Haloarchaea as Cell Factories to Produce Bioplastics
Abstract
1. Introduction
2. Description of Bioplastics; PHA, PHB, PHV, and PHVB as Examples
2.1. Chemical Structures
- PHA containing aliphatic fatty acids, such as PHB.
- PHAs containing aromatic fatty acids, such as poly(3-hydroxy-5-phenylvalerate) PHPV.
- PHA heteropolymers containing both aliphatic and aromatic fatty acids (i.e., P(3HB-co-3MP)).
- Homopolymers: composed of a single type of monomer, such as poly 4-hydroxybutyrate (P4HB), poly 3-hydroxypropionate (P3HP), poly 3-hydroxyvalerate (PHV), poly 3-hydroxy-4-pentenoate (P3H4P), poly 3-hydroxyhexanoate(P3HH), or poly 3-hydroxyheptanoate (P3HH).
- Random copolymers: composed of more than one type of randomly distributed monomers. The wide range of commercially produced PHA as random copolymers include poly(3HP-co-4HB), poly(3HB-co-3HP), poly(3HB-co-3HV) (PHBV), and poly(3HB-co-4HB) (P3HB4HB).
- Block copolymers: these molecules are characterized by chemically distinct monomer units grouped in discrete blocks along the polymer chain. Different di-block copolymers have been produced by means of regulating the availability of fed substrates, such as PHB-b-P3HVHHp, PHB-b-P4HB, PHB-b-PHHx, P3HB-b-P3HP, P3HP-b-P4HB, and P3HHx-b-P(3HD-co-3HDD).
2.2. Physical Properties
- Biodegradability: unlike any other types of biodegradable plastics, PHAs can biodegrade under anaerobic conditions. In all cases, PHAs’ degradation rate can be accelerated by means of lowering crystallinity [36] and melting temperature [37]. PHA copolymers containing the 4HB monomer seem to degrade more rapidly than P(3HB) or P(3HB-co-3HV) copolymers [38].
- Mechanical properties: Young’s modulus, tensile strain and stress, and elastic modulus provide a measure of PHA’s stiffness. The latest varies from that of very stiff polymers, such as PLA and PP, and from much softer material, such as LDPE. The incorporation of 3HA units into the PHB polymer chain tends to decrease the stiffness and simultaneously increase the ultimate elongation of the material. These effects are more pronounced with mcl-3HA having longer side chains. Lower crystallinity and Tg lead to softer materials. The decrease in the size of the crystals in the crystalline phase also causes an improvement in the elongation to break and impact strength [39].
- Gas barrier properties: the lower the crystallinity, the lower the gas permeability of the material. Despite this, the gas barrier properties of PHAs are insufficient to explore potential applications at a large scale. Consequently, research is underway to improve this feature for packaging applications [40,41].
2.3. Improving PHAs’ Physicochemical Properties by Copolymerization to Promote Industrial Uses
3. Analysis Used for PHA Isolation and Characterization
3.1. Methods for Staining Cells to Distinguish PHA/PHB Producers and Non-Producers
3.2. Protocols for PHA Solation: Advantages and Disadvantages
3.3. Characterization of the Composition and Distribution of Monomers
3.4. Analysis of Molecular Weight Distribution
3.5. Characterization of Thermal Properties
3.6. Characterization of Crystallinity
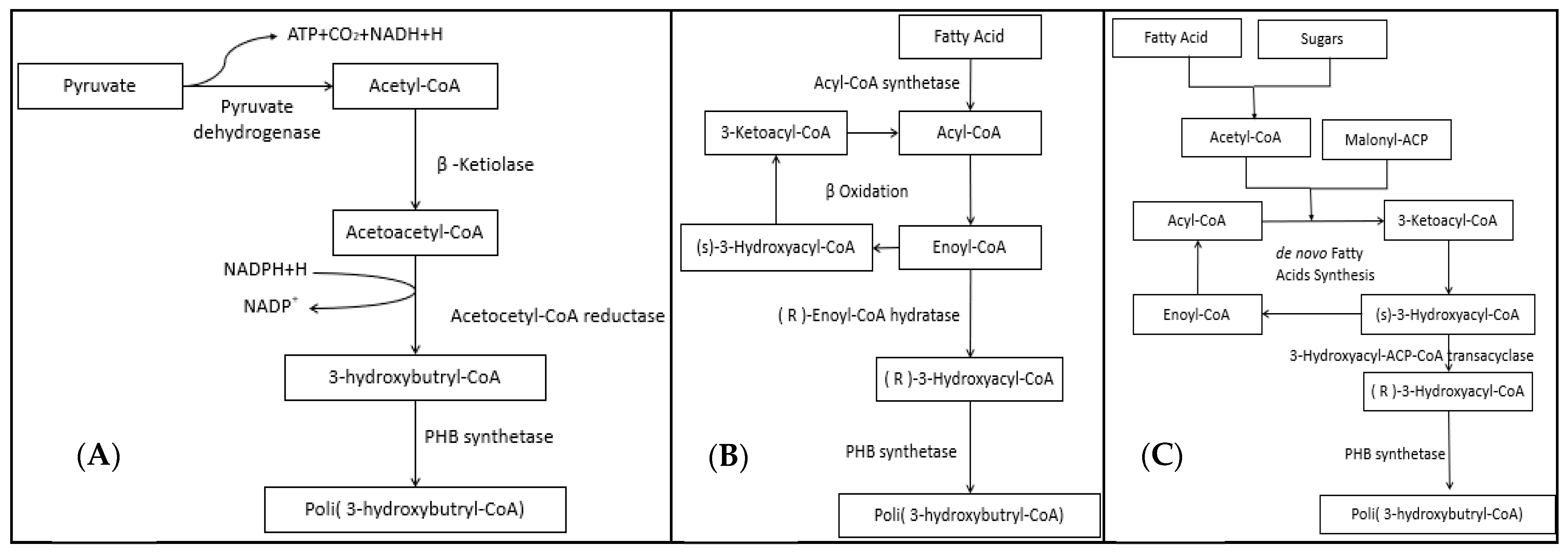
4. Organisms Producing Bioplastics and Their Use as Cell Factories for PHA Production
4.1. Bacteria
4.2. Archaea
