Effect of Oversulfation on the Composition, Structure, and In Vitro Anti-Lung Cancer Activity of Fucoidans Extracted from Sargassum aquifolium
Abstract
1. Introduction
2. Results
2.1. Preparation of Oversulfated Fucoidans (SCA-S1 and SCA-S2) and Compositional Analysis
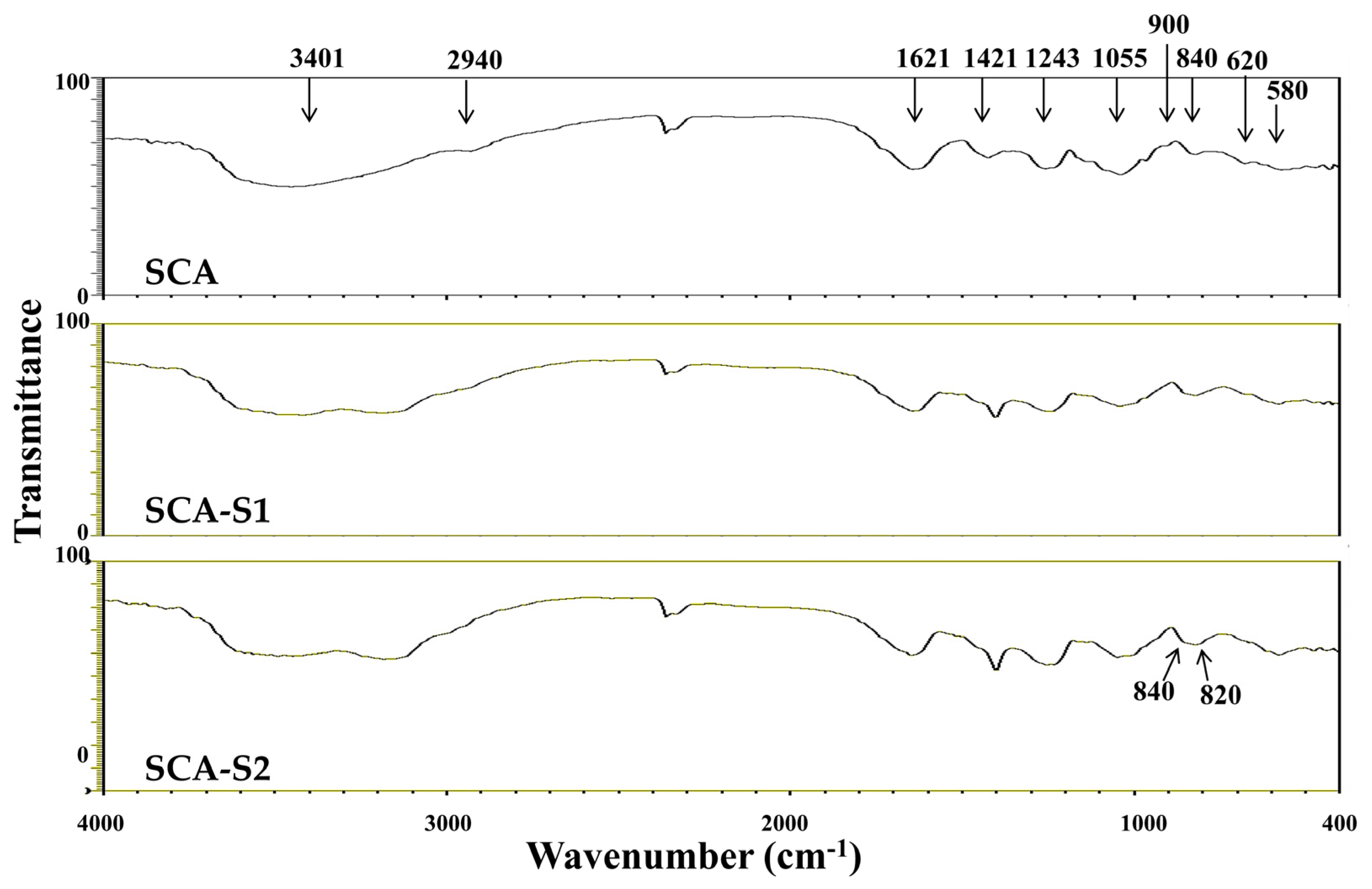
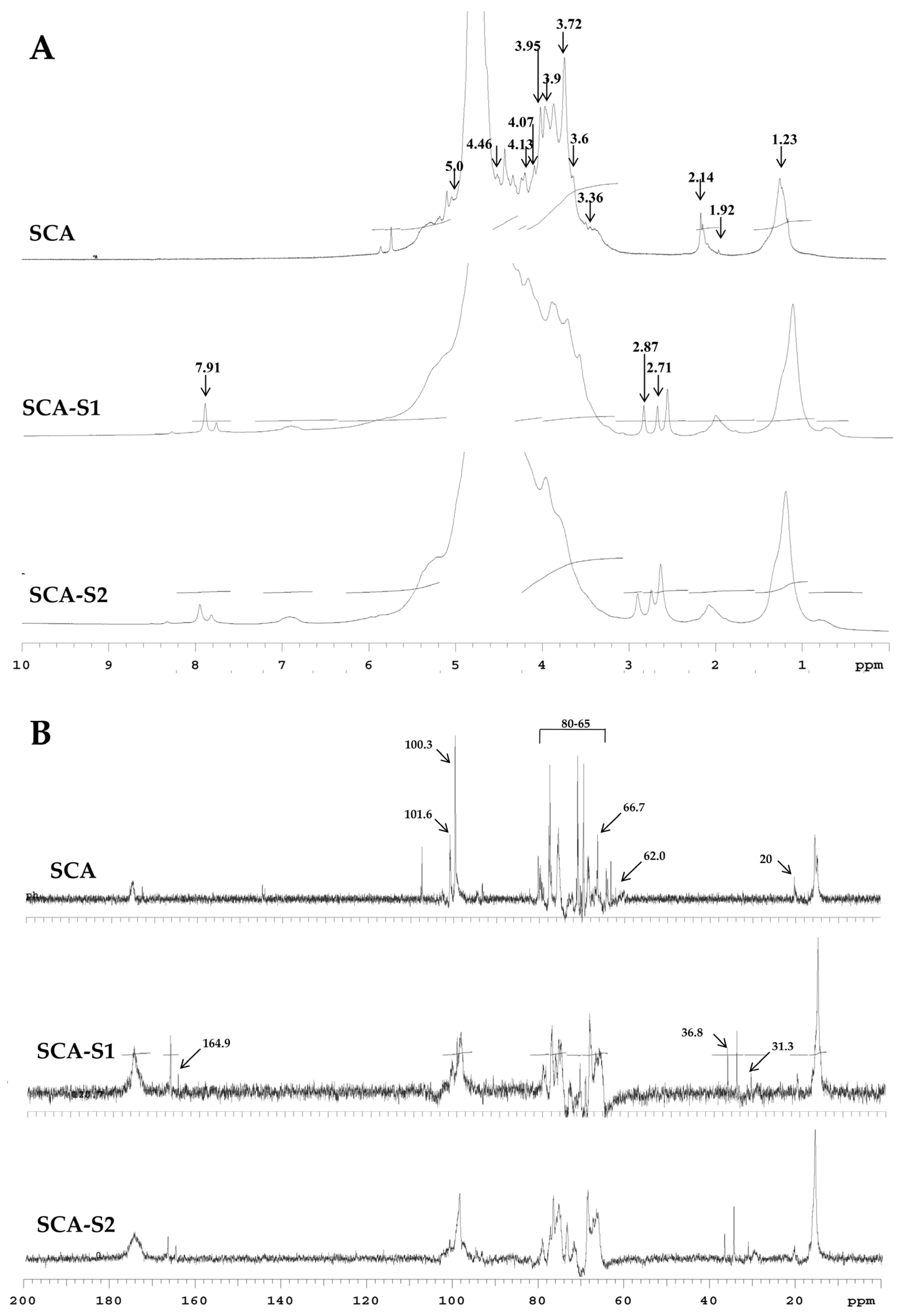
