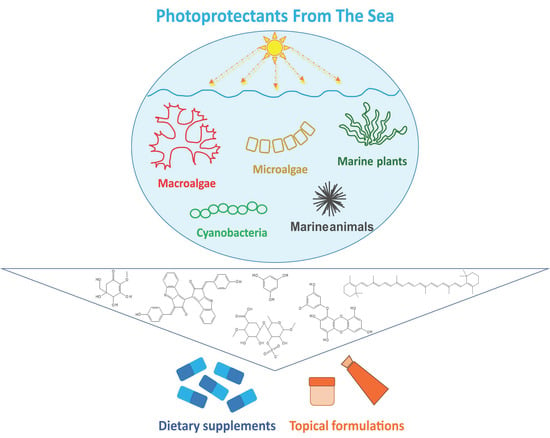
From Sea to Skin: Is There a Future for Natural Photoprotectants?
Abstract
1. Introduction
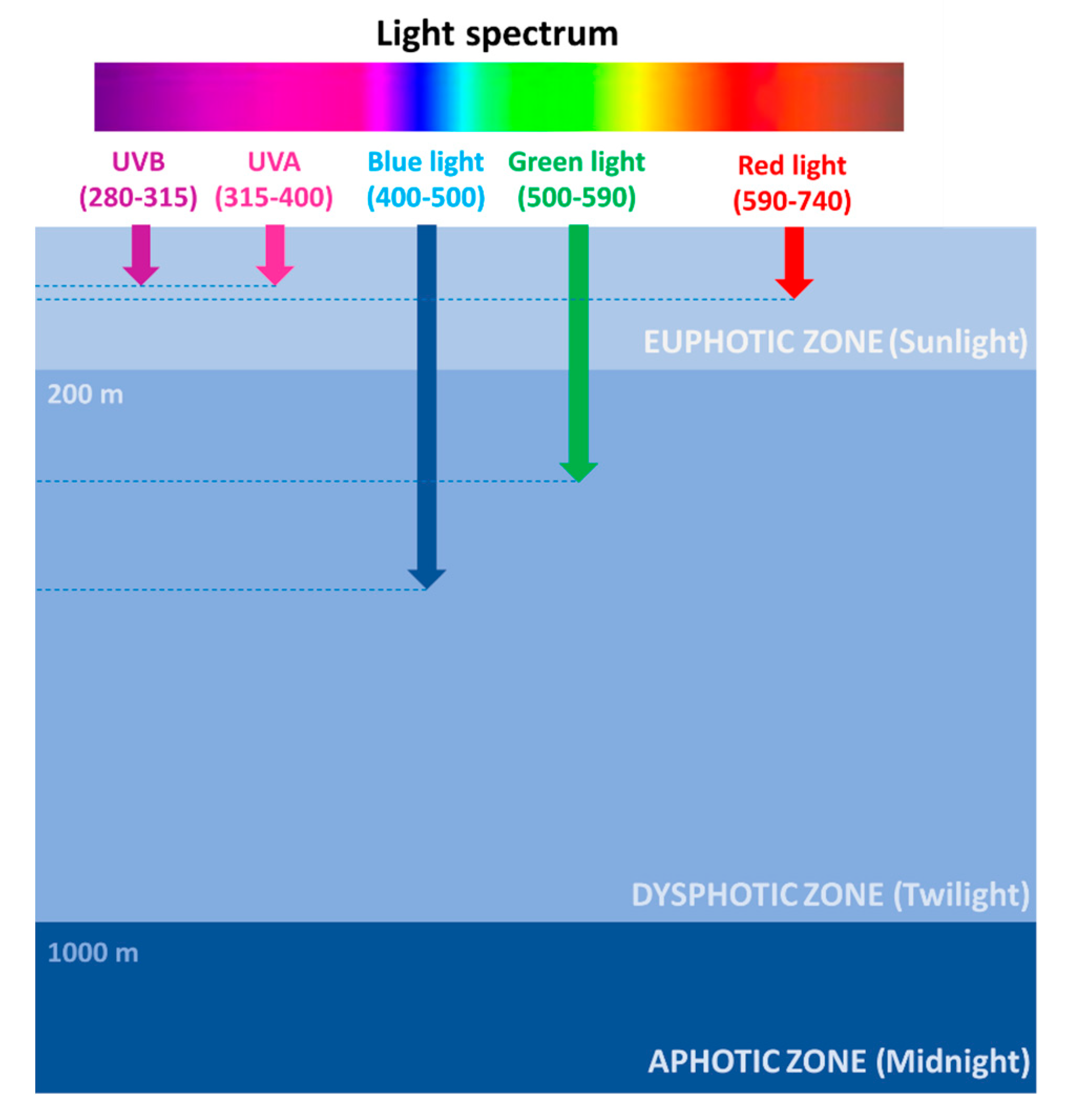
2. The Photic Zone: Where Light Fuels Life in the Ocean
3. Light Perception and Light-Driven Physiological Processes at Sea