4.3. Eukarya
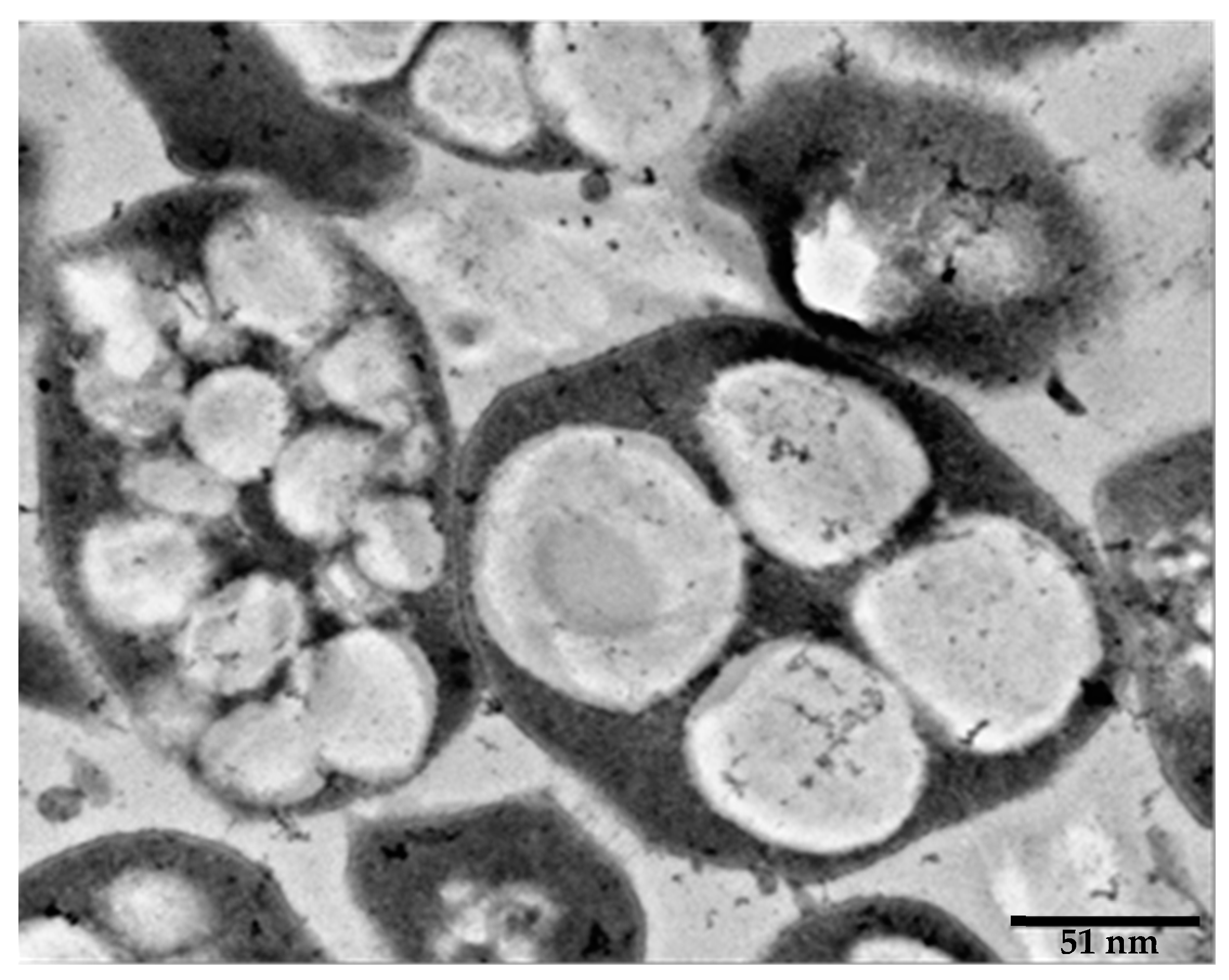
5. Advantages of Using Halophilic Microbes That Are Able to Produce PHA and PHB. Haloarchaea as Case of Study
5.1. Haloarchaeal Species Capable of Producing PHA, PHB and PHV
5.2. PHA-Related Genes in Haloarchaea
5.3. Effects of Cultivation Conditions on the Production of PHAs
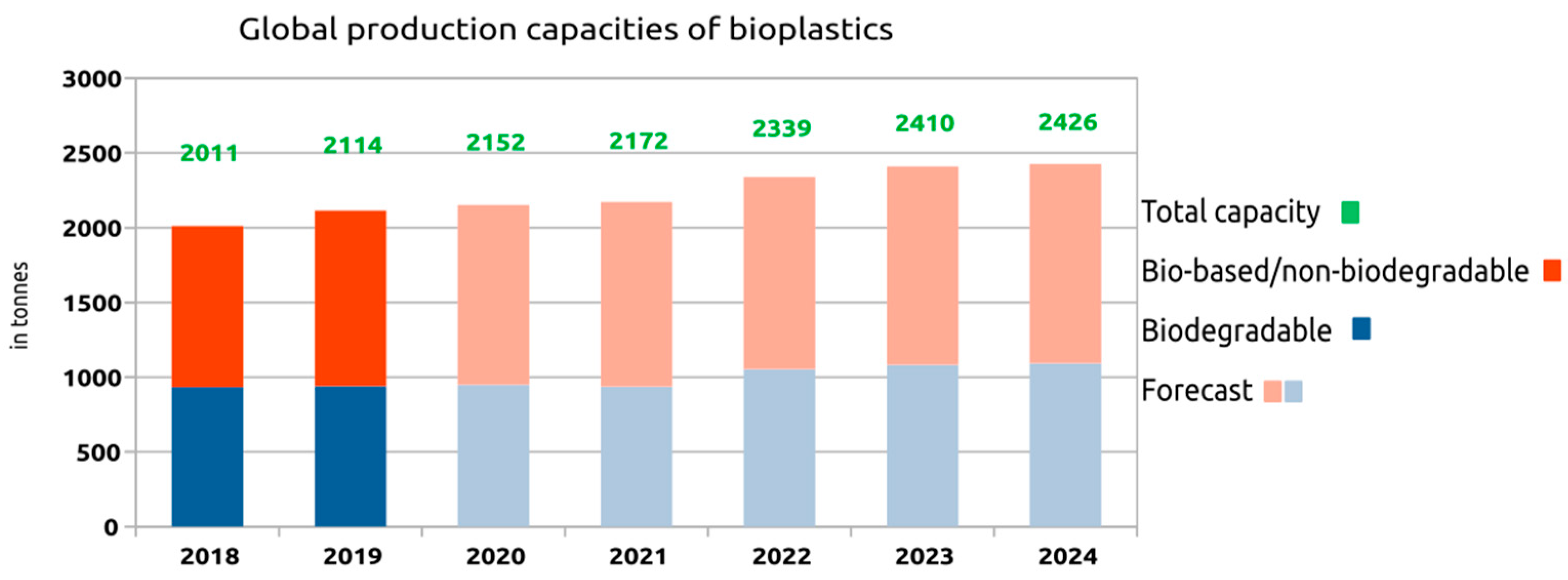
6. Global Market for Bioplastics
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beltrán-Sanahuja, A.; Casado-Coy, N.; Simó-Cabrera, L.; Sanz-Lázaro, C. Monitoring polymer degradation under different conditions in the marine environment. Environ. Pollut. 2020, 259, 113836. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2017, 24, 1405–1416. [Google Scholar] [CrossRef]
- Yang, C.Z.; Yaniger, S.I.; Jordan, V.C.; Klein, D.J.; Bittner, G.D. Most plastic products release estrogenic chemicals: A potential health problem that can be solved. Environ. Health Perspect. 2011, 119, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Jâms, I.B.; Windsor, F.M.; Poudevigne-Durance, T.; Ormerod, S.J.; Durance, I. Estimating the size distribution of plastics by animals. Nat. Commun. 2020, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef]
- Gong, J.; Xie, P. Research progress in sources, analytical methods, eco-environmental effects, and control measures of microplastics. Chemosphere 2020, 254, 126790. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Gutow, L.; Eckerlebe, A.; Gimenez, L.; Saborowski, R. Experimental evaluation of seaweeds as a vector for microplastics into marine food webs. Environ. Sci. Technol. 2015, 50, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L.; Morét-Ferguson, S.E.; Goodwin, D.S.; Zettler, E.R.; DeForce, E.; Kukulka, T.; Proskurowski, G. Distribution of surface plastic debris in the eastern Pacific Ocean from an 11—Year data set. Environ. Sci. Technol. 2014, 48, 4732–4738. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the awuatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S. State of the Science of Endocrine Disruptors. Environ. Health Perspect. 2013, 121, a107. [Google Scholar] [CrossRef]
- Jung, J.W.; Kang, J.S.; Choi, J.; Park, J.W. Chronic toxicity of endocrine disrupting chemicals used in plastic products in Korean resident species: Implications for aquatic ecological risk assessment. Ecotoxicol. Environ. Saf. 2020, 192, 110309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.; Pandey, A.; Gnansounou, E.; Lin, K.Y.A.; Tsang, D.C.W.; Thakur, I.S. Bacterial polyhydroxyalkanoates: Opportunities, challenges, and prospects. J. Clean. Prod. 2020, 263, 121500. [Google Scholar] [CrossRef]
- Castilho, L.R.; Mitchell, D.A.; Freire, D.M.G. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour. Technol. 2009, 100, 5996–6009. [Google Scholar] [CrossRef]
- Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Bioplastics with a green agenda. Curr. Opin. Microbiol. 2010, 13, 321–326. [Google Scholar] [CrossRef]
- Lemoigne, M. Produit de déshydratation et de polymérisation de l’acide b-oxybutyrique. Bull. Soc. Chim. Biol. 1926, 8, 770–782. [Google Scholar]
- National Library of Medicine. PubMed.gov: Plastic Pollution. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=plastic+pollution&filter=years.2010-2021 (accessed on 1 November 2020).
