A Novel Carotenoid with a Unique 2,6-Cyclo-ψ-End Group, Roretziaxanthin, from the Sea Squirt Halocynthia roretzi
Abstract
1. Introduction
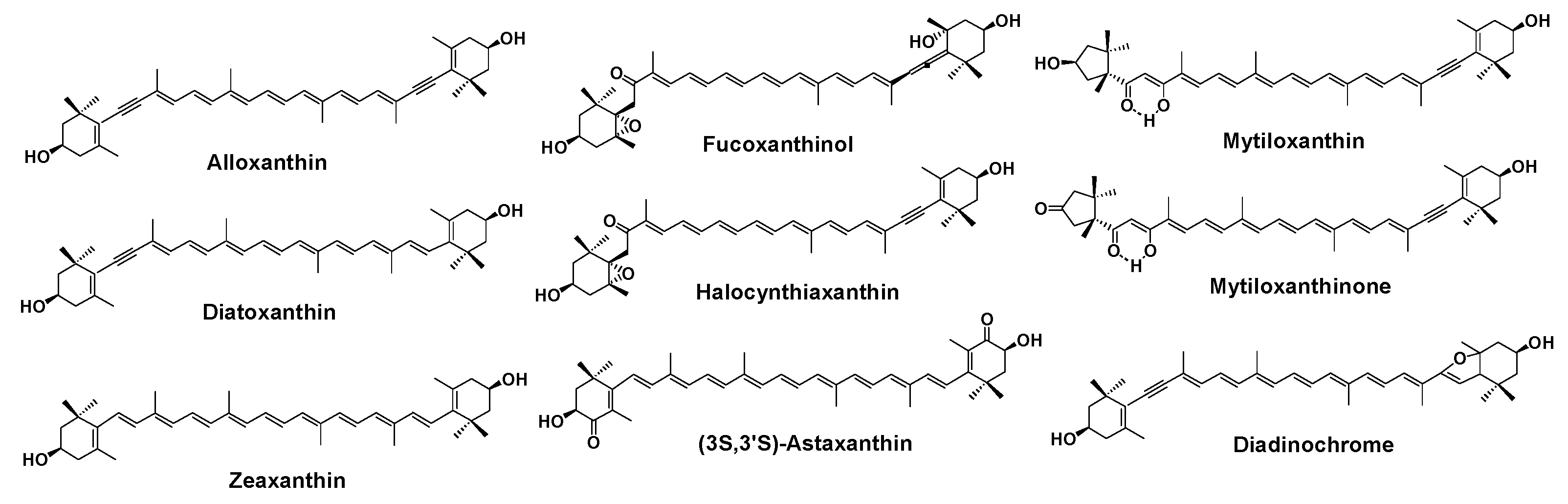
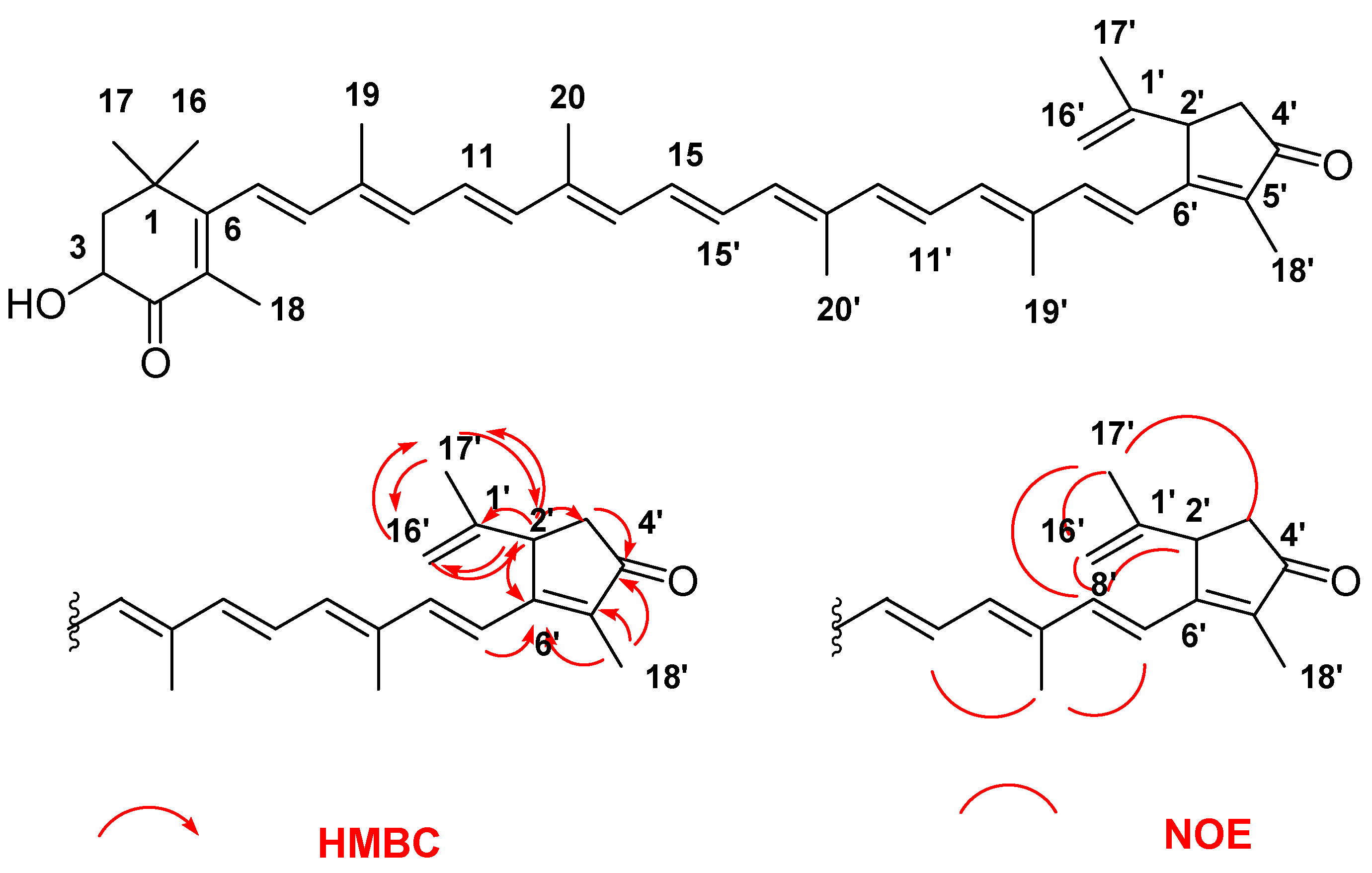
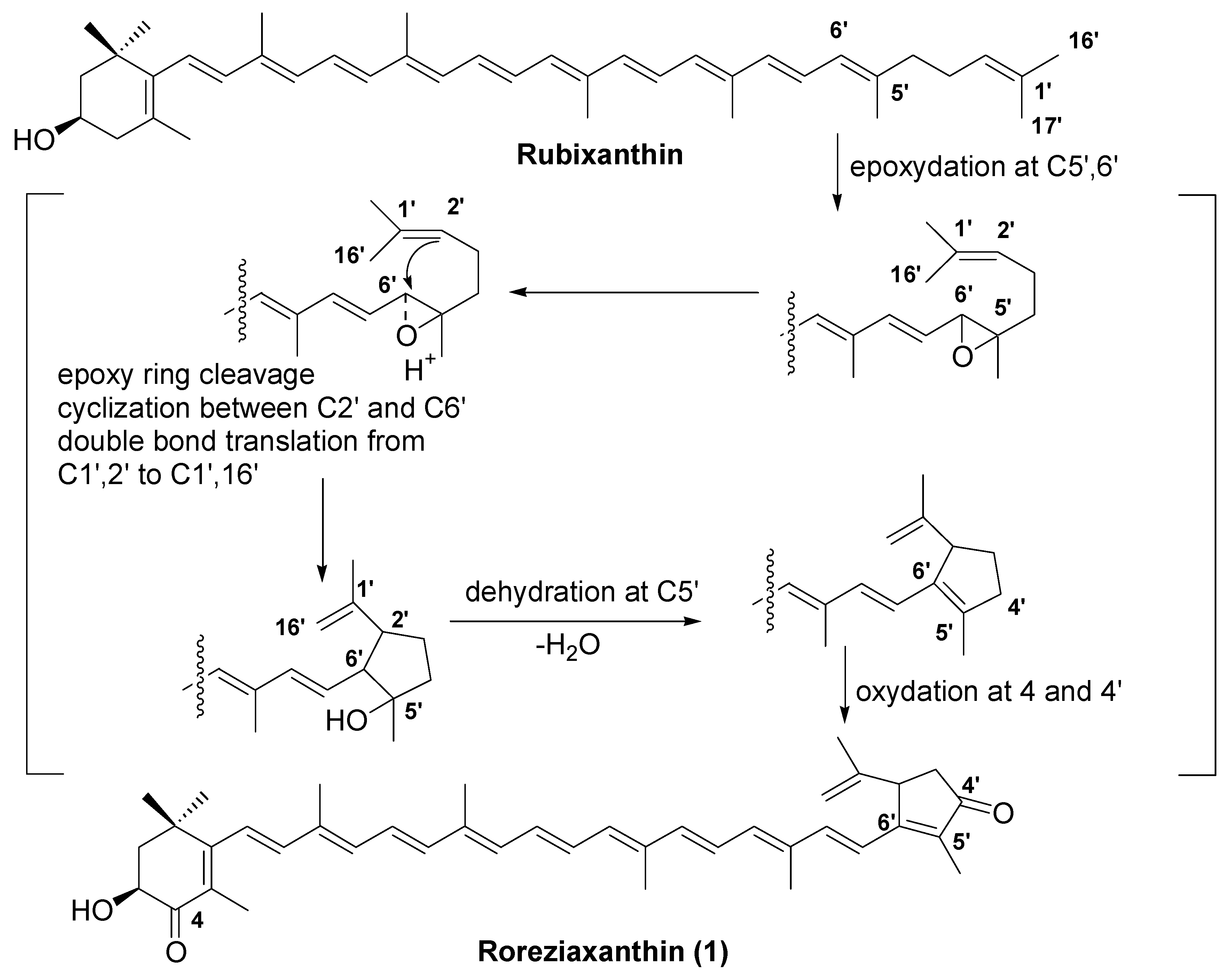
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Animal Material
3.3. Isolation of Roretziaxanthin from the Sea Squirt H. roretzi
3.4. Carotenoids from the Sea Squirt H. roretzi
3.5. Spectral Data of Roretziaxanthin (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Maoka, T.; Akimoto, N.; Tsushima, M.; Komemushi, S.; Mezaki, T.; Iwase, F.; Takahashi, Y.; Sameshima, N.; Mori, M.; Sakagami, Y. Carotenoids in Marine Invertebrates Living along the Kuroshio Current Coast. Mar. Drugs 2011, 9, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2019, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Ookubo, M.; Nishizawa, T.; Shimizu, I. Carotenoids of sea squirts. I. New marine carotenoids, halocynthiaxanthin and mytiloxanthinone from Halocynthia roretzi. Chem. Pharm. Bull. 1984, 32, 4309–4315. [Google Scholar] [CrossRef]
- Matsuno, T.; Ookubo, M.; Komori, T. Carotenoids of Tunicates, III. The Structural Elucidation of Two New Marine Carotenoids, Amarouciaxanthin A and B. J. Nat. Prod. 1985, 48, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Englert, G. NMR Spectroscopy in Carotenoids Vol. 1B; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1995; Volume 1B, pp. 147–260. [Google Scholar]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids Hand Book; Birkhäuser: Basel, Switzerland, 2014. [Google Scholar]
