Abstract
Five undescribed harziane-type diterpene derivatives, namely harzianol K (1), harzianol L (4), harzianol M (5), harzianol N (6), harzianol O (7), along with two known compounds, hazianol J (2) and harzianol A (3) were isolated from the deep-sea sediment-derived fungus Trichoderma sp. SCSIOW21. The relative configurations were determined by meticulous spectroscopic methods including 1D, 2D NMR spectroscopy, and HR-ESI-MS. The absolute configurations were established by the ECD curve calculations and the X-ray crystallographic analysis. These compounds (1, and 4–7) contributed to increasing the diversity of the caged harziane type diterpenes with highly congested skeleton characteristics. Harzianol J (2) exhibited a weak anti-inflammatory effect with 81.8% NO inhibition at 100 µM.
1. Introduction
The Trichoderma fungus, widely distributed in terrestrial and marine habitats, is a kind of important renewable natural resource with high economic value and application prospects. Among them, the species in the marine environment, together with Penicillium and Aspergillus, contributed to the discovery of more than half of the new terpenoids from marine fungi [1,2]. However, Trichoderma was rarely reported from deep marine ecosystems. During 2013 to 2019, a total of 151 novel compounds were reported from deep marine derived-fungi, of which 41.2% were from Penicillium, 28.1% were from Aspergillus, while only 1 Trichoderma was reported from the deep marine system [1].
Harziane-type diterpenes, containing unique tetracyclic 6-5-4-7 carbon skeleton with 5–6 contiguous stereocenters, are rarely encountered in other organisms. The unprecedented skeleton was initially discovered in 1992 from Trichoderma harzianum Rifai [3]. To date, only 44 harziane diterpenes have been reported, almost all of which were discovered solely from Trichoderma sp., except for heteroscyphsic acid A from Chinese liverwort Heteroscyphus coalitus [4]. These compounds exhibited extensive bioactivities, including anti-bacterial [5,6,7,8,9,10], cytotoxic [8,11,12,13], anti-inflammatory [13,14], anti-HIV [14], phytotoxic [15], algicidal [5,7,16,17], and marine zooplankton toxic activities [6,16] (Table S1 and Figure S1).
During our ongoing investigations on inhibitors from deep-sea fungi [18,19,20,21,22,23] against nitric oxide (NO) production induced by lipopolysaccharide (LPS), Trichoderma sp. SCSIOW21, which was isolated from sea sediment at a depth of over 1000 m, was found to be active. The subsequent cultivation of this strain resulted in the isolation of seven harziane diterpenes, including five new compounds. Herein, we report the isolation and identification procedures, as well as the anti-inflammatory, anti-fungal, and anti-bacterial activities of these compounds.
2. Results and Discussion
The fungus Trichoderma sp. SCSIOW21 was cultured at room temperature under static conditions. The BuOH extraction was fractioned and purified by silica gel, medium pressure ODS column chromatography, and semi-preparative HPLC to obtain seven harziane diterpenes (Figure 1).
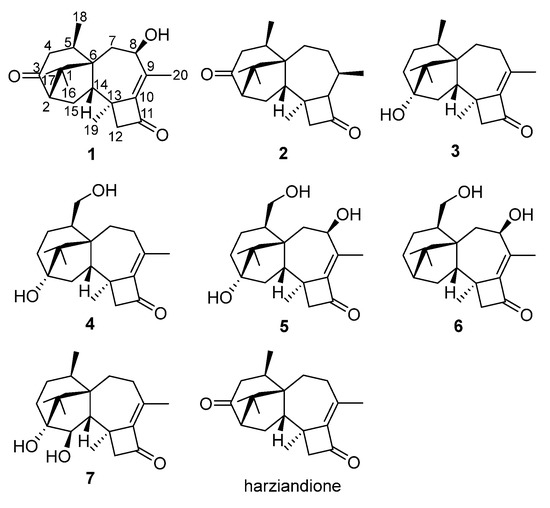
Figure 1.
Compounds 1–7 and harziandione.
Compound 1 was isolated as colorless crystal, with molecular formula as C20H28O3 using HRESIMS data. The IR spectrum showed strong absorption bands for two carbonyl groups at 1734 and 1695 cm−1, which was consistent with those reported for harziandione [3]. The 1H NMR and 13C NMR spectroscopy spectra along with HSQC data suggested five methyls, four methylenes, four methines, and seven quaternary carbon atoms (Table 1 and Table 2). The above NMR spectroscopy signal pattern was similar to the prior report for harziandione [3], except for 3 major differences: an additional hydroxy group at δ 5.31, an absent methylene group, and an extra hydroxy group at δ 4.24 compared with harziandione. The up-field shifts of H-8 to δ 4.24 and C-8 to δ 72.4 suggested this group connected to C-8 (Table 1 and Table 2). 1H-1H COSY correlations between 8-OH and H-8, H-8 and H-7, as well as HMBC correlations from 8-OH to C-8 and C-7 also confirmed the elucidation (Figure 2). This conclusion was further secured by careful analysis of 1D, 2D NMR spectroscopy data, and compound 1 was named as harzianol K, with the molecular framework shown in Figure 2.

