The Inhibition Effect of the Seaweed Polyphenol, 7-Phloro-Eckol from Ecklonia Cava on Alcohol-Induced Oxidative Stress in HepG2/CYP2E1 Cells
Abstract
1. Introduction
2. Results
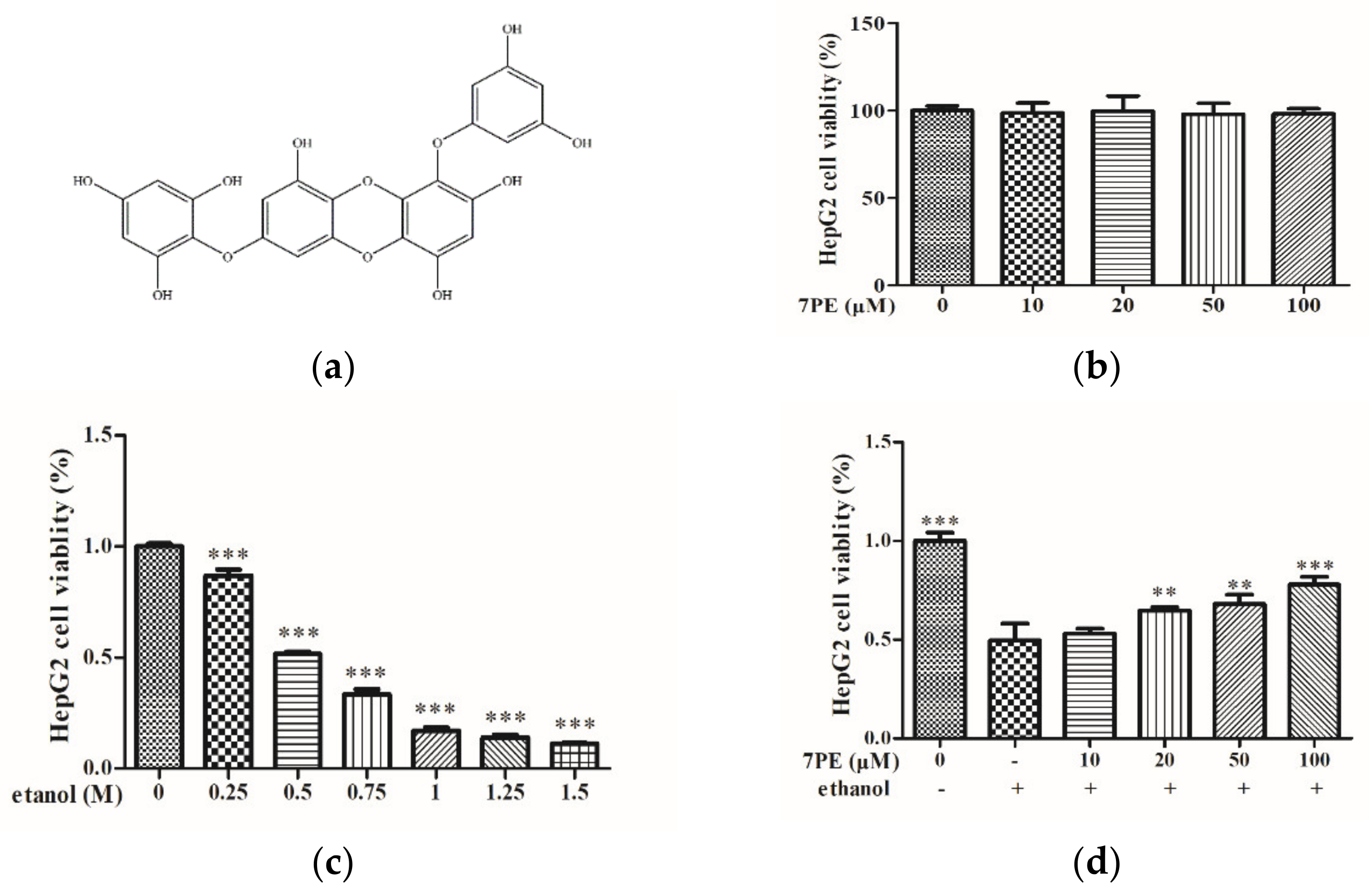
2.1. Effects of 7PE on Cell Viability of HepG2/CYP2E1 Cells
2.2. Determination of Intracellular ROS and NO
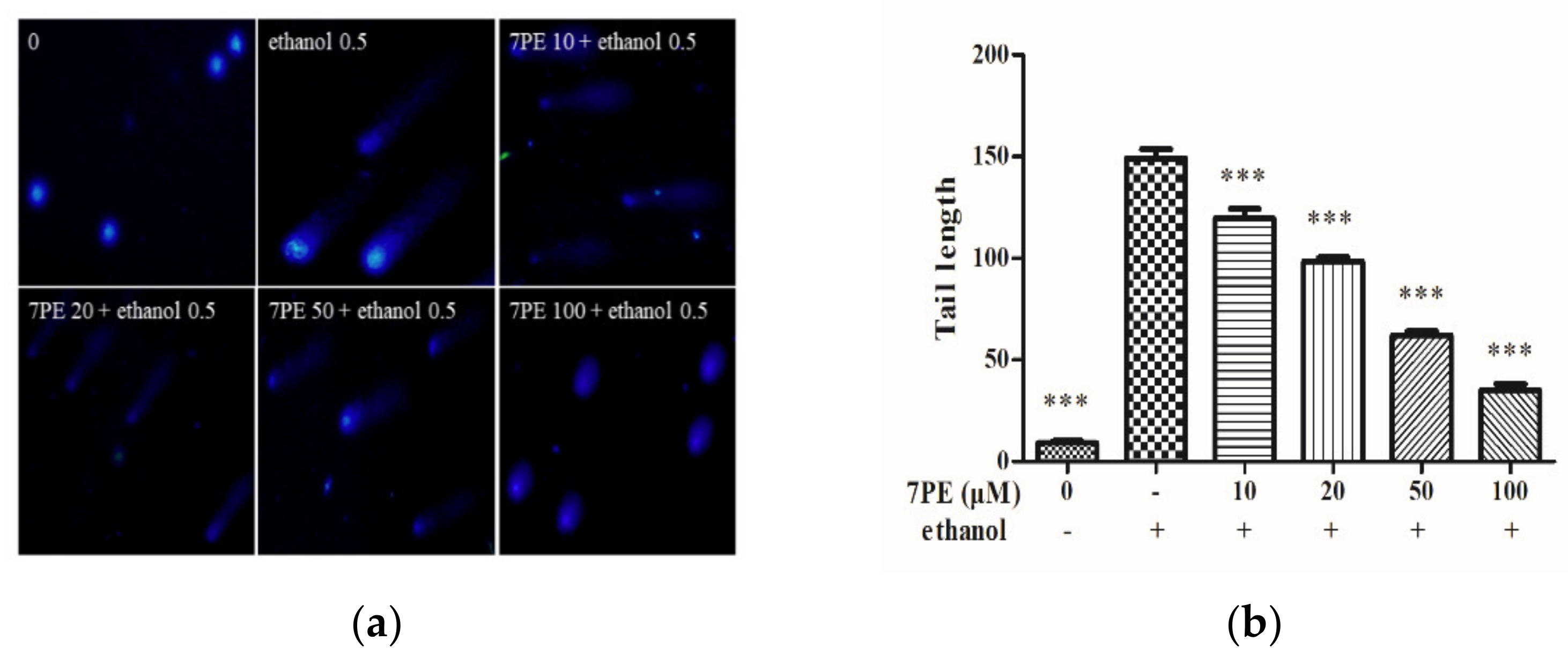
2.3. Determination of Intracellular DNA Damage
2.4. Effect of 7PE on the Level of Oxidative Stress-Related Proteins
2.5. Detection of Related Apoptosis Proteins
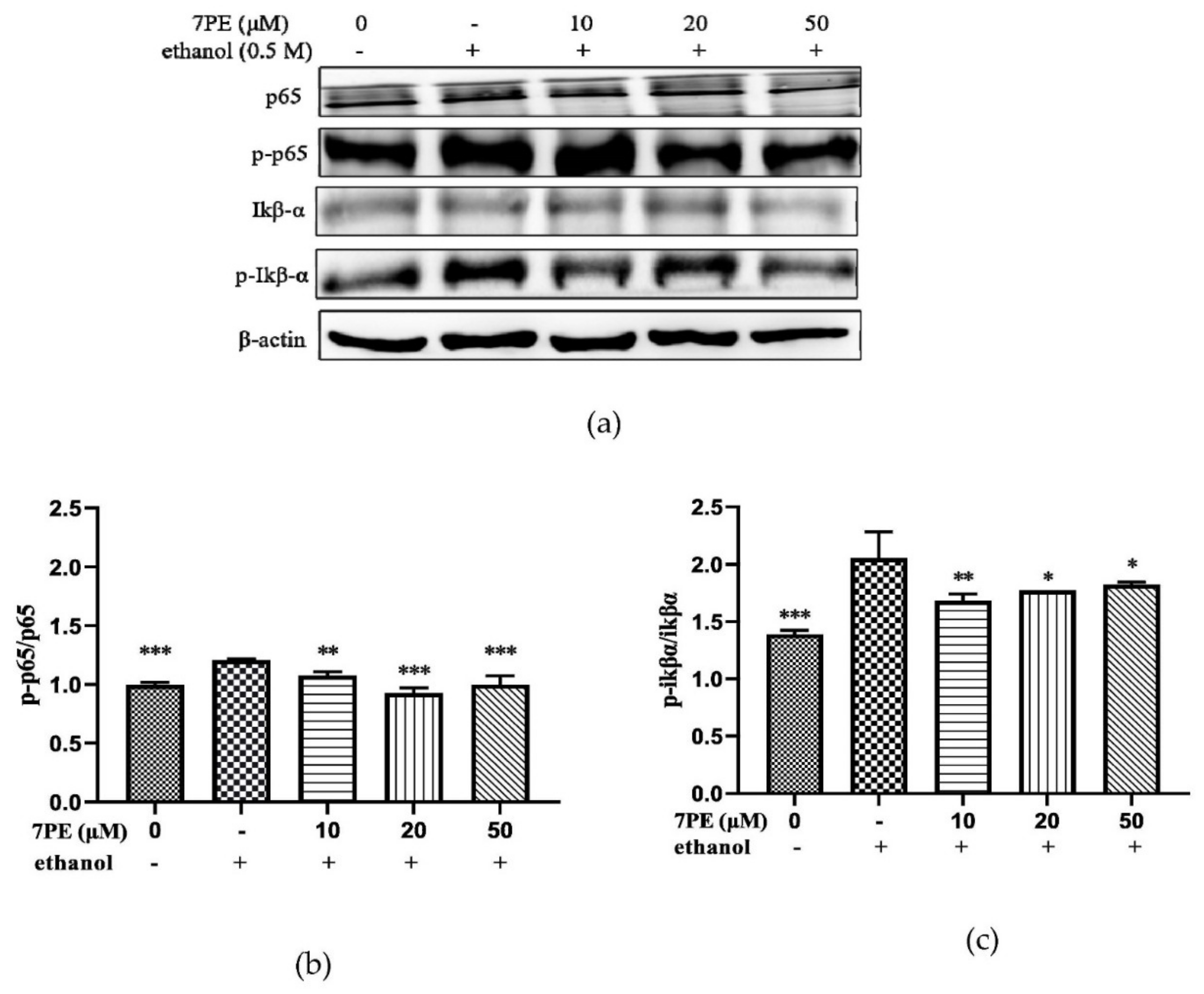
2.6. Effect of 7PE on the NF-κB Signal Pathway
2.7. JNK and p53 Protein Levels
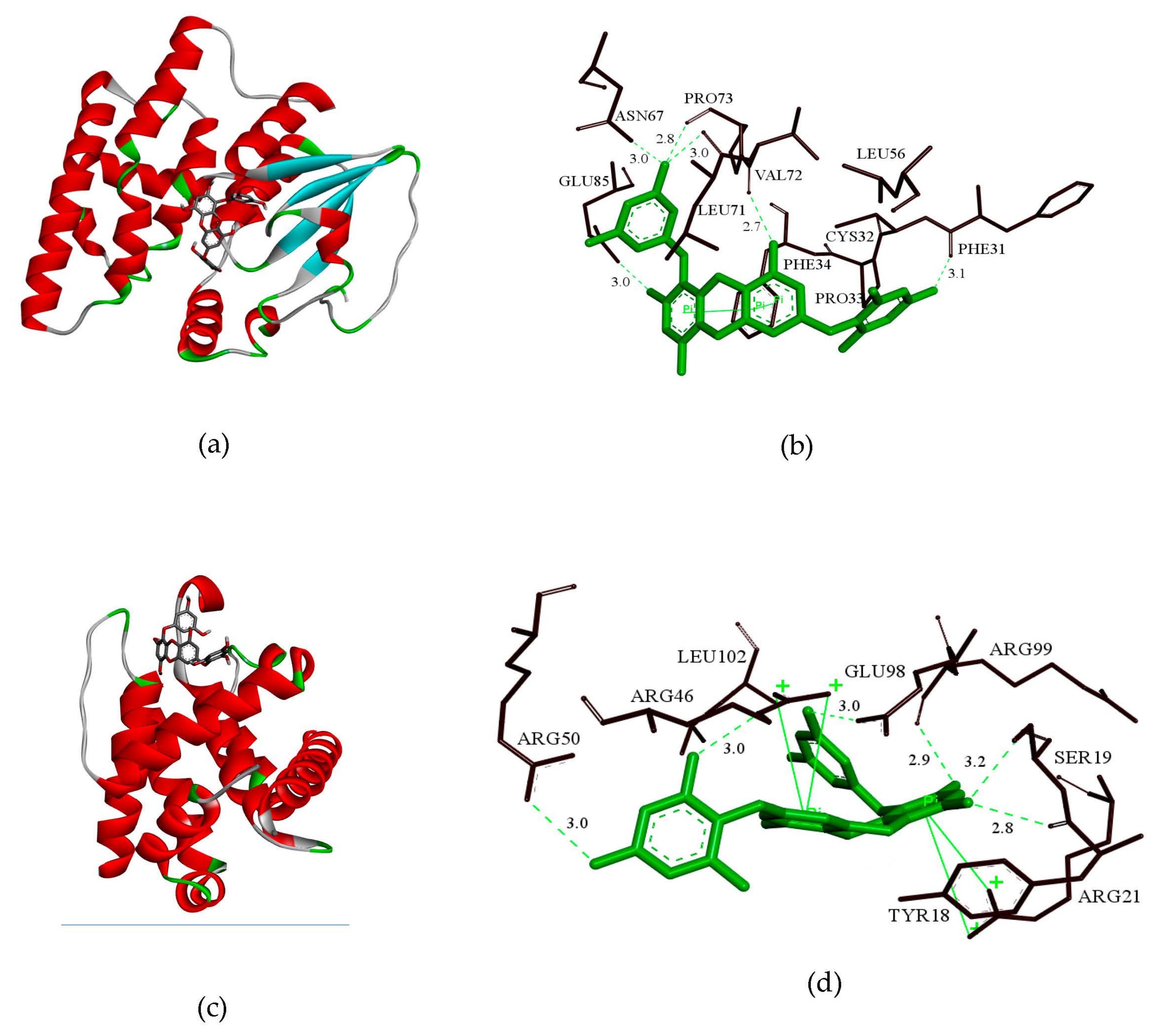
2.8. GSH and bcl-2 Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Cell ROS Analysis
4.5. Cell NO Analysis
4.6. Comet Assay
4.7. Western Blot
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Samir, Z. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 2006, 29, 245–254. [Google Scholar]
- Lieber, C.S. Alcohol and the liver: 1994 update. Gastroenterology 1994, 106, 1085–1105. [Google Scholar] [CrossRef]
- Wang, F.S.; Fan, J.G.; Zhang, Z.; Gao, B.; Wang, H.Y. The global burden of liver disease: The major impact of China. Hepatology 2014, 60, 2099–2108. [Google Scholar] [CrossRef]
- Orman, E.S.; Odena, G.; Bataller, R. Alcoholic liver disease: Pathogenesis, management, and novel targets for therapy. J. Gastroenterol. Hepatol. 2013, 28, 77–84. [Google Scholar] [CrossRef]
- Yang, S.T.; Chen, M.F.; Ryu, B.; Chen, J.; Xiao, Z.; Hong, P.Z.; Sun, S.L.; Wang, D.; Qian, Z.J.; Zhou, C.X. The Protective Effect of the Polysaccharide Precursor, D-Isofloridoside, from Laurencia undulata on Alcohol-Induced Hepatotoxicity in HepG2 Cells. Molecules 2020, 25, 1024. [Google Scholar] [CrossRef] [PubMed]