2.2. Structural Analysis of SCA, SCA-S1, and SCA-S2
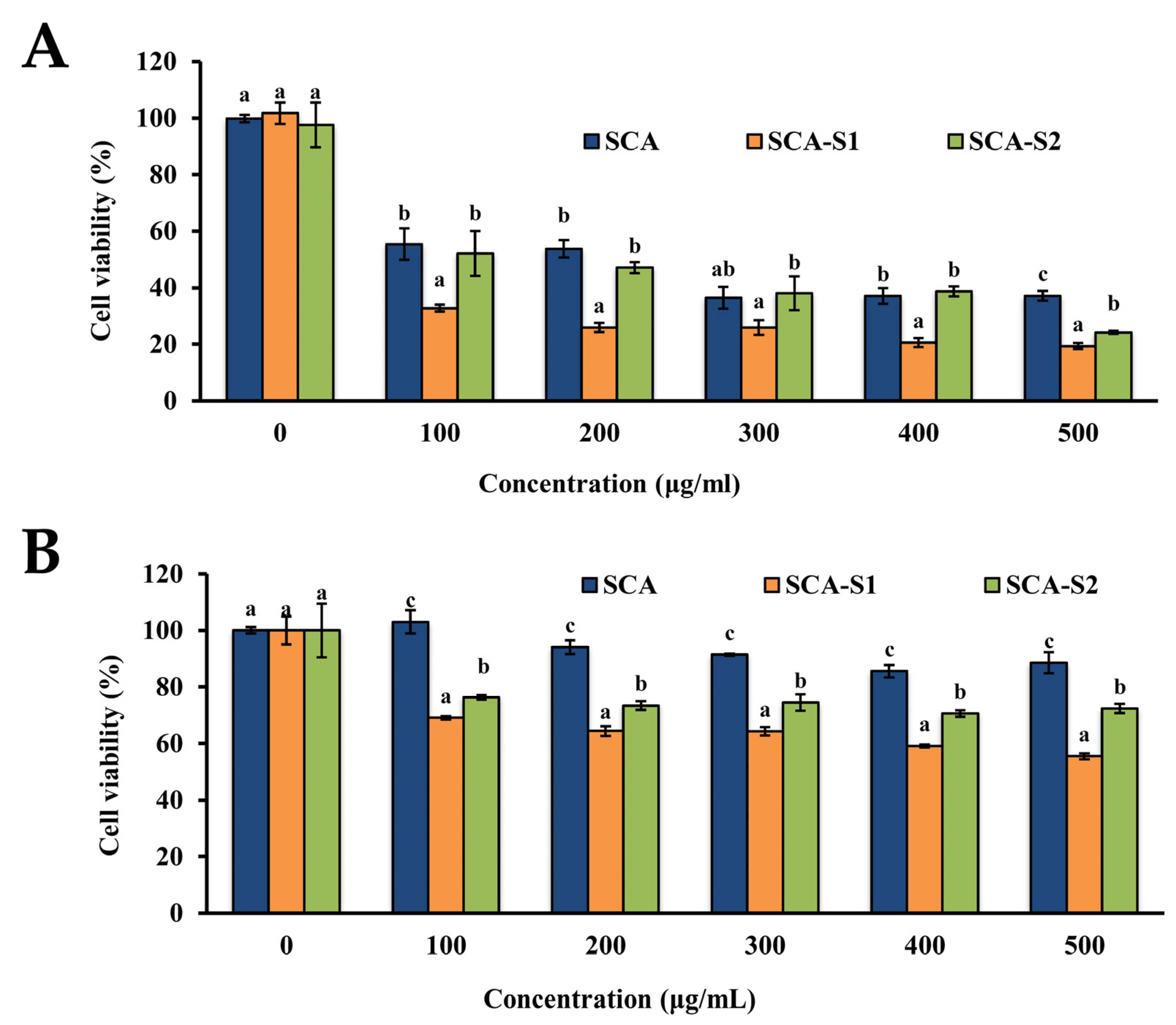
2.3. SCA, SCA-S1, and SCA-S2 Exhibited Cytotoxic Effects on A-549 Cells
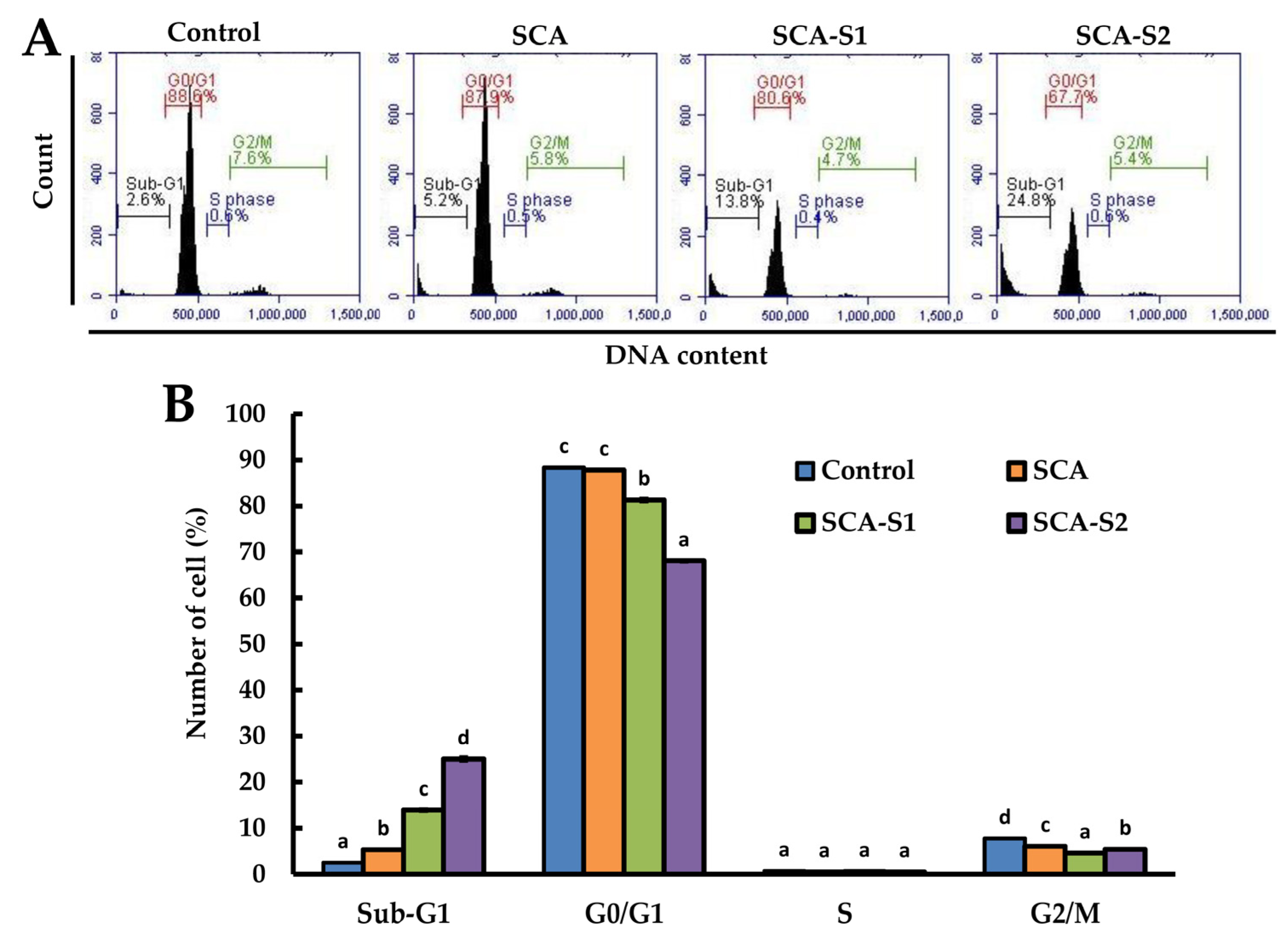
2.4. Effects of SCA, SCA-S1, and SCA-S2 on Cell Cycle Profile of A-549 Cells
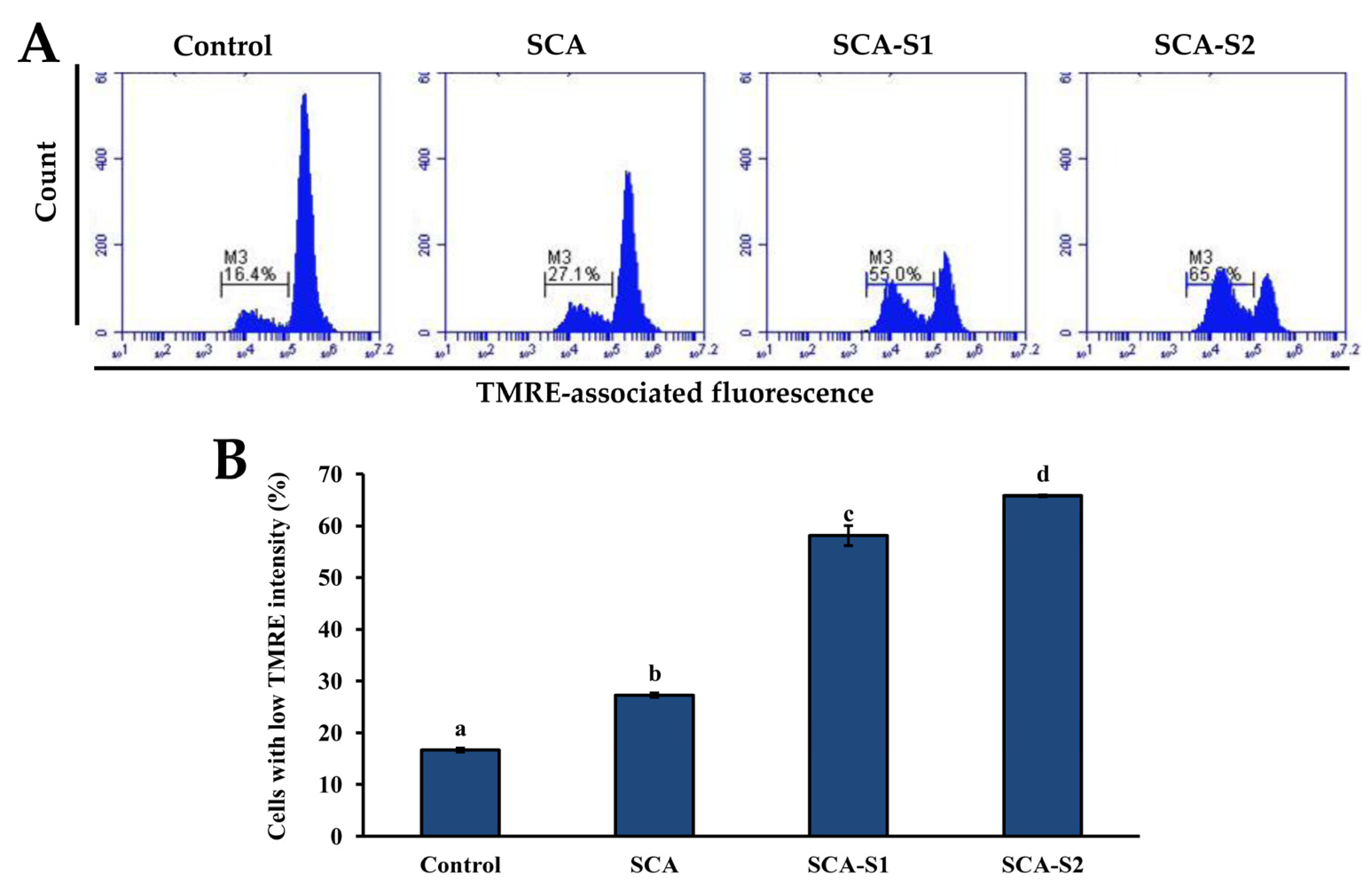
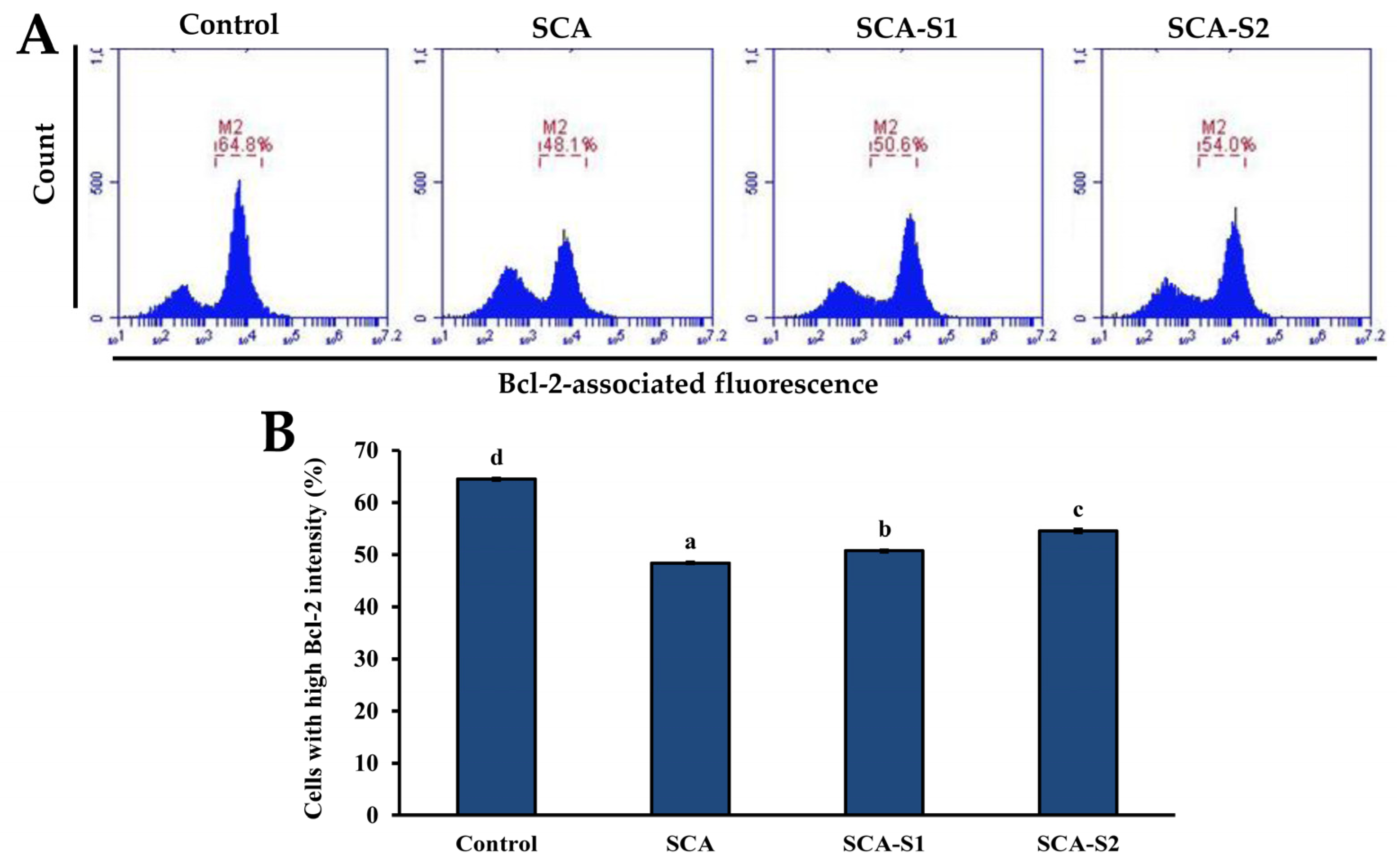
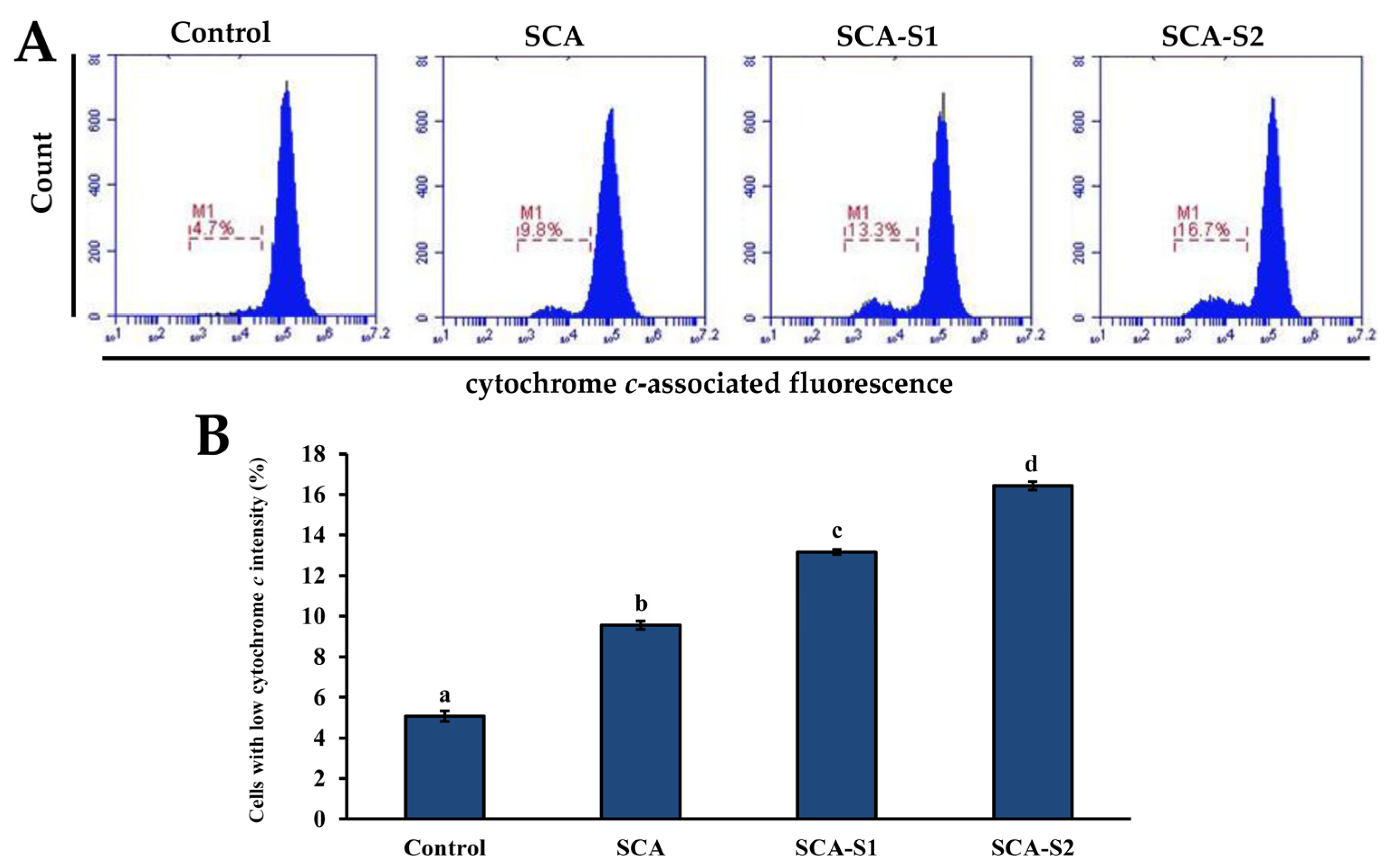
