 1036552. [Google Scholar] [CrossRef] [PubMed]
- Madar, I.H.; Sultan, G.; Tayubi, I.A.; Hasan, A.N.; Pahi, B.; Rai, A.; Sivanandan, P.K.; Loganathan, T.; Begum, M.; Rai, S. Identification of Marker Genes in Alzheimer’s Disease Using a Machine-Learning Model. Bioinformation 2021, 17, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Mittal, A.K.; Kalonia, H.; Madan, S.; Ghosh, S.; Sinha, J.K.; Rajput, S.K. SIRT1 Promotes Neuronal Fortification in Neurodegenerative Diseases through Attenuation of Pathological Hallmarks and Enhancement of Cellular Lifespan. Curr. Neuropharmacol. 2021, 19, 1019–1037. [Google Scholar] [CrossRef]
- Sachdeva, B.; Sachdeva, P.; Ghosh, S.; Ahmad, F.; Sinha, J.K. Ketamine as a Therapeutic Agent in Major Depressive Disorder and Posttraumatic Stress Disorder: Potential Medicinal and Deleterious Effects. Ibrain 2023, 9, 90–101. [Google Scholar] [CrossRef]
- Sinha, J.K.; Ghosh, S.; Raghunath, M. DNA Damage in Brain May Lead to Cognitive Dysfunctions and Reduced Longevity in WNIN/Ob Obese Rats. Alzheimer’s Dement. 2021, 17, e057963. [Google Scholar] [CrossRef]
- Ghosh, S.; Durgvanshi, S.; Agarwal, S.; Raghunath, M.; Sinha, J.K. Current Status of Drug Targets and Emerging Therapeutic Strategies in the Management of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 883–903. [Google Scholar] [CrossRef]
- Sotgiu, S.; Musumeci, S.; Marconi, S.; Gini, B.; Bonetti, B. Different Content of Chitin-like Polysaccharides in Multiple Sclerosis and Alzheimer’s Disease Brains. J. Neuroimmunol. 2008, 197, 70–73. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan Nanoparticles as a Promising Tool in Nanomedicine with Particular Emphasis on Oncological Treatment. Cancer Cell. Int. 2021, 21, 318. [Google Scholar] [CrossRef]
- Mohammed, M.; Syeda, J.; Wasan, K.; Wasan, E. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Argüelles-Monal, W.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.; Montiel-Herrera, M. Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials. Polymers 2018, 10, 342. [Google Scholar] [CrossRef]
- Parhi, R. Drug Delivery Applications of Chitin and Chitosan: A Review. Env. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Petrova, V.A.; Romanov, D.P.; Skorik, Y.A. PH-Sensitive Drug Delivery System Based on Chitin Nanowhiskers–Sodium Alginate Polyelectrolyte Complex. Materials 2022, 15, 5860. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Zainol Abidin, N.A.; Kormin, F.; Zainol Abidin, N.A.; Mohamed Anuar, N.A.F.; Abu Bakar, M.F. The Potential of Insects as Alternative Sources of Chitin: An Overview on the Chemical Method of Extraction from Various Sources. Int. J. Mol. Sci. 2020, 21, 4978. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; Kesari, K.K. Chitosan Nanoparticle Encapsulation of Antibacterial Essential Oils. Micromachines 2022, 13, 1265. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Özel, N.; Elibol, M. A Review on the Potential Uses of Deep Eutectic Solvents in Chitin and Chitosan Related Processes. Carbohydr. Polym. 2021, 262, 117942. [Google Scholar] [CrossRef]
- No, H.K.; Hur, E.Y. Control of Foam Formation by Antifoam during Demineralization of Crustacean Shell in Preparation of Chitin. J. Agric. Food Chem. 1998, 46, 3844–3846. [Google Scholar] [CrossRef]