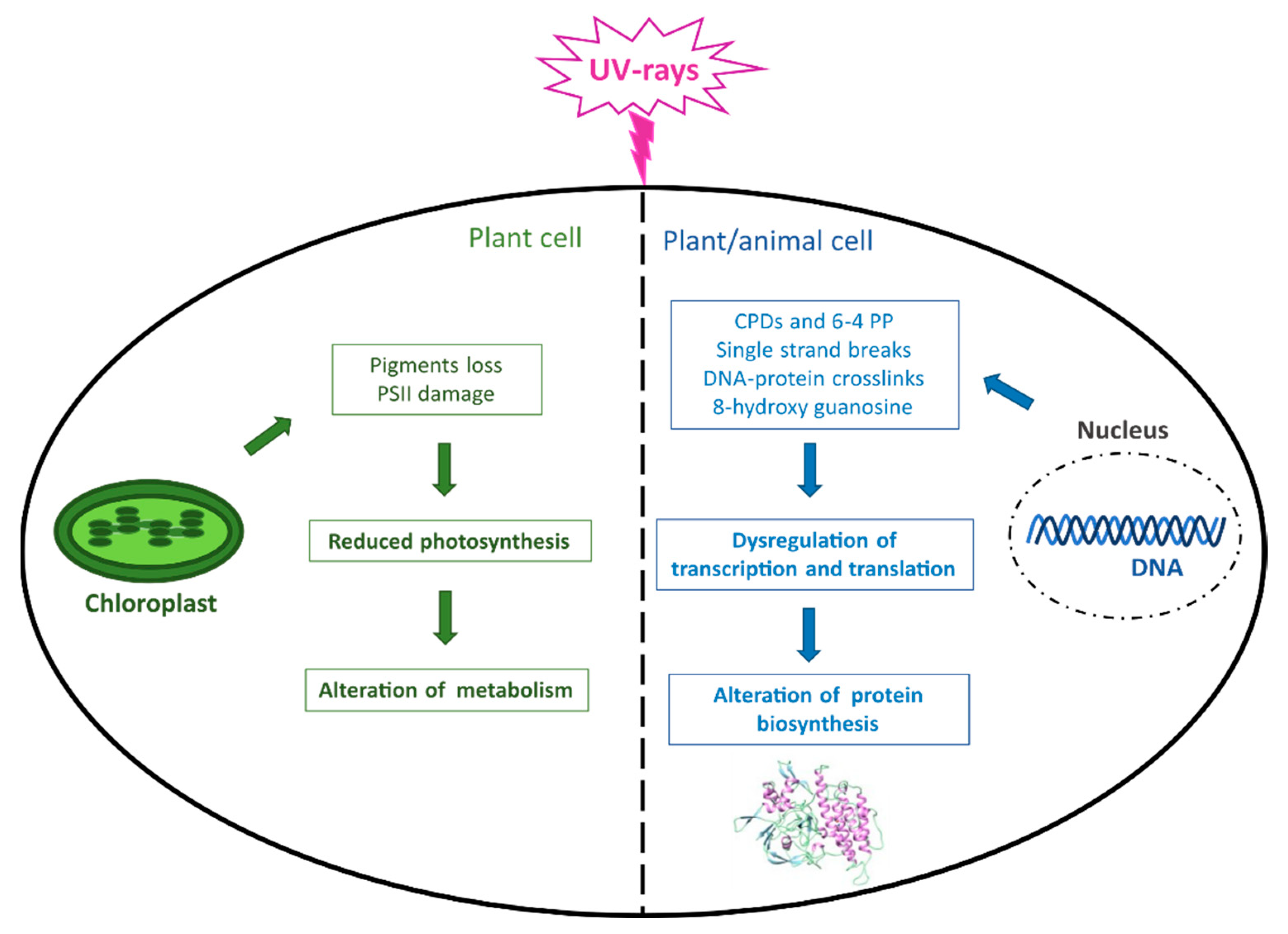
4. Photo-Damage/-Protection Mechanisms in Marine Organisms
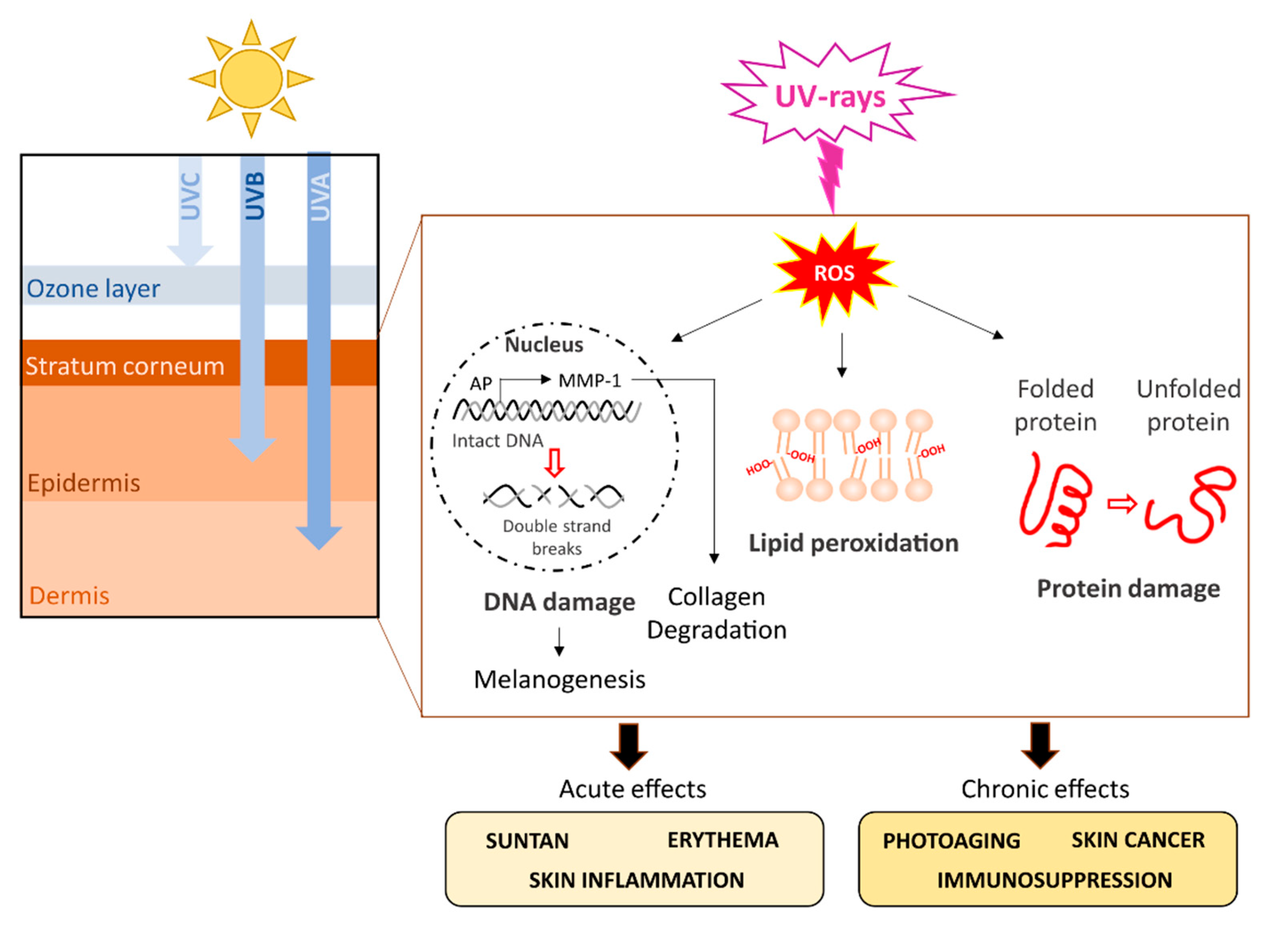
5. UVR Detrimental Effects on Humans
6. UV Filter Properties
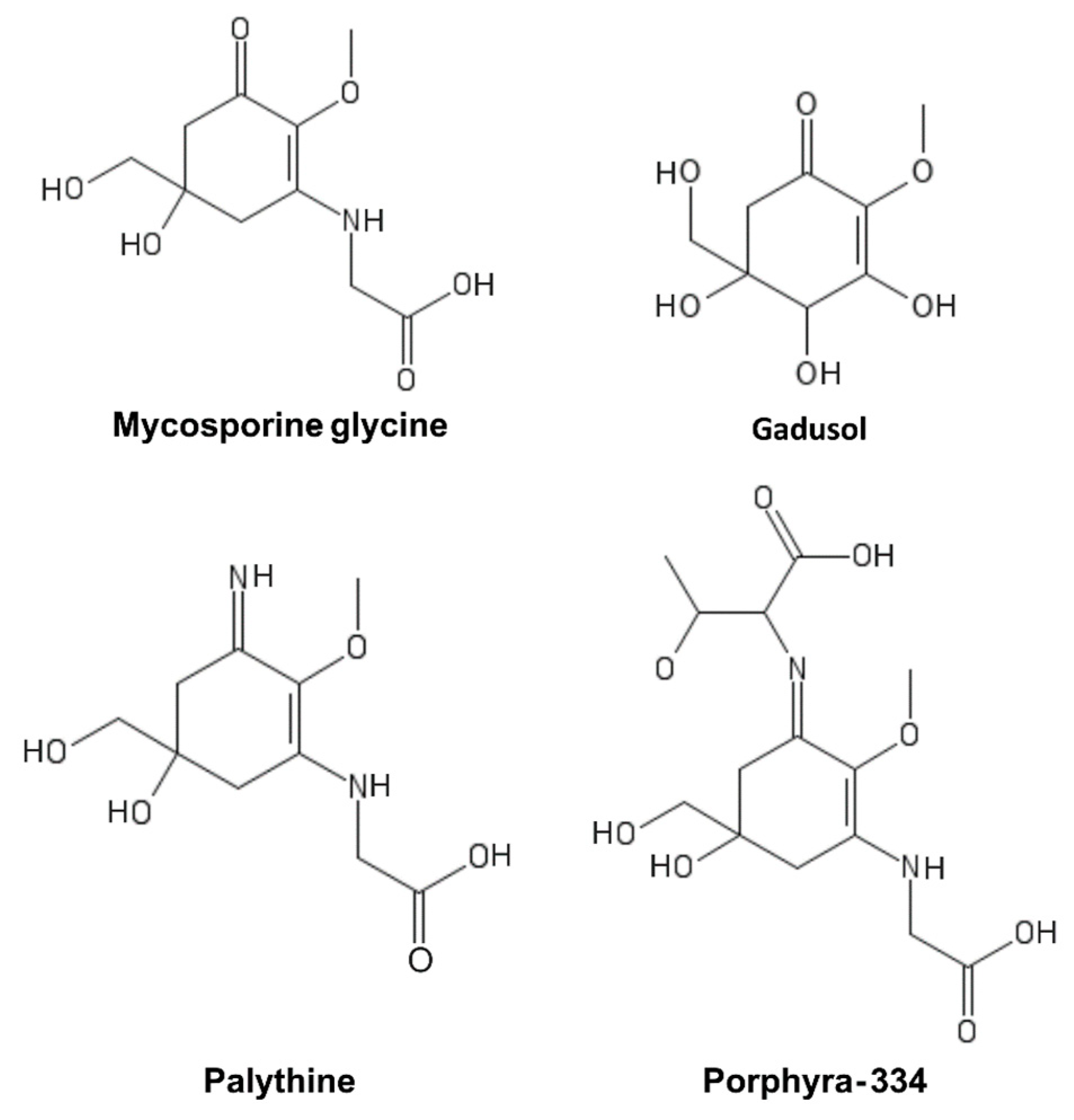