2.5. Effects of SCA, SCA-S1, and SCA-S2 on Mitochondrial Membrane Potential, Bcl-2 Expression, and Cytochrome c Release of A-549 Cells
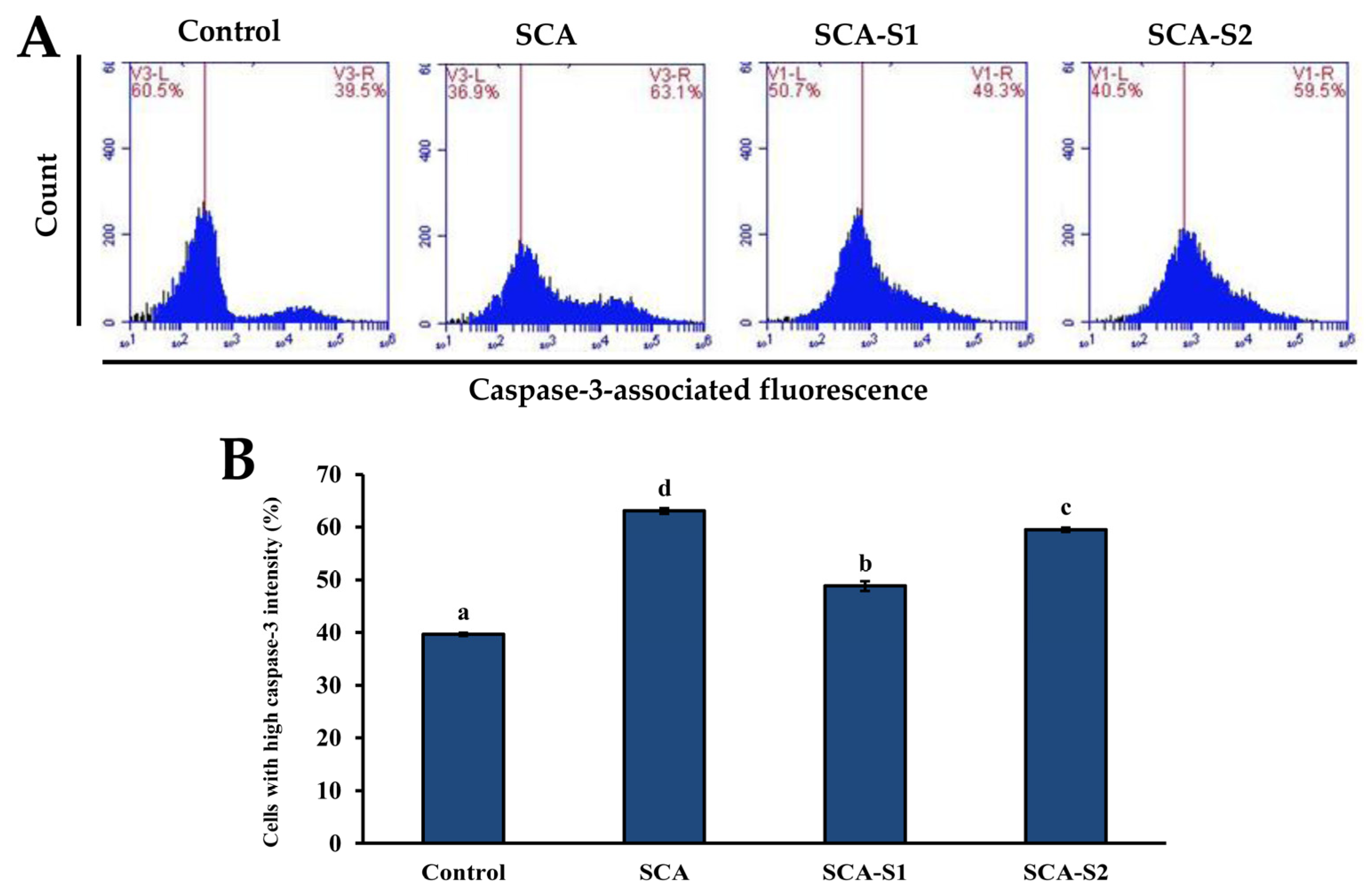
2.6. Effects of SCA, SCA-S1, and SCA-S2 on Activation of Caspase-3 and DNA Fragmentation of A-549 Cells
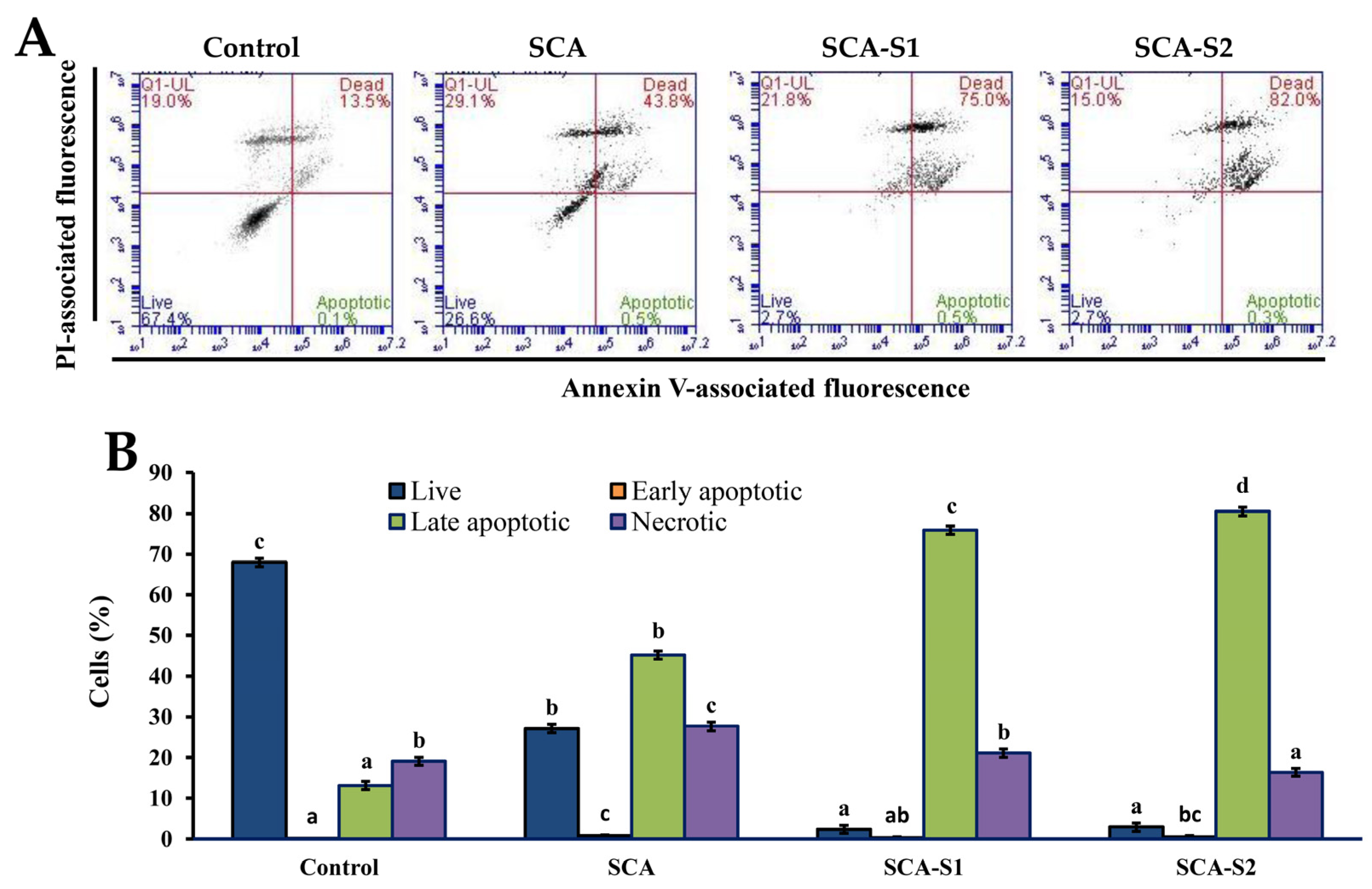