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- RameshKumar, S.; Shaiju, P.; O’Connor, K.E.; Babu, R. Bio-based and biodegradable polymers-State-of-the-art, challenges and emerging trends. Curr. Opin. Green Sustain. Chem. 2020, 21, 75–81. [Google Scholar] [CrossRef]
- Muhammadi; Shabina; Afzal, M.; Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77. [Google Scholar] [CrossRef]
- Winnacker, M. Polyhydroxyalkanoates: Recent Advances in Their Synthesis and Applications. Eur. J. Lipid Sci. Technol. 2019, 121, 1900101. [Google Scholar] [CrossRef]
- Tan, G.Y.A.; Chen, C.L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.Y. Start a Research on Biopolymer Polyhydroxyalkanoate (PHA): A Review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Salgaonkar, B.B.; Bragança, J.M. Biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Halogeometricum borinquense strain E3. Int. J. Biol. Macromol. 2015, 78, 339–346. [Google Scholar] [CrossRef]
- Salgaonkar, B.B.; Mani, K.; Bragança, J.M. Accumulation of polyhydroxyalkanoates by halophilic archaea isolated from traditional solar salterns of India. Extremophiles 2013, 17, 787–795. [Google Scholar] [CrossRef]
- Poltronieri, P.; Kumar, P. Polyhydroxyalkanoates (PHAs) in industrial applications. In Handbook of Ecomaterials; Torres Martínez, L., Kharissova, O., Kharisov, B., Eds.; Springer: Cham, Sweden, 2017; pp. 1–30. [Google Scholar]
- Kynadi, A.; Suchithra, T. Polyhydroxyalkanoates: Biodegradable plastics for environmental conservation. In Industrial & Environmental Biotechnology, 1st ed.; Pramanik, K., Patra, J., Eds.; Studium Press: New Delhi, India, 2014; pp. 1–15. [Google Scholar] [CrossRef]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes 2016, 9, 509. [Google Scholar] [CrossRef]
- Luzi, F.; Torre, L.; Kenny, J.M.; Puglia, D. Bio- and fossil-based polymeric blends and nanocomposites for packaging: Structure-property relationship. Materials 2019, 12, 471. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Yamane, T.; Chen, X.; Ueda, S. Growth-associated production of poly(3-hydroxyvalerate) from n-pentanol by a methylotrophic bacterium, Paracoccus denitrificans. Appl. Environ. Microbiol. 1996, 62, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef]
- Grigore, M.E.; Grigorescu, R.M.; Iancu, L.; Ion, R.M.; Zaharia, C.; Andrei, E.R. Methods of synthesis, properties and biomedical applications of polyhydroxyalkanoates: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Akaraonye, E.; Keshavarz, T.; Roy, I. Production of polyhydroxyalkanoates: The future green materials of choice. J. Chem. Technol. Biotechnol. 2010, 85, 732–743. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Xu, P.; Yang, W.; Niu, D.; Yu, M.; Du, M.; Dong, W.; Chen, M.; Jan Lemstra, P.; Ma, P. Multifunctional and robust polyhydroxyalkanoate nanocomposites with superior gas barrier, heat resistant and inherent antibacterial performances. Chem. Eng. J. 2020, 382, 122864. [Google Scholar] [CrossRef]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- Chodak, I. Polyhydroxyalkanoates: Origin, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources, 1st ed.; Belgacem, M., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 451–477. [Google Scholar] [CrossRef]
- Zhao, C.; Li, J.; He, B.; Zhao, L. Fabrication of hydrophobic biocomposite by combining cellulosic fibers with polyhydroxyalkanoate. Cellulose 2017, 24, 2265–2274. [Google Scholar] [CrossRef]
- Barouti, G.; Guillaume, S.M. Polyhydroxybutyrate (PHB)-based triblock copolymers: Synthesis of hydrophobic PHB/poly(benzyl β-malolactonate) and amphiphilic PHB/poly(malic acid) analogues by ring-opening polymerization. Polym. Chem. 2016, 7, 4603–4608. [Google Scholar] [CrossRef]
- Volova, T.G.; Zhila, N.O.; Shishatskaya, E.I.; Mironov, P.V.; Vasil’Ev, A.D.; Sukovatyi, A.G.; Sinskey, A.J. The physicochemical properties of polyhydroxyalkanoates with different chemical structures. Polym. Sci. Ser. A 2013, 55, 427–437. [Google Scholar] [CrossRef]
- Rai, R.; Keshavarz, T.; Roether, J.A.; Boccaccini, A.R.; Roy, I. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater. Sci. Eng. R. Rep. 2011, 72, 29–47. [Google Scholar] [CrossRef]
- Barrett, J.S.F. Physical and Biochemical Strategies for Improving the Yield and Material Properties of Polyhydroxyalkanoate Biopolymers. Ph.D. Thesis, Philosophy-University of Minnesota, Minneapolis, MN, USA, 20 October 2014. [Google Scholar]
- Chan, C.M.; Vandi, L.J.; Pratt, S.; Halley, P.; Ma, Y.; Chen, G.Q.; Richardson, D.; Werker, A.; Laycock, B. Understanding the effect of copolymer content on the processability and mechanical properties of polyhydroxyalkanoate (PHA)/wood composites. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105437. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, J.; Smith, A.C.; Carvalho, C.W.P.; Wellner, N.; Yakimets, I. Characterization of polyhydroxybutyrate-hydroxyvalerate (PHB-HV)/maize starch blend films. J. Food Eng. 2008, 89, 361–369. [Google Scholar] [CrossRef]
- Sagong, H.Y.; Son, H.F.; Choi, S.Y.; Lee, S.Y.; Kim, K.J. Structural Insights into Polyhydroxyalkanoates Biosynthesis. Trends Biochem. Sci. 2018, 43, 790–805. [Google Scholar] [CrossRef]
- Zhila, N.; Shishatskaya, E. Properties of PHA bi-, ter-, and quarter-polymers containing 4-hydroxybutyrate monomer units. Int. J. Biol. Macromol. 2018, 111, 1019–1026. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- Volova, T.G.; Vinogradova, O.N.; Zhila, N.O.; Kiselev, E.G.; Peterson, I.V.; Vasil’ev, A.D.; Sukovatyi, A.G.; Shishatskaya, E.I. Physicochemical properties of multicomponent polyhydroxyalkanoates: Novel aspects. Polym. Sci. 2017, 59, 98–106. [Google Scholar] [CrossRef]
- Noda, I.; Lindsey, S.; Caraway, D. NodaxTM class PHA copolymers: Their properties and applications. In Plastics from Bacteria; Chen, G., Ed.; Springer: Berlin, Germany, 2010; Volume 14, pp. 237–255. [Google Scholar] [CrossRef]
- Huong, K.H.; Mohd Yahya, A.R.; Amirul, A.A. Pronounced synergistic influence of mixed substrate cultivation on single step copolymer P (3HB-co-4HB) biosynthesis with a wide range of 4HB monomer composition. J. Chem. Technol. Biotechnol. 2014, 89, 1023–1029. [Google Scholar] [CrossRef]
- Schlegel, H.; Lafferty, R.; Krauss, I. The isolation of mutants nor accumulating poly-β-hydroxybutyric acid. Arch. Mikrobiol. 1970, 71, 283–294. [Google Scholar] [CrossRef]
- Kranz, R.G.; Gabbert, K.K.; Madigan, M.T. Positive selection systems for discovery of novel polyester biosynthesis genes based on fatty acid detoxification. Appl. Environ. Microbiol. 1997, 63, 3010–3013. [Google Scholar] [CrossRef] [PubMed]
- Porras, M.A.; Villar, M.A.; Cubitto, M.A. Novel spectrophotometric technique for rapid determination of extractable PHA using Sudan black dye. J. Biotechnol. 2017, 255, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.H.; Chen, W.C.; Huang, C.K.; Wu, H.S.; Sun, Y.M.; Lo, C.W.; Janarthanan, O.M. Screening and evaluation of polyhydroxybutyrate-producing strains from indigenous isolate Cupriavidus taiwanensis strains. Int. J. Mol. Sci. 2011, 12, 252–265. [Google Scholar] [CrossRef]
- Pielesz, A.; Baranowska, A.; Rybak, A.; Wlochowicz, A. Detection and determination of aromatic amines as products of reductive splitting from selected azo dyes. Ecotoxicol. Environ. Saf. 2002, 53, 42–47. [Google Scholar] [CrossRef]
- Lopez, M.I.; Ruisanchez, I.; Callao, M.P. Figures of merit of a SERS method for Sudan I determination at traces levels. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 111, 237–241. [Google Scholar] [CrossRef]
- Martinez, V.; Henary, M. Nile Red and Nile Blue: Applications and Syntheses of Structural Analogues. Chem. Eur. J. 2016, 22, 1–20. [Google Scholar] [CrossRef]
- Ostle, A.G.; Holt, J.G. Nile blue A as a fluorescent stain for poly-β-hydroxybutyrate. Appl. Environ. Microbiol. 1982, 44, 238–241. [Google Scholar] [CrossRef]
- Shrivastav, A.; Sanjiv, K.M.; Sandhya, M. Polyhydroxyalkanoate (PHA) synthesis by Spirulina subsalsa from Gujarat coast of India. Int. J. Biol. Macromol. 2010, 46, 255–260. [Google Scholar] [CrossRef]