7. Mycosporine-Like Amino Acids (MAAs)
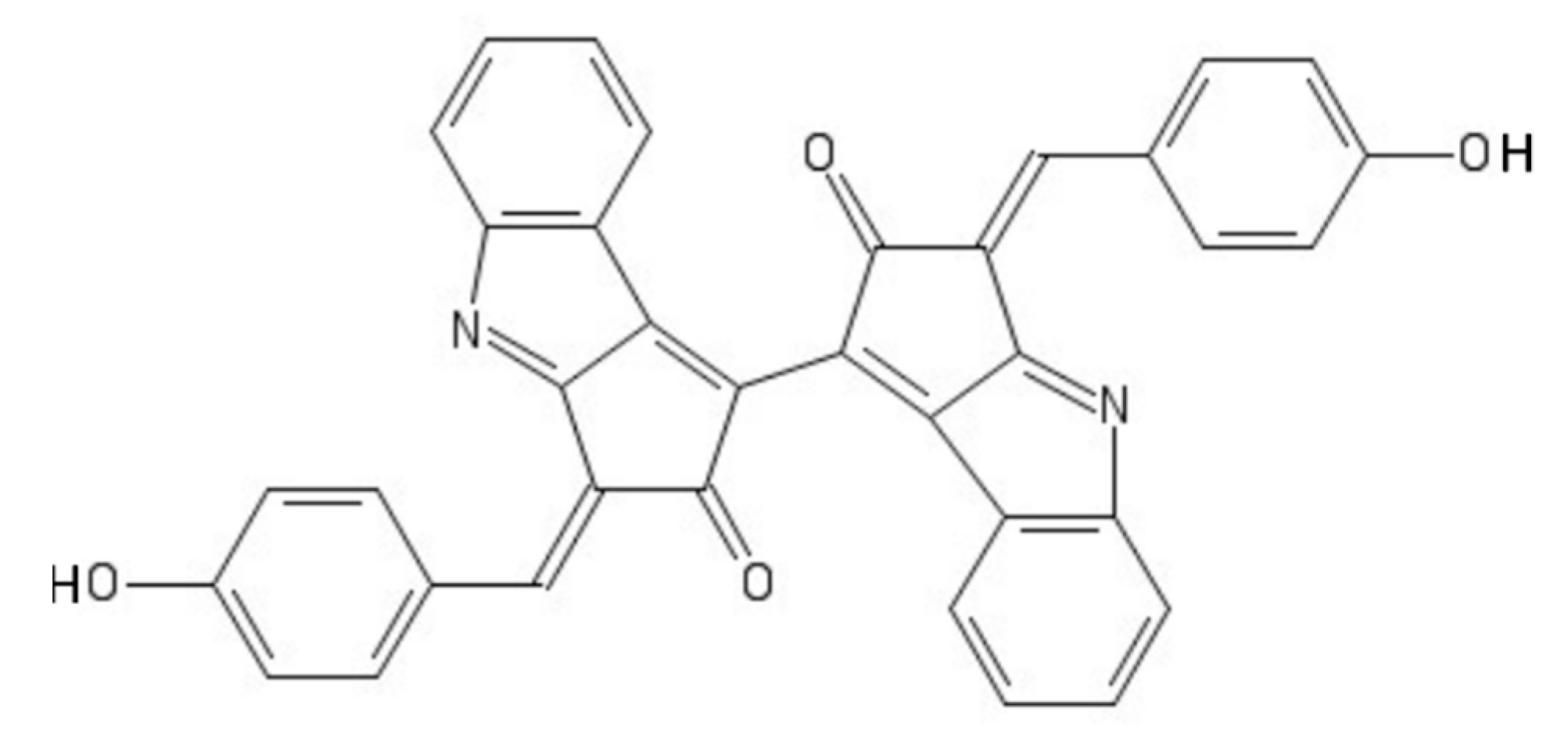
8. Scytonemin
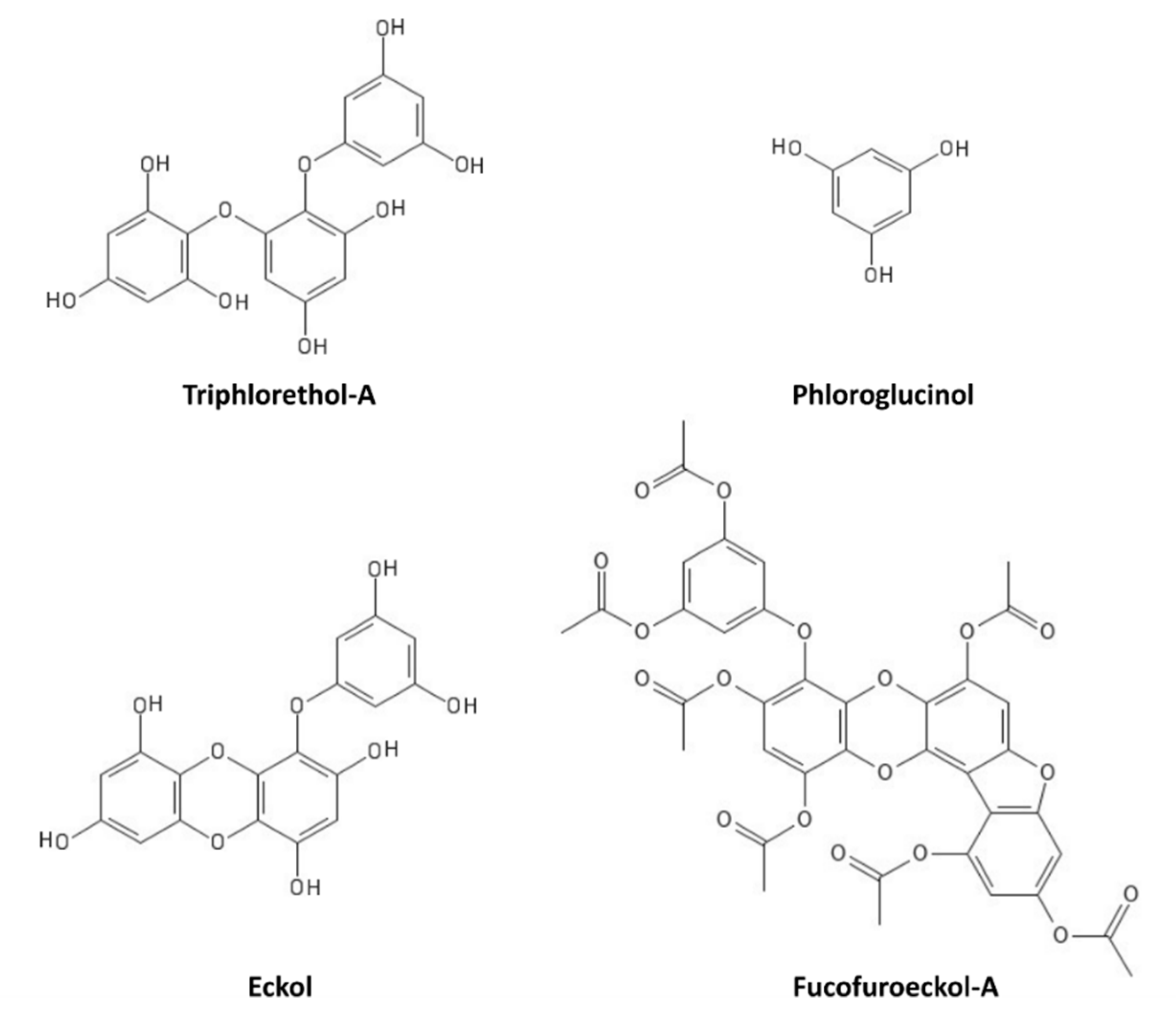
9. Polyphenols
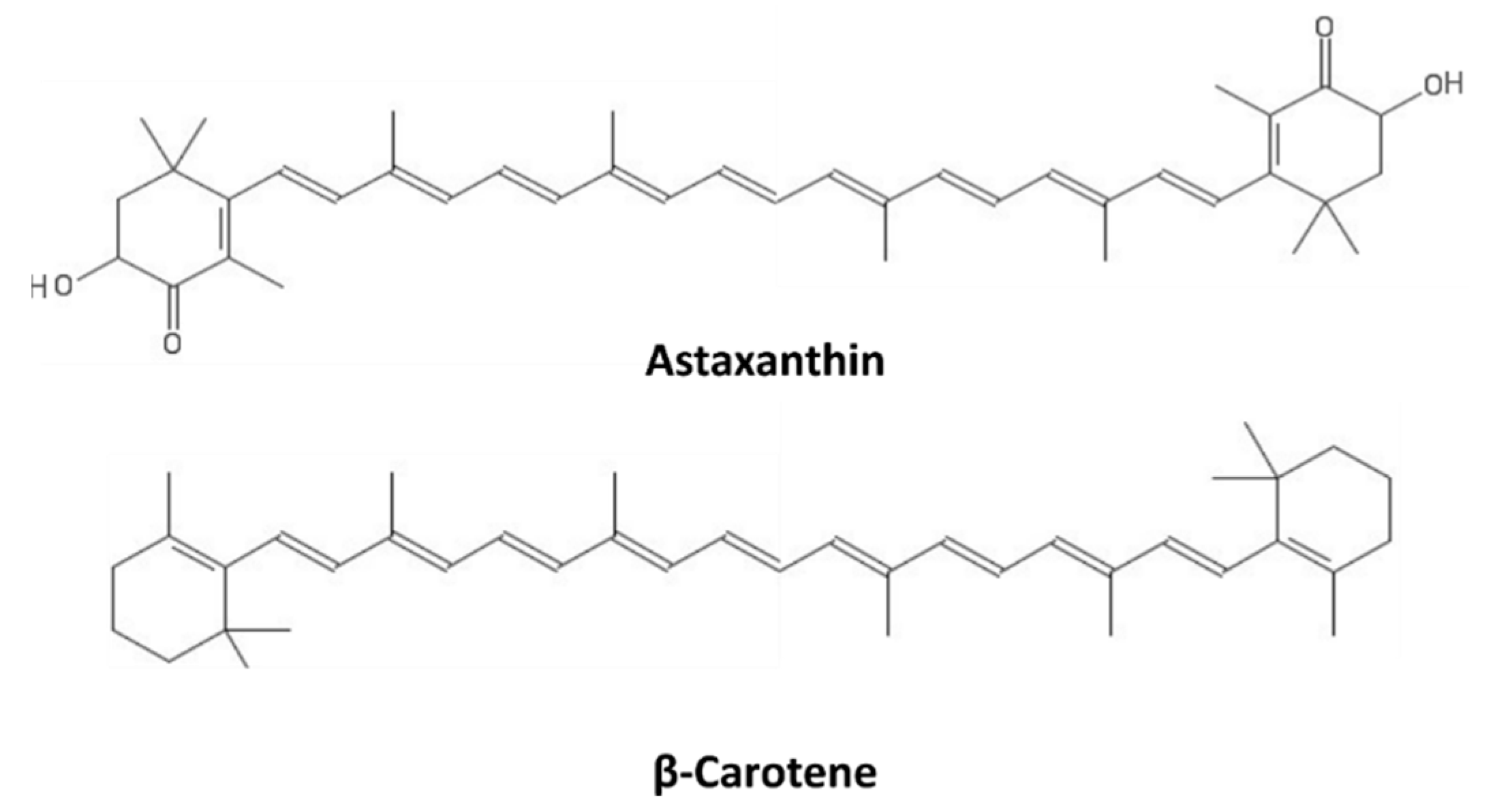
10. Carotenoids
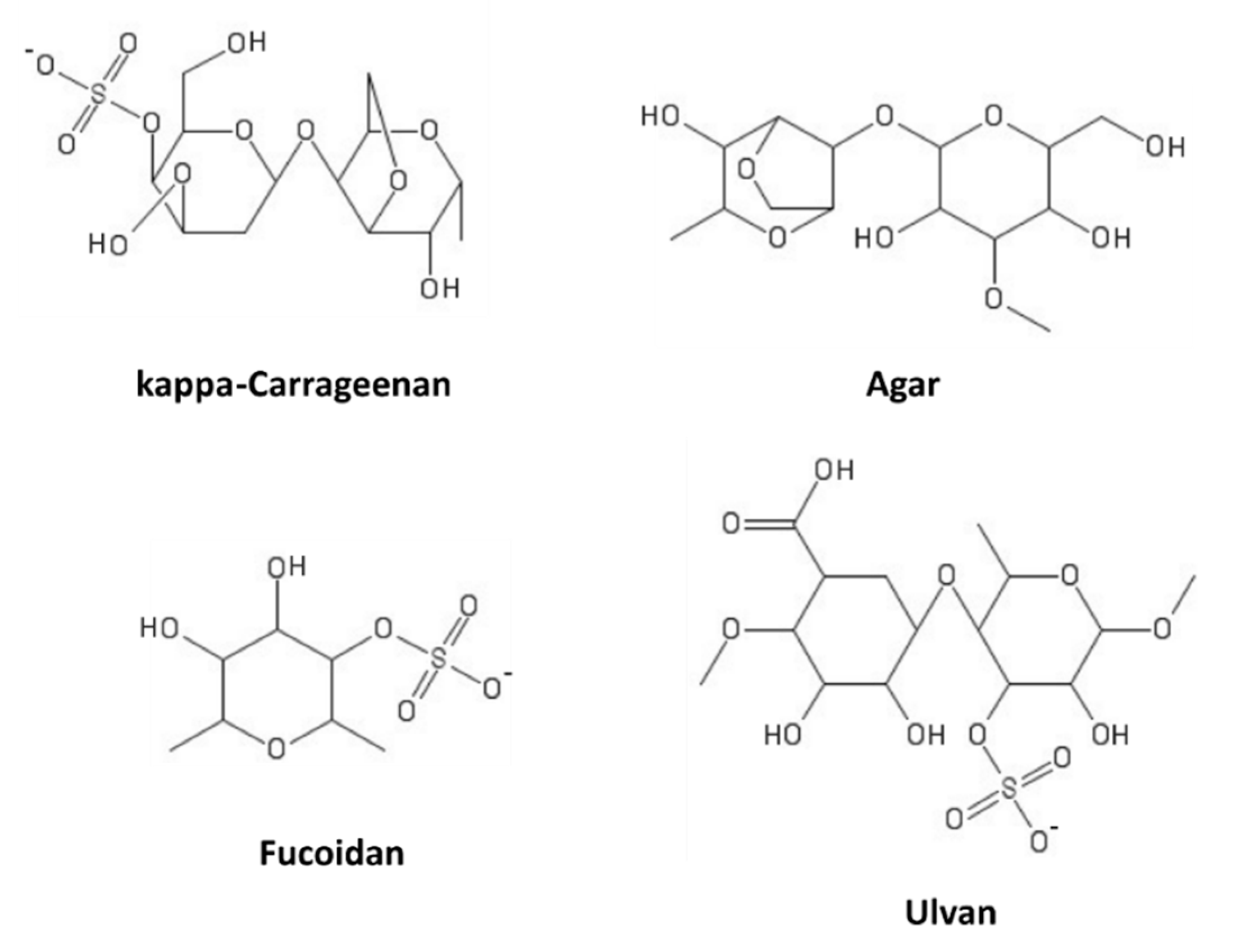
11. Sulfated Polysaccharides
12. Other Potential Photoprotective Compounds of Marine Origin
13. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- WHO. Available online: https://www.who.int/news-room/q-a-detail/radiation-sun-protection (accessed on 10 May 2021).