2.7. Effects of SCA, SCA-S1, and SCA-S2 on the Induction of Apoptosis in A-549 Cells
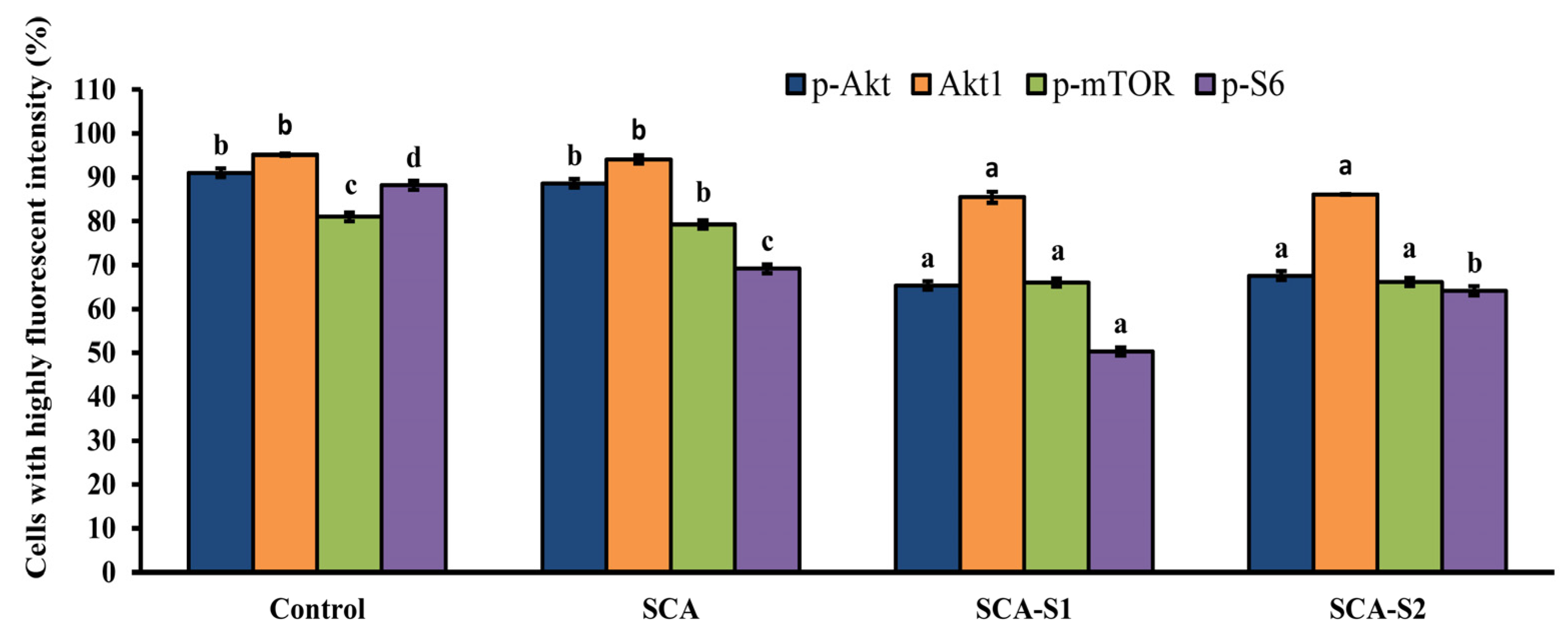
2.8. SCA, SCA-S1, and SCA-S2 Induced Dephosphorylation of Akt, mTOR, and S6 in A-549 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sulfation of Fucoidan
4.3. Analytical Methods
4.4. Monosaccharide Composition Analysis
4.5. FTIR Spectroscopy
4.6. NMR Spectroscopy
4.7. Cell Culture
4.8. Evaluation of Cytotoxic Activity
4.9. Flow Cytometry-Based Analyses
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frión-Herrera, Y.; Díaz-García, A.; Ruiz-Fuentes, J.; Rodríguez-Sánchez, H.; Sforcin, J.M. Brazilian green propolis induced apoptosis in human lung cancer A549 cells through mitochondrial-mediated pathway. J. Pharm. Pharmacol. 2015, 67, 1448–1456. [Google Scholar] [CrossRef]
- Islami, F.; Torre, L.A.; Jemal, A. Global trends of lung cancer mortality and smoking prevalence. Transl. Lung Cancer Res. 2015, 4, 327–338. [Google Scholar]
- Ministry of Health and Welfare. Taiwan, Statistics of Causes of Death. Available online: https://dep.mohw.gov.tw/DOS/lp-4927-113.html (accessed on 15 March 2021).