- Mohandas, S.P.; Balan, L.; Lekshmi, N.; Cubelio, S.S.; Philip, R.; Bright-Singh, I.S. Production and characterization of polyhydroxybutyrate from Vibrio harveyi MCCB 284 utilizing glycerol as carbon source. J. Appl. Microbiol. 2017, 122, 698–707. [Google Scholar] [CrossRef]
- Legat, A.; Gruber, C.; Zangger, K.; Wanner, G.; Stan-Lotter, H. Identification of polyhydroxyalkanoates in Halococcus and other haloarcheal species. Appl. Microbiol. Biotechnol. 2010, 87, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, P.; Rehm, B.H.A.; Kalscheuer, R.; Baumeister, D.; Steinbüchel, A. A sensitive, viable colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 1999, 171, 73–80. [Google Scholar] [CrossRef]
- Zuriani, R.; Vigneswari, S.; Azizan, M.N.M.; Majid, M.I.A.; Amirul, A.A. A high throughput Nile red fluorescence method for rapid quantification of intracellular bacterial polyhydroxyalkanoates. Biotechnol. Bioproc. E 2013, 18, 472–478. [Google Scholar] [CrossRef]
- Hu, S.; McDonald, A.G.; Coats, E.R. Characterization of polyhydroxybutyrate biosynthesized from crude glycerol waste using mixed microbial consortia. J. Appl. Polym. Sci. 2013, 129, 1314–1321. [Google Scholar] [CrossRef]
- Volova, T.; Shishatskaya, E.; Sevastianov, V.; Efremov, S.; Mogilnaya, O. Results of biomedical investigations of PHB and PHB/PHV fibers. Biochem. Eng. J. 2003, 16, 125–133. [Google Scholar] [CrossRef]
- Madkour, M.H.; Heinrich, D.; Alghamdi, M.A.; Shabbaj, I.I.; Steinbüchel, A. PHA recovery from biomass. Biomacromolecules 2013, 14, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Kachrimanidou, V.; Kopsahelis, N.; Vlysidis, A.; Papanikolaou, S.; Kookos, I.K.; Monje-Martínez, B.; Escrig-Rondán, M.C.; Koutinas, A.A. Downstream separation of poly(hydroxyalkanoates) using crude enzyme consortia produced via solid state fermentation integrated in a biorefinery concept. Food Bioprod. Process. 2016, 100, 323–334. [Google Scholar] [CrossRef]
- Koller, M.; Niebelschütz, H.; Braunegg, G. Strategies for recovery and purification of poly[(R)-3-hydroxyalkanoates] (PHA) biopolyesters from surrounding biomass. Eng. Life Sci. 2013, 13, 549–562. [Google Scholar] [CrossRef]
- Melanie, S.; Winterburn, J.B.; Devianto, H. Production of biopolymer polyhydroxyalkanoates (PHA) by extreme halophilic marine archaea Haloferax mediterranei in medium with varying phosphorus concentration. J. Eng. Technol. Sci. 2018, 50, 255–271. [Google Scholar] [CrossRef]
- Pais, J.; Serafim, L.S.; Freitas, F.; Reis, M.A.M. Conversion of cheese whey into poly(3- hydroxybutyrate-co-3-hydroxyvalerate) by Haloferax mediterranei. New Biotechnol. 2016, 33, 224–230. [Google Scholar] [CrossRef]
- Muangwong, A.; Boontip, T.; Pachimsawat, J.; Napathorn, S.C. Medium chain length polyhydroxyalkanoates consisting primarily of unsaturated 3-hydroxy-5-cis-dodecanoate synthesized by newly isolated bacteria using crude glycerol. Microb. Cell Fact. 2016, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.; Bassler, G.; Morrill, T. Spectrometric identification of organic compounds. In Organic Mass Spectrometry, 5th ed.; Chizov, O., Ed.; Wiley: New York, NY, USA, 1991; Volume 26, pp. 729–814. [Google Scholar] [CrossRef]
- Pradhan, S.; Dikshit, P.; Moholkar, V. Production, characterization, and applications of biodegradable polymer: Polyhydroxyalkanoates. In Advances in Sustainable Polymers: Synthesis, Fabrication and Characterization, 1st ed.; Katiyar, V., Kumar, A., Mulchandani, N., Eds.; Springer Nature: Singapore, 2020; pp. 51–94. [Google Scholar] [CrossRef]
- Godbole, S. Methods for identification, quantification and characterization of polyhydroxyalkanoates—A review. Int. J. Bioassays 2016, 5, 4977. [Google Scholar] [CrossRef]
- Ehrenstein, G.W.; Riedel, G.; Trawiel, P. Thermal Analysis of Plastics: Theory and Practice; Hanser: Munich, Germany, 2004; p. 399. [Google Scholar]
- Patel, J.; Parsania, P. Characterization, testing, and reinforcing materials of biodegradable composites. In Biodegradable and Biocompatible Polymer Composites, 1st ed.; Shimpi, N., Ed.; Elsevier: Cambridge, UK, 2018; pp. 55–79. [Google Scholar] [CrossRef]
- Sharma, P.K.; Munir, R.I.; Blunt, W.; Dartiailh, C.; Cheng, J.; Charles, T.C.; Levin, D.B. Synthesis and physical properties of polyhydroxyalkanoate polymers with different monomer compositions by recombinant Pseudomonas putida LS46 expressing a novel PHA synthase (PhaC116) enzyme. Appl. Sci. 2017, 7, 242. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Martino, V.; Pollet, E.; Avérous, L.; Reis, M.A.M. Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)-rich streams: Effect of substrate composition and feeding regime on PHA productivity, composition and properties. J. Biotechnol. 2011, 151, 66–76. [Google Scholar] [CrossRef]
- Bengtsson, S.; Pisco, A.R.; Johansson, P.; Lemos, P.C.; Reis, M.A.M. Molecular weight and thermal properties of polyhydroxyalkanoates produced from fermented sugar molasses by open mixed cultures. J. Biotechnol. 2010, 147, 172–179. [Google Scholar] [CrossRef]
- Prieto, M.A.; Kellerhals, M.B.; Bozzato, G.B.; Radnovic, D.; Witholt, B.; Kessler, B. Engineering of stable recombinant bacteria for production of chiral medium-chain-length poly-3-hydroxyalkanoates. Appl. Environ. Microbiol. 1999, 65, 3265–3271. [Google Scholar] [CrossRef]
- González García, Y.; Meza Contreras, J.C.; González Reynoso, O.; Córdova López, J.A. Síntesis y biodegradación de polihidroxialcanoatos: Plásticos de origen microbiano. Rev. Int. Contam. Ambient. 2013, 29, 77–115. [Google Scholar]
- Anderson, A.; Wynn, J. Microbial polyhydroxyalkanoates, polysaccharides and lipids. In Basic Biotechnology, 2nd ed.; Ratledge, C., Kristiansen, B., Eds.; Cambridge University Press: New York, NY, USA, 2001; pp. 325–333. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Pötter, M.; Madkour, M.H.; Mayer, F.; Steinbüchel, A. Regulation of phasin expression and polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha H16. Microbiology 2002, 148, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Arenas, T.; González-Contreras, M.; Anaya-Reza, O.; Sales-Cruz, M. Analysis of the fermentation strategy and its impact on the economics of the production process of PHB (polyhydroxybutyrate). Comput. Chem. Eng. 2017, 107, 140–150. [Google Scholar] [CrossRef]
- Findlay, R.H.; White, D.C. Polymeric beta-hydroxyalkanoates from environmental samples and Bacillus megaterium. Appl. Environ. Microbiol. 1983, 45, 71–78. [Google Scholar] [CrossRef]
- Shah, F.A.; Mahmood, Q.; Shah, M.M.; Pervez, A.; Asad, S.A. Microbial ecology of anaerobic digesters: The key players of anaerobiosis. Sci. World J. 2014, 2014, 1–21. [Google Scholar] [CrossRef]
- Hassan, M.A.; Shirai, Y.; Kusubayashi, N.; Abdul Karim, M.I.; Nakanishi, K.; Hashimoto, K. The production of poly-hydroxyalkanoate from anaerobically treated palm oil mill effluent by Rhodobacter sphaeroides. J. Ferment. Bioeng. 1997, 83, 420–423. [Google Scholar] [CrossRef]
- Jau, M.H.; Yew, S.P.; Toh, P.S.; Chong, A.S.; Chu, W.L.; Phang, S.M.; Najimudin, N.; Sudesh, K. Biosynthesis and mobilization of poly(3-hydroxybutyrate) [P(3HB)] by Spirulina platensis. Int. J. Biol. Macromol. 2005, 36, 144–151. [Google Scholar] [CrossRef]
- Arshad, U.M.; Jamil, N.; Naheed, N.; Hasnain, S. Analysis of bacterial strains from contaminated and non-contaminated sites for the production of biopolymers. Afr. J. Biotechnol. 2007, 6, 1115–1121. [Google Scholar] [CrossRef]
- Tombolini, R.; Nuti, M.P. Poly(β-hydroxyalkanoate) biosynthesis and accumulation by different Rhizobium species. FEMS Microbiol. Lett. 1989, 60, 299–304. [Google Scholar] [CrossRef]
- Senior, P.J.; Beech, G.A.; Ritchie, G.A.F.; Dawes, E.A. The role of oxygen limitation in the formation of poly-3-hydroxybutyrateduring batch and continuous culture of Azotobacter beijerinckii. Biochem. J. 1972, 128, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Stockdale, H.; Ribbons, D.W.; Dawes, E.A. Occurrence of poly-3-hydroxybutyrate in the Azotobacteriaceae. J. Bacteriol. 1968, 5, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Page, W.J.; Knosp, O. Hyperproduction of poly-beta-hydroxybutyrate during exponential growth of Azotobacter vinelandii UWD. Appl. Environ. Microbiol. 1989, 55, 1334–1339. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Recent advances in microbial polyhydroxyalkanoates. Process. Biochem. 2005, 40, 607–619. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamane, T.; Shimizu, S. Mass production of poly-β-hydroxybutyric acid by fully automatic fed-batch culture of methylotroph. Appl. Microbiol. Biotechnol. 1986, 23, 322–329. [Google Scholar] [CrossRef]