- Percot, A.; Viton, C.; Domard, A. Characterization of Shrimp Shell Deproteinization. Biomacromolecules 2003, 4, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Synowiecki, J. Isolation and Characterization of Nutrients and Value-Added Products from Snow Crab (Chionoecetes opilio) and Shrimp (Pandalus borealis) Processing Discards. J. Agric. Food Chem. 1991, 39, 1527–1532. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Saber, W.I.A.; Zweil, A.M.; Bashir, S.I. An Innovative Green Synthesis Approach of Chitosan Nanoparticles and Their Inhibitory Activity against Phytopathogenic Botrytis Cinerea on Strawberry Leaves. Sci. Rep. 2022, 12, 3515. [Google Scholar] [CrossRef] [PubMed]
- Synowiecki, J.; Al-Khateeb, N.A. Production, Properties, and Some New Applications of Chitin and Its Derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Extraction of a Novel Fungal Chitin from Hericium Erinaceus Residue Using Multistep Mild Procedures. Int. J. Biol. Macromol. 2020, 156, 1279–1286. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Schimpf, C.; Rafaja, D.; Brendler, E.; Viehweger, C.; Żółtowska-Aksamitowska, S.; Petrenko, I.; et al. Spider Chitin: An Ultrafast Microwave-Assisted Method for Chitin Isolation from Caribena Versicolor Spider Molt Cuticle. Molecules 2019, 24, 3736. [Google Scholar] [CrossRef]
- Rao, M.B.; Tanksale, A.M.; Ghatge, M.S.; Deshpande, V.V. Molecular and Biotechnological Aspects of Microbial Proteases. Microbiol. Mol. Biol. Rev. 1998, 62, 597–635. [Google Scholar] [CrossRef]
- Smolen, V.F. Bioavailability and Pharmacokinetic Analysis of Drug Responding Systems. Annu. Rev. Pharmacol. Toxicol. 1978, 18, 495–522. [Google Scholar] [CrossRef]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug Delivery Systems: Advanced Technologies Potentially Applicable in Personalized Treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M. Review Paper: Chitosan Derivatives as Promising Materials for Controlled Drug Delivery. J. Biomater. Appl. 2008, 23, 5–36. [Google Scholar] [CrossRef]
- Sabitha, M.; Sanoj Rejinold, N.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Development and Evaluation of 5-Fluorouracil Loaded Chitin Nanogels for Treatment of Skin Cancer. Carbohydr. Polym. 2013, 91, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Panonnummal, R.; Jayakumar, R.; Sabitha, M. Comparative Anti-Psoriatic Efficacy Studies of Clobetasol Loaded Chitin Nanogel and Marketed Cream. Eur. J. Pharm. Sci. 2017, 96, 193–206. [Google Scholar] [CrossRef]
- Gupta, H.; Velpandian, T.; Jain, S. Ion- and PH-Activated Novel in-Situ Gel System for Sustained Ocular Drug Delivery. J. Drug. Target. 2010, 18, 499–505. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and Evaluation of Naringenin-Loaded Sulfobutylether-β-Cyclodextrin/Chitosan Nanoparticles for Ocular Drug Delivery. Carbohydr. Polym. 2016, 149, 224–230. [Google Scholar] [CrossRef]
- Santhi, K.; Muralidharan, S.; Yee, Y.H.; Min, F.M.; Ting, C.Z.; Devi, D. In-Vitro Characterization of Chitosan Nanoparticles of Fluconazole as a Carrier for Sustained Ocular Delivery. NANOASIA 2017, 7, 41–50. [Google Scholar] [CrossRef]
- Ruge, C.A.; Kirch, J.; Lehr, C.-M. Pulmonary Drug Delivery: From Generating Aerosols to Overcoming Biological Barriers—Therapeutic Possibilities and Technological Challenges. Lancet Respir. Med. 2013, 1, 402–413. [Google Scholar] [CrossRef]
- Rawal, T.; Parmar, R.; Tyagi, R.K.; Butani, S. Rifampicin Loaded Chitosan Nanoparticle Dry Powder Presents an Improved Therapeutic Approach for Alveolar Tuberculosis. Coll. Surf. B Biointerfaces 2017, 154, 321–330. [Google Scholar] [CrossRef]
