Cytotoxic Polyketide Metabolites from a Marine Mesophotic Zone Chalinidae Sponge-Associated Fungus Pleosporales sp. NBUF144
Abstract
1. Introduction
2. Results and Discussion
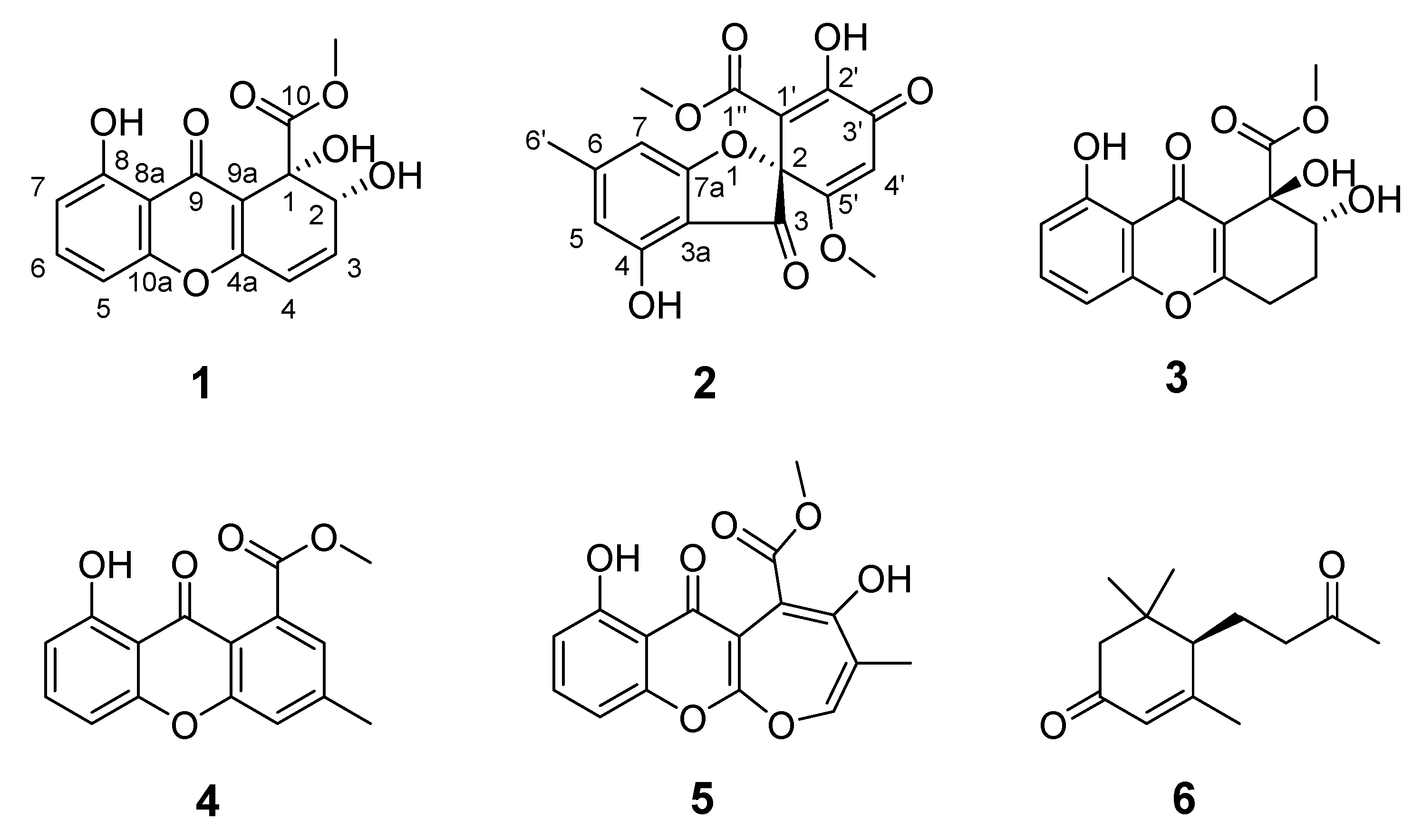
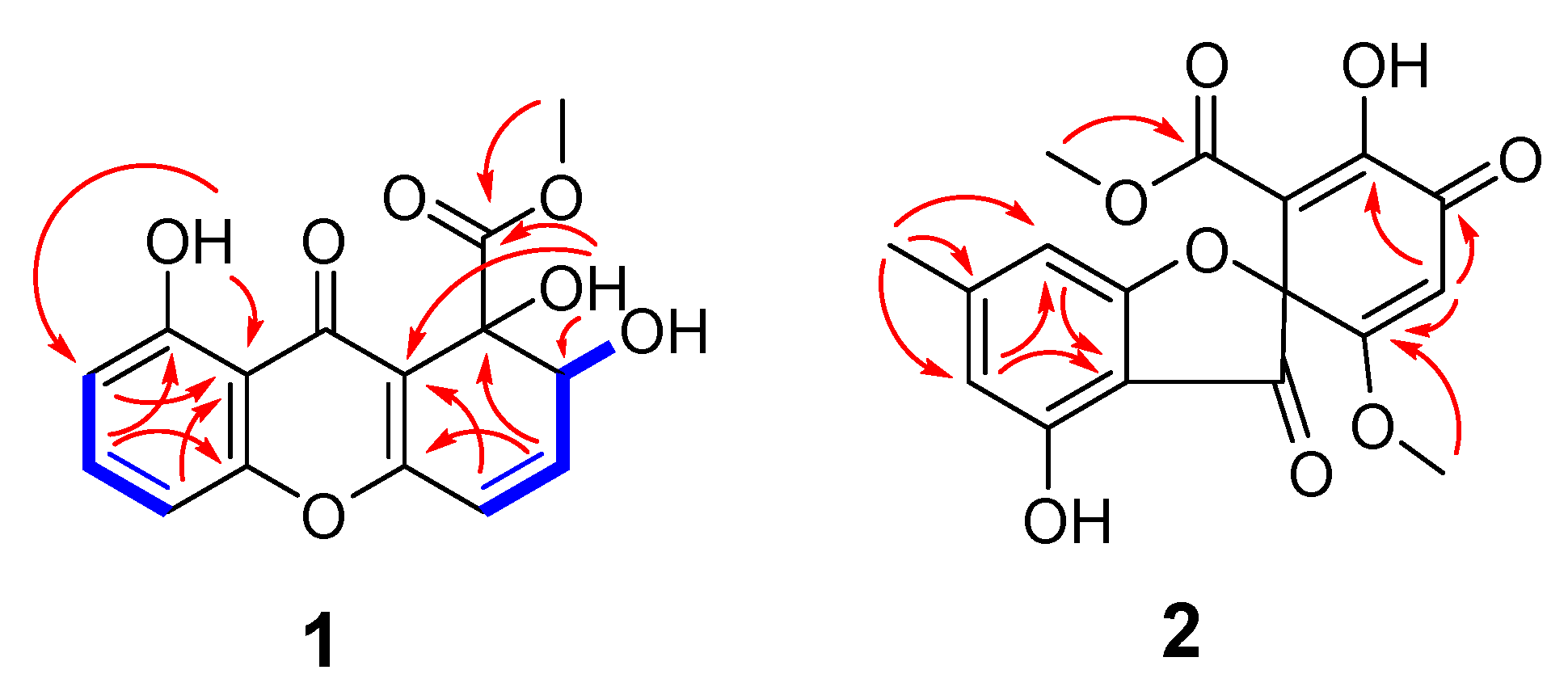
2.1. Structure Elucidation and Identification of Compounds
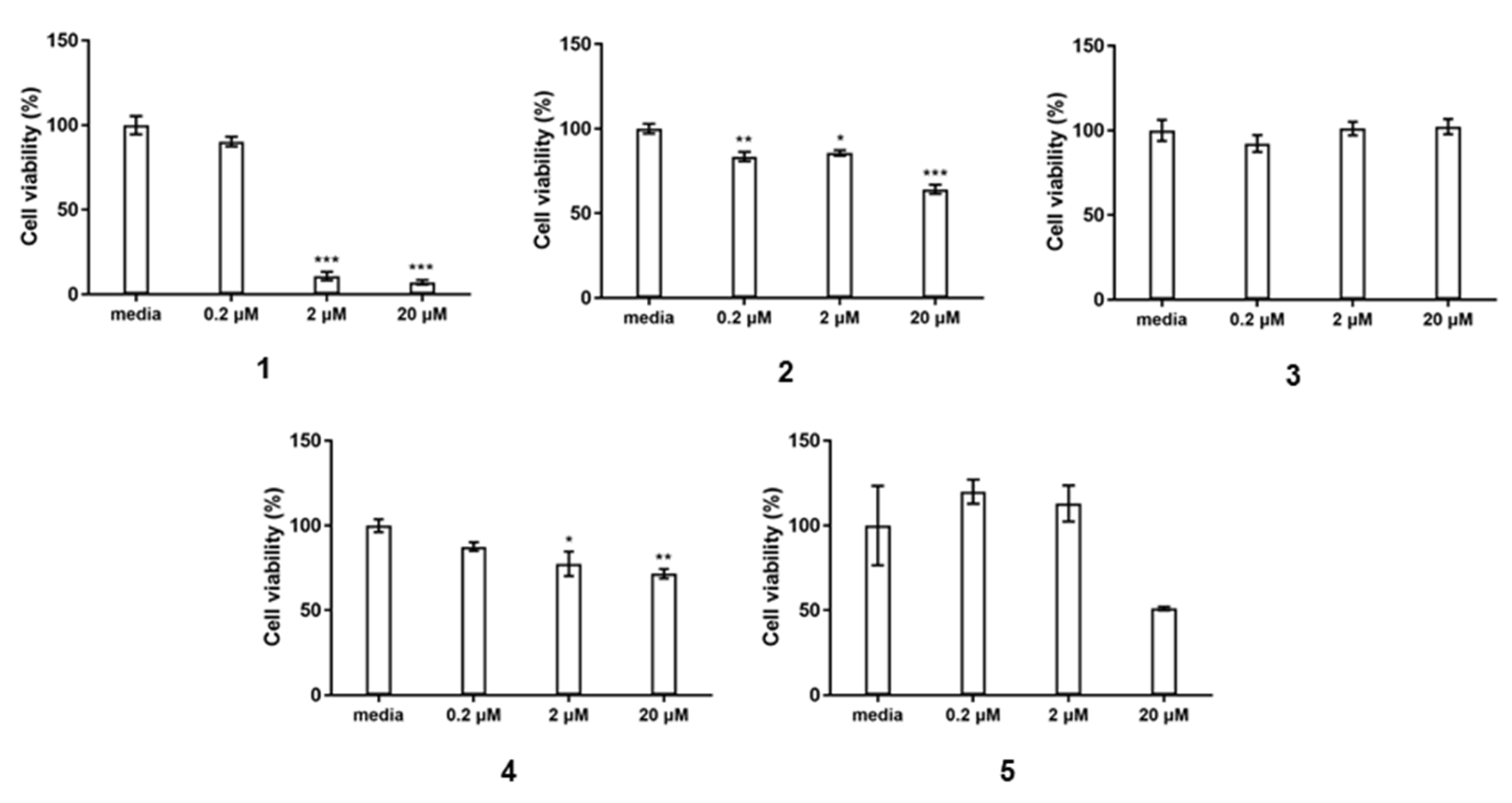
2.2. Bioactivity Assay
3. Materials and Methods
3.1. General
3.2. Fungal Strains
3.3. Cultivation of the Fungi
3.4. Extraction and Isolation
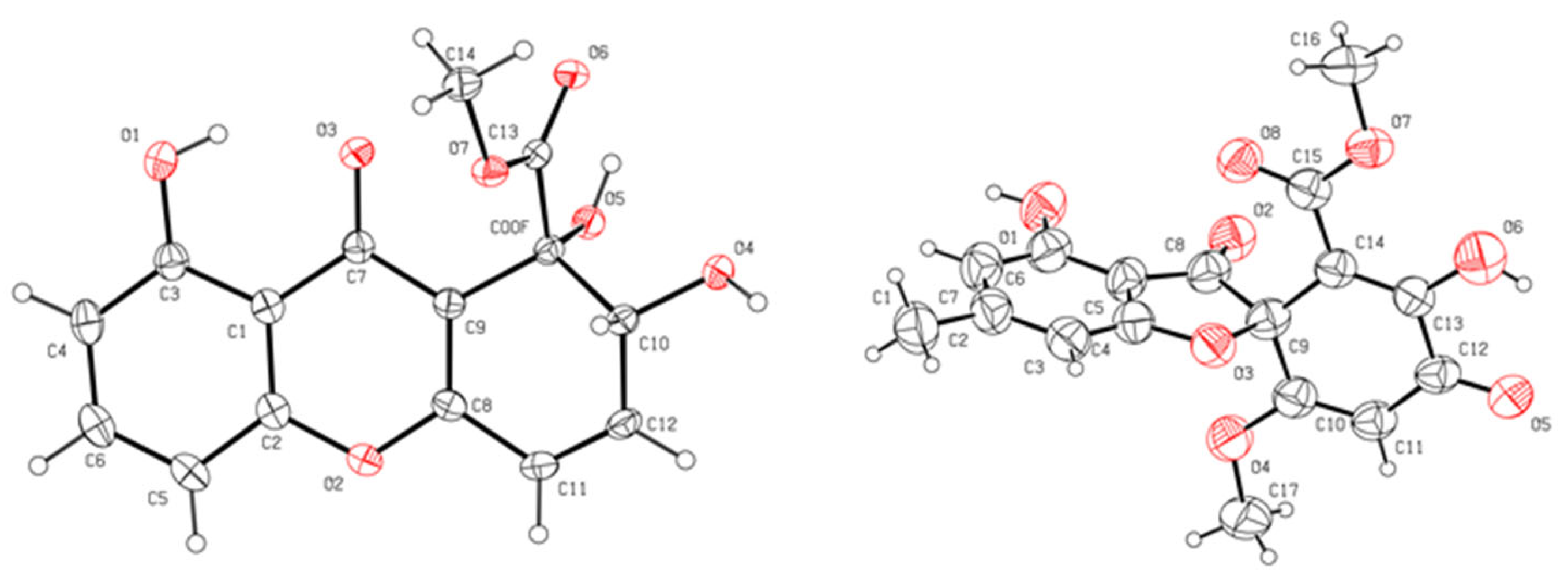
3.5. Single-Crystal X-ray Diffraction Analysis
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahidin, I.; Sabandar, C.W.; Wahyuni; Hamsidi, R.; Mardikasari, S.A.; Zubaydah, W.O.S.; Sadarun, B.; Musnina, W.O.S.; Darmawan, A.; Sundowo, A. Investigation of Compounds and Biological Activity of Selected Indonesian Marine Sponges. Nat. Prod. J. 2020, 10, 312–321. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Haidari, R.A.A.; Mohamed, G.A. Megaspinoxide A: New Norterpene Cyclic Peroxide from the Sponge Diacarnus megaspinorhabdosa. Nat. Prod. J. 2014, 4, 38–42. [Google Scholar]
- Rangnekar, S.S.; Khan, T. Novel Anti-inflammatory Drugs from Marine Microbes. Nat. Prod. J. 2015, 5, 206–218. [Google Scholar] [CrossRef]
- Mayer, A.M.; Rodríguez, A.D.; Berlinck, R.G.; Fusetani, N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Hamann, M.T. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 283–308. [Google Scholar]
- Kiran, G.S.; Sekar, S.; Ramasamy, P.; Thinesh, T.; Hassan, S.; Lipton, A.N.; Ninawe, A.S.; Selvin, J. Marine sponge microbial association: Towards disclosing unique symbiotic interactions. Mar. Environ. Res. 2018, 140, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S.; Thomas, T. The sponge hologenome. mBio 2016, 7, e00135-16. [Google Scholar] [CrossRef]