- Zen Life and Travel. Available online: https://www.zenlifeandtravel.com/sunscreen-bans/ (accessed on 10 May 2021).
- Schlumpf, M.; Schmid, P.; Durrer, S.; Conscience, M.; Maerkel, K.; Henseler, M.; Gruetter, M.; Herzog, I.; Reolon, S.; Ceccatelli, R.; et al. Endocrine activity and developmental toxicity of cosmetic UV filters—An update. Toxicology 2004, 205, 113–122. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2020, 134, 111161. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 3rd ed.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Brunet, C.; Chandrasekaran, R.; Barra, L.; Giovagnetti, V.; Corato, F.; Ruban, A.V. Spectral radiation dependent photoprotective mechanism in the diatom Pseudo-nitzschia multistriata. PLoS ONE 2014, 9, e87015. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, A.E.; Jaubert, M.; Enomoto, G.; Bouly, J.P.; Raniello, R.; Thaler, M.; Malviya, S.; Bernardes, J.S.; Rappaport, F.; Gentili, B.; et al. Diatom Phytochromes Reveal the Existence of Far-Red-Light-Based Sensing in the Ocean. Plant Cell 2016, 28, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.L.; Sancar, A. Photolyase/cryptochrome blue-light photoreceptors use photon energy to repair DNA and reset the circadian clock. Oncogene 2002, 21, 9043–9056. [Google Scholar] [CrossRef]
- Fortunato, A.E.; Annunziata, R.; Jaubert, M.; Bouly, J.P.; Falciatore, A. Dealing with light: The widespread and multitasking cryptochrome/photolyase family in photosynthetic organisms. J. Plant Physiol. 2015, 172, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Jaubert, M.; Bouly, J.P.; Ribera d’ Alcalà, M.; Falciatore, A. Light sensing and responses in marine microalgae. Curr. Opin. Plant Biol. 2017, 37, 70–77. [Google Scholar] [CrossRef]
- Marshall, J. Vision and lack of vision in the ocean. Curr. Biol. 2017, 27, R494–R502. [Google Scholar] [CrossRef]
- Baylor, D. How photons start vision. Proc. Natl. Acad. Sci. USA 1996, 93, 560–565. [Google Scholar] [CrossRef]
- Yokoyama, S. Evolution of Dim-Light and Color Vision Pigments. Annu. Rev. Genomics Hum. Genet. 2008, 9, 259–282. [Google Scholar] [CrossRef]
- Porter, M.L.; Blasic, J.R.; Bok, M.J.; Cameron, E.G.; Pringle, T.; Cronin, T.W.; Robinson, P.R. Shedding new light on opsin evolution. Proc. Biol. Sci. 2012, 279, 3–14. [Google Scholar] [CrossRef]
- Musilova, Z.; Cortesi, F.; Matschiner, M.; Davies, W.I.L.; Patel, J.S.; Stieb, S.M.; de Busserolles, F.; Malmstrøm, M.; Tørresen, O.K.; Brown, C.J.; et al. Vision using multiple distinct rod opsins in deep-sea fishes. Science 2019, 364, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, B.R. Water column optics and penetration of UVR. In UV Effects in Aquatic Organisms and Ecosystems; Helbling, E.W., Zagarese, H., Eds.; The Royal Society of Chemistry: London, UK, 2003; Volume 1, pp. 59–106. [Google Scholar]
- Morris, D.P.; Zagarese, H.; Williamson, C.E.; Balseiro, E.G.; Hargreaves, B.R.; Modenutti, B.; Moeller, R.; Queimalinos, C. The attenuation of solar UV radiation in lakes and the role of dissolved organic carbon. Limnol. Oceanogr. 1995, 40, 1381–1391. [Google Scholar] [CrossRef]
- Dring, M.J. Photocontrol of development in algae. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 157–174. [Google Scholar] [CrossRef]
- Gao, K.; Wu, Y.; Li, G.; Wu, H.; Villafañe, V.E.; Helbling, E.W. Solar UV radiation drives CO2 fixation in marine phytoplankton: A double-edged sword. Plant Physiol. 2007, 144, 54–59. [Google Scholar] [CrossRef]
- Johnsen, S.; Widder, E. Ultraviolet absorption in transparent zooplankton and its implications for depth distribution and visual predation. Mar. Biol. 2001, 138, 717–730. [Google Scholar] [CrossRef]
- Cronin, T.W.; Bok, M.J. Photoreception and vision in the ultraviolet. J. Exp. Biol. 2016, 219, 2790–2801. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K.; Truong, T.B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 2013, 16, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.-W. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Rochaix, J.-D. The Dynamics of the Photosynthetic Apparatus in Algae. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms; Larkum, A., Grossman, A., Raven, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 45, pp. 57–82. [Google Scholar]
- Goiris, K.; Muylaert, K.; De Cooman, L. Microalgae as a Novel Source of Antioxidants for Nutritional Applications. In Handbook of Marine Microalgae; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2015; Chapter 17; pp. 269–280. [Google Scholar]
- Milito, A.; Castellano, I.; Burn, R.; Seebeck, F.P.; Brunet, C.; Palumbo, A. First evidence of ovothiol biosynthesis in marine diatoms. Free Rad. Biol. Med. 2020, 152, 680–688. [Google Scholar] [CrossRef]
- Milito, A.; Orefice, I.; Smerilli, A.; Castellano, I.; Napolitano, A.; Brunet, C.; Palumbo, A. Insights into the Light Response of Skeletonema marinoi: Involvement of Ovothiol. Mar. Drugs 2020, 18, 477. [Google Scholar] [CrossRef] [PubMed]
- Buma, A.G.J.; Boelen, P.; Jeffrey, W.H. UVR- induced DNA damage in aquatic organisms. In UV Effects in Aquatic Organisms and Ecosystems; Helbling, E.W., Zagarese, H.E., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2003; Volume 1, pp. 289–327. [Google Scholar]
- Heraud, P.; Beardall, J. Changes in chlorophyll fluorescence during exposure of Dunaliella tertiolecta to UV radiation indicate a dynamic interaction between damage and repair processes. Photosynth. Res. 2000, 63, 123–134. [Google Scholar] [CrossRef]
- Gao, K.S.; Guan, W.C.; Helbling, E.W. Effects of solar ultraviolet radiation on photosynthesis of the marine red tide alga Heterosigma akashiwo (Raphidophyceae). J. Photochem. Photobiol. B Biol. 2007, 86, 140–148. [Google Scholar] [CrossRef]
- Harrison, J.W.; Smith, R.E. Effects of ultraviolet radiation on the productivity and composition of freshwater phytoplankton communities. Photochem. Photobiol. Sci. 2009, 8, 1218–1232. [Google Scholar] [CrossRef]
- Jiang, H.B.; Qiu, B.S. Inhibition of photosynthesis by UV-B exposure and its repair in the bloom-forming cyanobacterium Microcystis aeruginosa. J. Appl. Phycol. 2011, 23, 691–696. [Google Scholar] [CrossRef]
- Häder, D.P.; Williamson, C.E.; Wängberg, S.Å.; Rautio, M.; Rose, K.C.; Gao, K.; Helbling, E.W.; Sinha, R.P.; Worrest, R. Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors. Photochem. Photobiol. Sci. 2015, 14, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Dahms, H.-U.; Lee, J.-S. UV radiation in marine ectotherms: Molecular effects and responses. Aquat. Toxicol. 2010, 97, 3–14. [Google Scholar] [CrossRef]
- Alves, R.N.; Agustí, S. Effect of ultraviolet radiation (UVR) on the life stages of fish. Rev. Fish Biol. Fish. 2020, 30, 335–372. [Google Scholar] [CrossRef]