- Debnath, S.K.; Saisivam, S.; Debanth, M.; Omri, A. Development and Evaluation of Chitosan Nanoparticles Based Dry Powder Inhalation Formulations of Prothionamide. PLoS ONE 2018, 13, e0190976. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Gilani, K.; Moazeni, E.; Ghazi-Khansari, M.; Najafabadi, A.R.; Mohajel, N. Development of Chitosan-Based Nanoparticles for Pulmonary Delivery of Itraconazole as Dry Powder Formulation. Powder Technol. 2012, 222, 65–70. [Google Scholar] [CrossRef]
- Wang, J.; Tauchi, Y.; Deguchi, Y.; Morimoto, K.; Tabata, Y.; Ikada, Y. Positively Charged Gelatin Microspheres as Gastric Mucoadhesive Drug Delivery System for Eradication of H. pylori. Drug. Deliv. 2000, 7, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Dodane, V. Effect of Chitosan on Epithelial Permeability and Structure. Int. J. Pharm. 1999, 182, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Piazzini, V.; Landucci, E.; D’Ambrosio, M.; Tiozzo Fasiolo, L.; Cinci, L.; Colombo, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Luceri, C.; Bergonzi, M.C. Chitosan Coated Human Serum Albumin Nanoparticles: A Promising Strategy for Nose-to-Brain Drug Delivery. Int. J. Biol. Macromol. 2019, 129, 267–280. [Google Scholar] [CrossRef]
- Chatzitaki, A.-T.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.G.; Borges, O. Chitosan-Coated PLGA Nanoparticles for the Nasal Delivery of Ropinirole Hydrochloride: In Vitro and Ex Vivo Evaluation of Efficacy and Safety. Int. J. Pharm. 2020, 589, 119776. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Guy, R.H. Transdermal Drug Delivery. In Drug. Delivery; Schäfer-Korting, M., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 197, pp. 399–410. ISBN 978-3-642-00476-6. [Google Scholar]
- Nair, S.S. Chitosan-Based Transdermal Drug Delivery Systems to Overcome Skin Barrier Functions. J. Drug. Deliv. Ther. 2019, 9, 266–270. [Google Scholar] [CrossRef]
- Nawaz, A.; Wong, T.W. Microwave as Skin Permeation Enhancer for Transdermal Drug Delivery of Chitosan-5-Fluorouracil Nanoparticles. Carbohydr. Polym. 2017, 157, 906–919. [Google Scholar] [CrossRef]
- Abd-Allah, H.; Abdel-Aziz, R.T.A.; Nasr, M. Chitosan Nanoparticles Making Their Way to Clinical Practice: A Feasibility Study on Their Topical Use for Acne Treatment. Int. J. Biol. Macromol. 2020, 156, 262–270. [Google Scholar] [CrossRef]
- Daeschlein, G. Antimicrobial and Antiseptic Strategies in Wound Management. Int. Wound J. 2013, 10 (Suppl. S1), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound Healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a Starting Material for Wound Healing Applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-Rad, E.; Moslehi, M.; Ganji, A. Wound Healing Performance of PCL/Chitosan Based Electrospun Nanofiber Electrosprayed with Curcumin Loaded Chitosan Nanoparticles. Carbohydr. Polym. 2021, 259, 117640. [Google Scholar] [CrossRef]
- Sachdeva, P.; Ghosh, S.; Ghosh, S.; Han, S.; Banerjee, J.; Bhaskar, R.; Sinha, J.K. Childhood Obesity: A Potential Key Factor in the Development of Glioblastoma Multiforme. Life 2022, 12, 1673. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, Cell Cycle and Apoptosis in Cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Jolly, R.D.; Thompson, K.G.; Winchester, B.G. Bovine Mannosidosis--a Model Lysosomal Storage Disease. Birth Defects Orig. Artic. Ser. 1975, 11, 273–278. [Google Scholar]
