Marine Terpenic Endoperoxides
Abstract
:1. Introduction
2. Marine Endoperoxide Norterpenes
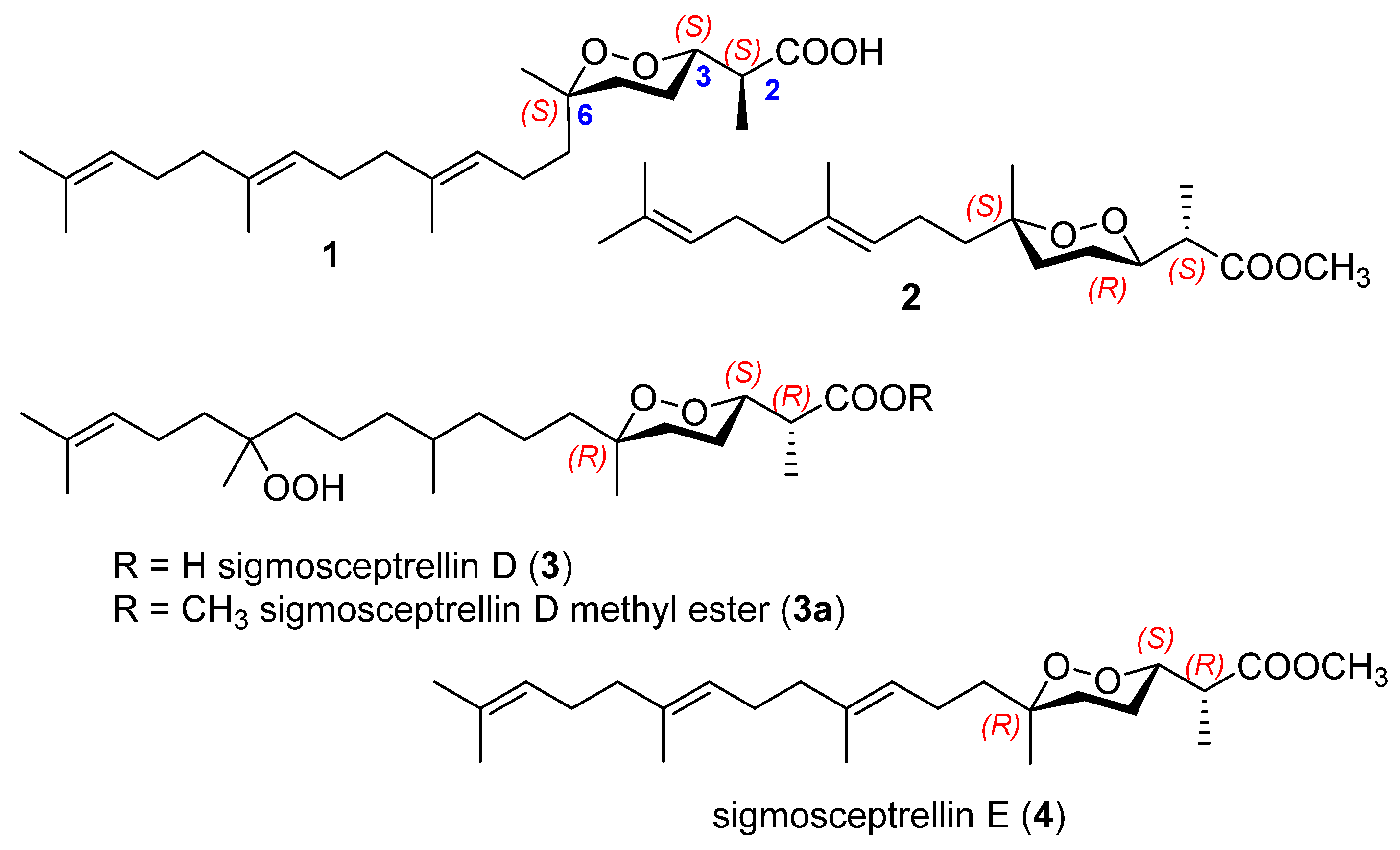
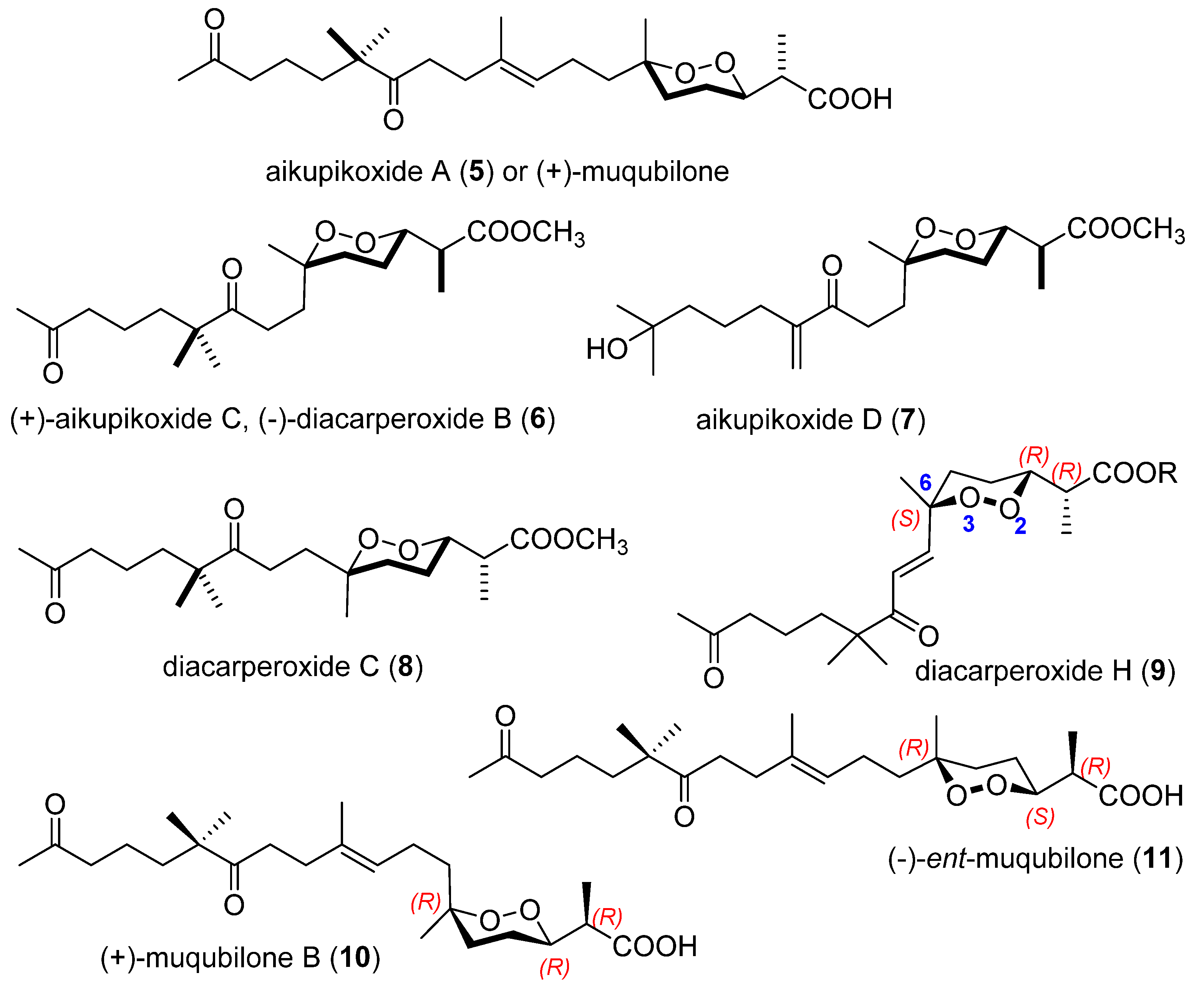
2.1. Endoperoxide Norterpenes with an Acyclic Carbon Skeleton
2.2. Endoperoxide Norterpenes with a Monocyclic Carbon Skeleton
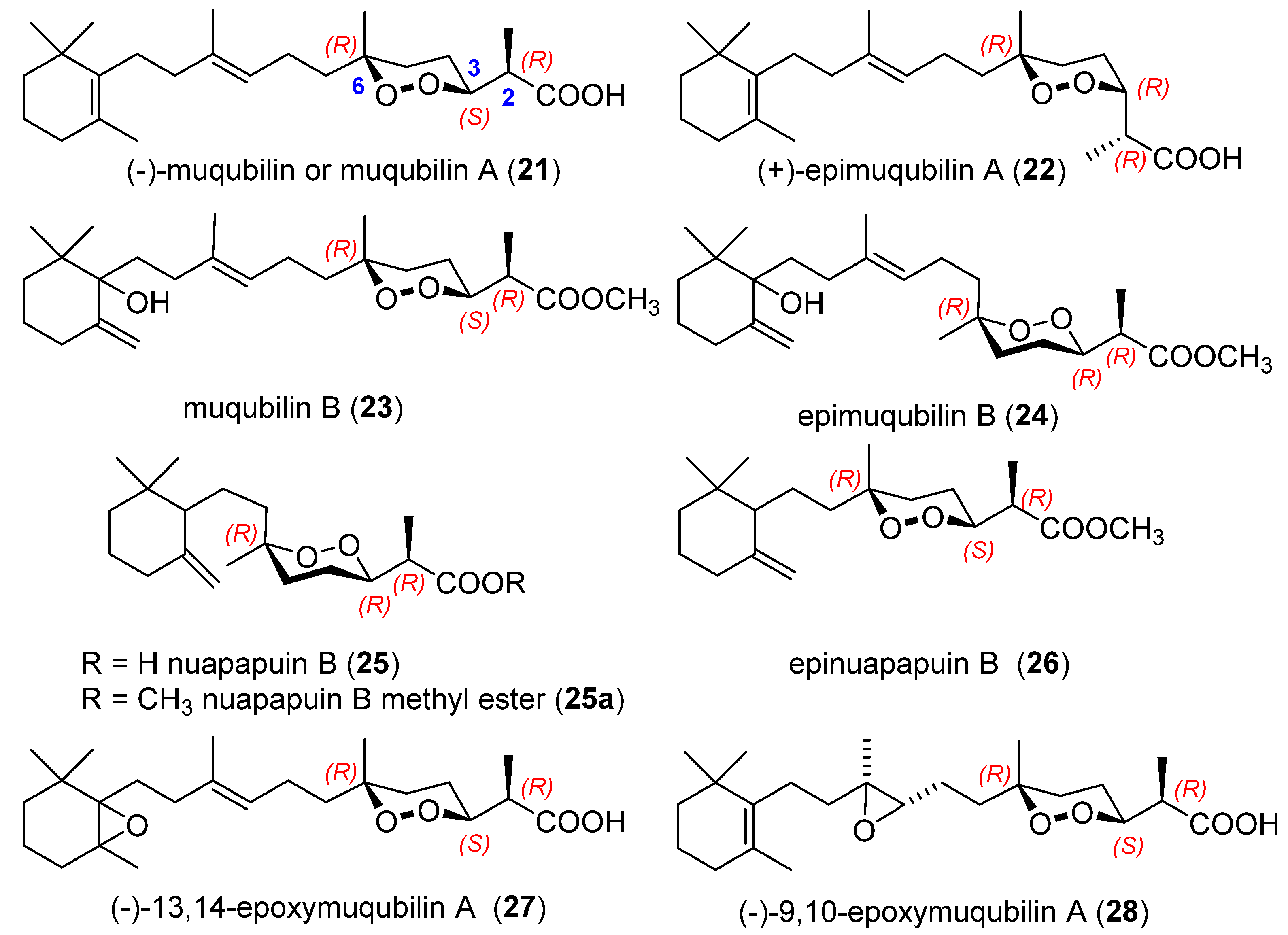
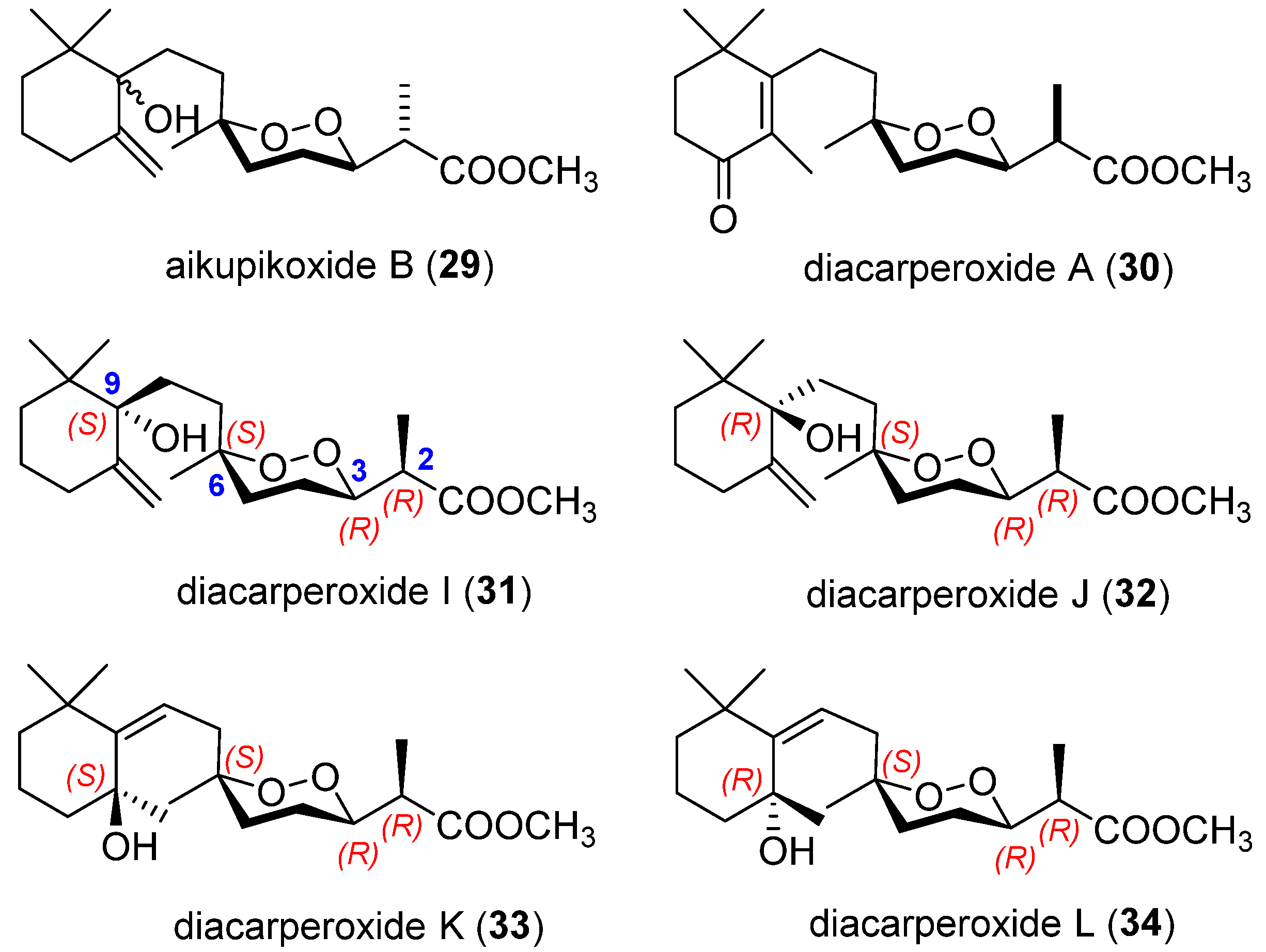
2.3. Endoperoxide Norterpenes with a Bicyclic Carbon Skeleton
3. Marine Endoperoxide Terpenes
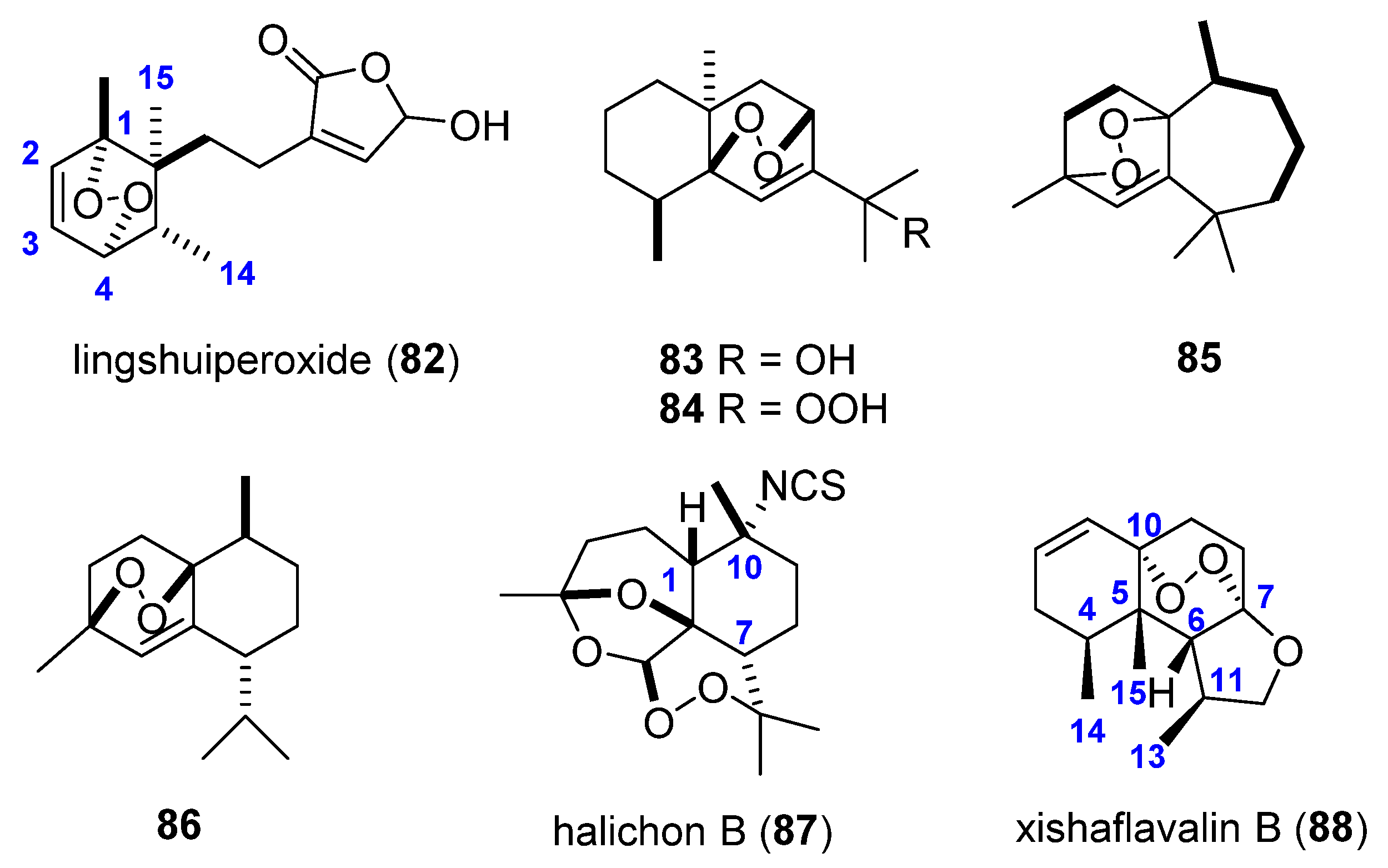
3.1. Endoperoxide Sesquiterpenes
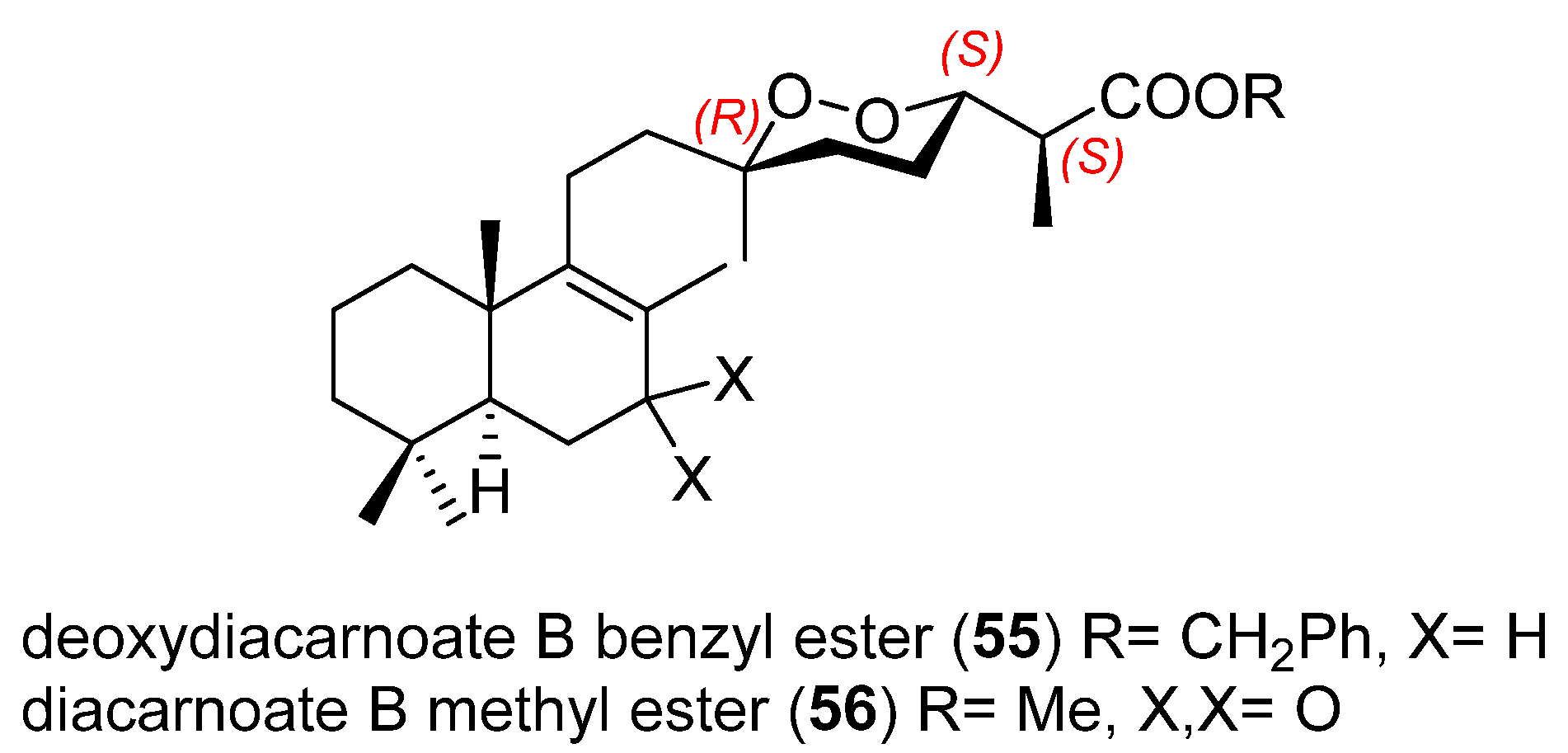
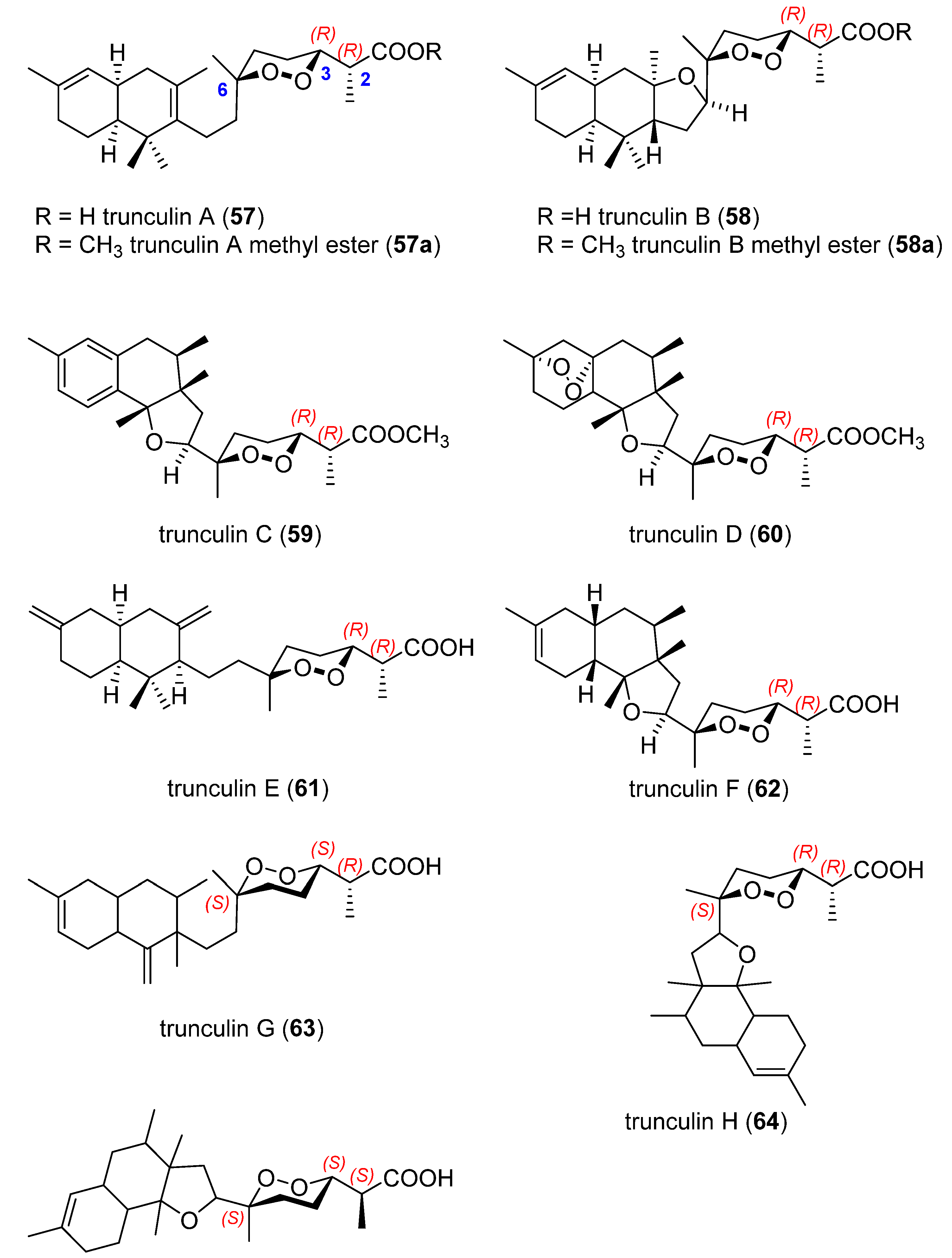
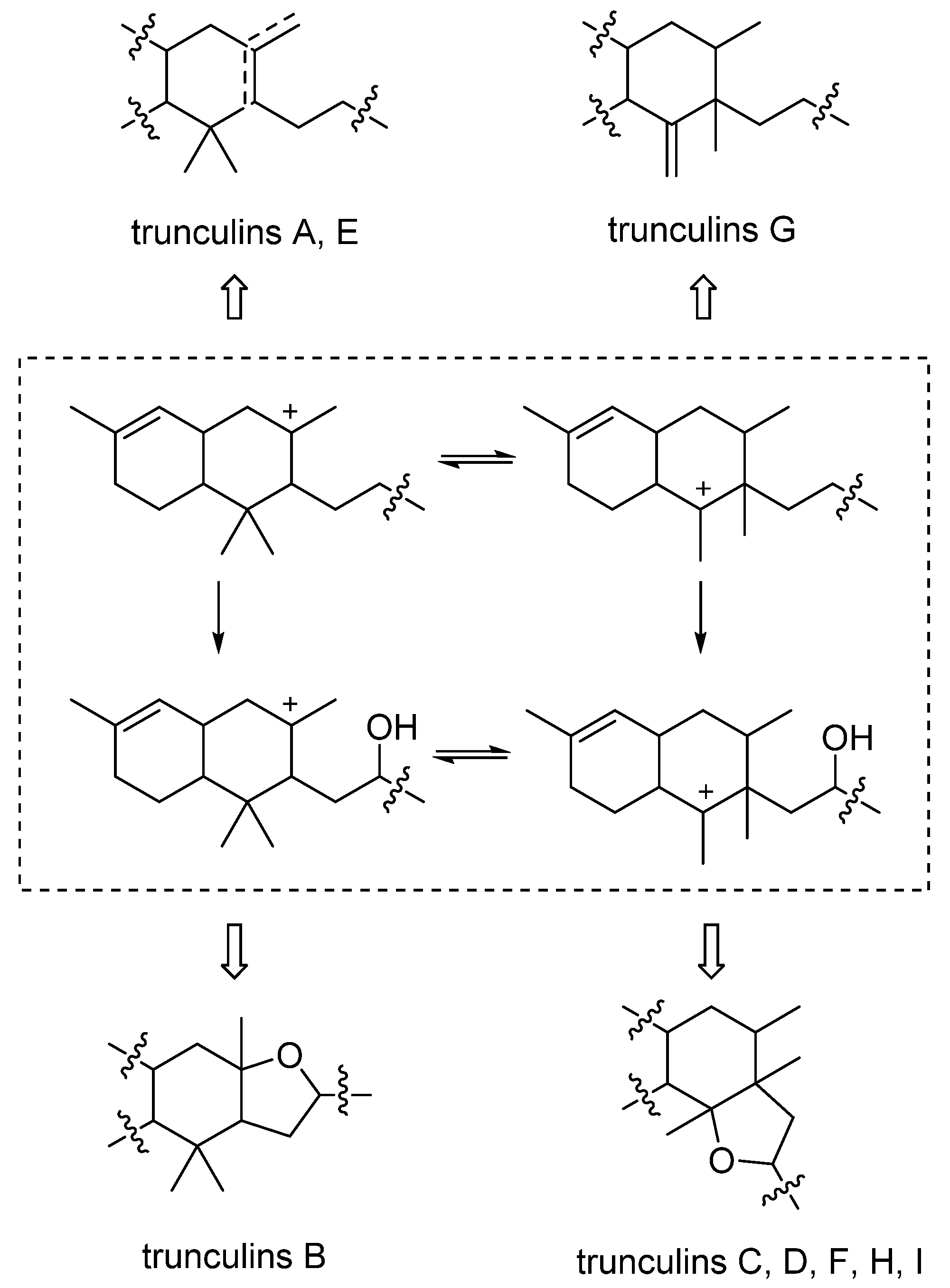
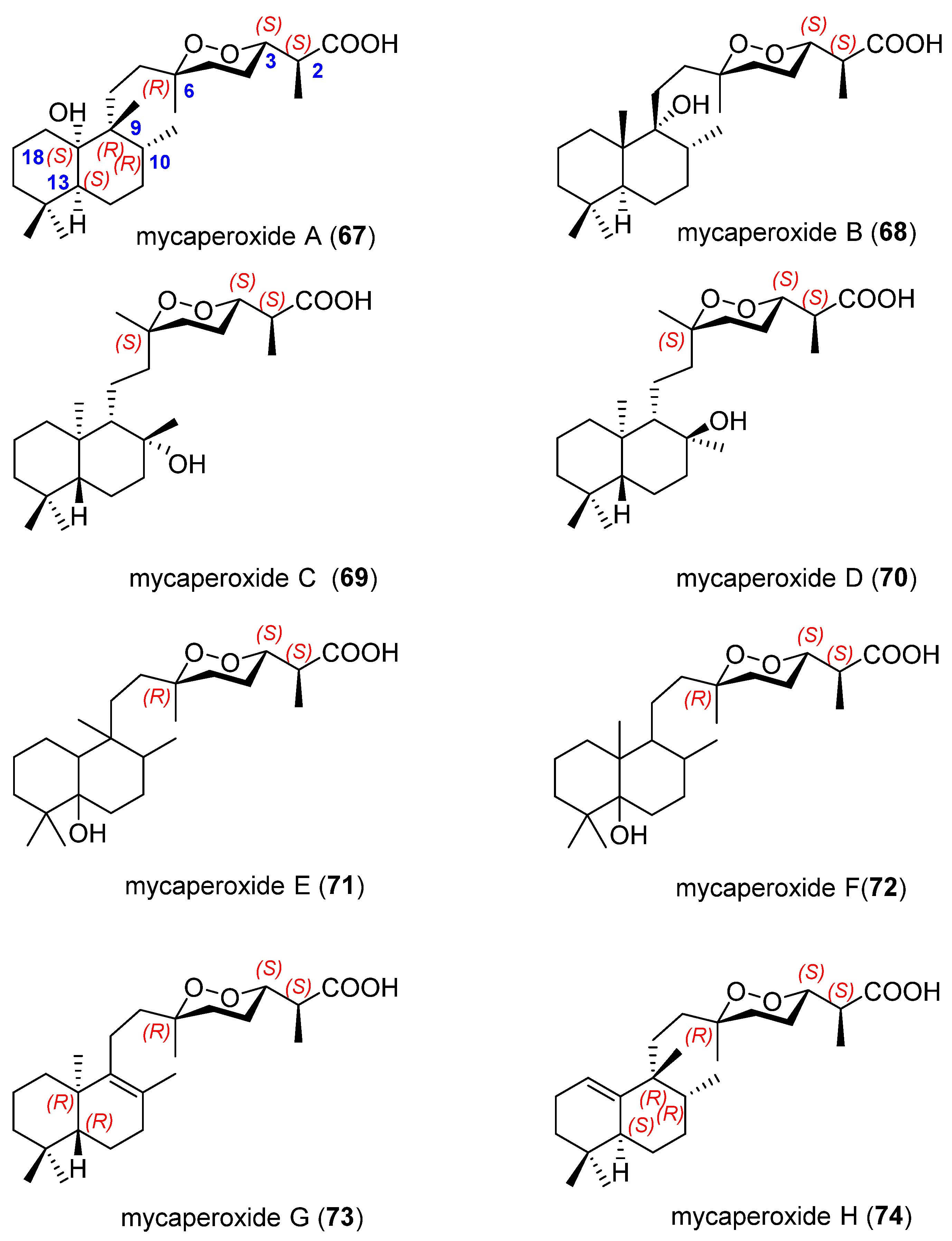
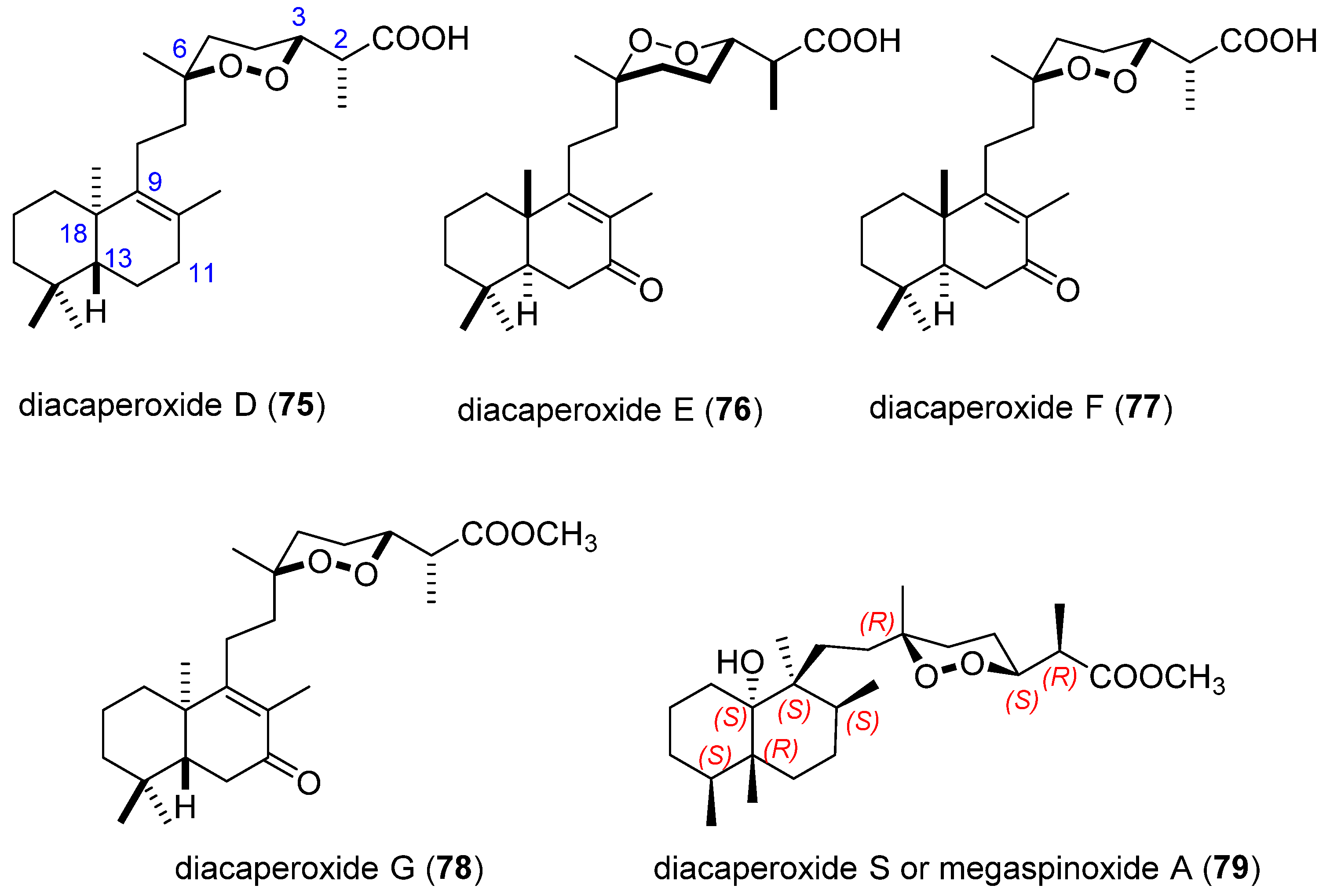
3.2. Endoperoxide Diterpenes
4. Biological Activity Overview
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sweetlove, L. Number of Species on Earth Tagged at 8.7 Million. Nature 2011. Available online: https://doi.org/10.1038/news.2011.498 (accessed on 19 November 2021). [CrossRef]
- Sato, A. The Search for New Drugs from Marine Organisms. J. Toxicol. Toxin Rev. 1996, 15, 171–198. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Natural peroxy anticancer agents. Mini-Rev. Med. Chem. 2007, 7, 571–589. [Google Scholar] [CrossRef]
- He, Q.; Miao, S.; Ni, N.; Man, Y.; Gong, K. A Review of the Secondary Metabolites From the Marine Sponges of the Genus Aaptos. Nat. Prod. Commun. 2020, 15, 1934578X20951439. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A Review of Terpenes from Marine-Derived Fungi: 2015–2019. Mar. Drugs 2020, 18, 321. [Google Scholar] [CrossRef]
- Capon, R.J.; Macleod, J.K. Structural and stereochemical studies on marine norterpene cyclic peroxides. Tetrahedron 1985, 41, 3391–3404. [Google Scholar] [CrossRef]
- Albericci, M.; Collart-Lempereur, M.; Braekman, J.C.; Daloze, D.; Tursch, B.; Declercq, J.P.; Germain, G.; Van Meerssche, M. Chemical studies of marine invertebrates. XLI. Sigmosceptrellin-A methyl ester, a nor-sesterterpenoid peroxide from the sponge Sigmosceptrella laevis. Tetrahedron Lett. 1979, 20, 2687–2690. [Google Scholar] [CrossRef]
- Horeau, A. Determination of the configuration of secondary alcohols by partial resolution. Fundam. Methods 1977, 3, 51–94. [Google Scholar]
- Ovenden, S.P.B.; Capon, R.J. Nuapapuin A and Sigmosceptrellins D and E: New Norterpene Cyclic Peroxides from a Southern Australian Marine Sponge, Sigmosceptrella sp. J. Nat. Prod. 1999, 62, 214–218. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Yoshida, W.Y.; Kelly, M.; Scheuer, P.J. Cytotoxic cyclic norterpene peroxides from a Red Sea sponge Diacarnus erythraenus. J. Nat. Prod. 2001, 64, 1332–1335. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Hamann, M.T.; Hashish, N.E.; Shier, W.T.; Kelly, M.; Khan, A.A. Antimalarial, antiviral, and antitoxoplasmosis norsesterterpene peroxide acids from the Red Sea sponge Diacarnus erythraeanus. J. Nat. Prod. 2001, 64, 522–524. [Google Scholar] [CrossRef]