- Castán, A.; Navarro, Y.; Sarría, L.; Larrosa, R.; Serradilla, M.; Serrablo, A. Radiological diagnosis of hepatocellular carcinoma in non-cirrhotic patients. Hepatoma Res. 2017, 3, 1–17. [Google Scholar] [CrossRef]
- Ron, M. Oxidative stress, antioxidants and stress tolerance. Trends Plant. Sci. 2002, 7, 405–410. [Google Scholar]
- Liu, Y.; Wang, J.; Li, L.; Hu, W.; Qu, Y.; Ding, Y.; Meng, L.; Teng, L.; Wang, D. Hepatoprotective Effects of Antrodia cinnamomea: The Modulation of Oxidative Stress Signaling in a Mouse Model of Alcohol-Induced Acute Liver Injury. Oxid. Med. Cell Longev. 2017, 2017, 7841823. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Gong, F.; Zhang, Y.Y.; Li, C.Y.; Zhou, C.X.; Hong, P.Z.; Sun, S.L.; Qian, Z.J. Preventive Effect of YGDEY from Tilapia Fish Skin Gelatin Hydrolysates against Alcohol-Induced Damage in HepG2 Cells through ROS-Mediated Signaling Pathways. Nutrients 2019, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Zhang, Y.Y.; Di He, M.; Li, C.Y.; Zhou, C.X.; Hong, P.Z.; Qian, Z.J. Antioxidant Peptide Purified from Enzymatic Hydrolysates of Isochrysis Zhanjiangensis and Its Protective Effect against Ethanol Induced Oxidative Stress of HepG2 Cells. Biotechnol. Bioprocess Eng. 2019, 24, 308–317. [Google Scholar] [CrossRef]
- Singh, M.; Gupta, S.; Singhal, U.; Pandey, R.; Aggarwal, S.K. Evaluation of the oxidative stress in chronic alcoholics. J. Clin. Diagn. Res. 2013, 7, 1568–1571. [Google Scholar]
- Praetorius Bjork, M.; Johansson, B. Gamma-Glutamyltransferase (GGT) as a biomarker of cognitive decline at the end of life: Contrasting age and time to death trajectories. Int. Psychogeriatr. 2018, 30, 981–990. [Google Scholar] [CrossRef]