- Vacelet, J. Etude en microscopie electronique de l’association entre bacteries et spongiaires du genre Verongia (Dictyoceratida). J. Microsc. Biol. Cell 1975, 23, 271–288. [Google Scholar]
- Easson, C.G.; Thacker, R.W. Phylogenetic signal in the community structure of host-specific microbiomes of tropical marine sponges. Front. Microbiol. 2014, 5, 532. [Google Scholar] [CrossRef]
- Tian, R.M.; Wang, Y.; Bougouffa, S.; Gao, Z.M.; Cai, L.; Bajic, V.; Qian, P.Y. Genomic analysis reveals versatile heterotrophic capacity of a potentially symbiotic sulfur-oxidizing bacterium in sponge. Environ. Microbiol. 2014, 16, 3548–3561. [Google Scholar] [CrossRef]
- Li, W.; Jiao, F.W.; Wang, J.Q.; Shi, J.; Wang, T.T.; Khan, S.; Jiao, R.H.; Tan, R.X.; Ge, H.M.; Unguisin, G. A new kynurenine-containing cyclic heptapeptide from the spong-associated fungus Aspergillus candidus NF2412. Tetrahedron Lett. 2020, 61, 152322. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Lin, S.T.; Kumaravel, K.; Zhou, H.; Wang, S.Y.; Liu, Y.H. Polyketide-derived metabolites from the sponge-derived fungus Aspergillus sp. F40. Phytochem. Lett. 2018, 27, 74–77. [Google Scholar] [CrossRef]
- Özkaya, F.C.; Ebrahim, W.; El-Neketi, M.; Tanrıkul, T.T.; Kalscheuer, R.; Müller, W.E.G.; Guo, Z.Y.; Zou, K.; Liu, Z.; Proksch, P. Induction of new metabolites from sponge-associated fungus Aspergillus carneus by OSMAC approach. Fitoterapia 2018, 131, 9–14. [Google Scholar] [CrossRef]