Table 1.
1H NMR spectroscopy (600 MHz) a of compounds 1, 4–7.

Table 2.
13C NMR spectroscopy (150 MHz) a data of Compounds 1, 4–7.
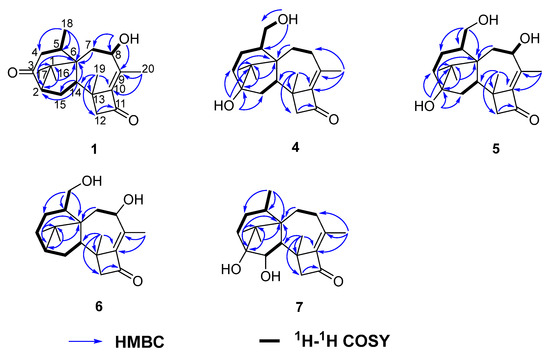
Figure 2.
Key 2D NMR spectroscopy correlations of compounds 1 and 4–7.
The relative configuration of 1 was determined by 1H-1H ROESY spectrum. The 1H-1H correlations—H-14 and H-2, H-14 and Me-16, H-5 and Me-19, Me-18 and 8-OH—indicated that H-2, H-14, Me-16, Me-17, and Me-18 were located on one side of the molecule, whereas Me-19 and H-5 were located on the opposite side (Figure 3).
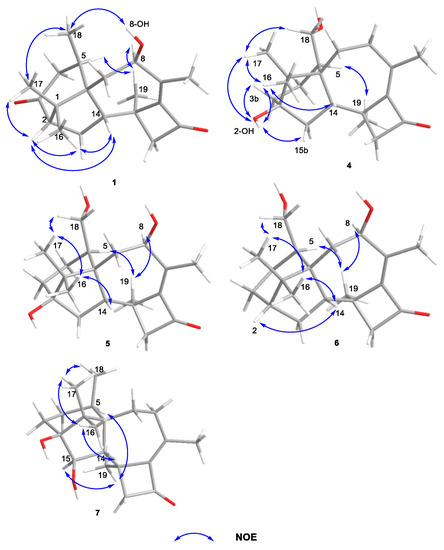
Figure 3.
Key NOE correlations of compounds 1 and 4–7.
The experimental CD spectrum of 1 was in accordance with the theoretically calculated ECD curve of the 2S, 5R, 6R, 8S, 13S, and 14S configuration. A total of 3 cotton effects were observed at 245 nm (negative), 292 nm (positive), and 351 nm (positive) (Figure 4a). Eventually, the stereocenters of 1 were determined as 2S, 5R, 6R, 8S, 13S, and 14S unambiguously through analysis of X-ray single-crystallography (Figure 5).
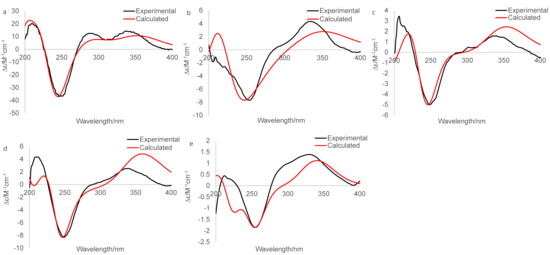
Figure 4.
Experimental and calculated (for 2S, 5R, 6R, 8S, 13S, 14S) ECD spectra of 1 (a), experimental and calculated (for 2S, 5R, 6R, 13S, 14S) ECD spectra of 4 (b), experimental and calculated (for 2S, 5R, 6R, 8S, 13S, 14S) ECD spectra of 5 (c), experimental and calculated (for 2S, 5R, 6R, 8S, 13S, 14S) ECD spectra of 6 (d), Experimental and calculated (for 2S, 5R, 6R, 13S, 14S, 15S) ECD spectra of 7 (e).
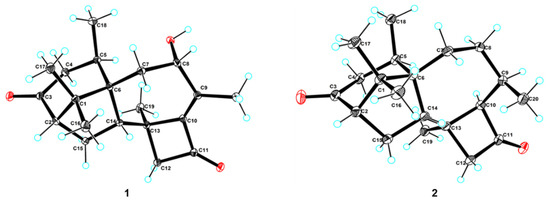
Figure 5.