- Closa, D.; Folch Puy, E. Oxygen free radicals and the systemic inflammatory response. IUBMB Life 2004, 56, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Tatsuya, K.; Junya, M.; Tsuyoshi, T.; Tetsuo, N.; Kazuhiro, H.; Jun, N. Prognostic significance of the immunohistochemical staining of cleaved caspase-3, an activated form of caspase-3, in gliomas. Clin. Cancer Res. 2007, 13, 3868–3874. [Google Scholar]
- Yi, G.; Li, H.; Li, Y.; Zhao, F.; Ying, Z.; Liu, M.; Zhang, J.; Liu, X. The protective effect of soybean protein-derived peptides on apoptosis via the activation of PI3K-AKT and inhibition on apoptosis pathway. Food Sci. Nutr. 2020, 8, 4591–4600. [Google Scholar] [CrossRef]
- Jiang, T.; Tian, F.; Zheng, H.T.; Whitman Samantha, A.; Lin, Y.F.; Zhang, Z.G.; Zhang, N.; Zhang, D.N. Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB mediated inflammatory response. Kidney Int. 2014, 85, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Kluwe, J.; Pradere, J.P.; Gwak, G.Y.; Mencin, A.; De Minicis, S.; Osterreicher, C.H.; Colmenero, J.; Bataller, R.; Schwabe, R.F. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology 2010, 138, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Wali, A.F.; Majid, S.; Rasool, S.; Shehada, S.B.; Abdulkareem, S.K.; Firdous, A.; Beigh, S.; Shakeel, S.; Mushtaq, S.; Akbar, I.; et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. 2019, 27, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef]
- Scieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Zargarzadeh, M.; Amaral, A.J.R.; Custodio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef]
- Zenthoefer, M.; Geisen, U.; Hofmann Peiker, K.; Fuhrmann, M.; Kerber, J.; Kirchhöfer, R.; Hennig, S.; Peipp, M.; Geyer, R.; Piker, L.; et al. Isolation of polyphenols with anticancer activity from the Baltic Sea brown seaweed Fucus vesiculosus using bioassay-guided fractionation. J. Appl. Phycol. 2017, 29, 1007. [Google Scholar] [CrossRef]