- Huang, L.M.; Ding, L.J.; Li, X.H.; Wang, N.; Yan, Y.S.; Yang, M.X.; Cui, W.; Naman, C.B.; Cheng, K.J.; Zhang, W.Y.; et al. A new lateral root growth inhibitor from the sponge-derived fungus Aspergillus sp. LS45. Bioorg. Med. Chem. Lett. 2019, 29, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.Z.; Peng, S.; Yang, J.; Cong, Z.W.; Lin, X.P.; Liao, S.R.; Yang, B.; Zhou, X.F.; Zhou, X.J.; Liu, Y.H.; et al. Structurally diverse sesquiterpenoids and polyketides from a sponge-associated fungus Aspergillus sydowii SCSIO41301. Fitoterapia 2019, 135, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, D.; Proksch, P.; Zhou, D.; Lin, W.H. Truncateols, O-V, further isoprenylated cyclohexanols from the sponge-associated fungus Truncatella angustata with antiviral activities. Phytochemistry 2018, 155, 61–68. [Google Scholar] [CrossRef]
- Lei, H.; Lin, X.P.; Han, L.; Ma, J.; Dong, K.L.; Wang, X.B.; Zhong, J.L.; Mu, Y.; Liu, Y.H.; Huang, X.S. Polyketide derivatives from a marine-sponge-associated fungus Pestalotiopsis heterocornis. Phytochemistry 2017, 142, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, S.; Chen, Y.; Han, X.; Gu, Q.; Yin, Y.; Ma, Z. The microtubule end-binding protein FgEB1 regulates polar growth and fungicide sensitivity via different interactors in Fusarium graminearum. Environ. Microbial. 2017, 19, 1791–1807. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhao, J.; Ding, L.J.; Huang, C.; Naman, C.B.; He, S.; Wu, B.; Zhu, P.; Luo, Q.J.; Gerwick, W.H.; et al. 5-Hydroxycyclopenicillone, a new β-amyloid fibrillization inhibitor from a sponge-derived fungus Trichoderma sp. HPQJ-34. Mar. Drugs 2017, 15, 260. [Google Scholar] [CrossRef]
- Weiss, K.R. Into the twilight zone. Science 2017, 355, 900–904. [Google Scholar] [CrossRef]