X-ray single-crystallography structures of 1 and 2. The ellipsoids of non-hydrogen atoms are shown at 30% probability levels for crystal structures.
Compounds 2 and 3 were confirmed as known compounds, namely harzianol J [8] and harzianol A [13], by comparing their NMR spectroscopy data with those reported in the literature (Tables S2 and S3) [8]. Nevertheless, the absolute configuration of 2 was not determined previously. Herein we report it as 2S, 5R, 6R, 13S, and 14S by X-ray diffraction (Figure 5).
Compounds 4–7 were all purified as colorless gum or amorphous solids. The molecular formulas of 4–7 were established as C20H30O3, C20H30O4, C20H30O3, and C20H30O3 based on HRESIMS data, respectively.
The IR spectrum of 4 showed strong absorption band for carbonyl group at 1716 cm−1. The 1H and 13C NMR spectra of 4 (Table 1 and Table 2) were similar to those of harzianol A (3) [13] except for two major differences: the lack of a methyl group and the presence of an extra hydroxy methylene group. The δH signals at 3.41, 3.28, 4.39 (OH) and the δC signal at 63.9 suggested that one methyl group was hydroxylated. The 1H-1H COSY cross-peaks between the hydroxy proton and methylene proton, methylene proton and H-5 (δH 2.13), along with the HMBC correlations from the hydroxy proton to C-5 (δC 40.1) and C-18 (δC 63.9), proved the hydroxy group connected to C-18 unambiguously. The molecular framework of 4 was consequently elucidated as harzianol L (Figure 1 and Figure 2). The relative configuration of 4 was determined by ROESY spectra which showed the same correlation patterns as those of 1 (Figure 3). The absolute configuration of 4 was determined as 2R, 5S, 6R, 13S, and 14S by comparison of experimental CD spectrum with its calculated ECD data (Figure 4b).
The IR spectrum of 5 showed strong absorption band for carbonyl group at 1732 cm−1. The NMR spectroscopy data of 5 was almost consistent with those of 4, except that a methylene group was missing, whereas an extra oxygenated methine group (δH 4.21 and δC 73.5) was detected. The signals suggested that one methylene group was oxygenated (Table 1 and Table 2). 1H-1H COSY correlations between the hydroxy proton and H-8, between H-8 and H-7, confirmed the connection of the hydroxy group to C-8. The structure was then determined as harzianol M by a detailed analysis of 2D NMR data (Figure 1 and Figure 2). In the ROESY spectra, H-8 showed correlations with Me-19, indicating the β configuration of the 8-hydroxy group (Figure 3). The absolute configurations of 5 were established as 2R, 5S, 6R, 8S, 13S, and 14S based on ECD calculation (Figure 4c).
The IR spectrum of 6 showed a strong absorption band for carbonyl group at 1734 cm−1. The NMR spectroscopy spectra of 6 matched well with those of 5, with just 1 more extra methine group (δH 2.26 and δC 51.8) and 1 less oxygenated quaternary carbon signal (Table 1 and Table 2). 1H-1H COSY correlations between the methine proton and H-3, H-15 suggested the methine group was located at C-3. The molecular framework of 6 was consequently established as harzianol N through a detailed analysis of 2D NMR spectroscopy spectra (Figure 1 and Figure 2). The absolute configurations of 6 were determined as 2S, 5S, 6R, 8S, 13S, and 14S through detailed analysis of ROESY spectra and ECD calculation (Figure 3 and Figure 4d).
The IR spectrum of 7 showed strong absorption band for carbonyl group at 1718 cm−1. The 1H and 13C NMR spectroscopy data of 7 were similar to those reported for harzianol A (3) (Table S3) [13], with an extra oxygenated methine group (δH 3.65 and δC 73.5) and a disappeared methylene group, indicating the oxygenation of the methylene group (Table 1 and Table 2). The molecular framework was confirmed as harzianol O (Figure 1 and Figure 2) through a detailed analysis of 2D NMR spectroscopy data, including the key COSY correlation between the methine proton and H-14 (δH 2.07), which suggested the hydroxy group connected to C-15. The ROESY correlations between H-15 and Me-19 suggested the β configuration of the 15-hydroxy group (Figure 3). The absolute configurations of 7 were determined as 2S, 5R, 6R, 13S, 14S, and 15R by ECD calculation.