- Khachik, F.; Beecher, G.R.; Goli, M.B.; Lusby, W.R.; Smith, J.C., Jr. Separation and identification of carotenoids and their oxidation products in the extracts of human plasma. Anal. Chem. 1992, 64, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Steck, A.; Niggli, U.A.; Pfander, H. Partial Synthesis and Structural Elucidation of the Oxidative Metabolites of Lycopene Identified in Tomato Paste, Tomato Juice, and Human Serum. J. Agric. Food Chem. 1998, 46, 4874–4884. [Google Scholar] [CrossRef]
- Khachik, F.; Pfander, H.; Traber, B. Proposed Mechanisms for the Formation of Synthetic and Naturally Occurring Metabolites of Lycopene in Tomato Products and Human Serum. J. Agric. Food Chem. 1998, 46, 4885–4890. [Google Scholar] [CrossRef]
- Lu, Y.; Etoh, H.; Watanabe, N.; Ina, K.; Ukai, N.; Oshima, S.; Ojima, F.; Sakamoto, H.; Ishiguro, Y. A New Carotenoid, Hydrogen Peroxide Oxidation Products from Lycopene. Biosci. Biotechnol. Biochem. 1995, 59, 2153–2155. [Google Scholar] [CrossRef]
- Yokota, T.; Etoh, H.; Ukai, N.; Oshima, S.; Sakamoto, H.; Ishiguro, Y. 1,5-Dihydroxyiridanyl-lycopene in Tomato Puree. Biosci. Biotechnol. Biochem. 1997, 61, 549–550. [Google Scholar] [CrossRef]
- Yokota, T.; Etoh, H.; Oshima, S.; Hayakawa, K.; Ishiguro, Y. Oxygenated Lycopene and Dehydrated Lutein in Tomato Puree. Biosci. Biotechnol. Biochem. 2003, 67, 2644–2647. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Yamano, Y.; Wada, A.; Etho, T.; Terada, Y.; Tokuda, H.; Nishino, H. Oxidative Metabolites of Lycopene and γ-Carotene in Gac (Momordica cochinchinensis). J. Agric. Food Chem. 2015, 63, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Brown, D.J.; Goodwin, T.W.; Leuenberger, F.J.; Schocher, A.J. The carotenoids of Flavobacterium strain R1560. Arch. Microbiol. 1977, 113, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.K.; White, D.C. Carotenoid Formation by Staphylococcus aureus. J. Bacteriol. 1970, 103, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Setiyono, E.; Heriyanto; Pringgenies, D.; Shioi, Y.; Kanesaki, Y.; Awai, K.; Brotosudarmo, T.H.P. Sulfur-Containing Carotenoids from A Marine Coral Symbiont Erythrobacter flavus Strain KJ5. Mar. Drugs 2019, 17, 349. [Google Scholar] [CrossRef] [PubMed]
| Position | 13C-NMR | 1H-NMR | Position | 13C-NMR | 1H-NMR | ||
|---|---|---|---|---|---|---|---|
| δ | δ | Mult.J (Hz) | δ | δ | Mult.J (Hz) | ||
| 1 | 36.80 | dd (13.5, 13.5) | 1’ | 146.0 | |||
| 2 | 45.38 | 1.81 | dd (13.5, 5.5) | 2’ | 46.23 | 3.78 | d (7.5) |
| 2.15 | ddd (13.5, 5.5, 2.0) | ||||||
| 3 | 69.19 | 4.32 | 3’ | 41.46 | 2.20 | dd (18.5, 2.0) | |
| 2.71 | dd (18.5, 7.5) | ||||||
| 4 | 200.42 | 4’ | 207.79 | ||||
| 5 | 126.78 | 5’ | 136.92 | ||||
| 6 | 162.25 | 6’ | 164.60 | ||||
| 7 | 123.33 | 6.22 | d (16.0) | 7’ | 120.59 | 6.62 | d (15.5) |
| 8 | 142.35 | 6.43 | d (16.0) | 8’ | 141.39 | 6.95 | d (15.5) |
| 9 | 134.66 | 9’ | 135.25 | ||||
| 10 | 135.16 | 6.31 | d (11.0) | 10’ | 137.51 | 6.37 | d (11.5) |
| 11 | 124.73 | 6.69~6.63 | overlapped | 11’ | 124.73 | 6.69~6.63 | overlapped |