- Al-Tarabeen, M.; El-Neketi, M.; Albohy, A.; Mueller, W.E.G.; Rasheed, M.; Ebrahim, W.; Proksch, P.; Ebada, S.S. Isolation and Molecular Docking of Cytotoxic Secondary Metabolites from Two Red Sea Sponges of the Genus Diacarnus. ChemistrySelect 2021, 6, 217–220. [Google Scholar] [CrossRef]
- Chao, C.-H.; Chou, K.-J.; Wang, G.-H.; Wu, Y.-C.; Wang, L.-H.; Chen, J.-P.; Sheu, J.-H.; Sung, P.-J. Norterpenoids and Related Peroxides from the Formosan Marine Sponge Negombata corticata. J. Nat. Prod. 2010, 73, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Ebel, R.; Wray, V.; Muller, W.E.G.; Edrada-Ebel, R.; Proksch, P. Diacarperoxides, Norterpene Cyclic Peroxides from the Sponge Diacarnus megaspinorhabdosa. J. Nat. Prod. 2008, 71, 1358–1364. [Google Scholar] [CrossRef]
- Yang, F.; Zou, Y.; Wang, R.-P.; Hamann, M.T.; Zhang, H.-J.; Jiao, W.-H.; Han, B.-N.; Song, S.-J.; Lin, H.-W. Relative and absolute stereochemistry of diacarperoxides: Antimalarial norditerpene endoperoxides from marine sponge Diacarnus megaspinorhabdosa. Mar. Drugs 2014, 12, 4399–4416. [Google Scholar] [CrossRef] [Green Version]
- Rubio, B.K.; Tenney, K.; Ang, K.-H.; Abdulla, M.; Arkin, M.; McKerrow, J.H.; Crews, P. The Marine Sponge Diacarnus bismarckensis as a Source of Peroxiterpene Inhibitors of Trypanosoma brucei, the Causative Agent of Sleeping Sickness. J. Nat. Prod. 2009, 72, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashman, Y.; Rotem, M. Muqubilin, a new C24-isoprenoid from a marine sponge. Tetrahedron Lett. 1979, 20, 1707–1708. [Google Scholar] [CrossRef]
- Yunker, M.B.; Scheuer, P.J. Mokupalides, three novel hexaprenoids from a marine sponge. J. Am. Chem. Soc. 1978, 100, 307–309. [Google Scholar] [CrossRef]
- Higgs, M.D.; Faulkner, D.J. Plakortin, an antibiotic from Plakortis halichondrioides. J. Org. Chem. 1978, 43, 3454–3457. [Google Scholar] [CrossRef]
- Sokoloff, S.; Halevy, S.; Usieli, V.; Colorni, A.; Sarel, S. Prianicin A and B, nor-sesterterpenoid peroxide antibiotics from Red Sea sponges. Experientia 1982, 38, 337–338. [Google Scholar] [CrossRef]
- Manes, L.V.; Bakus, G.J.; Crews, P. Bioactive marine sponge norditerpene and norsesterterpene peroxides. Tetrahedron Lett. 1984, 25, 931–934. [Google Scholar] [CrossRef]
- Miao, X.-X.; Chen, M.-X.; Zhu, H.-R.; Sun, F.; Hong, L.-L.; Wu, W.-H.; Lin, H.-W.; Yang, F. New polyketides and norterpenoids from the marine sponge Diacarnus megaspinorhabdosa. Tetrahedron 2020, 76, 131062. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Dunbar, D.C.; Goins, D.K.; Cordova, C.R.; Perry, T.L.; Wesson, K.J.; Sanders, S.C.; Janus, S.A.; Hamann, M.T. The marine environment: A resource for prototype antimalarial agents. J. Nat. Toxins 1996, 5, 261–285. [Google Scholar]
- Laurent, D.; Pietra, F. Antiplasmodial marine natural products in the perspective of current chemotherapy and prevention of malaria. A Review. Mar. Biotechnol. 2006, 8, 433–447. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, K.A.; Dunbar, D.C.; Perry, T.L.; Wilkins, S.P.; Hamann, M.T.; Greenplate, J.T.; Wideman, M.A. Marine natural products as prototype insecticidal agents. J. Agric. Food Chem. 1997, 45, 2735–2739. [Google Scholar] [CrossRef]