The anti-inflammatory activity of compounds 1–7 was measured by NO production inhibitory assay [20]. The cytotoxicity of these compounds was tested to avoid false-positive results due to cell death, and none of them showed cytotoxicity at the concentrations of 25–100 µM (Figure 6). Hazianol J (2), harzianol A (3) and harzianol O (7) exhibited the strongest NO production inhibitory activity at 100 µM with inhibitory rates at 81.8%, 46.8%, and 50.5%, respectively. The IC50 of Hazianol J (2) was 66.7 µM, while harzianol L (4) and harzianol K (1) only showed weak inhibition at the highest concentration of 100 µM (Figure 6). Compounds without “top” hydroxy groups at C-8 and C-18 (2,3, and 7) exhibited higher NO production inhibitory activities compared to the compounds with more hydroxy groups (1, 4, 5, and 6). These hydroxy groups may reduce the membrane permeability and reduced the activities.
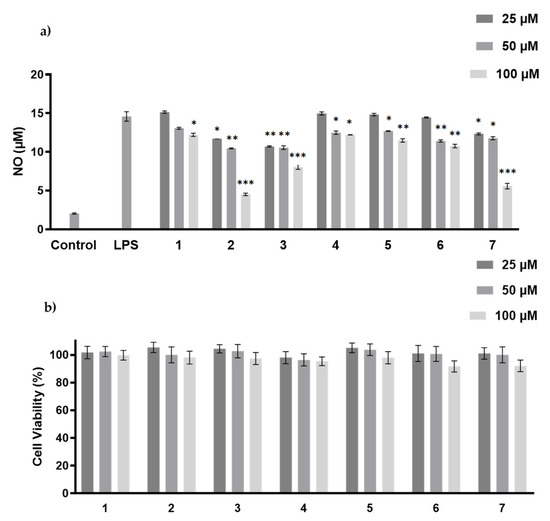
Figure 6.
LPS-induced NO production (a), and viability (b) of RAW 264.7 macrophages by 1–7 treatment. The values represent the mean ± SEM of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. control.
All of the compounds were examined for their activities against plant pathogenic fungi (Helminthosporium maydis, Gibberella sanbinetti, Botrytis cinerea Pers, Fusarium oxysporum f. sp. cucumerinum, Penicillium digitatum). None of the compounds exhibited obvious activities at the test concentration of 100 μg/mL. Since fungi from Trichoderma sp. are widely used as bio-control agents, many harziane diterpenes were investigated against plant pathogenic fungi [3,9,10,16,24]. However, the results were controversial. Although harziandione and isoharziandione, the structure of which was latterly revised as harziandione [10], were mentioned as antifungal agents, the activities of the pure compounds were not clarified in the original literature [3,24]. Harzianone was found to be inactive against Colletotrichum lagenarium and Fusarium oxysporum at 30 µg/disk using a disk diffusion assay [10]. Deoxytrichodermaerin and harzianol A were not active against Botrytis cinerea, Fusarium oxysporum, Glomerella cingulata, and Phomopsis asparagi at 40 µg/disk [16]. Harzianone E was not active against Candida albicans by traditional broth dilution assay [9]. According to the previous studies and our results, harziane diterpenes did not show anti-fungal activity.
3. Materials and Methods
3.1. General Experimental Procedures
The NMR spectroscopy spectra were obtained on the Bruker ASCEND 600 MHz NMR spectrometer equipped with CryoProbe (Bruker Biospin GmbH, Rheinstetten, Germany). Optical rotations were recorded on an Anton Paar MCP-100 polarimeter (Anton Paar GmbH, Austria), with MeOH as solvent. UV spectra were recorded on a UV-1800 spectrometer (Shimadzu Co., Kyoto, Japan). IR spectra were measured on the Nicolet 6700 spectrometer (Thermo, Madison, WI, USA). CD spectra were measured on a J-815 spectropolarimeter (Jasco Co., Japan). Crystallographic data was collected on an XtaLAB Pro: Kappa single four-circle diffractometer using Cu Kα radiation (Rigaku Co., Tokyo, Japan). HRESIMS spectra data were recorded on a MaXis quadrupole-time-of-flight mass spectrometer (Bruker Biospin GmbH, Rheinstetten, Germany). Normal and reverse phase column chromatography (C. C.) was performed using silica gel (200–300 mesh, Qingdao Haiyang Chemical, Qingdao, China) and ODS (YMC Co., Ltd., Kyoto, Japan), respectively. Normal and reverse phase thin-layer chromatography (TLC) was conducted using silica gel 60 F254 and RP-18 F254 (Merck Millipore Co., Darmstadt, Germany). HPLC was performed using Shimadzu LC-16P system (Shimadzu Co., Kyoto, Japan) with YMC-ODS-A C18 Column (20 × 250 mm, 5 µm) for separation. Analytical and HPLC grade reagents (Macklin Co., Shanghai, China) were used for isolation procedures.