- Lesser, M.P.; Lamare, M.D.; Barker, M.F. Transmission of ultraviolet radiation through the Antarctic annual sea ice and its biological effects on sea urchin embryos. Limnol. Oceanogr. 2004, 49, 1957–1963. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Escassi, L.; Pérez-Rodríguez, E.; Korbee-Peinado, N.; Giles, A.D.; Johnsen, G. Effects of short-term irradiation on photoinhibition and accumulation of mycosporine-like amino acids in sun and shade species of the red algal genus Porphyra. J. Photochem. Photobiol. B Biol. 2003, 69, 21–30. [Google Scholar] [CrossRef]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photoprotective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharm. 2012, 3, 118–126. [Google Scholar]
- Young, A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef]
- Schulman, J.M.; Fisher, D.E. Indoor ultraviolet tanning and skin cancer: Health risks and opportunities. Curr. Opin. Oncol. 2009, 21, 144–149. [Google Scholar] [CrossRef]
- Agar, N.; Young, A.R. Melanogenesis: A photoprotective response to DNA damage? Mutat. Res. 2005, 571, 121–132. [Google Scholar]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Gallagher, R.P.; Lee, T.K. Adverse effects of ultraviolet radiation: A brief review. Prog. Biophys. Mol. Biol. 2006, 92, 119–131. [Google Scholar] [CrossRef]
- Agarwal, N. UVR and Role of Pigmentation in Skin Aging and Cancer. In Skin Aging & Cancer; Dwivedi, A., Agarwal, N., Ray, L., Tripathi, A., Eds.; Springer: Singapore, 2019; pp. 59–69. [Google Scholar]
- Hart, P.H.; Norval, M. Ultraviolet radiation-induced immunosuppression and its relevance for skin carcinogenesis. Photochem. Photobiol. Sci. 2018, 17, 1872–1884. [Google Scholar] [CrossRef]
- Damiani, E.; Ullrich, S.E. Understanding the connection between platelet-activating factor, a UV-induced lipid mediator of inflammation, immune suppression and skin cancer. Prog. Lipid Res. 2016, 63, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Osterwalder, U.; Sohn, M.; Herzog, B. Global state of sunscreens. Photodermatol. Photoimmunol. Photomed. 2014, 30, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Sunscreens as a source of hydrogen peroxide production in coastal waters. Environ. Sci. Technol. 2014, 48, 9037–9042. [Google Scholar] [CrossRef] [PubMed]
- Shaath, N. Sunscreens: Regulations and Commercial Development, 3rd ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; Chapter 13; p. 222. [Google Scholar]
- Shaath, N.A. Ultraviolet filters. Photochem. Photobiol. Sci. 2010, 9, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Sánchez-Quiles, D.; Blasco, J.; Moreno-Garrido, I.; Lubián, L.M.; Pérez-García, S.; Tovar-Sánchez, A. Effects of TiO2 nanoparticles and sunscreens on coastal marine microalgae: Ultraviolet radiation is key variable for toxicity assessment. Environ. Int. 2017, 98, 62–68. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Damiani, E.; Marcellini, F.; Falugi, C.; Tiano, L.; Brugè, F.; Danovaro, R. Sunscreen products impair the early developmental stages of the sea urchin Paracentrotus lividus. Sci. Rep. 2017, 7, 7815. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci. Total Environ. 2018, 637, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef]
- Amador-Castro, F.; Rodriguez-Martinez, V.; Carrillo-Nieves, D. Robust natural ultraviolet filters from marine ecosystems for the formulation of environmental friendlier bio-sunscreens. Sci. Total Environ. 2020, 749, 141576. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-Like Amino Acids: Relevant Secondary Metabolites. Chemical and Ecological Aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Pathak, J.; Pandey, A.; Singh, V.; Rajneesh, H.A.; Kumar, D.; Sinha, R.P. Ultraviolet-screening compound mycosporine-like amino acids in cyanobacteria: Biosynthesis, functions, and applications. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 15; pp. 219–233. [Google Scholar]
- Xiong, F.; Kopecky, J.; Nedbal, L. The occurrence of UV-B absorbing mycosporine-like amino acids in freshwater and terrestrial microalgae (Chlorophyta). Aquat. Bot. 1999, 63, 37–49. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Zhou, J.; Dong, S.; Zhang, X.; Guo, L.; Guo, G. Distribution, Contents, and Types of Mycosporine-Like Amino Acids (MAAs) in Marine Macroalgae and a Database for MAAs Based on These Characteristics. Mar. Drugs 2020, 18, 43. [Google Scholar] [CrossRef]
- Rosic, N.N.; Dove, S. Mycosporine-Like Amino Acids from Coral Dinoflagellates. Appl. Environ. Microbiol. 2011, 77, 8478–8486. [Google Scholar] [CrossRef]
- Sinha, R.P.; Singh, S.P.; Häder, D.-P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B 2007, 89, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Shick, J.M.; Romaine-Lioud, S.; Ferrier-Pages, C.; Gattuso, J.P. Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral Stylophora pistillata despite decreases in its population of symbiotic dinoflagellates. Limnol. Oceanogr. 1999, 44, 1667–1682. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.J.; Dunlap, W.C.; Nicol, S.; Ritz, D. Antarctic krill (Euphausia superba) acquire a UV-absorbing mycosporine-like amino acid from dietary algae. J. Exp. Mar. Biol. Ecol. 2000, 255, 93–110. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, L.M.; Szmant, A.M. Biosynthesis of ‘essential’ amino acids by scleractinian corals. Biochem. J. 1997, 322, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Starcevic, A.; Akthar, S.; Dunlap, W.C.; Shick, J.M.; Hranueli, D.; Cullum, J.; Long, P.F. Enzymes of the shikimic acid pathway encoded in the genome of a basal metazoan, Nematostella vectensis, have microbial origins. Proc. Natl. Acad. Sci. USA 2008, 105, 2533–2537. [Google Scholar] [CrossRef]
- Shinzato, C.; Shoguchi, E.; Kawashima, T.; Hamada, M.; Hisata, K.; Tanaka, M.; Fujie, M.; Fujiwara, M.; Koyanagi, R.; Ikuta, T.; et al. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 2011, 476, 320–323. [Google Scholar] [CrossRef]
- Osborn, A.R.; Almabruk, K.H.; Holzwarth, G.; Asamizu, S.; LaDu, J.; Kean, K.M.; Karplus, P.A.; Tanguay, R.L.; Bakalinsky, A.T.; Mahmud, T. De novo synthesis of a sunscreen compound in vertebrates. eLife 2015, 4, e05919. [Google Scholar] [CrossRef]
- Brotherton, C.A.; Balskus, E.P. Shedding light on sunscreen biosynthesis in zebrafish. eLife 2015, 4, e07961. [Google Scholar] [CrossRef]
- De la Coba, F.; Aguilera, J.; Korbee, N.; de Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs). Mar. Drugs 2019, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Schmid, D.; Schürch, C.; Zülli, F. Mycosporine-like amino acids from red algae protect against premature skin-aging. Euro Cosmet. 2006, 9, 1–4. [Google Scholar]
- Cheewinthamrongrod, V.; Kageyama, H.; Palaga, T.; Takabe, T.; Waditee-Sirisattha, R. DNA damage protecting and free radical scavenging properties of mycosporine-2-glycine from the Dead Sea cyanobacterium in A375 human melanoma cell lines. J. Photochem. Photobiol. B Biol. 2016, 164, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.W. An Enzymatic Route to Sunscreens. ChemBioChem 2011, 12, 363–365. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef]
- Rosic, N.N. Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef] [PubMed]
- Proteau, P.J.; Gerwick, W.H.; Garcia-Pichel, F.; Castenholz, R. The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia 1993, 49, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Büdel, B.; Karsten, U.; Garcia-Pichel, F. Ultraviolet-absorbing scytonemin and mycosporine-like amino acid derivatives in exposed, rock-inhabiting cyanobacterial lichens. Oecologia 1997, 112, 165–172. [Google Scholar] [CrossRef]