- Pyle, R.L.; Copus, J.M.; Loya, Y.; Puglise, K.A.; Bridge, T. Mesophotic Coral Ecosystems: Introduction and Overview. In Mesophotic Coral Ecosystems; Springer: New York, NY, USA, 2019; pp. 3–27. [Google Scholar]
- Wang, T.T.; Wei, Y.J.; Ge, H.M.; Jiao, R.H.; Tan, R.X. Acaulide, an osteogenic macrodiolide from Acaulium sp. H-JQSF, an isopod-associated Fungus. Org. Lett. 2018, 20, 1007–1010. [Google Scholar] [CrossRef]
- Wang, T.T.; Wei, Y.J.; Ge, H.M.; Jiao, R.H.; Tan, R.X. Acaulins A and B, trimeric macrodiolides from Acaulium sp. H-JQSF. Org. Lett. 2018, 20, 2490–2493. [Google Scholar] [CrossRef]
- Höller, U.; Wright, A.D.; Matthee, G.F.; Konig, G.M.; Draeger, S.; Aust, H.J.; Schulz, B. Fungi from marine sponges: Diversity, biological activity and secondary metabolites. Mycol. Res. 2000, 104, 1354–1365. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Z.; Liu, D.Z.; Yu, Z.F. Memnoniella sinensis sp. nov. A new species from China and a key to species of the genus. Int. J. Syst. Evol. Microbiol. 2019, 69, 3161–3169. [Google Scholar] [CrossRef]
- Wang, T.T.; Zhou, J.; Zou, J.B.; Shi, Y.T.; Zhou, W.L.; Shao, P.; Yu, T.Z.; Cui, W.; Li, X.H.; Wu, X.X.; et al. Discovery of cymopolyphenols A-F from a marine mesophotic zone Aaptos sponge-associated fungus Cymostachys sp. NBUF082. Front. Microbiol. 2021, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, E.M.K.; Turbyville, T.J.; Fritz, A.; Whitesellb, L.; Gunatilaka, A.A.L. A new dihydroxanthenone from a plant-associated strain of the fungus Chaetomium globosum demonstrates anticancer activity. Bioorg. Med. Chem. 2006, 14, 7917–7923. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Krohn, K.; Floerke, U.; Schulz, B.; Draeger, S.; Pescitelli, G.; Antus, S.; Kurtán, T. Absolute configurations of globosuxanthone A and secondary metabolites from Microdiplodia sp.—A novel solid-state CD/TDDFT approach. Eur. J. Org. Chem. 2007, 292–295. [Google Scholar] [CrossRef]