3.2. Fungal Strain and Fermentation
The fungal strain, which was isolated from the South China deep-sea sediment sample (2134 m depth), was identified as Trichoderma sp. SCSIOW21 by ITS sequencing and morphology analysis. Its sequence data was deposited at GenBank (accession number: KC569351.1) and the strain was deposited at the Laboratory of Microbial Natural Products, Shenzhen University, China. The fungal strain was activated on potato dextrose agar dishes containing 3% sea salt at 28 °C for 3 days and cultured in modified rice broth (rice 50.0 g sprayed with 3% sea salt water 60.0 mL for each 500 mL flask) statically at room temperature for 30 days.
3.3. Extraction and Isolation
A total of 100 mL of water saturated BuOH were added in each of the Erlenmeyer flasks which contained fermentation broth. The BuOH extract was collected after 12 h and evaporated under vacuum. The extraction was repeated three times and the total yield was 12.9 g.
The BuOH extract was subjected to a silica gel chromatography with a gradient of CH2Cl2-MeOH-Water (100:0:0, 50:1:0, 20:1:0, 10:1:0, 5:1:0.1, 3:1:0.1, 1:1:0.1, and 0:0:100, v/v/v, 2.0 L each) to give 8 fractions (A–H). Fraction B and C were combined and subjected to a medium pressure ODS column with a gradient of MeOH-Water (5:5, 6:4, 7:3, 8:2, and 9:1) to give 5 subfractions. Subfraction 2 was separated by a semi-preparative HPLC column (Acetonitrile (ACN)-Water, 40:60) to give compound 1 (tR 52.1 min, 9.0 mg). Subfraction 3 was purified by a semi-preparative HPLC (ACN-Water, 47:53) to give compounds 2 (tR 49.2 min, 3.0 mg), 7 (tR 32.8 min, 0.8 mg), and 6 (tR 34.1 min, 0.8 mg). Subfraction 5 was separated by a semi-preparative HPLC (ACN-Water, 70:30) to give compound 3 (tR 15.9 min, 1.0 mg). Fraction D was subjected to a medium pressure liquid chromatography YMC-ODS-A C18 Column with a gradient of MeOH-Water (1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1) to give 14 subfractions. Subfraction 7 was purified by a semi-preparative HPLC (ACN-Water, 18:82) to give compound 5 (tR 29.6 min, 1.6 mg). Subfraction 9 was purified by a semi-preparative HPLC (ACN-Water, 23:77) to give compound 4 (tR 41.5 min, 1.0 mg).
3.4. Spectral Data of the Compounds
Harzianol K (1): colorless crystal; [α]25D +64.1 (c 0.36, MeOH); UV (MeOH) λmax (log ε) 252 (3.77) nm; ECD (0.12 mg/mL, MeOH) λmax (∆ε) 245 (−36.7), 292 (+7.4), 351 (+10.6) nm; IR (KBr) vmax 3402 (s), 2927 (m), 1734 (s), 1695 (s), 1190 (m), 1043 (m) cm−1; 1H NMR and 13C NMR spectroscopy data (DMSO-d6, 600 and 150 MHz), see Table 1 and Table 2; HREIMS m/z: 317.2115 [M + H]+ (calcd for C20H29O3, 317.2117).
Harzianol L (4): colorless gum; [α]25D +15.3 (c 0.28, MeOH); UV (MeOH) λmax (log ε) 256 (3.89) nm; ECD (0.14 mg/mL, MeOH) λmax (∆ε) 247 (−36.7), 349 (+2.7) nm; IR (KBr) vmax 3371(s), 2922 (m), 1716 (s), 1653 (m), 1149 (m), 1056 (m) cm−1; 1H NMR and 13C NMR spectroscopy data (DMSO-d6, 600 and 150 MHz), see Table 1 and Table 2; HREIMS m/z: 319.2278 [M + H]+ (calcd for C20H31O3, 319.2273).
HarzianolM (5): colorless gum; [α]25D +14.1 (c 0.15, MeOH); UV (MeOH) λmax (log ε) 251 (4.07) nm; ECD (0.15 mg/mL, MeOH) λmax (∆ε) 245 (−8.2), 358 (+4.8) nm; IR (KBr) vmax 3360 (s), 2922 (m), 1732 (s), 1668 (m), 1122 (m), 1024 (m) cm−1; 1H NMR and 13C NMR spectroscopy data (DMSO-d6, 600 and 150 MHz), see Table 1 and Table 2; HREIMS m/z: 335.2229 [M + H]+ (calcd for C20H31O4, 335.2222).