- Cao, J.; Wang, J.; Wang, S.; Xu, X. Porphyra Species: A Mini-Review of Its Pharmacological and Nutritional Properties. J. Med. Food 2016, 19, 111–119. [Google Scholar] [CrossRef]
- Peres, J.C.F.; de Carvalho, L.R.; Goncalez, E.; Berian, L.O.S.; Felicio, J.D. Evaluation of antifungal activity of seaweed extracts. Ciência Agrotecnologia 2012, 36, 294–299. [Google Scholar] [CrossRef]
- Bhadury, P.; Wright, P.C. Exploitation of marine algae: Biogenic compounds for potential antifouling applications. Planta 2004, 219, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Mhadhebi, L.; Mhadhebi, A.; Robert, J.; Bouraoui, A. Antioxidant, Anti-inflammatory and Antiproliferative Effects of Aqueous Extracts of Three Mediterranean Brown Seaweeds of the Genus Cystoseira. Iran J. Pharm. Res. 2014, 13, 207–220. [Google Scholar] [PubMed]
- Smyrniotopoulos, V.; de Andrade Tomaz, A.C.; Vanderlei de Souza, M.F.; Leitao da Cunha, E.V.; Kiss, R.; Mathieu, V.; Ioannou, E.; Roussis, V. Halogenated Diterpenes with In Vitro Antitumor Activity from the Red Alga Sphaerococcus corono-pifolius. Mar. Drugs 2019, 18, 29. [Google Scholar] [CrossRef]
- Montero, L.; Del Pilar Sanchez Camargo, A.; Ibanez, E.; Gilbert Lopez, B. Phenolic Compounds from Edible Algae: Bioactivity and Health Benefits. Curr. Med. Chem. 2018, 25, 4808–4826. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, Z.J.; Ryu, B.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, W.; Zhao, S.; Li, S.; Yan, D.; Wang, J. Eckol inhibits Reg3A-induced proliferation of human SW1990 pan-creatic cancer cells. Exp. Ther. Med. 2019, 18, 2825–2832. [Google Scholar] [PubMed]