- Kawata, Y.; Aiba, S.I. Poly (3-hydroxybutyrate) production by isolated Halomonas sp. KM-1 using waste glycerol. Biosci. Biotechnol. Biochem. 2010, 74, 175. [Google Scholar] [CrossRef]
- Tekin, E.; Ates, M.; Ilikkan, O.K. Poly-3-hydroxybutyrate-producing extreme halophilic archaeon: Haloferax sp. MA10 isolated from Çamaltı Saltern, İzmir. Turk. J. Biol. 2012, 36, 303–312. [Google Scholar] [CrossRef]
- Giani, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaeal Carotenoids: Healthy Novel Compounds from Extreme Environments. Mar. Drugs 2019, 17, 524. [Google Scholar] [CrossRef]
- García Lillo, J.; Rodríguez-Valera, F. Effects of culture conditions on poly (3-Hydroxybutyric Acid) production by Haloferax mediterranei. Appl. Environ. Microbiol. 1990, 56, 2517–2521. [Google Scholar] [CrossRef]
- Han, J.; Hou, J.; Liu, H.; Cai, S.; Feng, B.; Zhou, J.; Xiang, H. Wide distribution among halophilic archaea of a novel polyhydroxyalkanoate synthase subtype with homology to bacterial type III synthases. Appl. Environ. Microbiol. 2010, 76, 7811–7819. [Google Scholar] [CrossRef]
- Madison, L.L.; Huisman, G. Metabolic engineering of poly(3-hydroxyalkanoates): From DNA to plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar] [CrossRef]
- Portugal-Nunes, D.J.; Pawar, S.S.; Lidén, G.; Gorwa-Grauslund, M.F. Effect of nitrogen availability on the poly-3-D-hydroxybutyrate accumulation by engineered Saccharomyces cerevisiae. AMB Expr. 2017, 7, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Soto, L.R.; Byrne, E.; van Niel, E.W.J.; Sayed, M.; Villanueva, C.C.; Hatti-Kaul, R. Hydrogen and polyhydroxybutyrate production from wheat straw hydrolysate using Caldicellulosiruptor species and Ralstonia eutropha in a coupled process. Bioresour. Technol. 2019, 272, 259–266. [Google Scholar] [CrossRef]
- Snell, K.D.; Singh, V.; Brumbley, S.M. Production of novel biopolymers in plants: Recent technological advances and future prospects. Curr. Opin. Biotechnol. 2015, 32, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Borah, A.J.; Poddar, M.K.; Dikshit, P.K.; Rohidas, L.; Moholkar, V.S. Microbial production, ultrasound-assisted extraction and characterization of biopolymer polyhydroxybutyrate (PHB) from terrestrial (P. hysterophorus) and aquatic (E. crassipes) invasive weeds. Bioresour. Technol. 2017, 242, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Saratale, G.D.; Cho, S.K.; Kim, D.S.; Ghodake, G.S.; Kadam, A.; Kumar, G.; Bharagava, R.N.; Banu, R.; Shin, H.S. Pretreatment of kenaf (Hibiscus cannabinus L.) biomass feedstock for polyhydroxybutyrate (PHB) production and characterization. Bioresour. Technol. 2019, 282, 75–80. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.C.; Ma, Y.; Chen, G.Q. Engineering biosynthesis of polyhydroxyalkanoates (PHA) for diversity and cost reduction. Metab. Eng. 2020, 58, 82–93. [Google Scholar] [CrossRef]
- Zhao, Y.; Rao, Z.; Xue, Y. Biosynthesis, property comparison, and hemocompatibility of bacterial and haloarchaeal poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Sci. Bull. 2015, 60, 1901–1910. [Google Scholar] [CrossRef]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal production of polyhydroxyalkanoate (PHA) Co- and terpolyesters from biodiesel industry-derived by-products. Archaea 2013, 2013, 129268. [Google Scholar] [CrossRef]
- Poli, A.; Donato, P.D.; Abbamondi, G.R.; Nicolaus, B. Synthesis, Production, and Biotechnological Applications of Exopolysaccharides and Polyhydroxyalkanoates by Archaea. Archaea 2011, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lynch, E.A.; Langille, G.I.; Darling, A.; Wilbanks, E.G.; Haltiner, C.; Shao, K.S.Y.; Starr, M.O.; Teiling, C.; Harkins, T.T.; Edwards, R.A.; et al. Sequencing of seven haloarchaeal genomes reveals patterns of genomic flux. PLoS ONE. 2012, 7, e41389. [Google Scholar] [CrossRef]
- Rodríguez-Valera, F.; Lillo, J.A.G. Halobacteria as producers of polyhydroxyalkanoates. FEMS Microbiol. Lett. 1992, 103, 181–186. [Google Scholar] [CrossRef]
- Kirk, R.G.; Ginzburg, M. Ultrastructure of two species of halobacterium. J. Ultrastruct. Res. 1972, 41, 80–94. [Google Scholar] [CrossRef]
- Fernandez-Castillo, R.; Rodriguez-Valera, F.; Gonzalez-Ramos, J.; Ruiz-Berraquero, F. Accumulation of poly (β-hydroxybutyrate) by halobacteria. Appl. Environ. Microbiol. 1986, 51, 214–216. [Google Scholar] [CrossRef]
- Altekar, W.; Rajagopalan, R. Ribulose bisphosphate carboxylase activity in halophilic Archaebacteria. Arch. Microbiol. 1990, 153, 169–174. [Google Scholar] [CrossRef]
- Nicolaus, B.; Lama, L.; Esposito, E.; Manca, M.C.; Improta, R.; Bellitti, M.R.; Duckworth, A.W.; Grant, W.D.; Gambacorta, A. Haloarcula spp able to biosynthesize exo- and endopolymers. J. Ind. Microbiol. Biotechnol. 1999, 23, 489–496. [Google Scholar] [CrossRef]
- Hezayen, F.F.; Rehm, B.H.; Eberhardt, R.; Steinbüchel, A. Polymer production by two newly isolated extremely halophilic archaea: Application of a novel corrosion-resistant bioreactor. Appl. Microbiol. Biotechnol. 2000, 54, 319–325. [Google Scholar] [CrossRef]
- Hezayen, F.F.; Gutiérrez, M.C.; Steinbüchel, A.; Tindall, B.J.; Rehm, B.H. Halopiger aswanensis sp. nov., a polymer-producing and extremely halophilic archaeon isolated from hypersaline soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 633–637. [Google Scholar] [CrossRef]
- Burns, D.G.; Janssen, P.H.; Itoh, T.; Kamekura, M.; Li, Z.; Jensen, G.; RodriguezValera, F.; Bolhuis, H.; Dyall-Smith, M.L. Haloquadratum walsbyi gen. nov., sp. nov., the square haloarchaeon of Walsby, isolated from saltern crystallizers in Australia and Spain. Int. J. Syst. Evol. Microbiol. 2007, 57, 387–392. [Google Scholar] [CrossRef]
- Hezayen, F.F.; Tindall, B.J.; Steinbüchel, A.; Rehm, B.H. Characterization of a novel halophilic archaeon, Halobiforma haloterrestris gen. nov., sp. nov., and transfer of Natronobacterium nitratireducens to Halobiforma nitratireducens comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2271–2280. [Google Scholar] [CrossRef]
- Romano, I.; Poli, A.; Finore, I.; Huertas, F.J.; Gambacorta, A.; Pelliccione, S.; Nicolaus, G.; Lama, L.; Nicolaus, B. Haloterrigena hispanica sp. nov., an extremely halophilic archaeon from Fuente de Piedra, southern Spain. Int. J. Syst. Evol. Microbiol. 2007, 57, 1499–1503. [Google Scholar] [CrossRef]
- Han, J.; Li, M.; Hou, J.; Wu, L.; Zhou, J.; Xiang, H. Comparison of four phaC genes from Haloferax mediterranei and their function in different PHBV copolymer biosynthesis in Haloarcula hispanica. Saline Syst. 2010, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.J.; Liao, Y.; Ye, J.; Kuchel, R.P.; Poljak, A.; Raftery, M.J.; Cavicchioli, R. Cold adaptation of the Antarctic haloarchaea Halohasta litchfeldiae and Halorubrum lacusprofundi. Environ. Microbiol. 2017, 19, 2210–2227. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Xue, Q.; Zhou, J.; Zhao, D.H.; Han, J.; Xiang, H. Engineering Haloferax mediterranei as an efficient platform for high level production of lycopene. Front. Microbiol. 2018, 9, 2893. [Google Scholar] [CrossRef] [PubMed]
- Don, T.M.; Chen, C.W.; Chan, T.H. Preparation and characterization of poly (hydroxyalkanoate) from the fermentation of Haloferax mediterranei. J. Biomater. Sci. Polym. Ed. 2006, 17, 1425–1438. [Google Scholar] [CrossRef]
- Han, J.; Wu, L.P.; Hou, J.; Zhao, D.; Xiang, H. Biosynthesis, characterization, and hemostasis potential of tailor-made poly(3-hydroxybutyrate-co3-hydroxyvalerate) produced by Haloferax mediterranei. Biomacromolecules 2015, 16, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Potential of various archae-and eubacterial strains as industrial polyhydroxyalkanoate producers from whey. Macromol. Biosci. 2007, 7, 218–226. [Google Scholar] [CrossRef]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Biosynthesis of high quality polyhydroxyalkanoate Co- And terpolyesters for potential medical application by the archaeon haloferax mediterranei. Macromol. Symp. 2007, 253, 33–39. [Google Scholar] [CrossRef]