- Yamazaki, H.; Rotinsulu, H.; Kaneko, T.; Murakami, K.; Fujiwara, H.; Ukai, K.; Namikoshi, M. A new dibenz [b, e] oxepine derivative, 1-hydroxy-10-methoxy-dibenz [b, e] oxepin-6, 11-dione, from a marine-derived fungus, Beauveria bassiana TPU942. Mar. Drugs 2012, 10, 2691–2697. [Google Scholar] [CrossRef]
- Flack, H.D.; Bernardinelli, G. Absolute structure and absolute configuration. Acta Cryst. 1999, 55, 908–915. [Google Scholar] [CrossRef]
- Mahmoodian, A.; Stickings, C.E. Studies in the biochemistry of micro-organisms. Biochem. J. 1964, 92, 369–378. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoshitake; Matsuzaki, K.; Zhong, C.L.; Yoshida, H.; Kawakubo, T.; Masuma, R.; Tanaka, H.; Omura, S. Dechlorogeodin and its new dihydro derivatives, fungal metabolites with herbicidal activity. J. Antibiot. 1996, 49, 1056–1059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- NordlÖv, H.; Gatenbeck, S. Enzymatic synthesis of (+)- and (−)-bisdechlorogeodin with sulochrin oxidase from Penicillium frequentans and Oospora sulphurea-ochracea. Arch. Microbiol. 1982, 131, 208–211. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Huang, Q.-L.; Chen, J.; Zhang, W.-J.; Jin, H.-X.; Wang, H.-B.; Naman, C.B.; Cao, Z.-Y. Phthalideisoquinoline hemiacetal alkaloids from Corydalis decumbens that inhibit spontaneous calcium oscillations, including alkyl derivatives of (+)-egenine that are strikingly levorotatory. J. Nat. Prod. 2019, 82, 2713–2720. [Google Scholar] [CrossRef]