- Zhang, M.Y.; Guo, J.; Hu, X.M.; Zhao, S.Q.; Li, S.L.; Wang, J. An in vivo anti-tumor effect of eckol from marine brown algae by improving the immune response. Food Funct. 2019, 10, 4361–4371. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhang, M.; Chen, Y.; Zhu, T.; Wang, J. Protective Effect of Eckol against Acute Hepatic Injury Induced by Carbon Tetrachloride in Mice. Mar. Drugs 2018, 16, 300. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Kim, K.N.; Kang, S.M.; Yang, X.; Kim, E.A.; Song, C.B.; Nah, J.W.; Jang, M.K.; Lee, J.S.; Jung, W.K.; et al. Protective effect of dieckol isolated from Ecklonia cava against ethanol caused damage in vitro and in zebrafish model. Environ. Toxicol. Pharmacol. 2013, 36, 1217–1226. [Google Scholar] [CrossRef]
- Kang, S.M.; Cha, S.H.; Ko, J.Y.; Kang, M.C.; Kim, D.; Heo, S.J.; Kim, J.S.; Heu, M.S.; Kim, Y.T.; Jung, W.K.; et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharmacol. 2012, 34, 96–105. [Google Scholar] [CrossRef]
- Kim, A.D.; Kang, K.A.; Piao, M.J.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Hyun, C.L.; Kang, H.K.; Lee, N.H.; et al. Cytoprotective effect of eckol against oxidative stress-induced mitochondrial dysfunction: Involvement of the FoxO3a/AMPK pathway. J. Cell Biochem. 2014, 115, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Ah Jung, H.; Roy, A.; Jung, J.H.; Choi, J.S. Evaluation of the inhibitory effects of eckol and dieckol isolated from edible brown alga Eisenia bicyclis on human monoamine oxidases A and B. Arch. Pharmacal Res. 2017, 40, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Qian, Z.J.; Ryu, B.; Karadeniz, F.; Kim, D.; Kim, S.K. Antioxidant peptides from protein hydrolysate of microalgae Navicula incerta and their protective effects in HepG2/CYP2E1 cells induced by ethanol. Phytother. Res. 2012, 26, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Qian, Z.