- Wang, J.; Zhang, Q.; Li, S.; Chen, Z.; Tan, J.; Yao, J.; Duan, D. Low molecular weight fucoidan alleviates diabetic nephropathy by binding fibronectin and inhibiting ECM-receptor interaction in human renal mesangial cells. Int. J. Biol. Macromol. 2020, 150, 304–314. [Google Scholar] [CrossRef]
- Soeda, S.; Sakaguchi, S.; Shimeno, H.; Nagamatsu, A. Fibrinolytic and anticoagulant activities of highly sulfated fucoidan. Biochem. Pharmacol. 1992, 43, 1853–1858. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Cho, M.L.; Lee, B.Y.; You, S.G. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules 2011, 16, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Hong, Y.H.; Tsai, Y.H.; Hsieh, S.L.; Huang, R.H.; Kuo, C.H.; Huang, C.Y. Degradation of Sargassum crassifolium fucoidan by ascorbic acid and hydrogen peroxide, and compositional, structural, and in vitro anti-Lung cancer analyses of the degradation products. Mar. Drugs 2020, 18, 334. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Shao, P.; Pei, Y.P.; Fang, Z.X.; Sun, P.L. Effects of partial desulfation on antioxidant and inhibition of DLD cancer cell of Ulva fasciata polysaccharide. Int. J. Biol. Macromol. 2014, 65, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Kuo, C.H.; Chen, P.W. Compressional-puffing pretreatment enhances neuroprotective effects of fucoidans from the brown seaweed Sargassum hemiphyllum on 6-hydroxydopamine-induced apoptosis in SH-SY5Y cells. Molecules 2018, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Bleha, R.; Synytsya, A.; Pohl, R.; Hayashi, K.; Yoshinaga, K.; Nakano, T.; Hayashi, T. Mekabu fucoidan: Structural complexity and defensive effects against avian influenza A viruses. Carbohydr. Polym. 2014, 111, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Tako, M.; Nakada, T.; Hongou, F. Chemical characterization of fucoidan from commercially cultured Nemacystus decipiens (Itomozuku). Biosci. Biotechnol. Biochem. 1999, 63, 1813–1815. [Google Scholar] [CrossRef] [PubMed]
- Immanuel, G.; Sivagnanavelmurugan, M.; Marudhupandi, T.; Radhakrishnan, S.; Palavesam, A. The effect of fucoidan from brown seaweed Sargassum wightii on WSSV resistance and immune activity in shrimp Penaeus monodon (Fab). Fish Shellfish Immunol. 2012, 32, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Jégou, C.; Kervarec, N.; Cérantola, S.; Bihannic, I.; Stiger-Pouvreau, V. NMR use to quantify phlorotannins: The case of Cystoseira tamariscifolia, a phloroglucinol-producing brown macroalga in Brittany (France). Talanta 2015, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 2004, 339, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.V.; Lakshmanasenthil, S.; Geetharamani, D.; Marudhupandi, T.; Suja, G.; Suganya, P. Fucoidan—A α-d-glucosidase inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int. J. Biol. Macromol. 2015, 72, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Imbs, T.I.; Ermakova, S.P.; Malyarenko, O.S.; Isakov, V.V.; Zvyagintseva, T.N. Structural elucidation of polysaccharide fractions from the brown alga Coccophora langsdorfii and in vitro investigation of their anticancer activity. Carbohydr. Polym. 2016, 135, 162–168. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Zhang, Q.; Zhang, Z.; Shi, X.; Li, P. Synthesized different derivatives of low molecular fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Int. J. Biol. Macromol. 2009, 44, 379–384. [Google Scholar] [CrossRef]
- Chen, H.M.; Lee, M.J.; Kuo, C.Y.; Tsai, P.L.; Liu, J.Y.; Kao, S.H. Ocimum gratissimum aqueous extract induces apoptotic signalling in lung adenocarcinoma cell A549. Evid. Based Complement. Altern. Med. 2011, 2011, 739093. [Google Scholar] [CrossRef]
- Ma, L.; Qin, C.; Wang, M.; Gan, D.; Cao, L.; Ye, H.; Zeng, X. Preparation, preliminary characterization and inhibitory effect on human colon cancer HT-29 cells of an acidic polysaccharide fraction from Stachys floridana Schuttl. ex Benth. Food Chem. Toxicol. 2013, 60, 269–276. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef]
- Penninger, J.M.; Kroemer, G. Mitochondria, AIF and caspases—Rivaling for cell death execution. Nat. Cell Biol. 2003, 5, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Tissot, B.; Salpin, J.-Y.; Martinez, M.; Gaigeot, M.-P.; Daniel, R. Differentiation of the fucoidan sulfated l-fucose isomers constituents by CE-ESIMS and molecular modeling. Carbohydr. Res. 2006, 341, 598–609. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, T.C.; Hsieh, S.L.; Tsai, Y.H.; Yeh, C.W.; Huang, C.Y. Antioxidant activity and growth inhibition of human colon cancer cells by crude and purified fucoidan preparations extracted from Sargassum cristaefolium. J. Food Drug Anal. 2015, 23, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, X. Cytochrome-c-mediated apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Enari, M.; Sakahira, H.; Yokoyama, H.; Okawa, K.; Iwamatsu, A.; Nagata, S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 1998, 391, 43–50. [Google Scholar] [CrossRef]
- Liu, X.S.; Li, P.; Widlak, P.; Zou, H.; Luo, X.; Garrard, W.T.; Wang, X.D. The 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis. Proc. Natl. Acad. Sci. USA 1998, 95, 8461–8466. [Google Scholar] [CrossRef] [PubMed]