- Koo, M.M.; Swann, R.; McPhail, S.; Abel, G.A.; Elliss-Brookes, L.; Rubin, G.P.; Lyratzopoulos, G. Presenting Symptoms of Cancer and Stage at Diagnosis: Evidence from a Cross-Sectional, Population-Based Study. Lancet Oncol. 2020, 21, 73–79. [Google Scholar] [CrossRef]
- Wu, L.; Qu, X. Cancer Biomarker Detection: Recent Achievements and Challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Wyld, L.; Audisio, R.A.; Poston, G.J. The Evolution of Cancer Surgery and Future Perspectives. Nat. Rev. Clin. Oncol. 2015, 12, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-Infiltrating Lymphocytes in the Immunotherapy Era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T. New Chemotherapy Strategies for Gastric Cancer. In Vivo 2008, 22, 273–278. [Google Scholar]
- Nasir, A.; Khan, A.; Li, J.; Naeem, M.; Khalil, A.A.K.; Khan, K.; Qasim, M. Nanotechnology, A Tool for Diagnostics and Treatment of Cancer. Curr. Top. Med. Chem. 2021, 21, 1360–1376. [Google Scholar] [CrossRef]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.-J.; He, C.-H.; Takyar, S.; Elias, J.A. Role of Chitin and Chitinase/Chitinase-Like Proteins in Inflammation, Tissue Remodeling, and Injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef]
- Timoshenko, A.V. Chitin Hydrolysate Stimulates VEGF-C Synthesis by MDA-MB-231 Breast Cancer Cells. Cell. Biol. Int. 2011, 35, 281–286. [Google Scholar] [CrossRef]
- Solairaj, D.; Rameshthangam, P.; Arunachalam, G. Anticancer Activity of Silver and Copper Embedded Chitin Nanocomposites against Human Breast Cancer (MCF-7) Cells. Int. J. Biol. Macromol. 2017, 105, 608–619. [Google Scholar] [CrossRef]
- Sachdeva, P.; Mehdi, I.; Kaith, R.; Ahmad, F.; Anwar, M.S. Potential Natural Products for the Management of Autism Spectrum Disorder. Ibrain 2022, 8, 365–376. [Google Scholar] [CrossRef]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Curcumin Loaded Chitin Nanogels for Skin Cancer Treatment via the Transdermal Route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Min, M.; Du, N.; Gu, Y.; Hode, T.; Naylor, M.; Chen, D.; Nordquist, R.E.; Chen, W.R. Chitin, Chitosan, and Glycated Chitosan Regulate Immune Responses: The Novel Adjuvants for Cancer Vaccine. Clin. Dev. Immunol. 2013, 2013, 387023. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.A.; Notananda, V.; Schepens, J. Studies on Malaria and Responses of Anopheles Balabacensis Balabacensis and Anopheles Minimus to DDT Residual Spraying in Thailand. Acta Trop. 1975, 32, 206–231. [Google Scholar]
- SUZUKI, K.; OKAWA, Y.; HASHIMOTO, K.; SUZUKI, S.; SUZUKI, M. Protecting Effect of Chitin and Chitosan on Experimentally Induced Murine Candidiasis. Microbiol. Immunol. 1984, 28, 903–912. [Google Scholar] [CrossRef] [PubMed]
- van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan for Mucosal Vaccination. Adv. Drug Deliv. Rev. 2001, 52, 139–144. [Google Scholar] [CrossRef]
- McNeela, E.A.; Jabbal-Gill, I.; Illum, L.; Pizza, M.; Rappuoli, R.; Podda, A.; Lewis, D.J.M.; Mills, K.H.G. Intranasal Immunization with Genetically Detoxified Diphtheria Toxin Induces T Cell Responses in Humans: Enhancement of Th2 Responses and Toxin-Neutralizing Antibodies by Formulation with Chitosan. Vaccine 2004, 22, 909–914. [Google Scholar] [CrossRef]