Harzianol N (6): amorphous solid; [α]25D +10.1 (c 0.18, MeOH); UV (MeOH) λmax (log ε) 252 (4.19) nm; ECD (0.18 mg/mL, MeOH) λmax (∆ε) 220 (+1.7), 245 (−4.9), 353 (+2.4) nm; IR (KBr): vmax 3379 (s), 2924 (m), 1734 (s), 1647 (m), 1153 (m), 1049 (m) cm−1; 1H NMR and 13C NMR spectroscopy data (DMSO-d6, 600 and 150 MHz), see Table 1 and Table 2; HREIMS m/z: 341.2089 [M + Na]+ (calcd for C20H30NaO3, 341.2093).
HarzianolO (7): amorphous solid; [α]25D +12.0 (c 0.14, MeOH); UV (MeOH) λmax (log ε) 256 (4.11) nm; ECD (0.14 mg/mL, MeOH) λmax (∆ε) 255 (−1.8), 340 (+1.1) nm; IR (KBr) vmax 3360 (s), 2922 (m), 1718 (s), 1660 (m), 1147 (m), 1058 (m) cm−1; 1H NMR and 13C NMR spectroscopy data (DMSO-d6, 600 and 150 MHz), see Table 1 and Table 2; HREIMS m/z: 319.2269 [M + H]+ (calcd for C20H31O3, 319.2273).
3.5. X-ray Crystal Analysis of Compounds 1 and 2
The crystals of compounds 1 and 2 were obtained from concentrated MeOH solutions and 1 suitable crystal for each compound was selected. The crystals were scanned using Cu Kα radiation (λ = 1.54184 Å) on the XtaLAB AFC12 (RINC) Kappa single diffraction instrument, the structures of which were solved by the Olex2 software, the SHELXT [25], and the SHELXL [26] package with the parameters corrected by the least-squares minimization method.
The single-crystal data has been submitted to the Cambridge Crystallographic Data Centre database, with CCDC 2093540 for 1 and CCDC 2093541 for 2. The data can be downloaded for free from the website http://www.ccdc.cam.ac.uk/ (accessed on 7 November 2021).
X-ray crystal data of1: C20H28O3 (M = 316.42 g/mol): monoclinic, space group P21 (no. 4), a = 8.73030 (10) Å, b = 11.43810 (10) Å, c = 8.99520 (10) Å, β = 110.2970 (10)°, V = 842.468 (16) Å3, Z = 2, T = 100.01 (10) K, μ (Cu Kα) = 0.648 mm−1, Dcalc = 1.247 g/cm3, 8392 reflections measured (10.486° ≤ 2ϴ ≤ 148.666°), 3306 unique (Rint = 0.0193, Rsigma = 0.0222) which were used in all calculations. The final R1 was 0.0273 [I > 2σ(I)] and wR2 was 0.0705 (all data), Flack parameter 0.04 (5).
X-ray crystal data of2: C40H60O4 (M = 604.88 g/mol): monoclinic, space group P21 (no. 4), a = 7.84120 (10) Å, b = 9.31180 (10) Å, c = 23.1108 (2) Å, β = 93.9960 (10)°, V = 1683.35 (3) Å3, Z = 2, T = 100.01(10) K, μ(Cu Kα) = 0.576 mm−1, Dcalc = 1.193 g/cm3, 19,167 reflections measured (7.67° ≤ 2 ϴ ≤ 148.826°), 6578 unique (Rint = 0.0295, Rsigma = 0.0304) which were used in all calculations. The final R1 was 0.0343 [I > 2σ(I)] and wR2 was 0.0890 (all data), Flack parameter 0.03 (9).
3.6. ECD Computational Methods
The conformations of compounds 1 and 4–7 were searched by Marvin Sketch software (optimization limit = normal, diversity limit = 0.1) ignoring the rotation of methyl and hydroxy groups. Geometric optimization of the molecules in MeOH (Figures S49–S53) was carried out at 6-31G (d, p) level using DFT/B3LYP through Gaussian 09 software [27], within the 3 kcal/mol energy threshold from the global minimum [28]. The ECD curve was simulated based on TD-DFT calculations and drawn with sigma = 0.3 by SpecDis software (version 1.71, Berlin, Germany). The calculated data was also produced by Boltzmann’s weighting and magnetization based on experimental values.
3.7. MTT and NO Production Inhibitory Assay
The cytotoxicity and NO production inhibitory activity were examined using RAW 264.7 macrophages, and the detailed methods were reported previously [20].
