Regulation of p53 Activity by (+)-Epiloliolide Isolated from Ulva lactuca
Abstract
1. Introduction
2. Results
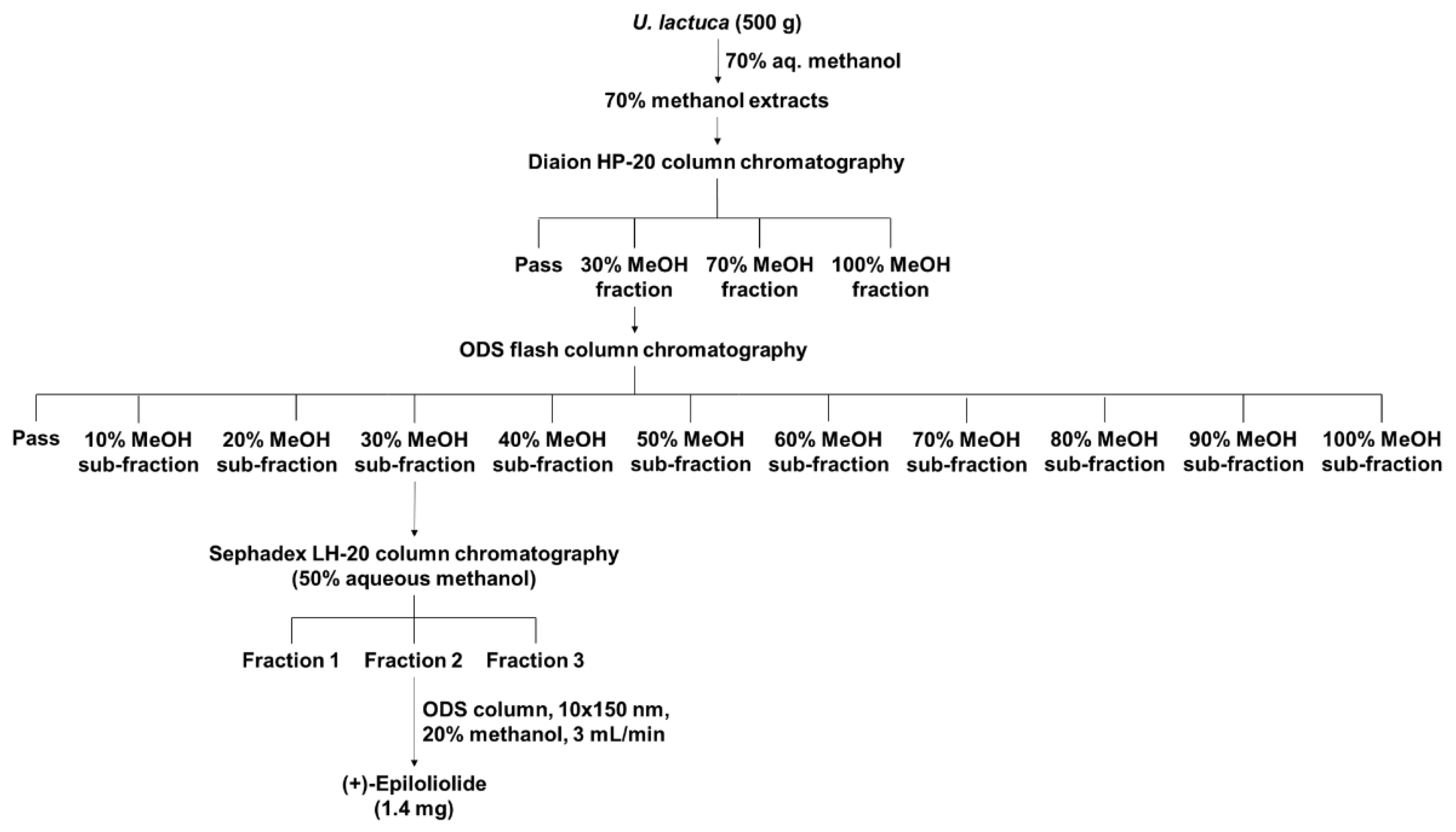
2.1. Isolation and Purification of (+)-Epiloliolide from U. lactuca Methanol Extracts
2.2. Cell Viability of (+)-Epiloliolide with or without UVB Irradiation
2.3. Gene Ontology (GO) and GSEA Pathway Analysis of (+)-Epiloliolide
2.4. Induction of p53 Nuclear Translocation and DNA Damage Reduction by (+)-Epiloliolide
2.5. Regulation of DNA Damage Response (DDR) and Apoptosis Related Proteins by (+)-Epiloliolide
2.6. Anti-Wrinkle Effects of (+)-Epiloliolide against UVB
3. Discussion
4. Materials and Methods
4.1. Algal Materials
4.2. Isolation of (+)-Epiloliolide from U. lactuca
4.3. Identification of (+)-Epiloliolide from U. lactuca
4.4. Cell Culture and Cell Viability Assay
4.5. Immunofluorescence
4.6. Immunodot Blot (IDB)
4.7. Comet Assay
4.8. Western Blotting
4.9. Reverse Transcription PCR (RT-PCR)
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yagura, T.; Makita, K.; Yamamoto, H.; Menck, C.F.M.; Schuch, A.P. Biological sensors for solar ultraviolet radiation. Sensors 2011, 11, 4277–4294. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H.J. Genome maintenance mechanisms for preventing cancer. Nature 2001, 411, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Potten, C.S.; Nikaido, O.; Parsons, P.G.; Boenders, J.; Ramsden, J.M.; Chadwick, C.A. Human melanocytes and keratinocytes exposed to UVB or UVA in vivo show comparable levels of thymine dimers. J. Investig. Dermatol. 1998, 111, 936–940. [Google Scholar] [CrossRef]
- Pfeifer, G.P.; Besaratinia, A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem. Photobiol. Sci. 2012, 11, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.; Bernstein, H.; Payne, C.M.; Garewal, H. DNA repair / pro-apoptotic dual-role proteins in five major DNA repair pathways: Fail-safe protection against carcinogenesis. Mutat. Res. 2002, 511, 145–178. [Google Scholar] [CrossRef]
- Lo, H.L.; Nakajima, S.; Ma, L.; Walter, B.; Yasui, A.; Ethell, D.W.; Owen, L.B. Differential biologic effects of CPD and 6-4PP UV-induced DNA damage on the induction of apoptosis and cell-cycle arrest. BMC Cancer 2005, 5, 135. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, A.R.; Galandáková, A.; Sianská, J.; Dolezal, D.; Lichnnova, J.; Vostalova, J. DNA damage after acute exposure of mice skin to physiological doses of UVB and UVA light. Arch. Dermatol. Res. 2012, 304, 407–412. [Google Scholar] [CrossRef]
- Fortini, P.; Parlanti, E.; Sidorkina, O.M.; Laval, J.; Dogliotti, E. The type of DNA glycosylase determines the base excision repair pathway in mammalian cells. J. Biol. Chem. 1999, 274, 15230–15236. [Google Scholar] [CrossRef]