- Krohn, K.; Kouam, S.F.; Kuigoua, G.M.; Hussain, H.; Cludius-Brandt, S.; Flörke, U.; Kurtán, T.; Pescitelli, G.; Di Bari, L.; Draeger, S.; et al. Xanthones and oxepino[2, 3-b]chromones from three endophytic fungi. Chem. Eur. J. 2010, 15, 12121–12132. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zhang, J.; Shao, C.L.; Ding, W.J.; She, Z.G.; Lin, Y.C. A new xanthone derivative from the co-culture broth of two marine fungi (strain No. E33 and K38). Chem. Nat. Comp. 2011, 47, 382–384. [Google Scholar] [CrossRef]
- Xiao, W.L.; Chen, W.H.; Zhang, J.Y.; Song, X.P.; Chen, G.Y.; Han, C.R. Ionone-type sesquiterpenoids from the stems of Ficus Pumila. Chem. Nat. Comp. 2011, 52, 531–533. [Google Scholar] [CrossRef]
- Williams, R.B.; Martin, S.M.; Lawrence, J.A.; Norman, V.L.; O’Neil-Johnson, M.; Eldridge, G.R.; Starks, C.M. Isolation and identification of the novel tubulin polymerization inhibitor bifidenon. J. Nat. Prod. 2017, 80, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, P.D.; Byrum, T.; Liu, W.T.; Dorrestein, P.C.; Gerwick, W.H. Viequeamide A, a cytotoxic member of the kulolide superfamily of cyclic depsipeptides from a marine button Cyanobacterium. J. Nat. Prod. 2012, 75, 1560–1570. [Google Scholar] [CrossRef]
| Pos. | 1 | Pos. | 2 | ||
|---|---|---|---|---|---|
| δC, mult | δH, Mult (J in Hz) | δC, mult | δH, Mult (J in Hz) | ||
| 1 | 73.2, C | 1 | |||
| 2 | 71.5, CH | 4.90 d (9.7) | 2 | 93.4, C | |
| 3 | 143.8, CH | 6.50 d (9.8) | 3 | 198.1, C | |
| 4 | 120.5, CH | 6.32 d (9.8) | 3a | 108.2, C | |
| 4a | 160.9, C | 4 | 171.8, C | ||
| 5 | 107.3, CH | 6.90 d (8.3) | 5 | 104.6, CH | 6.40, s |
| 6 | 135.8, CH | 7.52 t (8.3) | 6 | 152.7, C | |
| 7 | 112.1, CH | 6.80 d (8.2) | 7 | 109.0, CH | 6.35, s |
| 8 | 160.8, C | 7a | 155.8, C | ||