- Peng, J.; Shen, X.; El Sayed, K.A.; Dunbar, D.C.; Perry, T.L.; Wilkins, S.P.; Hamann, M.T.; Bobzin, S.; Huesing, J.; Camp, R.; et al. Marine natural products as prototype agrochemical agents. J. Agric. Food Chem. 2003, 51, 2246–2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ambrosio, M.; Guerriero, A.; Debitus, C.; Waikedre, J.; Pietra, F. Relative contributions to antitumoral activity of lipophilic vs. polar reactive moieties in marine terpenoids. Tetrahedron Lett. 1997, 38, 6285–6288. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Guerriero, A.; Deharo, E.; Debitus, C.; Munoz, V.; Pietra, F. New types of potentially antimalarial agents. Epidioxy-substituted norditerpene and norsesterterpenes from the marine sponge Diacarnus levii. Helv. Chim. Acta 1998, 81, 1285–1292. [Google Scholar] [CrossRef]
- Festa, C.; Cassiano, C.; D’Auria, M.V.; Debitus, C.; Monti, M.C.; De Marino, S. Scalarane sesterterpenes from Thorectidae sponges as inhibitors of TDP-43 nuclear factor. Org. Biomol. Chem. 2014, 12, 8646–8655. [Google Scholar] [CrossRef]
- Sperry, S.; Valeriote, F.A.; Corbett, T.H.; Crews, P. Isolation and Cytotoxic Evaluation of Marine Sponge-Derived Norterpene Peroxides. J. Nat. Prod. 1998, 61, 241–247. [Google Scholar] [CrossRef]
- Lefranc, F.; Nuzzo, G.; Hamdy, N.A.; Fakhr, I.; Moreno, Y.; Banuls, L.; Van Goietsenoven, G.; Villani, G.; Mathieu, V.; van Soest, R.; et al. In Vitro Pharmacological and Toxicological Effects of Norterpene Peroxides Isolated from the Red Sea Sponge Diacarnus erythraeanus on Normal and Cancer Cells. J. Nat. Prod. 2013, 76, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, E.; Iannotti, F.A.; Falkenberg, L.G.; Martella, A.; Gentile, A.; De Maio, F.; Ciavatta Maria, L.; Gavagnin, M.; Waxman, J.S.; Di Marzo, V.; et al. In Silico Identification and Experimental Validation of (-)-Muqubilin A, a Marine Norterpene Peroxide, as PPARα/γ-RXRα Agonist and RARα Positive Allosteric Modulator. Mar. Drugs 2019, 17, 110. [Google Scholar] [CrossRef] [Green Version]
- Cheenpracha, S.; Park, E.-J.; Rostama, B.; Pezzuto, J.M.; Chang, L.C. Inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 cells by the norsesterterpene peroxide, epimuqubilin A. Mar. Drugs 2010, 8, 429–437. [Google Scholar] [CrossRef]
- Park, E.-J.; Cheenpracha, S.; Chang, L.C.; Pezzuto, J.M. Suppression of cyclooxygenase-2 and inducible nitric oxide synthase expression by epimuqubilin A via IKK/IκB/NF-κB pathways in lipopolysaccharide-stimulated RAW 264.7 cells. Phytochem. Lett. 2011, 4, 426–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, D.T.A. Tasnemoxides A-C, new cytotoxic cyclic norsesterterpene peroxides from the Red Sea sponge Diacarnus erythraenus. J. Nat. Prod. 2004, 67, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liu, Y.; Zhou, Y.-D.; Nagle, D.G. Hypoxia-Selective Antitumor Agents: Norsesterterpene Peroxides from the Marine Sponge Diacarnus levii Preferentially Suppress the Growth of Tumor Cells under Hypoxic Conditions. J. Nat. Prod. 2007, 70, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.-A.; Seifert, K. Towards the Total Synthesis of the Norsesterterpene Diacarnoxide C. Eur. J. Org. Chem. 2017, 2017, 6739–6746. [Google Scholar] [CrossRef]