3.8. Anti-Fungal Activities
The anti-fungal activities were tested on a 96-well plate by mycelial growth inhibitory assay [29], using actidione as the positive control. Five plant pathogenic fungal species (Helminthosporium maydis, Gibberella sanbinetti, Botrytis cinerea Pers, Fusarium Oxysporum f. sp. cucumerinum, Penicillium digitatum) were donated by CAS Key Laboratory of Tropical Marine Bio-resources and Ecology, Chinese Academy of Sciences.
4. Conclusions
Herein, we reported the isolation, structure elucidation, and biological activities of seven harziane diterpenes, including five new compounds from a deep-sea derived fungus, Trichoderma sp. SCSIOW21. The stereo configurations of the new compounds, harzianol K (1), harzianol L (4), harzianol M (5), harzianol N (6), and harzianol O (7) were characterized by ECD calculations. Hazianol K (1) and harzianol J (2) were unambiguously determined by X-ray single crystallographic analysis. Hazianol J (2), harzianol A (3), and harzianol O (7) exhibited weak NO production inhibitory activity. All of the compounds did not show any anti-fungal activities.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/md19120689/s1, including detailed 1D and 2D NMR data, ECD calculations, HRESIMS spectra for compounds 1–7, as well as a brief summary of reported literatures about harziane type diterpenes from 1992 to 2021.
Author Contributions
The contributions of the respective authors are as follows: H.L. contributed to fermentation, extraction, structure elucidation, and manuscript preparation; X.L. (Xinyi Liu) contributed to isolation and data acquisition; X.L. (Xiaofan Li) contributed to the evaluation of bioactivities and manuscript revision; Z.H. contributed to manuscript revision; L.W. contributed to the experimental design, manuscript preparation, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Key Research and Development Project 2019YFC0312501 and 2018YFA0902504; the Science and Technology Project of Shenzhen City, Shenzhen Bureau of Science, Technology, and Information under Grant JCYJ20180305123659726, JCYJ20190808114415068 and by the Interdisciplinary Innovation Team Project of Shenzhen University.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Instrumentation Analysis Center of Shenzhen University for the measurement of NMR spectra and MS data. We also thank the Analysis Center, college of life sciences and oceanography, Shenzhen University for the measurement of IR and CD spectra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zain Ul Arifeen, M.; Ma, Y.N.; Xue, Y.R.; Liu, C.H. Deep-sea fungi could be the new arsenal for bioactive molecules. Mar. Drugs 2019, 18, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef]
- Ghisalberti, E.L.; Hockless, D.C.R.; Rowland, C.; White, A.H. Harziandione, a new class of diterpene from Trichoderma harzianum. J. Nat. Prod. 1992, 55, 1690–1694. [Google Scholar] [CrossRef]
- Wang, X.; Jin, X.Y.; Zhou, J.C.; Zhu, R.X.; Qiao, Y.N.; Zhang, J.Z.; Li, Y.; Zhang, C.Y.; Chen, W.; Chang, W.Q.; et al. Terpenoids from the Chinese liverwort Heteroscyphus coalitus and their anti-virulence activity against Candida albicans. Phytochemistry 2020, 174, 112324. [Google Scholar] [CrossRef]
- Song, Y.P.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Diterpenes and sesquiterpenes from the marine algicolous fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018, 81, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.P.; Liu, X.H.; Shi, Z.Z.; Miao, F.P.; Fang, S.T.; Ji, N.Y. Bisabolane, cyclonerane, and harziane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Phytochemistry 2018, 152, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.P.; Miao, F.P.; Liang, X.R.; Yin, X.L.; Ji, N.Y. Harziane and cadinane terpenoids from the alga-endophytic fungus Trichoderma asperellum A-YMD-9-2. Phytochem. Lett. 2019, 32, 38–41. [Google Scholar] [CrossRef]
- Li, W.Y.; Liu, Y.; Lin, Y.T.; Liu, Y.C.; Guo, K.; Li, X.N.; Luo, S.H.; Li, S.H. Antibacterial harziane diterpenoids from a fungal symbiont Trichoderma atroviride isolated from Colquhounia coccinea var. mollis. Phytochemistry 2020, 170, 112198. [Google Scholar] [CrossRef]
- Shi, T.; Shao, C.L.; Liu, Y.; Zhao, D.L.; Cao, F.; Fu, X.M.; Yu, J.Y.; Wu, J.S.; Zhang, Z.K.; Wang, C.Y. Terpenoids from the coral-derived fungus Trichoderma harzianum (XS-20090075) induced by chemical epigenetic manipulation. Front. Microbiol. 2020, 11, 572. [Google Scholar] [CrossRef]