- Lu, Q.; Han, J.; Zhou, L.; Zhou, J.; Xiang, H. Genetic and biochemical characterization of the poly (3-hydroxybutyrate-co-3-hydroxyvalerate) synthase in Haloferax mediterranei. J. Bacteriol. 2008, 190, 4173–4180. [Google Scholar] [CrossRef] [PubMed]
- Salgaonkar, B.; Bragança, J. Utilization of sugarcane bagasse by Halogeometricum borinquense strain E3 for biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering 2017, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Danis, O.; Ogan, A.; Tatlican, P.; Attar, A.; Cakmakci, E.; Mertoglu, B.; Birbir, M. Preparation of poly(3-hydroxybutyrate-co-hydroxyvalerate) flms from halophilic archaea and their potential use in drug delivery. Extremophiles 2015, 19, 515–524. [Google Scholar] [CrossRef]
- Mahansaria, R.; Dhara, A.; Saha, A.; Haldar, S.; Mukherjee, J. Production enhancement and characterization of the polyhydroxyalkanoate produced by Natrinema ajinwuensis (as synonym) ≡ Natrinema altunense strain RM-G10. Int. J. Biol. Macromol. 2018, 107, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Rao, Z.M.; Xue, Y.F.; Gong, P.; Ji, Y.Z.; Ma, Y.H. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) production by Haloarchaeon Halogranum amylolyticum. Appl. Microbiol. Biotechnol. 2015, 99, 7639–7649. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Xiang, H.; Han, J. Current developmentson polyhydroxyalkanoates synthesis by usinghalophiles as a promising cell factory. Microb. Cell Fact. 2020, 19, 86–115. [Google Scholar] [CrossRef]
- Torregrosa-Crespo, J.; González-Torres, P.; Bautista, V.; Esclapez, J.M.; Pire, C.; Camacho, M.; Bonete, M.J.; Richardson, D.J.; Watmough, N.J.; Martínez-Espinosa, R.M. Analysis of multiple haloarchaeal genomes suggests that the quinone-dependent respiratory nitric oxide reductase is an important source of nitrous oxide in hypersaline environments. Environ. Microbiol. Rep. 2017, 9, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lu, Q.; Zhou, L.; Zhou, J.; Xiang, H. Molecular characterization of the phaECHm genes, required for biosynthesis of poly(3-hydroxybutyrate) in the extremely halophilic archaeon Haloarcula marismortui. Appl. Environ. Microbiol. 2007, 73, 6058–6065. [Google Scholar] [CrossRef]
- Rehm, B.H.A. Biogenesis of microbial polyhydroxyalkanoate granules: A platform technology for the production of tailor-made bioparticles. Curr. Issues Mol. Biol. 2007, 9, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Taran, M. Utilization of petrochemical wastewater for the production of poly(3- hydroxybutyrate) by Haloarcula sp. IRU1. J. Hazard. Mater. 2011, 188, 26–28. [Google Scholar] [CrossRef]
- Ferre-Guell, A.; Winterburn, J. Biosynthesis and characterization of polyhydroxyalkanoates with controlled composition and microstructure. Biomacromolecules 2018, 19, 996–1005. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pramanik, A.; Maji, S.K.; Haldar, S.; Mukhopadhyay, U.K. Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Express 2012, 2, 34. [Google Scholar] [CrossRef]
- Alsafadi, D.; Al-Mashaqbeh, O. A one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill wastewater by Haloferax mediterranei. N. Biotechnol. 2017, 34, 47–53. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Jana, K.; Haldar, S.; Bhowmic, A.; Mukhopadhyay, U.K.; De, S.; Mukherjee, J. Integration of poly-3-(hydroxybutyrate-co-hydroxyvalerate) production by Haloferax mediterranei through utilization of stillage from rice-based ethanol manufacture in India and its techno-economic analysis. World J. Microbiol. Biotechnol. 2015, 31, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Duan, K.J.; Shih-yow, H.; Chen, C.W. Production of polyhydroxyalkanoates from inexpensive extruded rice bran and starch by Haloferax mediterranei. J. Ind. Microbiol. Biotechnol. 2006, 33, 701–706. [Google Scholar] [CrossRef]
- Raho, S.; Carofiglio, V.E.; Montemurro, M.; Miceli, V.; Centrone, D.; Stufano, P.; Schioppa, M.; Pontonio, E.; Rizzello, C.G. Production of the polyhydroxyalkanoate PHBV from ricotta cheese exhausted whey by Haloferax mediterranei fermentation. Foods 2020, 9, 1459. [Google Scholar] [CrossRef] [PubMed]
- Alsafadi, D.; Ibrahim, M.I.; Alamry, K.A.; Hussein, M.A.; Mansour, A. Utilizing the crop waste of date palm fruit to biosynthesize polyhydroxyalkanoate bioplastics with favorable properties. Sci. Total Environ. 2020, 737, 139716. [Google Scholar] [CrossRef]
- Koller, M. Recycling of waste streams of the biotechnological poly(hydroxyalkanoate) production by Haloferax mediterranei on whey. Int. J. Polym. Sci. 2015, 2015, 370164. [Google Scholar] [CrossRef]
- Ghosh, S.; Gnaim, R.; Greiserman, S.; Fadeev, L.; Gozin, M.; Golberg, A. Macroalgal biomass subcritical hydrolysates for the production of polyhydroxyalkanoate (PHA) by Haloferax mediterranei. Bioresour. Technol. 2019, 271, 166–173. [Google Scholar] [CrossRef]
- Pramanik, A.; Mitra, A.; Arumugam, M.; Bhattacharyya, A.; Sadhukhan, S.; Ray, A.; Haldar, S.; Mukhopadhyay, U.K.; Mukherjee, J. Utilization of vinasse for the production of polyhydroxybutyrate by Haloarcula marismortui. Folia Microbiol. 2012, 57, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Taran, M.; Amirkhani, H. Strategies of poly (3-hydroxybutyrate) synthesis by Haloarcula sp. IRU1 utilizing glucose as carbon source: Optimization of culture conditions by Taguchi methodology. Int. J. Biol. Macromol. 2010, 47, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Biodegradable Polymers: A Review; Technical Report; Environmental and Plastics Industry Council: Ottawa, ON, Canada, 2000.
- ASTM D6400-19, D20 Committee. Standard Specification for Labeling of Plastics Designed to be Aerobically Composted in Municipal or Industrial Facilities; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Gestión Ambiental, UNE—Asociación Española de Normalización y Certificación. CTN 49/GT 1—Envases y Embalajes. Available online: https://www.une.org/Paginas/Normalizacion/Ficha-CTN.aspx?n=2&c=CTN%2049/GT%201 (accessed on 10 June 2020).
- Hayes, D.; Dharmalingam, S.; Wadsworth, L.; Leonas, K.; Miles, C.; Inglis, D. Biodegradable agricultural mulches derived from biopolymers. In Degradable Polymers and Materials: Principles and Practice, 2nd ed.; Khemani, K., Scholz, C., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Volume 1114, pp. 201–223. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Girdhar, A.; Bhatia, M.; Nagpal, S.; Kanampalliwar, A.; Tiwari, A. Process parameters for influencing polyhydroxyalkanoate producing bacterial factories: An overview. J. Pet. Environ. Biotechnol. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Market Data: PlasticsEurope. Available online: https://www.plasticseurope.org/en/resources/market-data (accessed on 28 May 2020).
- Market—European Bioplastics e.V. Available online: https://www.european-bioplastics.org/market (accessed on 28 May 2020).
- Kaneka Biodegradable Polymer PHBH. Available online: http://www.kaneka.be/new-business/kaneka-biodegradable-polymer-phbh (accessed on 23 November 2020).
- TianAn Biopolymer. Nature’s Eco-Friendly Solution. ENMAT. Available online: http://www.tianan-enmat.com/ (accessed on 23 November 2020).
- Bio-on. Available online: http://www.bio-on.it (accessed on 23 November 2020).
- Mirel. Bioplastics by Telles. Available online: www.mirelplastics.com (accessed on 23 November 2020).
- GREENBIO. Available online: http://www.tjgreenbio.com/en/ (accessed on 23 November 2020).
- Danimer Scientific a Biotechnology Company. Available online: https://danimerscientific.com (accessed on 23 November 2020).
- Bluepha. PHA Supplier. Available online: http://en.bluepha.com/pha-bioplastic (accessed on 23 November 2020).
- CjBio. Available online: https://www.cjbio.net/en/products/cjPha.do (accessed on 23 November 2020).
- Full Cycle. The Circular Revolution is Here—Brought to You by Full Cycle. Available online: http://fullcyclebioplastics.com/ (accessed on 23 November 2020).