- Balskus, E.P.; Case, R.J.; Walsh, C.T. The biosynthesis of cyanobacterial sunscreen scytonemin in microbial mat communities. FEMS Microbiol. Ecol. 2011, 77, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Nazifi, E.; Hirai, Y.; Wada, N.; Matsugo, S.; Sakamoto, T. The cyanobacterial UVabsorbing pigment scytonemin displays radical scavenging activity. J. Gen. Appl. Microbiol. 2012, 58, 137–144. [Google Scholar] [CrossRef]
- Pathak, J.; Sonker, A.S.; Richa, R.; Rajneesh, R.; Kannaujiya, V.K.; Singh, V.; Ahmed, H.; Sinha, R.P. Screening and partial purification of photoprotective pigment, scytonemin from cyanobacteria dominated biological crusts dwelling on the historical monuments in and around Varanasi, India. Microbiol. Res. 2017, 8, 6559. [Google Scholar] [CrossRef]
- Simeonov, A.; Michaelian, K. Properties of cyanobacterial UV-absorbing pigments suggest their evolution was driven by optimizing photon dissipation rather than photoprotection. arXiv Prepr. 2017, arXiv:1702.03588. [Google Scholar]
- Pandey, A.; Pathak, J.; Singh, D.K.; Ahmed, H.; Singh, V.; Kumar, D.; Sinha, R.P. Photoprotective role of UV-screening pigment scytonemin against UV-B-induced damages in the heterocyst-forming cyanobacterium Nostoc sp. strain HKAR-2. Braz. J. Bot. 2020, 43, 67–80. [Google Scholar] [CrossRef]
- Pathak, J.; Pandey, A.; Maurya, P.K.; Rajneesh, R.; Sinha, R.P.; Singh, S.P. Cyanobacterial Secondary Metabolite Scytonemin: A Potential Photoprotective and Pharmaceutical Compound. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 467–481. [Google Scholar] [CrossRef]
- Sinha, R.P.; Pathak, J.; Ahmed, R.H.; Pandey, A.; Singh, P.R.; Mishra, S.; Häder, D.-P. Cyanobacterial photoprotective compounds: Characterization and utilization in human welfare. In Natural Bioactive Compounds; Sinha, R.P., Häder, D.-P., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 5; pp. 83–114. [Google Scholar]
- Pandey, A.; Sinha, R.P. Biophysical Characterization of Scytonemin: A Sunsreening Pigment in Cyanobacterial Biofilms. JIAPS 2019, 23, 411–424. [Google Scholar]
- Kang, M.R.; Jo, S.A.; Lee, H.; Yoon, Y.D.; Kwon, J.-H.; Yang, J.-W.; Choi, B.J.; Park, K.H.; Lee, M.Y.; Lee, C.W.; et al. Inhibition of Skin Inflammation by Scytonemin, an Ultraviolet Sunscreen Pigment. Mar. Drugs 2020, 18, 300. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.S.; Capper, E.A.; Roshak, A.K.; Marquez, B.; Eichman, C.; Jackson, J.R.; Mattern, M.; Gerwick, W.H.; Jacobs, R.S.; Marshall, L.A. The identification and characterization of the marine natural product scytonemin as a novel antiproliferative pharmacophore. J. Pharm. Exp. Ther. 2002, 303, 858–866. [Google Scholar] [CrossRef]
- Itoh, T.; Tsuzuki, R.; Tanaka, T.; Ninomiya, M.; Yamaguchi, Y.; Takenaka, H.; Ando, M.; Tsukamasa, Y.; Koketsu, M. Reduced scytonemin isolated from Nostoc commune induces autophagic cell death in human T-lymphoid cell line Jurkat cells. Food Chem. Toxicol. 2013, 60, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Koketsu, M.; Yokota, N.; Touho, S.; Ando, M.; Tsukamasa, Y. Reduced scytonemin isolated from Nostoc commune suppresses LPS/IFNc-induced NO production in murine macrophage RAW264 cells by inducing hemeoxygenase-1 expression via the Nrf2/ARE pathway. Food Chem. Toxicol. 2014, 69, 330–338. [Google Scholar] [CrossRef]
- Helms, G.L.; Moore, R.E.; Niemczura, W.P.; Patterson, G.M.L.; Tomer, K.B.; Gross, M.L. Scytonemin A, a novel calcium antagonist from a blue-green alga. J. Org. Chem. 1988, 53, 1298–1307. [Google Scholar] [CrossRef]
- Edwards, H.G.; Sadooni, F.; Vítek, P.; Jehlička, J. Raman spectroscopy of the Dukhan sabkha: Identification of geological and biogeological molecules in an extreme environment. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3099–3107. [Google Scholar] [CrossRef]
- Varnali, T.; Edwards, H.G.M. Reduced and oxidised scytonemin: Theoretical protocol for Raman spectroscopic Cyanobacterial Secondary Metabolite Scytonemin: A Potential Photoprotective and identification of potential key biomolecules for astrobiology. Spectrochim. Acta A 2014, 117, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L.; Spagnuolo, C.; Russo, M.; Tedesco, I.; Moccia, S.; Cervellera, C. Mechanisms of aging and potential role of selected polyphenols in extending healthspan. Biochem. Pharmacol. 2020, 173, 113719. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef]
- Martins, B.T.; Correia da Silva, M.; Pinto, M.; Cidade, H.; Kijjoa, A. Marine natural flavonoids: Chemistry and biological activities. Nat. Prod. Res. 2019, 33, 3260–3272. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar]
- Piao, M.J.; Zhang, R.; Lee, N.H.; Hyun, J.W. Protective effect of triphlorethol-A against ultraviolet B-mediated damage of human keratinocytes. J. Photochem. Photobiol. B 2012, 106, 74–80. [Google Scholar] [CrossRef]
- Piao, M.J.; Zhang, R.; Lee, N.H.; Hyun, J.W. Phloroglucinol attenuates ultraviolet B radiation-induced matrix metalloproteinase-1 production in human keratinocytes via inhibitory actions against mitogen-activated protein kinases and activator protein-1. Photochem. Photobiol. 2012, 88, 381–388. [Google Scholar] [CrossRef]
- Piao, M.J.; Lee, N.H.; Chae, S.; Hyun, J.W. Eckol inhibits ultraviolet B-induced cell damage in human keratinocytes via a decrease in oxidative stress. Biol. Pharm. Bull. 2012, 35, 873–880. [Google Scholar] [CrossRef][Green Version]
- Ko, S.-C.; Cha, S.-H.; Heo, S.-J.; Lee, S.-H.; Kang, S.-M.; Jeon, Y.-J. Protective effect of Ecklonia cava on UVB-induced oxidative stress: In vitro and in vivo zebrafish model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.-K.; Ryu, B.; Ngo, D.H.; Yoon, N.-Y.; Bach, L.G.; Hang, N.T.N.; Ngo, D.N. The Suppressive Activity of Fucofuroeckol-A Derived from Brown Algal Ecklonia stolonifera Okamura on UVB-Induced Mast Cell Degranulation. Mar. Drugs 2018, 16, 1. [Google Scholar] [CrossRef]
- Sotiropoulou, G.; Zingkou, E.; Pampalakis, G. Redirecting drug repositioning to discover innovative cosmeceuticals. Exp. Dermatol. 2021, 30, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Merhan, O. The Biochemistry and Antioxidant Properties of Carotenoids. In Carotenoids; Cvetkovic, D.J., Nikolic, G.S., Eds.; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and Photosynthesis. In Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 2016; Volume 79, pp. 111–139. [Google Scholar]
- Rastogi, R.P.; Richa, R.; Sinha, R.P.; Singh, S.P.; Häder, D.-P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef] [PubMed]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Photoprotection by dietary carotenoids: Concept, mechanisms, evidence and future development. Mol. Nutr. Food Res. 2012, 56, 287–295. [Google Scholar] [CrossRef]
- Wertz, K.; Hunziker, P.B.; Seifert, N.; Riss, G.; Neeb, M.; Steiner, G.; Hunziker, W.; Goralczyk, R. beta-Carotene interferes with ultraviolet light A-induced gene expression by multiple pathways. J. Investig. Dermatol. 2005, 124, 428–434. [Google Scholar] [CrossRef]