- Lee, C.G.; Da Silva, C.A.; Lee, J.-Y.; Hartl, D.; Elias, J.A. Chitin Regulation of Immune Responses: An Old Molecule with New Roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Hance, K.W.; Rogers, C.J.; Schlom, J.; Greiner, J.W. Intratumoral Immunotherapy of Established Solid Tumors with Chitosan/IL-12. J. Immunother. 2010, 33, 697–705. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Priya, K.; Ilavenil, S.; Janani, B.; Vedarethinam, V.; Ramesh, T.; Arasu, M.V.; Al-Dhabi, N.A.; Kim, Y.-O.; Kim, H.-J. Shrimp Shells Extracted Chitin in Silver Nanoparticle Synthesis: Expanding Its Prophecy towards Anticancer Activity in Human Hepatocellular Carcinoma HepG2 Cells. Int. J. Biol. Macromol. 2020, 165, 1402–1409. [Google Scholar] [CrossRef]
- Kamalabadi-Farahani, M.; Vasei, M.; Ahmadbeigi, N.; Ebrahimi-Barough, S.; Soleimani, M.; Roozafzoon, R. Anti-Tumour Effects of TRAIL-Expressing Human Placental Derived Mesenchymal Stem Cells with Curcumin-Loaded Chitosan Nanoparticles in a Mice Model of Triple Negative Breast Cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1011–1021. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Lan, Y.; He, X.; Liu, K.; Liang, Y. Antitumor Effect of Hyaluronic-Acid-Modified Chitosan Nanoparticles Loaded with SiRNA for Targeted Therapy for Non-Small Cell Lung Cancer. Int. J. Nanomed. 2019, 14, 5287–5301. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic Acid-Chitosan Nanoparticles for Co-Delivery of MiR-34a and Doxorubicin in Therapy against Triple Negative Breast Cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Kavaz, D.; Kirac, F.; Kirac, M.; Vaseashta, A. Low Releasing Mitomycin C Molecule Encapsulated with Chitosan Nanoparticles for Intravesical Installation. JBNB 2017, 08, 203–219. [Google Scholar] [CrossRef]
- Kumar, S.; Garg, P.; Pandey, S.; Kumari, M.; Hoon, S.; Jang, K.-J.; Kapavarapu, R.; Choung, P.-H.; Sobral, A.J.F.N.; Hoon Chung, J. Enhanced Chitosan-DNA Interaction by 2-Acrylamido-2-Methylpropane Coupling for an Efficient Transfection in Cancer Cells. J. Mater. Chem. B. 2015, 3, 3465–3475. [Google Scholar] [CrossRef]
- Komenek, S.; Luesakul, U.; Ekgasit, S.; Vilaivan, T.; Praphairaksit, N.; Puthong, S.; Muangsin, N. Nanogold-Gallate Chitosan-Targeted Pulmonary Delivery for Treatment of Lung Cancer. AAPS PharmSciTech 2017, 18, 1104–1115. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Song, S.; Lee, S.J.; Park, S.-G.; Kim, K.-S.; Kim, M.G.; Son, S.; Koo, H.; Kwon, I.C.; Jeong, J.H.; et al. Cancer-Targeted MDR-1 SiRNA Delivery Using Self-Cross-Linked Glycol Chitosan Nanoparticles to Overcome Drug Resistance. J. Control. Release 2015, 198, 1–9. [Google Scholar] [CrossRef]
- Sun, P.; Huang, W.; Jin, M.; Wang, Q.; Fan, B.; Kang, L.; Gao, Z. Chitosan-Based Nanoparticles for Survivin Targeted SiRNA Delivery in Breast Tumor Therapy and Preventing Its Metastasis. Int. J. Nanomed. 2016, 11, 4931–4945. [Google Scholar] [CrossRef]
- Darvishi, M.H.; Nomani, A.; Amini, M.; Shokrgozar, M.A.; Dinarvand, R. Novel Biotinylated Chitosan-Graft-Polyethyleneimine Copolymer as a Targeted Non-Viral Vector for Anti-EGF Receptor SiRNA Delivery in Cancer Cells. Int. J. Pharm. 2013, 456, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Fan, J.-C.; Le, Y.-G.; Zeng, F.; Cheng, H.; Hu, X.; Cao, J.-Q. Construction of METHFR ShRNA/5-Fluorouracil Co-Loaded Folate-Targeted Chitosan Polymeric Nanoparticles and Its Anti-Carcinoma Effect on Gastric Cells Growth. J. Nanopart. Res. 2016, 18, 105. [Google Scholar] [CrossRef]
- Soofiyani, S.R.; Hallaj-Nezhadi, S.; Lotfipour, F.; Hosseini, A.M.; Baradaran, B. Gene Therapy Based on Interleukin-12 Loaded Chitosan Nanoparticles in a Mouse Model of Fibrosarcoma. Iran. J. Basic. Med. Sci. 2016, 19, 1238–1244. [Google Scholar]