| 12 | 139.67 | 6.45 | d (15.5) | 12’ | 140.49 | 6.49 | d (11.5) |
| 13 | 136.78 | 13’ | 136.92 | ||||
| 14 | 133.81 | 6.33~6.31 | overlapped | 14’ | 134.34 | 6.33~6.31 | overlapped |
| 15 | 130.65 | 6.69~6.63 | overlapped | 15’ | 130.95 | 6.69~6.63 | overlapped |
| 16 | 29.15 | 1.32 | s | 16’ | 112.90 | 4.88 | t (2.0) |
| 4.95 | s | ||||||
| 17 | 30.75 | 1.21 | s | 17’ | 18.08 | 1.52 | s |
| 18 | 14.04 | 1.95 | s | 18’ | 8.40 | 1.88 | d (2.0) |
| 19 | 12.85 | 2.01 | s | 19’ | 2.48/12.81 | 2.00/2.01 | s |
| 20 | 12.59 | 1.99 | s | 20’ | 2.48/12.81 | 2.00/2.01 | s |
| OH | 3.69 | d (2.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maoka, T.; Tode, C. A Novel Carotenoid with a Unique 2,6-Cyclo-ψ-End Group, Roretziaxanthin, from the Sea Squirt Halocynthia roretzi. Mar. Drugs 2022, 20, 732. https://doi.org/10.3390/md20120732
Maoka T, Tode C. A Novel Carotenoid with a Unique 2,6-Cyclo-ψ-End Group, Roretziaxanthin, from the Sea Squirt Halocynthia roretzi. Marine Drugs. 2022; 20(12):732. https://doi.org/10.3390/md20120732
Chicago/Turabian StyleMaoka, Takashi, and Chisato Tode. 2022. "A Novel Carotenoid with a Unique 2,6-Cyclo-ψ-End Group, Roretziaxanthin, from the Sea Squirt Halocynthia roretzi" Marine Drugs 20, no. 12: 732. https://doi.org/10.3390/md20120732
APA StyleMaoka, T., & Tode, C. (2022). A Novel Carotenoid with a Unique 2,6-Cyclo-ψ-End Group, Roretziaxanthin, from the Sea Squirt Halocynthia roretzi. Marine Drugs, 20(12), 732. https://doi.org/10.3390/md20120732