- PolyFerm Canada. Renewable & Biodegradable Polymers for Engineered Solutions. Available online: https://www.polyfermcanada.com/versamer_products.html (accessed on 23 November 2020).
- Mangomaterials. Nature’s Solution to Plastic. Available online: https://www.mangomaterials.com/products/ (accessed on 23 November 2020).
- Biomer Biodegradable Polymers. Injection Molded Articles Made of Renewable Raw Materials. Available online: http://www.biomer.de/IndexE.html (accessed on 23 November 2020).
- Newlight. From Greenhouse Gas to Regenerative Materials that Improve the World. Available online: https://www.newlight.com/ (accessed on 23 November 2020).
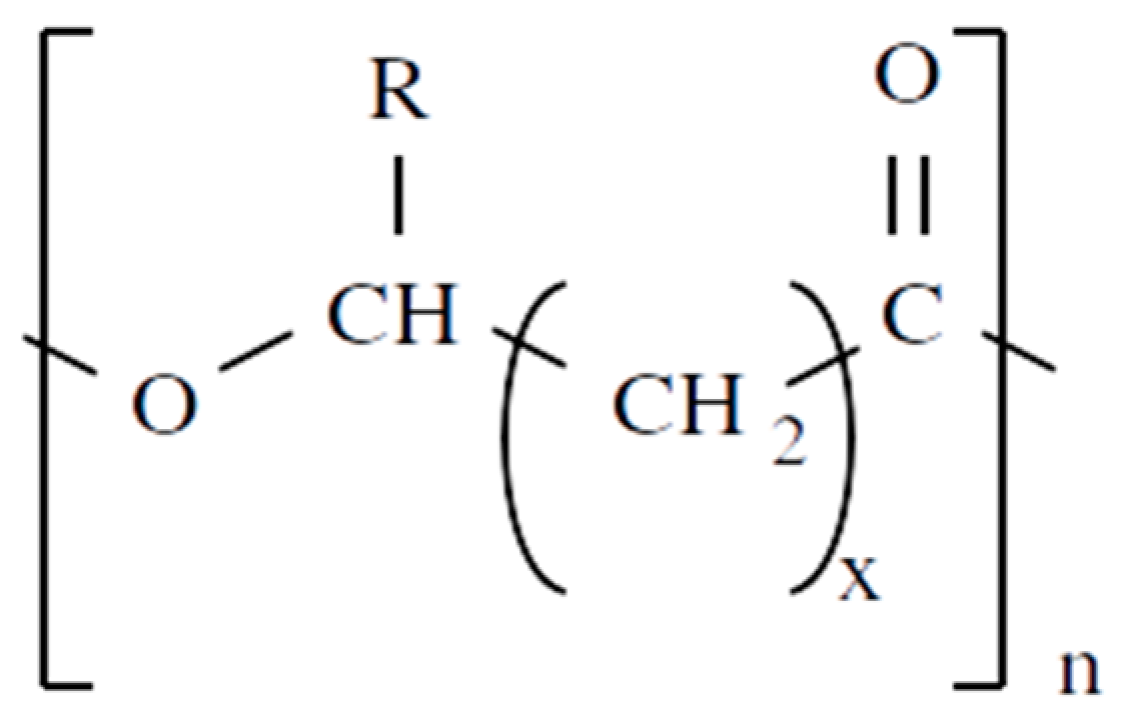

| Monomer | Abbreviation | Group (R) | N° of Carbons | Chemical Structure |
|---|---|---|---|---|
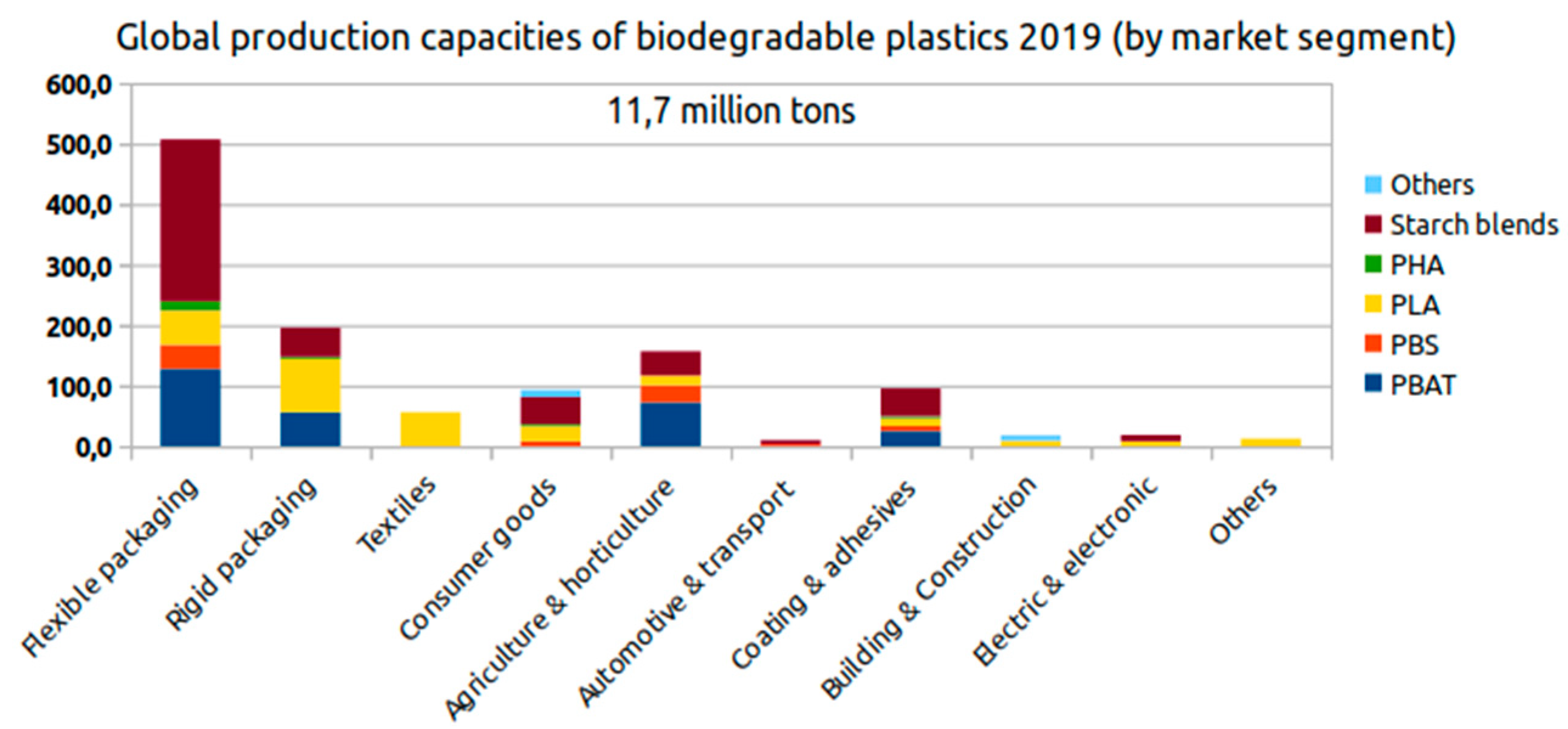
| 3-hydroxypropionic acid | 3HP | Hydrogen | 3 | 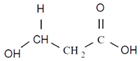 |
| 3-hydroxybutyric acid | 3HB | Methyl | 4 |  |
| 3-hydroxyvaleric acid | 3HV | Ethyl | 5 | 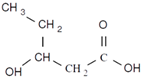 |
| 3-hydroxyhexanoic acid | 3HHx | Propyl | 6 | 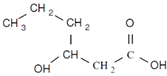 |
| 4-hydroxybutiric acid | 4HB | Hydrogen | 4 |  |
| 4-hydroxyvaleric acid | 4HV | Methyl | 5 |  |
| Name | Chemical Structure | Physicochemical Properties |
|---|---|---|
| Polyhydroxyalkanoate (PHA) |  | Water-insoluble; UV resistance; poor resistance to acids and bases; soluble in chloroform and other chlorinated hydrocarbons. |
| Polyhydroxybutyrate (PHB) |  | High crystallinity degree; brittle. |
| Polyhydroxyvalerate (PHV) | 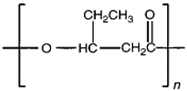 | PHB co-polymer; lower crystallinity degree and more flexibility than PHB. |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) | 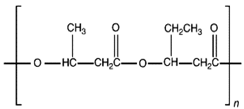 | Brittle; high elastic modulus; low tensile strength. |
| Strain | Carbon Source | Type of PHA (mol %) | Cultivation Mode | DCW (g L−1) | PHA (g L−1) | PHA/CDW (%) | YPHA/Subst | Productivity (g L−1 h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Haloferax mediterranei DSM 1411 | 25% pretreated vinasse | PHBV (12.4%3HV) | Flask | 19.7 | 70.0 | 0.87 | 0.21 | [146] | |
| Haloferax mediterranei DSM 1411 | 50% pretreated vinasse | PHBV (14.1%3HV) | Flask | 17.4 | 66.0 | 0.52 | 0.18 | [146] | |
| Haloferax mediterranei DSM 1411 | Hydrolyzed whey | PHBV (6.0% 3HV) | Batch-42L Bioreactor | 12.2 | 72.8 | 0.29 | 0.09 | [134] | |
| Haloferax mediterranei DSM 1411 | Hydrolyzed whey + sodium valerate + Y-butyrolactone | P-(3HB-co-21.8%3Hvco-5.1%4HB | Batch-10L Bioreactor | 14.7 | 87.5 | 0.20 | 0.14 | [134] | |
| Haloferax mediterranei DSM 1411 | MST medium with 15% of olive mill wastewater | PHBV (6.5% 3HV) | Flask | 10 | 0.2 | 43.0 | n.r. | n.r | [147] |
| Haloferax mediterranei DSM 1411 | Rice-based ethanol stillage | PHBV (17.91%3HV) | 14 L tank | 13.2 | 63.0 | 0.27 | 0.14 | [148] | |
| Haloferax mediterranei DSM 1411 | Crude glycerol (biodiesel industry) | PHBV (10% 3HV) | Fed batch10L Bioreactor | 13.4 | 75.4 | 0.37 | 0.12 | [115] | |
| Haloferax mediterranei DSM 1411 | Hydrolyzed cheese whey | PHBV (98.5%HB–1.5%3HV) | Batch bioreactor | 7.6 | 7.92 | 53.0 | 0.78 | 0.17 | [75] |
| Haloferax mediterranei DSM 1411 | Glucose + galactose | PHBV | Flask | 6.8 | 6.7 | 46.0 | 0.66 | 0.055 | [75] |
| Haloferax mediterranei DSM 1411 | Enzymatic extruded starch | PHBV (10.4%3HV) | 6L Fed-batch Bioreactor | 39.4 | 20 | 50.8 | n.r. | n.r. | [149] |
| Haloferax mediterranei DSM 1411 | Butanoic: pentanoic VFAs (29:71) | PHBV (71.5%3HV) | Fed batch flask | 5.8 | 1.5 | 25.0 | 0.14 | n.r. | [145] |
| Haloferax mediterranei DSM 1411 | Butanoic: pentanoic VFAs (56:44) | PHBV (44.4%3HV) | Fed batch flask | 6.0 | 1.2 | 19.9 | 0.11 | n.r. | [145] |
| Haloferax mediterranei DSM 1411 | Butanoic: pentanoic VFAs (79:21) | PHBV (20.6%3HV) | Fed batch flask | 5.5 | 1.2 | 20.7 | 0.11 | n.r. | [145] |
| Haloferax mediterranei DSM 1411 | 0.5M pentanoic VFA | PHBV (99.5%3HV) | Fed batch flask | 5.5 | 1.5 | 27.1 | n.r. | n.r. | [145] |
| Haloferax mediterranei DSM 1411 | 0.5M propanoic VFA | PHBV (66.2%3HV) | Batch flask | 6.7 | 1.0 | 14.5 | n.r. | n.r. | [145] |