- Guo, Y.; Gavagnin, M.; Mollo, E.; Trivellone, E.; Cimino, G.; Hamdy, N.A.; Fakhr, I.; Pansini, M. A new norsesterterpene peroxide from a Red Sea sponge. Nat. Prod. Lett. 1996, 9, 105–112. [Google Scholar] [CrossRef]
- Yang, F.; Wang, R.-P.; Xu, B.; Yu, H.-B.; Ma, G.-Y.; Wang, G.-F.; Dai, S.-W.; Zhang, W.; Jiao, W.-H.; Song, S.-J.; et al. New antimalarial norterpene cyclic peroxides from Xisha Islands sponge Diacarnus megaspinorhabdosa. Bioorg. Med. Chem. Lett. 2016, 26, 2084–2087. [Google Scholar] [CrossRef]
- Albericci, M.; Braekman, J.C.; Daloze, D.; Tursch, B. Chemical studies of marine invertebrates. XLV. The chemistry of three norsesterterpene peroxides from the sponge Sigmosceptrella laevis. Tetrahedron 1982, 38, 1881–1890. [Google Scholar] [CrossRef]
- Minale, L.; Riccio, R.; Sodano, G. Avarol a novel sesquiterpenoid hydroquinone with a rearranged drimane skeleton from the sponge Disidea avara. Tetrahedron Lett. 1974, 15, 3401–3404. [Google Scholar] [CrossRef]
- de Rosa, S.; Minale, L.; Riccio, R.; Sodano, G. The absolute configuration of avarol, a rearranged sesquiterpenoid hydroquinone from a marine sponge. J. Chem. Soc. Perkin Trans. 1 1976, 1408–1414. [Google Scholar] [CrossRef]
- Piccinni-Leopardi, C.; Germain, G.; Van Meerssche, M.; Albericci, M.; Braekman, J.C.; Daloze, D.; Tursch, B. Chemical studies of marine invertebrates. Part 46. Confirmation of the molecular structure of sigmosceptrellin-B by X-ray diffraction analysis. J. Chem. Soc. Perkin Trans. 2 1982, 1523–1526. [Google Scholar] [CrossRef]
- Kelly-Borges, M.; Vacelet, J. A revision of Diacarnus Burton and Negombata de Laubenfels (Demospongiae: Latrunculiidae) with descriptions of new species from the west central Pacific and the Red Sea. Mem. Queensl. Mus. 1995, 38, 477–503. [Google Scholar]
- Capon, R.J.; MacLeod, J.K. Structural and stereochemical studies on marine norterpene cyclic peroxides. Part 2. J. Nat. Prod. 1987, 50, 225–229. [Google Scholar] [CrossRef]
- Capon, R.J.; MacLeod, J.K.; Willis, A.C. Trunculins A and B, norsesterterpene cyclic peroxides from a marine sponge, Latrunculia brevis. J. Org. Chem. 1987, 52, 339–342. [Google Scholar] [CrossRef]
- He, H.Y.; Faulkner, D.J.; Lu, H.S.M.; Clardy, J. Norsesterterpene peroxides from the sponge Latrunculia sp. J. Org. Chem. 1991, 56, 2112–2115. [Google Scholar] [CrossRef]
- Butler, M.S.; Capon, R.J. Trunculin-F and contrunculin-A and -B: Novel oxygenated norterpenes from a southern Australian marine sponge, Latrunculia conulosa. Aust. J. Chem. 1993, 46, 1363–1374. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Capon, R.J. Trunculins G-I: New norsesterterpene cyclic peroxides from a southern Australian marine sponge, Latrunculia sp. Aust. J. Chem. 1998, 51, 573–579. [Google Scholar] [CrossRef]