- Van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Ho, C.-T.; Zhang, K.; Liu, Q.; Zhao, H. Garcinol from Garcinia indica downregulates cancer stem-like cell biomarker ALDH1A1 in nonsmall cell lung cancer A549 cells through DDIT3 activation. J. Agric. Food Chem. 2017, 65, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.A.; Oster, C.G.; Mayer, M.M.; Avery, M.L.; Audus, K.L. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp. Cell Res. 1998, 243, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Q.; Feng, J.Q.; Chen, J.; Chen, P.X.; Zhi, J.L.; Cui, Y.; Guo, R.X.; Yu, H.M. Protection of oxidative preconditioning against apoptosis induced by H2O2 in PC12 cells: Mechanisms via MMP, ROS, and Bcl-2. Brain Res. 2005, 1057, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.; Lu, Y.; Wei, Y.; Liu, Y.; Shen, P. The role of ROS in microcystin-LR-induced hepatocyte apoptosis and liver injury in mice. Toxicology 2007, 232, 15–23. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta Bioenergy 2006, 1757, 639–647. [Google Scholar] [CrossRef]
- Campos, C.B.; Paim, B.A.; Cosso, R.G.; Castilho, R.F.; Rottenberg, H.; Vercesi, A.E. Method for monitoring of mitochondrial cytochrome c release during cell death: Immunodetection of cytochrome c by flow cytometry after selective permeabilization of the plasma membrane. Cytom. Part A J. Int. Soc. Anal. Cytol. 2006, 69, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.; Aubert, M. Flow cytometric detection of activated caspase. In Apoptosis and Cancer: Methods and Protocols, 2008 ed.; Mor, G., Alvero, A.B., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2008; pp. 47–56. [Google Scholar]
- Robertson, J.D.; Orrenius, S.; Zhivotovsky, B. Nuclear events in apoptosis. J. Struct. Biol. 2000, 129, 346–358. [Google Scholar] [CrossRef]
- Dienstmann, R.; Rodon, J.; Serra, V.; Tabernero, J. Picking the point of inhibition: A comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol. Cancer Ther. 2014, 13, 1021–1031. [Google Scholar] [CrossRef]
- Polivka, J., Jr.; Janku, F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol. Ther. 2014, 142, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.-S.; Kim, E. Fucoidan from seaweed Fucus vesiculosus inhibits migration and invasion of human lung cancer cell via PI3K-Akt-mTOR pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef] [PubMed]
- Haroun-Bouhedja, F.; Ellouali, M.; Sinquin, C.; Boisson-Vidal, C. Relationship between sulfate groups and biological activities of fucans. Thromb. Res. 2000, 100, 453–459. [Google Scholar] [CrossRef]
- Huang, C.Y.; Wu, S.J.; Yang, W.N.; Kuan, A.W.; Chen, C.Y. Antioxidant activities of crude extracts of fucoidan extracted from Sargassum glaucescens by a compressional-puffing-hydrothermal extraction process. Food Chem. 2016, 197, 1121–1129. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, C.H.; Lee, C.H. Antibacterial and antioxidant capacities and attenuation of lipid accumulation in 3T3-L1 adipocytes by low-molecular-weight fucoidans prepared from compressional-puffing-pretreated Sargassum crassifolium. Mar. Drugs 2018, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wu, T.C.; Wu, C.H.; Tsai, Y.H.; Chung, S.M.; Hong, Y.H.; Huang, C.Y. Antioxidant, anti-inflammatory, and HEP G2 cell growth-inhibitory effects of aqueous-ethanol extracts obtained from non-puffed and compressional-puffed Sargassum crassifolium. J. Mar. Sci. Technol. 2020, 28, 200–210. [Google Scholar]
- Yang, W.N.; Chen, P.W.; Huang, C.Y. Compositional characteristics and in vitro evaluations of antioxidant and neuroprotective properties of crude extracts of fucoidan prepared from compressional puffing-pretreated Sargassum crassifolium. Mar. Drugs 2017, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Shiao, W.C.; Kuo, C.H.; Tsai, Y.H.; Hsieh, S.L.; Kuan, A.W.; Hong, Y.H.; Huang, C.Y. In vitro evaluation of anti-colon cancer potential of crude extracts of fucoidan obtained from Sargassum glaucescens pretreated by compressional-puffing. Appl. Sci. 2020, 10, 3058. [Google Scholar] [CrossRef]
- Huang, C.Y.; Wu, T.C.; Hong, Y.H.; Hsieh, S.L.; Guo, H.R.; Huang, R.H. Enhancement of cell adhesion, cell growth, wound healing, and oxidative protection by gelatins extracted from extrusion-pretreated tilapia (Oreochromis sp.) fish scale. Molecules 2018, 23, 2406. [Google Scholar] [CrossRef] [PubMed]

| Chemical Composition | SCA 2 | SCA-S1 2 | SCA-S2 2 |
| Sulfate (%) 1 | 13.67 ± 2.19 a | 34.67 ± 3.73 b | 60.63 ± 3.69 c |
| Total sugar (%) 1 | 41.70 ± 0.91 c | 28.95 ± 0.24 a | 35.08 ± 0.21 b |
| Fucose (%) 1 | 35.22 ± 2.79 c | 20.36 ± 1.52 b | 12.58 ± 0.46 a |
| Monosaccharide Composition (Molar Ratio) | SCA | SCA-S1 | SCA-S2 |
| Fucose | 1 | 1 | 1 |
| Galactose | 0.30 | 0.28 | 0.27 |
| Glucuronic acid | 0.01 | ND 3 | ND |
| Galacturonic acid | 0.11 | ND | ND |
| Mannose | 0.05 | 0.05 | 0.05 |
| Xylose | 0.05 | ND | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, H.-H.; Wu, T.-C.; Tsai, Y.-H.; Kuo, C.-H.; Huang, R.-H.; Hong, Y.-H.; Huang, C.-Y. Effect of Oversulfation on the Composition, Structure, and In Vitro Anti-Lung Cancer Activity of Fucoidans Extracted from Sargassum aquifolium. Mar. Drugs 2021, 19, 215. https://doi.org/10.3390/md19040215
Hsiao H-H, Wu T-C, Tsai Y-H, Kuo C-H, Huang R-H, Hong Y-H, Huang C-Y. Effect of Oversulfation on the Composition, Structure, and In Vitro Anti-Lung Cancer Activity of Fucoidans Extracted from Sargassum aquifolium. Marine Drugs. 2021; 19(4):215. https://doi.org/10.3390/md19040215
Chicago/Turabian StyleHsiao, Hui-Hua, Tien-Chiu Wu, Yung-Hsiang Tsai, Chia-Hung Kuo, Ren-Han Huang, Yong-Han Hong, and Chun-Yung Huang. 2021. "Effect of Oversulfation on the Composition, Structure, and In Vitro Anti-Lung Cancer Activity of Fucoidans Extracted from Sargassum aquifolium" Marine Drugs 19, no. 4: 215. https://doi.org/10.3390/md19040215
APA StyleHsiao, H.-H., Wu, T.-C., Tsai, Y.-H., Kuo, C.-H., Huang, R.-H., Hong, Y.-H., & Huang, C.-Y. (2021). Effect of Oversulfation on the Composition, Structure, and In Vitro Anti-Lung Cancer Activity of Fucoidans Extracted from Sargassum aquifolium. Marine Drugs, 19(4), 215. https://doi.org/10.3390/md19040215