-J.; Ryu, B.; Kim, D.; Kim, S.K. Protective effects of protein hydrolysate from marine microalgae Navicula incerta on ethanol-induced toxicity in HepG2/CYP2E1 cells. Food Chem. 2012, 132, 677–685. [Google Scholar] [CrossRef]
- Yoshihito, O.; Akiko, I.; Ryuichiro, S.; Toru, O. A new phloroglucinol derivative from the brown alga Eisenia bicyclis: Potential for the effective treatment of diabetic complications. J. Nat. Prod. 2004, 67, 1318–1323. [Google Scholar]
- Athukorala, Y.; Jung, W.K.; Vasanthan, T.; Jeon, Y.J. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr. Polym. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.; Jeon, Y.J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: A review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Tang, Y.; Kim, Y.S.; Hwang, J.W.; Choi, E.J.; Lee, J.H.; Lee, S.H.; Jeon, Y.J.; Park, P.J. First evidence that Ecklonia cava-derived dieckol attenuates MCF-7 human breast carcinoma cell migration. Mar. Drugs 2015, 13, 1785–1797. [Google Scholar] [CrossRef] [PubMed]
- Strathearn, L.S.; Stepanov, A.I.; Font-Burgada, J. Inflammation in Primary and Metastatic Liver Tumorigenesis–Under the Influence of Alcohol and High-Fat Diets. Nutrients 2020, 12, 933. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.R.; Morris, N.L.; Hammer, A.M.; Curtis, B.; Remick, D.G.; Yeligar, S.M.; Poole, L.; Burnham, E.L.; Wyatt, T.A.; Molina, P.E.; et al. Alcohol and inflammatory responses: Highlights of the 2015 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol 2016, 54, 73–77. [Google Scholar] [CrossRef]
- Congcong, Z.; Yulin, L.; Yina, W.; Luya, W.; Xiaonan, W.; Jie, D. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J. Biol. Chem. 2013, 288, 1489–1499. [Google Scholar]