- Dale, J.A.; Mosher, H.S. Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and α-methoxy-α-trifluoromethylphenylacetate (MTPA) esters. J. Amer. Chem. Soc. 1973, 95, 512. [Google Scholar] [CrossRef]
- Tanaka, J.-I.; Higa, T.; Suwanborirux, K.; Kokpol, U.; Bernardinelli, G.; Jefford, C.W. Bioactive norsesterterpene 1,2-dioxanes from a Thai sponge, Mycale sp. J. Org. Chem. 1993, 58, 2999–3002. [Google Scholar] [CrossRef]
- Kusumi, T.; Fujita, Y.; Ohtani, I.; Kakisawa, H. Anomaly in the modified Mosher’s method: Absolute configurations of some marine cembranolides. Tetrahedron Lett. 1991, 32, 2923–2926. [Google Scholar] [CrossRef]
- Capon, R.J.; Rochfort, S.J.; Ovenden, S.P.B. Cyclic Peroxides and Related Norterpenes from a Southern Australian Marine Sponge, Mycale sp. J. Nat. Prod. 1997, 60, 1261–1264. [Google Scholar] [CrossRef]
- Capon, R.J. Two new norsesterterpene cyclic peroxides from a marine sponge, Mycale (Carmia) cf. spongiosa. J. Nat. Prod. 1991, 54, 190–195. [Google Scholar] [CrossRef]
- Capon, R.J.; Rochfort, S.J.; Ovenden, S.P.B.; Metzger, R.P. Mycaperoxides F and G and a Related Norterpene Ketone from Southern Australian Marine Sponges, Mycale species. J. Nat. Prod. 1998, 61, 525–528. [Google Scholar] [CrossRef]
- Butler, M.S.; Capon, R.J. Norterpene dienes from an Australian marine sponge Latrunculia brevis. Aust. J. Chem. 1991, 44, 77–85. [Google Scholar] [CrossRef]
- Butler, M.S.; Capon, R.J. The Luffarins (A-Z), Novel Terpenes From an Australian Marine Sponge, Luffariella geometrica. Aust. J. Chem. 1992, 45, 1705–1743. [Google Scholar] [CrossRef]
- Phuwapraisirisan, P.; Matsunaga, S.; Fusetani, N.; Chaitanawisuti, N.; Kritsanapuntu, S.; Menasveta, P. Mycaperoxide H, a new cytotoxic norsesterterpene peroxide from a Thai marine sponge Mycale sp. J. Nat. Prod. 2003, 66, 289–291. [Google Scholar] [CrossRef]
- Dave, K.; Panchal, H. Review on chemogenomics approach: Interpreting antagonist activity of secreted frizzled-related protein 1 in glaucoma disease with in-Silico docking. Curr. Top. Med. Chem. 2012, 12, 1834–1842. [Google Scholar] [CrossRef]
- Kondru, R.K.; Wipf, P.; Beratan, D.N. Theory-Assisted Determination of Absolute Stereochemistry for Complex Natural Products via Computation of Molar Rotation Angles. J. Am. Chem. Soc. 1998, 120, 2204–2205. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M. Diacarperoxide S, new norterpene cyclic peroxide from the sponge Diacarnus megaspinorhabdosa. Nat. Prod. Commun. 2012, 7, 9–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.R.M.; Al Haidari, R.A.; Mohamed, G.A. Megaspinoxide A: New Norterpene Cyclic Peroxide from the Sponge Diacarnus megaspinorhabdosa. Nat. Prod. J. 2014, 4, 38–42. [Google Scholar] [CrossRef]
- Erickson, K.L.; Beutler, J.A.; Gray, G.N.; Cardellina, J.H.; Boyd, M.R. Majapolene A, a Cytotoxic Peroxide, and Related Sesquiterpenes from the Red Alga Laurencia majuscula. J. Nat. Prod. 1995, 58, 1848–1860. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Suzuki, M.; Ishii, T.; Okino, T.; Abe, T.; Masuda, M. Antibacterial activity of halogenated sesquiterpenes from Malaysian Laurencia spp. Phytochemistry 2008, 69, 2490–2494. [Google Scholar] [CrossRef] [PubMed]