| Haloferax mediterranei ES1 (engineered strain) | 10 g/L glucose | PHBV (8.9%3HV) | Shake flask | 10.3 | 3.3 | 32.4 | n.r. | n.r. | [132] |
| Haloferax mediterranei ES1 (engineered strain) | 10 g/L glucose + 6.5 mM valerate | PHBV (20.8%3HV) | Shake flask | 11.6 | 4.0 | 34.4 | n.r. | n.r. | [132] |
| Haloferax mediterranei ES1 (engineered strain) | 10 g/L glucose + 15 mM valerate | PHBV (36.6%3HV) | Shake flask | 13.3 | 5.4 | 41.0 | n.r. | n.r. | [132] |
| Haloferax mediterranei DSM 1411 | Ricotta hydrolyzed cheese whey | PHBV | Batch 3 L bioreactor | 18.3 | 1.27 | n.r. | 0.1 | n.r. | [150] |
| Haloferax mediterranei DSM 1411 | Date palm sugars | PHBV (18%3HV) | 2 L Fed-batch bioreactor | 18 | 4.5 | 25.0 | n.r | n.r | [151] |
| Haloferax mediterranei DSM 1411 | Hydrolyzed whey permeate | PHBV (10%3HV) | 220 L bioreactor | n.r. | 7.2 | 66.0 | n.r | n.r | [152] |
| Haloferax mediterranei DSM1411 | 25% v/v of macroalgal hydrolyzate (Ulva sp.) | PHBV (8%3HV) | Shake flask | 3.8 | 2.2 | 0.55 | 0.035 | [153] | |
| Halogeometricum borinquense strain TN9 | 20 g/L glucose | PHB | Shake flask | 14.0 | [29] | ||||
| Natrinema ajinwuensis RM-G10 | Glucose | PHBV(13.9%3HV) | Batch flask | 61.7 | 0.211 | [138] | |||
| Halogeometricum borinquense E3 | 20 g/L glucose | PHBV(21.5%3HV) | Batch flask | 2.1 | n.r. | 75.2 | n.r. | 0.025 | [28] |
| Haloarcula marismortui MTCC1596 | 100% pretreated vinasse (ethanol industry) | PHB | Shake flask | 15 | 4.5 | 30.3 | 0.77 | 0.021 | [154] |
| Haloarcula sp. IRU1 | Glucose | PHB | Shake flask | 66.0 | [155] | ||||
| Halogeometricum borinquense E3 | Sugarcane bagasse 25% | PHBV (13.3%3HV) | n.r. | n.r. | n.r. | 50.0 | 0.44 | 0.0095 | [136] |
| Natrinema palladium 1TK1 | 2% whey nutrient broth | PHBV | Shake Flask | 0.41 | 0.20 | 47.7 | n.r. | n.r. | [137] |
| Natrinema palladium 1TK1 | 2% tomato nutrient broth | PHBV | Shake Flask | 2.8 | 0.87 | 31.2 | n.r. | n.r. | [137] |
| Natrinema palladium 2KYS1 | 2% melon nutrient broth | PHBV | Shake flask | 0.55 | 0.15 | 26.3 | n.r. | n.r. | [137] |
| Natrinema palladium 5TL6 | 2% corn starch nutrient Broth | PHBV | Shake flask | 0.43 | 0.18 | 41.4 | n.r. | n.r. | [137] |
| Haloarcula sp | Petrochemical wastewater | PHB | Shake flask | n.r. | n.r. | 46.6 | n.r. | n.r. | [144] |
| Manufacturer | PHA Type | Reference |
|---|---|---|
| Kaneka Belgium NV | PHBH | [164] |
| TianAn Biopolymer | PHBV | [165] |
| Bio-on S.p.A. | PHA | [166] |
| Telles (a joint venture of Metabolix and ADM) | PHBV | [167] |
| Tianjin GreenBio Materials Co. | PHA | [168] |
| Danimer Scientific | PHA | [169] |
| Bluepha | PHA | [170] |
| CJ CheilJedang Corp. | PHA | [171] |
| Full Cycle | PHA | [172] |
| PolyFerm Canada | PHA | [173] |
| Mango Materials | PHA | [174] |
| Biomer | PHB | [175] |
| Newlight Technologies | PHA | [176] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simó-Cabrera, L.; García-Chumillas, S.; Hagagy, N.; Saddiq, A.; Tag, H.; Selim, S.; AbdElgawad, H.; Arribas Agüero, A.; Monzó Sánchez, F.; Cánovas, V.; et al. Haloarchaea as Cell Factories to Produce Bioplastics. Mar. Drugs 2021, 19, 159. https://doi.org/10.3390/md19030159
Simó-Cabrera L, García-Chumillas S, Hagagy N, Saddiq A, Tag H, Selim S, AbdElgawad H, Arribas Agüero A, Monzó Sánchez F, Cánovas V, et al. Haloarchaea as Cell Factories to Produce Bioplastics. Marine Drugs. 2021; 19(3):159. https://doi.org/10.3390/md19030159
Chicago/Turabian StyleSimó-Cabrera, Lorena, Salvador García-Chumillas, Nashwa Hagagy, Amna Saddiq, Hend Tag, Samy Selim, Hamada AbdElgawad, Alejandro Arribas Agüero, Fuensanta Monzó Sánchez, Verónica Cánovas, and et al. 2021. "Haloarchaea as Cell Factories to Produce Bioplastics" Marine Drugs 19, no. 3: 159. https://doi.org/10.3390/md19030159
APA StyleSimó-Cabrera, L., García-Chumillas, S., Hagagy, N., Saddiq, A., Tag, H., Selim, S., AbdElgawad, H., Arribas Agüero, A., Monzó Sánchez, F., Cánovas, V., Pire, C., & Martínez-Espinosa, R. M. (2021). Haloarchaea as Cell Factories to Produce Bioplastics. Marine Drugs, 19(3), 159. https://doi.org/10.3390/md19030159