- Horiguchi, N.; Wang, L.; Mukhopadhyay, P.; Park, O.; Jeong, W.I.; Lafdil, F.; Osei Hyiaman, D.; Moh, A.; Fu, X.Y.; Pacher, P.; et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology 2008, 134, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Min, S.; Lee, Y.H.; Hwang, K.; Jun, W. Hepatoprotective effect of 10% ethanolic extract from Curdrania tricuspidata leaves against ethanol-induced oxidative stress through suppression of CYP2E1. Food Chem. Toxicol. 2017, 108, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, A.F.A.; Chan, C.K.; Mohamad, J.; Kadir, H.A. Dioscorea bulbifera induced apoptosis through inhibition of ERK 1/2 and activation of JNK signaling pathways in HCT116 human colorectal carcinoma cells. Biomed. Pharmacother. 2018, 104, 806–816. [Google Scholar] [CrossRef]
- Zou, H.; Henzel, W.J.; Liu, X.; Lutschg, A.; Wang, X. Apaf-1, a Human Protein Homologous to C. elegans CED-4, Participates in Cytochrome c–Dependent Activation of Caspase-3. Cell 1997, 90, 405–413. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta 2011, 1813, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, L.; Ma, H.G.; Sun, P.; Wang, Q.L.; Zhang, T.T.; Shen, Y.M.; Zhu, W.M.; Li, X. Bisindolylmaleimide alkaloid BMA-155Cl induces autophagy and apoptosis in human hepatocarcinoma HepG-2 cells through the NF-κB p65 pathway. Acta Pharmacol. Sin. 2017, 38, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xin, W.Y.; Yao, B.R.; Cong, W.; Wang, C.H.; Hou, G.G. N-phenylsulfonyl-3,5-bis(arylidene)-4-piperidone derivatives as activation NF-κB inhibitors in hepatic carcinoma cell lines. Eur. J. Med. Chem. 2018, 155, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Anning, L. Activation of the JNK signaling pathway: Breaking the brake on apoptosis. Bioessays 2003, 25, 17–24. [Google Scholar]