- Hemmati, K.; Ahmadi Nasab, N.; Hesaraki, S.; Nezafati, N. In Vitro Evaluation of Curcumin-Loaded Chitosan-Coated Hydroxyapatite Nanocarriers as a Potential System for Effective Treatment of Cancer. J. Biomater. Sci. Polym. Ed. 2021, 32, 1267–1287. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Mishra, N.; Khurana, B.; Kaur, S.; Arora, D. DoE Based Optimization and Development of Spray-Dried Chitosan-Coated Alginate Microparticles Loaded with Cisplatin for the Treatment of Cervical Cancer. Curr. Mol. Pharm. 2021, 14, 381–398. [Google Scholar] [CrossRef]
- Jensen, B.M.; Kühl, C.; Mølsted-Pedersen, L.; Saurbrey, N.; Fog-Pedersen, J. Preconceptional Treatment with Insulin Infusion Pumps in Insulin-Dependent Diabetic Women with Particular Reference to Prevention of Congenital Malformations. Acta Endocrinol. Suppl. 1986, 277, 81–85. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, Y.; Xu, Y.; Zhu, C.; Liu, T.; Wang, K. Chitosan Nanoparticles for Oral Photothermally Enhanced Photodynamic Therapy of Colon Cancer. Int. J. Pharm. 2020, 589, 119763. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Deng, D.; Xiao, Z.; Dong, Z.; Wang, Z.; Lei, Q.; Gao, S.; Huang, G.; Zhang, E.; et al. Fluorinated Chitosan to Enhance Transmucosal Delivery of Sonosensitizer-Conjugated Catalase for Sonodynamic Bladder Cancer Treatment Post-Intravesical Instillation. ACS Nano 2020, 14, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Pinto, M.L.; Pereira, C.L.; Serre, K.; Barbosa, M.A.; Vermaelen, K.; Gärtner, F.; Gonçalves, R.M.; De Wever, O.; Oliveira, M.J. Chitosan/γ-PGA Nanoparticles-Based Immunotherapy as Adjuvant to Radiotherapy in Breast Cancer. Biomaterials 2020, 257, 120218. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Viravaidya-Pasuwat, K. Development of Anti-HER2-Targeted Doxorubicin-Core-Shell Chitosan Nanoparticles for the Treatment of Human Breast Cancer. Int. J. Nanomed. 2019, 14, 4105–4121. [Google Scholar] [CrossRef]
- Duan, R.; Zhou, Z.; Su, G.; Liu, L.; Guan, M.; Du, B.; Zhang, Q. Chitosan-Coated Gold Nanorods for Cancer Therapy Combining Chemical and Photothermal Effects. Macromol. Biosci. 2014, 14, 1160–1169. [Google Scholar] [CrossRef]
- Yan, L.; Gao, S.; Shui, S.; Liu, S.; Qu, H.; Liu, C.; Zheng, L. Small Interfering RNA-Loaded Chitosan Hydrochloride/Carboxymethyl Chitosan Nanoparticles for Ultrasound-Triggered Release to Hamper Colorectal Cancer Growth in Vitro. Int. J. Biol. Macromol. 2020, 162, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Matos, B.N.; Pereira, M.N.; Bravo, M.D.O.; Cunha-Filho, M.; Saldanha-Araújo, F.; Gratieri, T.; Gelfuso, G.M. Chitosan Nanoparticles Loading Oxaliplatin as a Mucoadhesive Topical Treatment of Oral Tumors: Iontophoresis Further Enhances Drug Delivery Ex Vivo. Int. J. Biol. Macromol. 2020, 154, 1265–1275. [Google Scholar] [CrossRef]
- Park, Y.; Kang, E.; Kwon, O.-J.; Hwang, T.; Park, H.; Lee, J.M.; Kim, J.H.; Yun, C.-O. Ionically Crosslinked Ad/Chitosan Nanocomplexes Processed by Electrospinning for Targeted Cancer Gene Therapy. J. Control. Release 2010, 148, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, H.-S.; Kwon, J.; Oh, P.-S.; Park, H.S.; Lim, S.T.; Sohn, M.-H.; Jeong, H.-J. Chitosan-Based Hydrogel Microparticles for Treatment of Carcinoma in a Rabbit VX2 Liver Tumor Model. J. Vasc. Interv. Radiol. 2018, 29, 575–583. [Google Scholar] [CrossRef]