- Monde, K.; Taniguchi, T.; Miura, N.; Vairappan, C.S.; Suzuki, M. Absolute configurations of brominated sesquiterpenes determined by vibrational circular dichroism. Chirality 2006, 18, 335–339. [Google Scholar] [CrossRef]
- Monde, K.; Taniguchi, T.; Miura, N.; Vairappan, C.S.; Suzuki, M. Absolute configurations of endoperoxides determined by vibrational circular dichroism (VCD). Tetrahedron Lett. 2006, 47, 4389–4392. [Google Scholar] [CrossRef]
- Huang, X.-C.; Li, J.; Li, Z.-Y.; Shi, L.; Guo, Y.-W. Sesquiterpenes from the Hainan Sponge Dysidea septosa. J. Nat. Prod. 2008, 71, 1399–1403. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Dai, C.-F.; Duh, C.-Y. Sesquiterpenoids and Artificial 19-Oxygenated Steroids from the Formosan Soft Coral Nephthea erecta. J. Nat. Prod. 2007, 70, 1449–1453. [Google Scholar] [CrossRef]
- Ishii, T.; Ueoka, R.; Matsunaga, S.; Vairappan, C.S. Bioactive secondary metabolites from the Borneon soft corals of the genus Nephthea. Malays. J. Sci. 2010, 29, 262–268. [Google Scholar] [CrossRef]
- Ghandourah, M.A.; Alarif, W.M.; Abdel-Lateff, A.; Al-Lihaibi, S.S.; Ayyad, S.-E.N.; Basaif, S.A.; Badria, F.A. Two new terpenoidal derivatives: A himachalene-type sesquiterpene and 13,14-secosteroid from the soft coral Litophyton arboreum. Med. Chem. Res. 2015, 24, 4070–4077. [Google Scholar] [CrossRef]
- Narula, A.P.S.; Dev, S. Studies in sesquiterpenes—LI: β-Himachalene epoxide—Stereochemistry and solvolysis. Tetrahedron 1977, 33, 813–816. [Google Scholar] [CrossRef]
- Roy, P.K.; Ashimine, R.; Miyazato, H.; Taira, J.; Ueda, K. Endoperoxy and hydroperoxy cadinane-type sesquiterpenoids from an Okinawan soft coral, Sinularia sp. Arch. Pharm. Res. 2016, 39, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, H.; Taira, J.; Ueda, K. Hydrogen peroxide derived from marine peroxy sesquiterpenoids induces apoptosis in HCT116 human colon cancer cells. Bioorg. Med. Chem. Lett. 2016, 26, 4641–4644. [Google Scholar] [CrossRef]
- Prawat, H.; Mahidol, C.; Kaweetripob, W.; Prachyawarakorn, V.; Tuntiwachwuttikul, P.; Ruchirawat, S. Sesquiterpene isocyanides, isothiocyanates, thiocyanates, and formamides from the Thai sponge Halichondria sp. Tetrahedron 2016, 72, 4222–4229. [Google Scholar] [CrossRef]
- Sorek, H.; Zelikoff, A.L.; Benayahu, Y.; Kashman, Y. Axiplyns A–E, new sesquiterpene isothiocyanates from the marine sponge Axinyssa aplysinoides. Tetrahedron Lett. 2008, 49, 2200–2203. [Google Scholar] [CrossRef]
- Wu, Q.; Ye, F.; Li, X.-L.; Liang, L.-F.; Sun, J.; Sun, H.; Guo, Y.-W.; Wang, H. Uncommon Polyoxygenated Sesquiterpenoids from South China Sea Soft Coral Lemnalia flava. J. Org. Chem. 2019, 84, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Roethle, P.A.; Trauner, D. The chemistry of marine furanocembranoids, pseudopteranes, gersolanes, and related natural products. Nat. Prod. Rep. 2008, 25, 298–317. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.D.; Li, Y.; Dhasmana, H.; Barnes, C.L. New Marine Cembrane Diterpenoids Isolated from the Caribbean Gorgonian Eunicea mammosa. J. Nat. Prod. 1993, 56, 1101–1113. [Google Scholar] [CrossRef]