- Dianova, I.I.; Bohr, V.A.; Dianov, G.L. Interaction of human AP endonuclease 1 with flap endonuclease 1 and proliferating cell nuclear antigen involved in long-patch base excision repair. Biochemistry 2001, 40, 12639–12644. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Kumar, A.R.; Yuagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation UV-induced DNA damage and reapir. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Kusakabe, M.; Onishi, Y.; Tada, H.; Kurihara, F.; Kusao, K.; Furukawa, M.; Iwai, S.; Yokoil, M.; Sakail, W. Mechanism and regulation of DNA damage recognition in nucleotide excision repair. Gene Environ. 2019, 41, 414. [Google Scholar] [CrossRef]
- Yeo, J.E.; Khoo, A.; Fagbemi, A.F.; Schärer, O.D. The efficiencies of damage recognition and excision correlate with duplex destabilization induced by acetylaminofluorene adducts in human nucleotide excision repair. Chem. Res. Toxicol. 2012, 25, 2462–2468. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Fischer, E.S.; Yasuda, T.; Dohmae, N.; Iwai, S.; Mori, T.; Nishi, R.; Yoshino, K.; Sakai, W.; Hanaoka, F.; et al. Functional regulation of the DNA damage-recognition factor DDB2 by ubiquitination and interaction with xeroderma pigmentosum group C protein. Nucleic Acids Res. 2015, 43, 1700–1713. [Google Scholar] [CrossRef]
- Fousteri, M.; Vermeulen, W.; Zeeland, A.A.; Mullenders, L.H.F. Cockayne syndrome A and B proteins differentially regulate recruitment of chromatin remodeling and repair factors to stalled RNA polymerase II in vivo. Mol. Cell. 2006, 23, 471–482. [Google Scholar] [CrossRef]
- Kemp, M.G.; Sancar, A. DNA excision repair. Annu. Rev. Biochem. 1996, 65, 43–81. [Google Scholar] [CrossRef]
- Xiao, G.; Chicas, A.; Olivier, M.; Taya, Y.; Tyagi, S.; Kramer, F.R.; Bargonetti, J. A DNA damage signal is required for p53 to activate gadd45. Cancer Res. 2000, 60, 1711–1719. [Google Scholar]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold. Spring. Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Qin, J.; Srivenugopal, K.S.; Wang, M.; Zhang, R. The MDM2-p53 pathway revisited. J. Biomed. Res. 2013, 27, 254–271. [Google Scholar]
- Stadler, J.; Richly, H. Regulation of DNA Repair Mechanisms: How the Chromatin Environment Regulates the DNA Damage Response. Int. J. Mol. Sci. 2017, 18, 1715. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Chen, L.; Li, Z.; Lane, W.S.; Chen, J. ATM activates p53 by regulating MDM2 oligomerization and E3 processivity. EMBO J. 2009, 28, 3857–3867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jin, S.; Antinore, M.J.; Lung, F.D.; Fan, F.; Blanck, P.; Roller, P.; Fornace Jr, A.J.; Zhan, Q. The central region of Gadd45 is required for its interaction with p21/WAF1. Exp. Cell. Res. 2000, 258, 92–100. [Google Scholar] [CrossRef]
- Pustisek, N.; Situm, M. UV-radiation, apoptosis and skin. Coll. Antropol. 2011, 2, 339–341. [Google Scholar]