| 8a | 111.0, C | 1′ | 149.6, C | ||
| 9 | 180.7, C | 2′ | 148.3, C | ||
| 9a | 113.7, C | 3′ | 180.9, C | ||
| 10 | 174.6, C | 4′ | 102.5, CH | 5.82, s | |
| 10a | 155.5, C | 5′ | 171.2, C | ||
| 1-OH | 4.42 s | 6′ | 23.2, CH3 | 2.39, s | |
| 2-OH | 2.93 s | 1″ | 167.7, C | ||
| 8-OH | 12.13 s | 1″-OCH3 | 51.4, CH3 | 3.44, s | |
| 10-OCH3 | 54.4, CH3 | 3.92 s | 5′-OCH3 | 57.1, CH3 | 3.69, s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhang, H.; Ye, J.; Wu, X.; Wang, W.; Lin, H.; Yan, X.; Lazaro, J.E.H.; Wang, T.; Naman, C.B.; et al. Cytotoxic Polyketide Metabolites from a Marine Mesophotic Zone Chalinidae Sponge-Associated Fungus Pleosporales sp. NBUF144. Mar. Drugs 2021, 19, 186. https://doi.org/10.3390/md19040186
Zhou J, Zhang H, Ye J, Wu X, Wang W, Lin H, Yan X, Lazaro JEH, Wang T, Naman CB, et al. Cytotoxic Polyketide Metabolites from a Marine Mesophotic Zone Chalinidae Sponge-Associated Fungus Pleosporales sp. NBUF144. Marine Drugs. 2021; 19(4):186. https://doi.org/10.3390/md19040186
Chicago/Turabian StyleZhou, Jing, Hairong Zhang, Jing Ye, Xingxin Wu, Weiyi Wang, Houwen Lin, Xiaojun Yan, J. Enrico H. Lazaro, Tingting Wang, C. Benjamin Naman, and et al. 2021. "Cytotoxic Polyketide Metabolites from a Marine Mesophotic Zone Chalinidae Sponge-Associated Fungus Pleosporales sp. NBUF144" Marine Drugs 19, no. 4: 186. https://doi.org/10.3390/md19040186
APA StyleZhou, J., Zhang, H., Ye, J., Wu, X., Wang, W., Lin, H., Yan, X., Lazaro, J. E. H., Wang, T., Naman, C. B., & He, S. (2021). Cytotoxic Polyketide Metabolites from a Marine Mesophotic Zone Chalinidae Sponge-Associated Fungus Pleosporales sp. NBUF144. Marine Drugs, 19(4), 186. https://doi.org/10.3390/md19040186