- Catanzaro, E.; Bishayee, A.; Fimognari, C. On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer. Mar. Drugs 2020, 18, 544. [Google Scholar] [CrossRef] [PubMed]
- Hama, S.; Takahashi, K.; Inai, Y.; Shiota, K.; Sakamoto, R.; Yamada, A.; Tsuchiya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective effects of topical application of a poorly soluble antioxidant astaxanthin liposomal formulation on ultraviolet-induced skin damage. J. Pharm. Sci. 2012, 101, 2909–2916. [Google Scholar] [CrossRef]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive effect of dietary astaxanthin on UVA-induced skin photoaging in hairless mice. PLoS ONE 2017, 12, e0171178. [Google Scholar] [CrossRef]
- Li, X.; Matsumoto, T.; Takuwa, M.; Saeed Ebrahim Shaiku Ali, M.; Hirabashi, T.; Kondo, H.; Fujino, H. Protective effects of astaxanthin supplementation against ultraviolet-induced photoaging in hairless mice. Biomedicines 2020, 8, 18. [Google Scholar] [CrossRef]
- Ito, N.; Seki, S.; Ueda, F. The protective role of Astaxanthin for UV-induced skin deterioration in healthy people-a randomized, double-blind, placebo-controlled trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous Astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Cho, H.H.; Cho, S.; Lee, S.-R.; Shin, M.-H.; Chung, J.H. Supplementing with dietary astaxanthin combined with collagen hydrolysate improves facial elasticity and decreases matrix metalloproteinase-1 and -12 expression: A comparative study with placebo. J. Med. Food 2014, 17, 810–816. [Google Scholar] [CrossRef]
- Ng, Q.X.; De Deyn, M.L.Z.Q.; Loke, W.; Foo, N.X.; Chan, H.W.; Yeo, W.S. Effects of Astaxanthin supplementation on skin health: A systematic review of clinical studies. J. Diet. Suppl. 2020, 18, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Winberg, P.C.; Fitton, H.J.; Stringer, D.; Karpiniec, S.S.; Gardiner, V.-A. Controlling Seaweed Biology, Physiology and Metabolic Traits in Production for Commercially Relevant Bioactives in Glycobiology. Adv. Bot. Res. 2014, 71, 221–252. [Google Scholar]
- Aquino, R.S.; Grativol, C.; Mourão, P.A.S. Rising from the Sea: Correlations between Sulfated Polysaccharides and Salinity in Plants. PLoS ONE 2011, 6, e18862. [Google Scholar] [CrossRef]
- Zierer, M.S.; Mourão, P.A.S. A wide diversity of sulfated polysaccharides are synthesized by different species of marine sponges. Carbohydr. Res. 2000, 328, 209–216. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kang, N.; Ahn, G.; Jee, Y.; Kim, Y.; Jeon, Y. Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp in related to human health applications: A review. Food Hydrocoll. 2018, 81, 200–208. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef]
- Thevanayagam, H.; Mohamed, S.M.; Chu, W. Assessment of UVB-photoprotective and antioxidative activities of carrageenan in keratinocytes. J. Appl. Phycol. 2014, 26, 1813–1821. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.-J. Protective Effect of Sulfated Polysaccharides from Celluclast-Assisted Extract of Hizikia fusiforme Against Ultraviolet B-Induced Skin Damage by Regulating NF-κB, AP-1, and MAPKs Signaling Pathways In Vitro in Human Dermal Fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Yang, H.; Kim, H.S.; Jeon, Y.-J. Protective effect of sulfated polysaccharides from a Celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-induced photoaging in vitro in human keratinocytes and in vivo in zebrafish. Mar. Life Sci. Technol. 2019, 1, 104–111. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kim, D.; Park, J.S.; Park, H.J.; Shin, J.; Lee, S.K. Photoprotective Activity of Topsentin, A Bis(Indole) Alkaloid from the Marine Sponge Spongosorites genitrix, by Regulation of COX-2 and Mir-4485 Expression in UVB-Irradiated Human Keratinocyte Cells. Mar. Drugs 2020, 18, 87. [Google Scholar] [CrossRef]
- Castellano, I.; Seebeck, F. On ovothiol biosynthesis and biological roles: From life in the ocean to therapeutic potential. Nat. Prod. Rep. 2018, 35, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Sollitto, M.; Pallavicini, A.; Castellano, I. The complex evolutionary history of sulfoxide synthase in ovothiol biosynthesis. Proc. R. Soc. B. 2019, 286, 20191812. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Russo, M.; Masullo, M.; Palumbo, A.; Russo, G.L.; Castellano, I. Sulfur-containing histidine compounds inhibit γ-glutamyl transpeptidase activity in human cancer cells. J. Biol. Chem. 2019, 294, 14603–14614. [Google Scholar] [CrossRef] [PubMed]
- Milito, A.; Brancaccio, M.; Lisurek, M.; Masullo, M.; Palumbo, A.; Castellano, I. Probing the Interactions of Sulfur-Containing Histidine Compounds with Human Gamma-Glutamyl Transpeptidase. Mar. Drugs 2019, 17, 650. [Google Scholar] [CrossRef]
- Milito, A.; Brancaccio, M.; D’ Argenio, G.; Castellano, I. Natural Sulfur-Containing Compounds: An Alternative Therapeutic Strategy against Liver Fibrosis. Cells 2019, 8, 1356. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Castellano, I.; Napolitano, A. Ovothiol: A potent natural antioxidant from marine organisms. In Blue Biotechnology: Production and Use of Marine Molecules; Barre, S., Bates, S.S., Eds.; Wiley VCH: Weinheim, Germany, 2018; pp. 583–610. [Google Scholar]
- Russo, G.L.; Russo, M.; Castellano, I.; Napolitano, A.; Palumbo, A. Ovothiol isolated from sea urchin oocytes induces autophagy in the Hep-G2 cell line. Mar. Drugs 2014, 12, 4069–4085. [Google Scholar] [CrossRef]
- Castellano, I.; Migliaccio, O.; D’Aniello, S.; Merlino, A.; Napolitano, A.; Palumbo, A. Shedding light on ovothiol biosynthesis in marine metazoans. Sci. Rep. 2016, 6, 21506. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-Like Amino Acids for Skin Photoprotection. Curr. Med. Chem. 2018, 25, 5512–5527. [Google Scholar] [CrossRef]
- Helisch, H.; Keppler, J.; Detrell, G.; Belz, S.; Ewald, R.; Fasoulas, S.; Heyer, A.G. High density long-term cultivation of Chlorella vulgaris SAG 211-12 in a novel microgravity-capable membrane raceway photobioreactor for future bioregenerative life support in SPACE. Life Sci. Space Res. 2020, 24, 91–107. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations All authors have read and agreed to the published version of the manuscript.. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milito, A.; Castellano, I.; Damiani, E. From Sea to Skin: Is There a Future for Natural Photoprotectants? Mar. Drugs 2021, 19, 379. https://doi.org/10.3390/md19070379
Milito A, Castellano I, Damiani E. From Sea to Skin: Is There a Future for Natural Photoprotectants? Marine Drugs. 2021; 19(7):379. https://doi.org/10.3390/md19070379
Chicago/Turabian StyleMilito, Alfonsina, Immacolata Castellano, and Elisabetta Damiani. 2021. "From Sea to Skin: Is There a Future for Natural Photoprotectants?" Marine Drugs 19, no. 7: 379. https://doi.org/10.3390/md19070379
APA StyleMilito, A., Castellano, I., & Damiani, E. (2021). From Sea to Skin: Is There a Future for Natural Photoprotectants? Marine Drugs, 19(7), 379. https://doi.org/10.3390/md19070379