- Miao, F.P.; Liang, X.R.; Yin, X.L.; Wang, G.; Ji, N.Y. Absolute configurations of unique harziane diterpenes from Trichoderma species. Org. Lett. 2012, 14, 3815–3817. [Google Scholar] [CrossRef]
- Adelin, E.; Servy, C.; Martin, M.T.; Arcile, G.; Iorga, B.I.; Retailleau, P.; Bonfill, M.; Ouazzani, J. Bicyclic and tetracyclic diterpenes from a Trichoderma symbiont of Taxus baccata. Phytochemistry 2014, 97, 55–61. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.M.; Zhao, J.L.; Li, N.; Chen, R.D.; Xie, K.B.; Zhang, W.J.; Feng, K.P.; Yan, Z.; Wang, N.; et al. Two new diterpenoids from the endophytic fungus Trichoderma sp. Xy24 isolated from mangrove plant Xylocarpus granatum. Chin. Chem. Lett. 2016, 27, 957–960. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Chen, R.; Zhao, J.; Xie, K.; Chen, D.; Feng, K.; Dai, J. Microbial oxidation of harzianone by Bacillus sp. IMM-006. Tetrahedron 2017, 73, 7195–7199. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Chen, R.; Zhao, J.; Xie, K.; Chen, D.; Feng, K.; Dai, J. Two Furanharzianones with 4/7/5/6/5 ring system from microbial transformation of harzianone. Org. Lett. 2017, 19, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.L.; Yang, L.J.; Shi, T.; Wang, C.Y.; Shao, C.L.; Wang, C.Y. Potent phytotoxic harziane diterpenes from a soft coral-derived strain of the fungus Trichoderma harzianum XS-20090075. Sci. Rep. 2019, 9, 13345. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.X.; Song, Y.P.; Ji, N.Y. Deoxytrichodermaerin, a harziane lactone from the marine algicolous fungus Trichoderma longibrachiatum A-WH-20-2. Nat. Prod. Res. 2021, 35, 216–221. [Google Scholar] [CrossRef]
- Zou, J.X.; Song, Y.P.; Zeng, Z.Q.; Ji, N.Y. Proharziane and harziane derivatives from the marine algicolous fungus Trichoderma asperelloides RR-dl-6-11. J. Nat. Prod. 2021, 84, 1414–1419. [Google Scholar] [CrossRef]
- Li, X.; Xia, Z.; Tang, J.; Wu, J.; Tong, J.; Li, M.; Ju, J.; Chen, H.; Wang, L. Identification and biological evaluation of secondary metabolites from marine derived fungi-Aspergillus sp. SCSIOW3, cultivated in the presence of epigenetic modifying agents. Molecules 2017, 22, 1302. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, M.; Lin, Y.; Du, S.; Liu, Z.; Ju, J.; Suzuki, H.; Sawada, M.; Umezawa, K. Inhibition of cellular inflammatory mediator production and amelioration of learning deficit in flies by deep sea Aspergillus-derived cyclopenin. J. Antibiot. 2020, 73, 622–629. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Tang, J.; Li, X. Eremophilane sesquiterpenes from a deep marine-derived fungus, Aspergillus sp. SCSIOW2, cultivated in the presence of epigenetic modifying agents. Molecules 2016, 21, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Umezawa, K. Cellular signal transductions and their inhibitors derived from deep-sea organisms. Mar. Drugs. 2021, 19, 205. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, P.; Tang, J.; Wu, Z.; Li, X.; Li, S.; Wang, Y.; Liu, G.; He, Z.; Gou, D.; et al. A novel cyclic dipeptide from deep marine-derived fungus Aspergillus sp. SCSIOW2. Nat. Prod. Res. 2016, 30, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; He, J.; Wu, Y.; Du, N.; Li, X.; Ju, J.; Hu, Z.; Umezawa, K.; Wang, L. Isolation and characterization of new anti-inflammatory and antioxidant components from deep marine-derived fungus Myrothecium sp. Bzo-l062. Mar. Drugs 2020, 18, 597. [Google Scholar] [CrossRef]
- Mannina, L.; Segre, A.L.; Ritieni, A.; Fogliano, V.; Vinale, F.; Randazzo, G.; Maddau, L.; Bottalico, A. A new fungal growth inhibitor from Trichoderma viride. Tetrahedron 1997, 53, 3135–3144. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta. Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta. Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.T.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
- Zhai, M.M.; Qi, F.M.; Li, J.; Jiang, C.X.; Hou, Y.; Shi, Y.P.; Di, D.L.; Zhang, J.W.; Wu, Q.X. Isolation of secondary metabolites from the soil-derived fungus Clonostachys rosea YRS-06, a biological control agent, and evaluation of antibacterial activity. J. Agric. Food Chem. 2016, 64, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).