- Fernando, I.P.S.; Nah, J.W.; Jeon, Y.J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, D.; Jyoti, P.; Savan, D.; Suumitra, C. Pharmacognostic standardization of Chaetomorpha antennina and Ulva lactuca, green seaweeds from Gujarat coast. JPP 2018, 7, 3863–3870. [Google Scholar]
- Abirami, R.G.; Kowsalya, S. Nutirent and nutraceutical potentials of seaweed biomass Ulva lactuca and Kappaphycus alvarezii. JAST 2011, 5, 109–115. [Google Scholar]
- Dominguez, H.; Loret, E.P. Ulva lactuca, A source of troubles and potential riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.I.; Baky, H.H.; EI Baroty, G.S. Potential of macroalgae Ulva lactuca as a source feedstock for biodiesel production. Recent Pat. Food Nutr. Agric. 2017, 8, 199–204. [Google Scholar]
- El-baky, H.H.A.; El-baz, F.K.; El-baroty, G.S. Evaluation of marine alga Ulva lactuca L. as source of natural prservative ingredient. Am. Eurasian J. Agric. Environ. Sci. 2008, 3, 434–444. [Google Scholar]
- Li, H.; Lee, S.M.; Lee, D.G.; Lee, J.H.; Ha, B.J.; Jang, J.S.; Kim, W.S.; Ha, J.M. Antioxidant activities of Ulva lactuca extracts with different solvents. J. Life Sci. 2008, 17, 51–55. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Sivakumar, S.R. Antifungal activity of seaweed Ulva lactuca L. extracted crude protein against pathogenic fungi. Asian J. Pharm. Clin. Res. 2019, 12, 393–396. [Google Scholar]
- Abd-Ellatef, G.F.; Ahmed, O.M.; Abdel-Reheim, E.; Abdel-Hamid, A.Z. Ulva lactuca polysaccharides prevent Wistar rat breast carcinogenesis through the augmentation of apoptosis, enhancement of antioxidant defense system, and suppression of inflammation. Breast Cancer 2017, 27, 67–83. [Google Scholar]
- Erosa-Rejón, G.; Peña-Rodríguez, L.M.; Sterner, O. Secondary Metabolites from Heliotropium angiospermum. J. Mex. Chem. Soc. 2009, 53, 44–47. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Kim, H.S.; Han, E.J.; Kim, M.J. Effects of (–)-Loliolide against Fine Dust Preconditioned Keratinocyte Media-Induced Dermal Fibroblast Inflammation. Antioxidant 2021, 10, 675. [Google Scholar] [CrossRef]
- Gangadhar, K.M.; Rodrigues, M.J.; Pereira, H.; Gaspar, H.; Malcata, F.X.; Barreira, L.; Varela, J. Anti-Hepatocellular Carcinoma (HepG2) Activities of Monoterpene Hydroxy Lactones Isolated from the Marine Microalga Tisochrysis Lutea. Mar. Drugs 2020, 18, 567. [Google Scholar] [CrossRef]
- Jeong, S.; Chung, Y.; Park, J.K. Protective effects of Ulva lactuca methanol extracts against the ultraviolet B-induced DNA damage. Korean J. Food Nutr. 2020, 33, 309–316. [Google Scholar]
- Mahabir, S.; Wei, Q.; Barrera, S.L.; Dong, Y.Q.; Etzel, C.J.; Spitz, M.R.; Forman, M.R. Dietary magnesium and DNA repair capacity as risk factors for lung cancer. Carcinogenesis 2008, 29, 949–956. [Google Scholar] [CrossRef]