- Speidel, D. Transcription-independent p53 apoptosis: An alternative route to death. Trends Cell Biol. 2010, 20, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Li, J.; Liao, S.; Ma, S.; Li, F.; Zhong, C.; Li, G.; Wei, Y.; Huang, H.; Wei, Q.; et al. Go6983 attenuates titanium particle-induced osteolysis and RANKL mediated osteoclastogenesis through the suppression of NFkappaB/JNK/p38 pathways. Biochem. Biophys. Res. Commun. 2018, 503, 62–70. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Yang, S.; Xiao, Z.; Hong, P.; Sun, S.; Zhou, C.; Qian, Z.-J. The Inhibition Effect of the Seaweed Polyphenol, 7-Phloro-Eckol from Ecklonia Cava on Alcohol-Induced Oxidative Stress in HepG2/CYP2E1 Cells. Mar. Drugs 2021, 19, 158. https://doi.org/10.3390/md19030158
Lin L, Yang S, Xiao Z, Hong P, Sun S, Zhou C, Qian Z-J. The Inhibition Effect of the Seaweed Polyphenol, 7-Phloro-Eckol from Ecklonia Cava on Alcohol-Induced Oxidative Stress in HepG2/CYP2E1 Cells. Marine Drugs. 2021; 19(3):158. https://doi.org/10.3390/md19030158
Chicago/Turabian StyleLin, Liyuan, Shengtao Yang, Zhenbang Xiao, Pengzhi Hong, Shengli Sun, Chunxia Zhou, and Zhong-Ji Qian. 2021. "The Inhibition Effect of the Seaweed Polyphenol, 7-Phloro-Eckol from Ecklonia Cava on Alcohol-Induced Oxidative Stress in HepG2/CYP2E1 Cells" Marine Drugs 19, no. 3: 158. https://doi.org/10.3390/md19030158
APA StyleLin, L., Yang, S., Xiao, Z., Hong, P., Sun, S., Zhou, C., & Qian, Z.-J. (2021). The Inhibition Effect of the Seaweed Polyphenol, 7-Phloro-Eckol from Ecklonia Cava on Alcohol-Induced Oxidative Stress in HepG2/CYP2E1 Cells. Marine Drugs, 19(3), 158. https://doi.org/10.3390/md19030158