- Uchio, Y.; Eguchi, S.; Kuramoto, J.; Nakayama, M.; Hase, T. Denticulatolide, an ichthyotoxic peroxide-containing cembranolide from the soft coral Lobophytum denticulatum. Tetrahedron Lett. 1985, 26, 4487–4490. [Google Scholar] [CrossRef]
- Kusumi, T.; Ohtani, I.; Inouye, Y.; Kakisawa, H. Absolute configurations of cytotoxic marine cembranolides; Consideration of mosher’s method. Tetrahedron Lett. 1988, 29, 4731–4734. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Wang, S.-K.; Chung, S.-G.; Chou, G.-C.; Dai, C.-F. Cytotoxic Cembrenolides and Steroids from the Formosan Soft Coral Sarcophyton crassocaule. J. Nat. Prod. 2000, 63, 1634–1637. [Google Scholar] [CrossRef]
- Fukazawa, Y.; Usui, S.; Uchio, Y.; Shiobara, Y.; Kodama, M. Conformational study of the cembranolide diterpene denticulatolide by molecular mechanics method. Tetrahedron Lett. 1986, 27, 1825–1828. [Google Scholar] [CrossRef]
- Mason, R.P.; Kalyanaraman, B.; Tainer, B.E.; Eling, T.E. A carbon-centered free radical intermediate in the prostaglandin synthetase oxidation of arachidonic acid. Spin trapping and oxygen uptake studies. J. Biol. Chem. 1980, 255, 5019–5022. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishizaka, T.; Miura, N.; Mitsuhashi, H. Marine terpenes and terpenoids. III. Isolation and structures of two cembrane diols from the soft coral Sinularia mayi. Chem. Pharm. Bull. 1987, 35, 2314. [Google Scholar] [CrossRef] [Green Version]
- Kamada, T.; Kang, M.-C.; Phan, C.-S.; Zanil, I.I.; Jeon, Y.-J.; Vairappan, C.S. Bioactive Cembranoids from the Soft Coral Genus Sinularia sp. in Borneo. Mar. Drugs 2018, 16, 99. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.-H.; Wen, Z.-H.; Wu, Y.-C.; Yeh, H.-C.; Sheu, J.-H. Cytotoxic and Anti-inflammatory Cembranoids from the Soft Coral Lobophytum crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar] [CrossRef]
- Kapojos, M.M.; Lee, J.-S.; Oda, T.; Nakazawa, T.; Takahashi, O.; Ukai, K.; Mangindaan, R.E.P.; Rotinsulu, H.; Wewengkang, D.S.; Tsukamoto, S.; et al. Two unprecedented cembrene-type terpenes from an indonesian soft coral Sarcophyton sp. Tetrahedron 2010, 66, 641–645. [Google Scholar] [CrossRef]
- Wanzola, M.; Furuta, T.; Kohno, Y.; Fukumitsu, S.; Yasukochi, S.; Watari, K.; Tanaka, C.; Higuchi, R.; Miyamoto, T. Four New Cembrane Diterpenes Isolated from an Okinawan Soft Coral Lobophytum crassum with Inhibitory Effects on Nitric Oxide Production. Chem. Pharm. Bull. 2010, 58, 1203–1209. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhang, Y.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Cytotoxic Cembranoid Diterpenes from a Soft Coral Sinularia gibberosa. J. Nat. Prod. 2005, 68, 649–652. [Google Scholar] [CrossRef]