- Bardagjy, A.S.; Hu, Q.; Giebler, K.A.; Ford, A.; Steinberg, F.M. Effects of grape consumption on biomarkers of inflammation, endothelial function, and PBMC gene expression in obese subjects. Arch. Biochem. Biophys. 2018, 646, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Itoh, K.; Yamamoto, M.; Zweier, J.L.; Li, Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: Protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005, 579, 3029–3036. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. UV, stress and aging. Dermato-Endocrinology 2012, 4, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Greussing, R.; Hackl, M.; Charoentong, P.; Pauck, A.; Monteforte, R.; Cavinato, M.; Hofer, E.; Scheideler, M.; Neuhaus, M.; Micutkova, L.; et al. Identification of microRNA-mRNA functional interactions in UVB-induced senescence of human diploid fibroblasts. BMC Genom. 2013, 14, 224. [Google Scholar] [CrossRef]
- Waldera Lupa, D.M.; Kalfalah, F.; Safferling, K.; Boukamp, P.; Poschmann, G.; Volpi, E.; Götz-Rösch, C.; Bernerd, F.; Haag, L.; Huebenthal, U.; et al. Characterization of skin aging-associated secreted proteins (SAASP) produced by dermal fibroblasts isolated from intrinsically aged human skin. J. Investig. Dermatol. 2015, 135, 1954–1968. [Google Scholar] [CrossRef]
- Cavinato, M.; Jansen- Dürr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.B.; Chen, C.; Smeets, M.; Hengst, L.; Prives, C.; Reed, S.I. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol. Cell. Biol. 1998, 18, 629–643. [Google Scholar] [CrossRef]
- Byun, S.; Namba, T.; Lee, S.W. Losing p53 loosens up ER-stress. Aging 2015, 7, 895–896. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maeda, T.; Hanna, A.N.; Sim, A.B.; Chua, P.P.; Chong, M.T.; Tron, V.A. GADD45 regulates G2/M arrest, DNA repair, and cell death in keratinocytes following ultraviolet exposure. J. Investig. Dermatol. 2002, 119, 22–26. [Google Scholar] [CrossRef]
- Gilljam, K.M.; Müller, R.; Liabakk, N.B.; Otterlei, M. Nucleotide excision repair is associated with the replisome and its efficiency depends on a direct interaction between XPA and PCNA. PLoS ONE 2012, 7, e49199. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.; Jeong, S.; Lee, I.-K.; Yun, B.-S.; Lee, J.S.; Ro, S.; Park, J.K. Regulation of p53 Activity by (+)-Epiloliolide Isolated from Ulva lactuca. Mar. Drugs 2021, 19, 450. https://doi.org/10.3390/md19080450
Chung Y, Jeong S, Lee I-K, Yun B-S, Lee JS, Ro S, Park JK. Regulation of p53 Activity by (+)-Epiloliolide Isolated from Ulva lactuca. Marine Drugs. 2021; 19(8):450. https://doi.org/10.3390/md19080450
Chicago/Turabian StyleChung, Yuheon, Seula Jeong, In-Kyoung Lee, Bong-Sik Yun, Jung Sup Lee, Seungil Ro, and Jong Kun Park. 2021. "Regulation of p53 Activity by (+)-Epiloliolide Isolated from Ulva lactuca" Marine Drugs 19, no. 8: 450. https://doi.org/10.3390/md19080450
APA StyleChung, Y., Jeong, S., Lee, I.-K., Yun, B.-S., Lee, J. S., Ro, S., & Park, J. K. (2021). Regulation of p53 Activity by (+)-Epiloliolide Isolated from Ulva lactuca. Marine Drugs, 19(8), 450. https://doi.org/10.3390/md19080450