- Snima, K.S.; Jayakumar, R.; Unnikrishnan, A.G.; Nair, S.V.; Lakshmanan, V.-K. O-Carboxymethyl Chitosan Nanoparticles for Metformin Delivery to Pancreatic Cancer Cells. Carbohydr. Polym. 2012, 89, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Lee, N.; Kang, H.W.; Oh, J. In Vitro Study on Apoptotic Cell Death by Effective Magnetic Hyperthermia with Chitosan-Coated MnFe2O4. Nanotechnology 2016, 27, 115101. [Google Scholar] [CrossRef]
- Alkhader, E.; Billa, N.; Roberts, C.J. Mucoadhesive Chitosan-Pectinate Nanoparticles for the Delivery of Curcumin to the Colon. AAPS PharmSciTech 2017, 18, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, D.; Ranjithkumar, R.; Chandarshekar, B.; Bhuvaneshwari, V. Bio-Inspired Synthesis of Chitosan/Copper Oxide Nanocomposite Using Rutin and Their Anti-Proliferative Activity in Human Lung Cancer Cells. Int. J. Biol. Macromol. 2019, 141, 476–483. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Kim, T.; Hwang, E.J.; Kim, S.W.; Sonntag, K.-C.; Kim, D.H.; Koh, J.W. Reactive Oxygen Species-Sensitive Nanophotosensitizers of Aminophenyl Boronic Acid Pinacol Ester Conjugated Chitosan-g-Methoxy Poly(Ethylene Glycol) Copolymer for Photodynamic Treatment of Cancer. Biomed. Mater. 2020, 15, 055034. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, X.; Zhang, G.-L.; Hao, H.; Hou, H.-M.; Bi, J. Preparation of Chitosan-Cellulose-Benzyl Isothiocyanate Nanocomposite Film for Food Packaging Applications. Carbohydr. Polym. 2022, 285, 119234. [Google Scholar] [CrossRef]
- Li, Y.; Chi, Y.-Q.; Yu, C.-H.; Xie, Y.; Xia, M.-Y.; Zhang, C.-L.; Han, X.; Peng, Q. Drug-Free and Non-Crosslinked Chitosan Scaffolds with Efficient Antibacterial Activity against Both Gram-Negative and Gram-Positive Bacteria. Carbohydr. Polym. 2020, 241, 116386. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xia, W.; Jiang, Q.; Xu, Y.; Yu, P. Effect of Kojic Acid-Grafted-Chitosan Oligosaccharides as a Novel Antibacterial Agent on Cell Membrane of Gram-Positive and Gram-Negative Bacteria. J. Biosci. Bioeng. 2015, 120, 335–339. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Laffleur, F.; Hintzen, F.; Rahmat, D.; Shahnaz, G.; Millotti, G.; Bernkop-Schnürch, A. Enzymatic Degradation of Thiolated Chitosan. Drug. Dev. Ind. Pharm. 2013, 39, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, J.; Li, F.; Xiang, J. Chitin Synthesis and Degradation in Crustaceans: A Genomic View and Application. Mar. Drugs 2021, 19, 153. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Goyal, A. Chitin and Chitinase: Role in Pathogenicity, Allergenicity and Health. Int. J. Biol. Macromol. 2017, 97, 331–338. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan-a Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Filion, D.; Lavertu, M.; Buschmann, M.D. Ionization and Solubility of Chitosan Solutions Related to Thermosensitive Chitosan/Glycerol-Phosphate Systems. Biomacromolecules 2007, 8, 3224–3234. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural Differences between Chitin and Chitosan Extracted from Three Different Marine Sources. Int. J. Biol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Zhao, L.; Mehwish, H.M.; Wu, Y.; Mahmood, S. Chitosan and Its Derivatives: Synthesis, Biotechnological Applications, and Future Challenges. Appl. Microbiol. Biotechnol. 2019, 103, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Negi, A.; Bhattacharya, B.; Dey, T.; Mitra, P.; Preetam, S.; Kumar, L.; Kar, S.; Das, S.S.; Iqbal, D.; et al. Nanotheranostics to Target Antibiotic-resistant Bacteria: Strategies and Applications. OpenNano 2023, 100138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).