- Uchio, Y.; Eguchi, S.; Fukazawa, Y.; Kodama, M. 7-Epidenticulatolide, a New Cembranolide with a Cyclic Peroxide Function from the Soft Coral Lobophytum denticulatum. Bull. Chem. Soc. Jap. 1992, 65, 1182–1184. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Venkateswara Rao, C.; Anjaneyulu, V.; Satyanarayana, P.; Subba Rao, P.V.; Ward, R.S.; Pelter, A. New diterpenes from a new species of Lobophytum soft coral of the South Andaman Coast. Tetrahedron 1992, 48, 3111–3120. [Google Scholar] [CrossRef]
- Hambley, T.W.; Taylor, W.C.; Toth, S. The constituents of marine sponges. IX. New norditerpenoids from Aplysilla pallida. Aust. J. Chem. 1997, 50, 903–909. [Google Scholar] [CrossRef]
- Cimino, G.; De Rosa, D.; De Stefano, S.; Minale, L. Isoagatholactone, a diterpene of a new structural type from the sponge Spongia officinalis. Tetrahedron 1974, 30, 645–649. [Google Scholar] [CrossRef]
- Umeyama, A.; Machida, M.; Nozaki, M.; Arihara, S. Peroxypolasol and Mugipolasol: Two Novel Diterpenes from the Marine Sponge Epipolasis sp. J. Nat. Prod. 1998, 61, 1435–1436. [Google Scholar] [CrossRef] [PubMed]




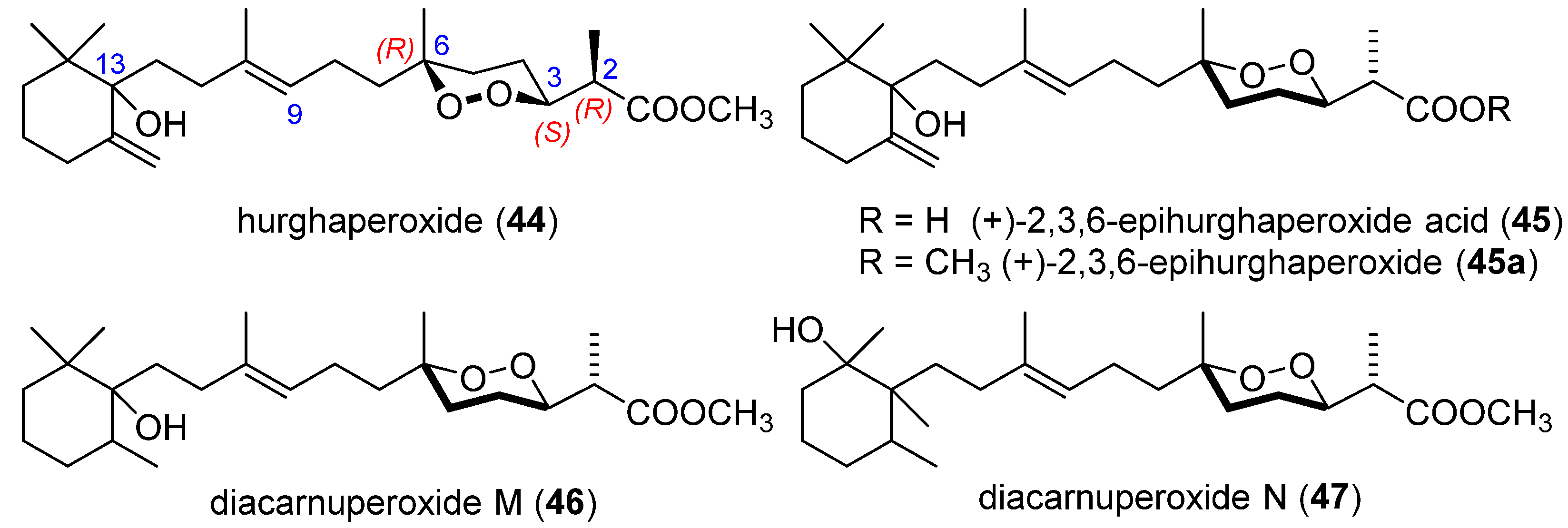
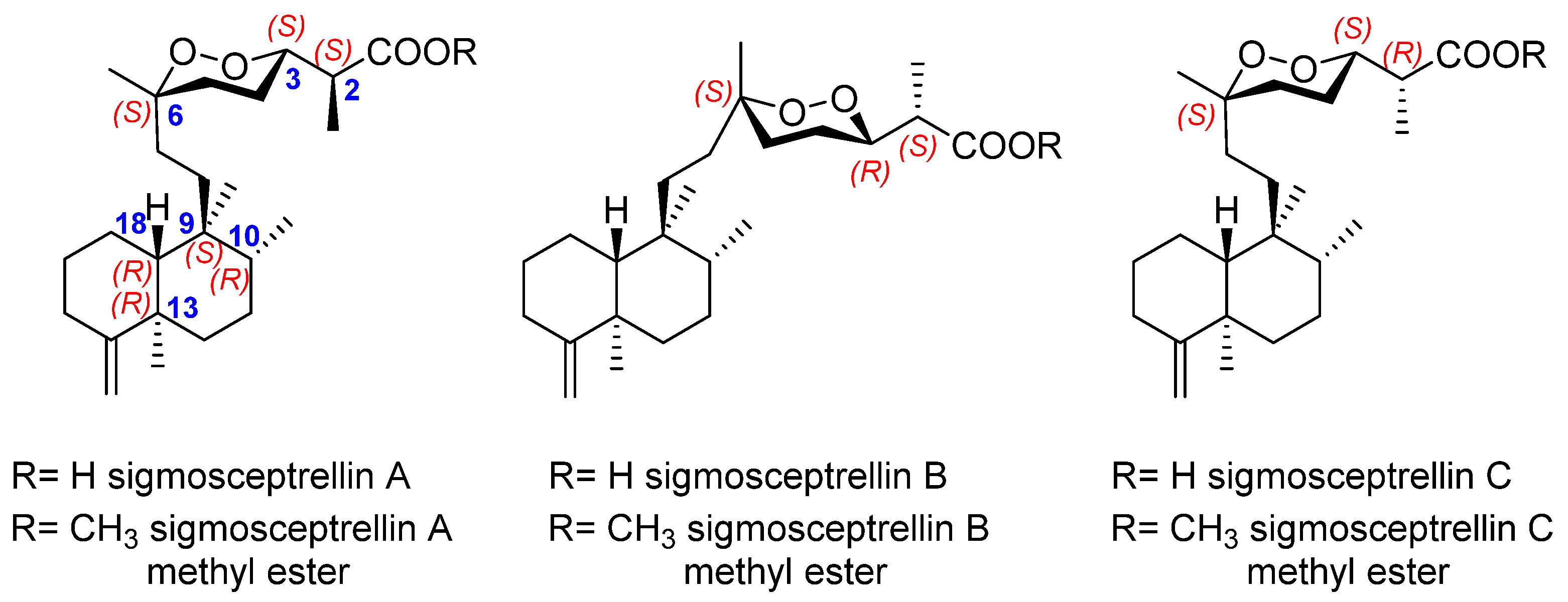
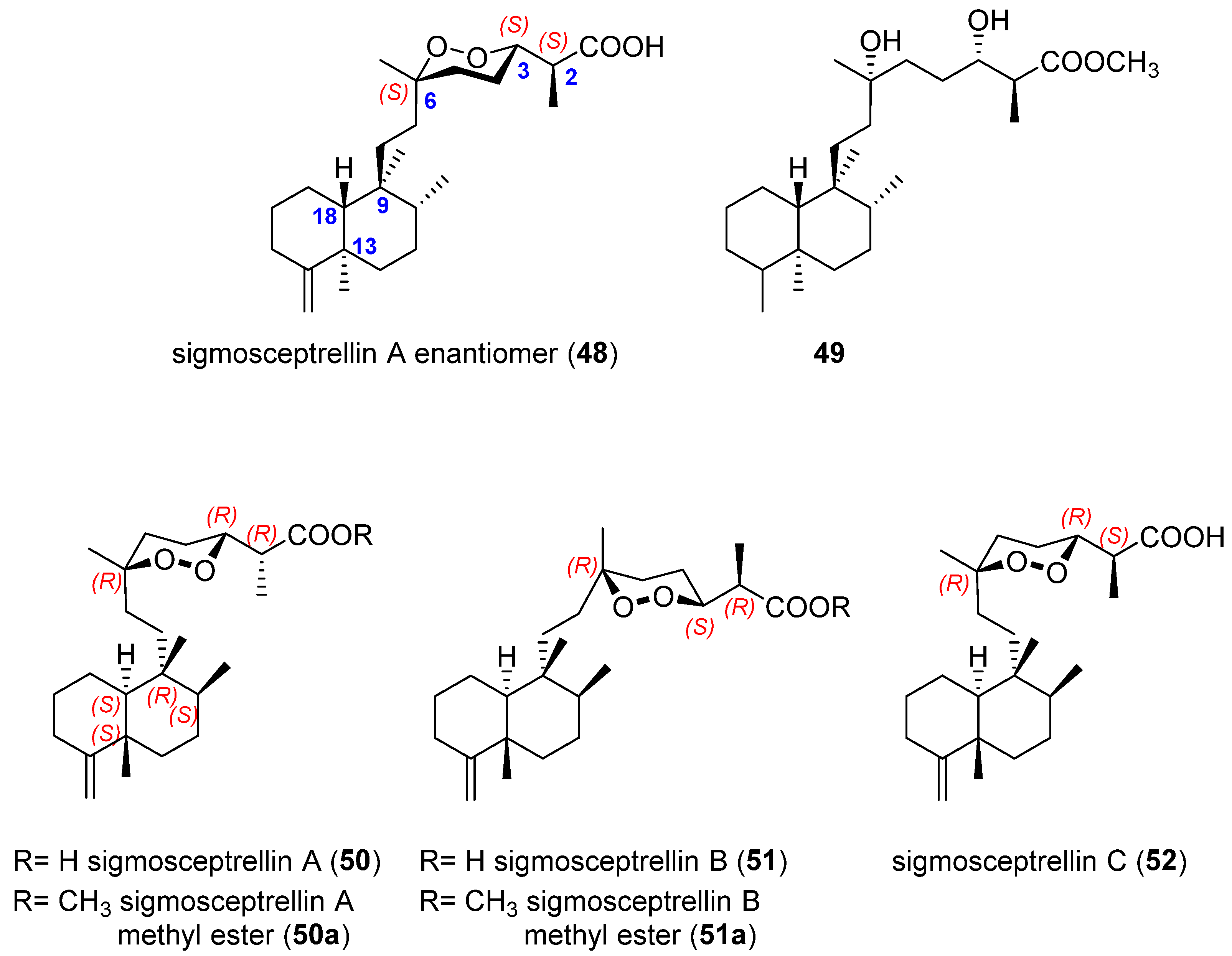

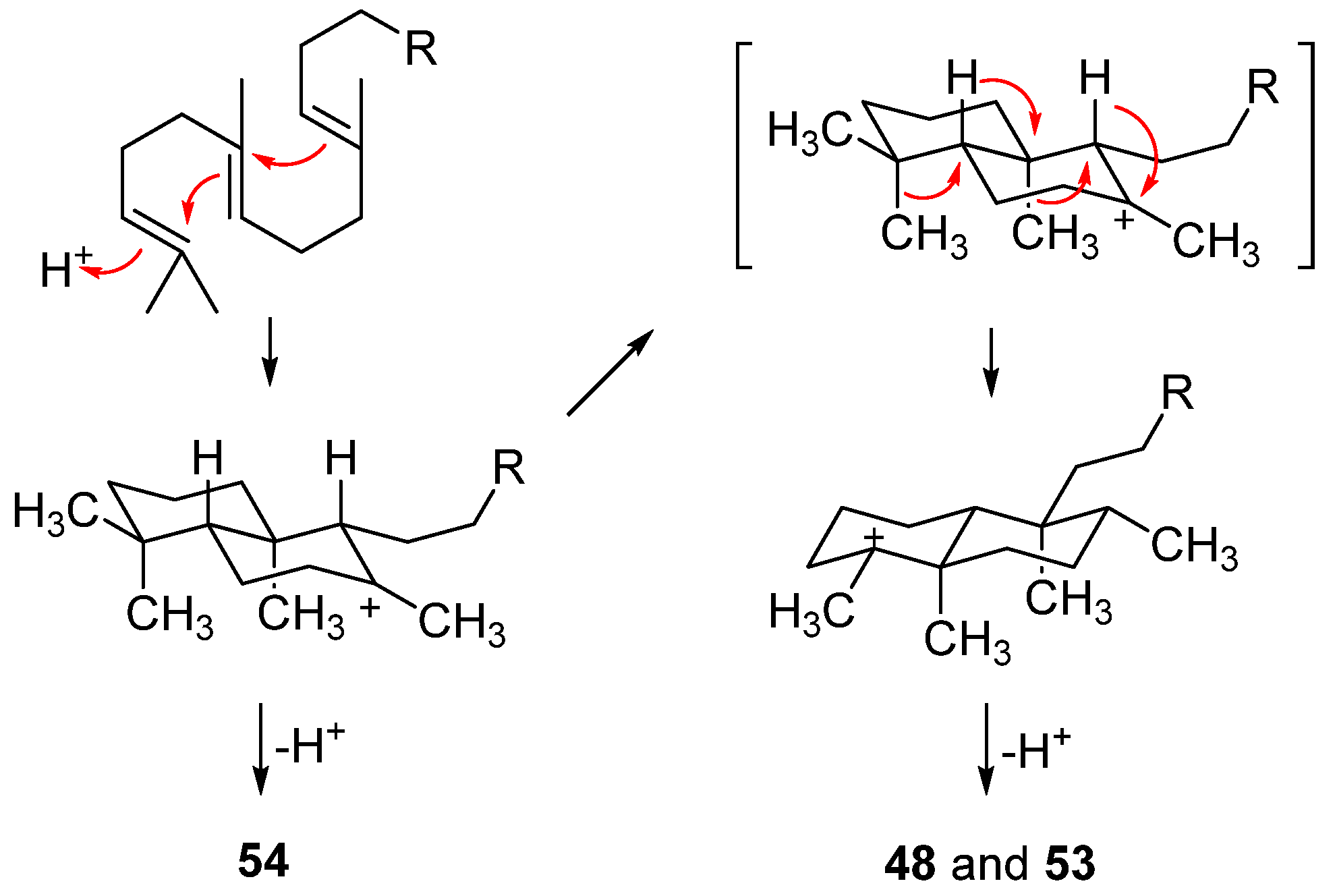




| Target | Norsesterterpenes | Norditerpenes d | Sesqui- and Diterpenes e | ||
|---|---|---|---|---|---|
| Acyclics a | Monocyclics b | Bicyclics c | |||
| Bacteria | 1 [6], 3a [9], 4 [9] | 12 [20] | 57 [46], 58 [46], 61 [47], 67 [51], 68 [51], 79 [61] | 80 [64], 81 [64], 84 [69], 86 [72] | |
| Yeasts | 1 [6] | 12 [20] | 57 [46], 58 [46], 61 [47], 79 [61] | ||
| Viruses | 5 [11] | 12 [11] | 67 [51], 68 [51] | ||
| Plasmodia | 12 [24], 15 [24,28], 20 [24,28], 21 [39], 45 [39], 45a [39], 46 [39], 47 [39] | 50 [23], 55 [28] | 8 [24,28], 9 [15], 13 [39], 19 [24,28], 30 [39], 31 [15], 32 [15] | ||
| Trypanosoma | 10 [16], 11 [16] | 22 [16] | 50 [16], 50a [16], 51 [16] | 26 [16] | |
| Toxoplasma | 12 [11] | 51 [11] | |||
| Inflammation | 22 [33,34] | 86 [72], 89 [84,85,86,87] | |||
| Citotoxicity | 5 [10,12] | 15 [27], 16 [27], 17 [27], 21 [31], 38 [36] | 50 [33], 51 [12], 51a [12], 67 [51], 68 [51], 74 [58], 75 [14], 76 [14], 77 [14], 78 [14], 79 [61] | 6 [10], 7 [10], 13 [22], 18 [12], 25 [13] | 80 [63], 83 [69], 84 [68,69], 86 [72,73], 89 [79,80,84,85,86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-García, I.; López-Martínez, J.L.; Muñoz-Dorado, M.; Rodríguez-García, I.; Álvarez-Corral, M. Marine Terpenic Endoperoxides. Mar. Drugs 2021, 19, 661. https://doi.org/10.3390/md19120661
Torres-García I, López-Martínez JL, Muñoz-Dorado M, Rodríguez-García I, Álvarez-Corral M. Marine Terpenic Endoperoxides. Marine Drugs. 2021; 19(12):661. https://doi.org/10.3390/md19120661
Chicago/Turabian StyleTorres-García, Irene, Josefa L. López-Martínez, Manuel Muñoz-Dorado, Ignacio Rodríguez-García, and Miriam Álvarez-Corral. 2021. "Marine Terpenic Endoperoxides" Marine Drugs 19, no. 12: 661. https://doi.org/10.3390/md19120661
APA StyleTorres-García, I., López-Martínez, J. L., Muñoz-Dorado, M., Rodríguez-García, I., & Álvarez-Corral, M. (2021). Marine Terpenic Endoperoxides. Marine Drugs, 19(12), 661. https://doi.org/10.3390/md19120661






