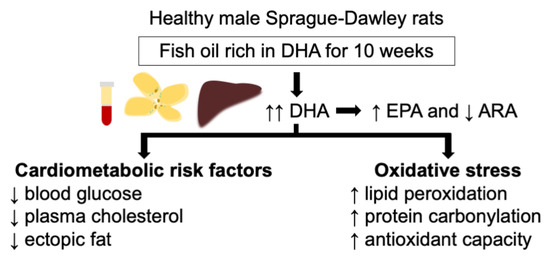
Effects of a Fish Oil Rich in Docosahexaenoic Acid on Cardiometabolic Risk Factors and Oxidative Stress in Healthy Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biometric Data, Glucose Metabolism, Plasma Lipid Profile, Ectopic Fat and Transaminases
2.2. Fatty Acid Composition of Erythrocytes and Tissues
2.3. Membrane Fluidity of Erythrocytes
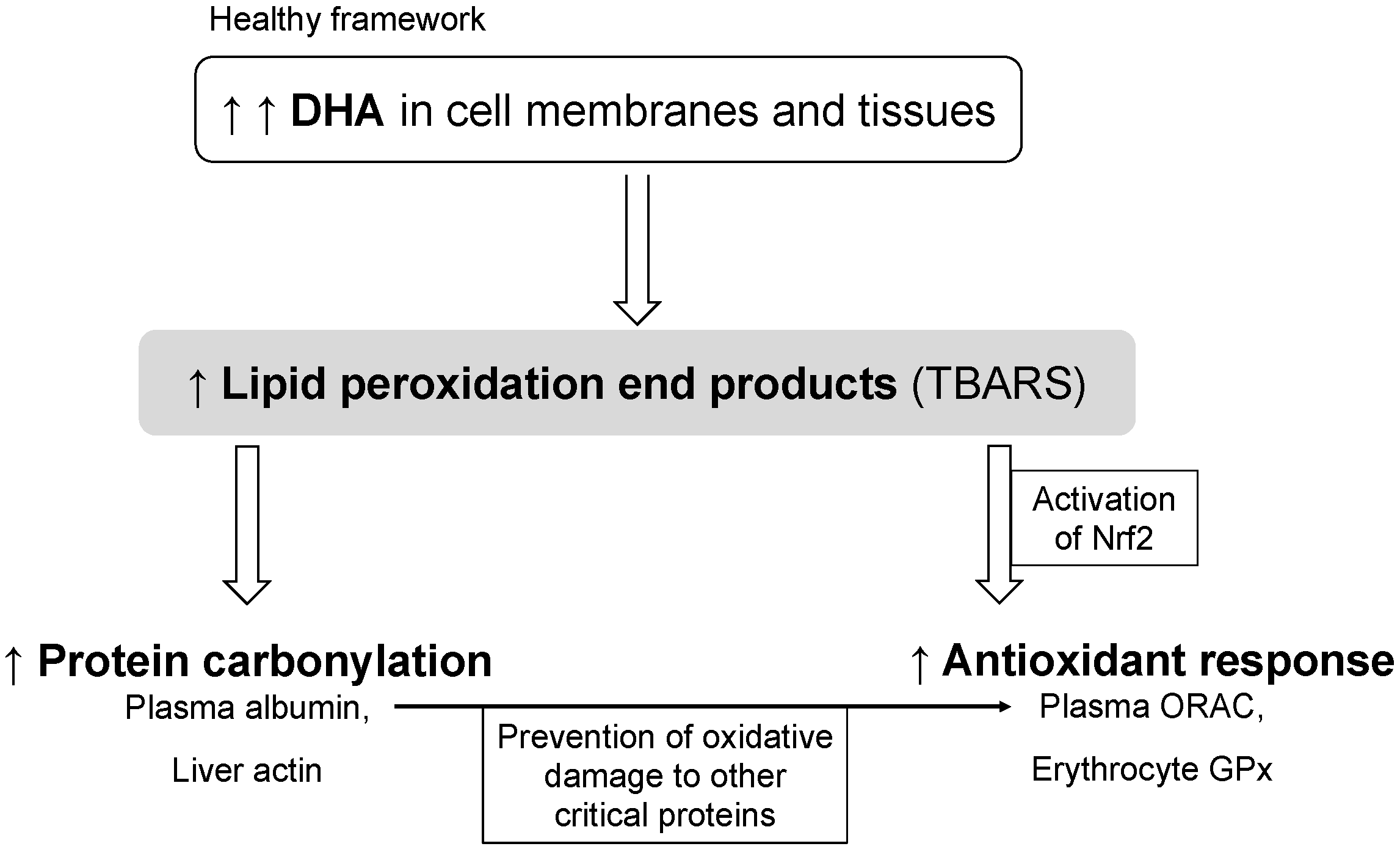
2.4. Biomarkers of Inflammation and Oxidative Stress
3. Materials and Methods
3.1. Ethics Statement
3.2. Animals and Experimental Design
3.3. Fatty Acid Composition and Peroxide Content of the Oils
3.4. Feed Intake and Biometric Data
3.5. Measurement of Blood Glucose
3.6. Sample Processing
3.7. Measurements of Total Fat and Fatty Acids in Blood and Tissues
3.8. Measurement of Erythrocyte Membrane Fluidity
3.9. Measurements of Glycated Hemoglobin and Total hemoglobin
3.10. Measurements of Lipid Profile, Transaminases and Inflammatory Biomarkers
3.11. Histological Analysis of the Liver
3.12. Measurements of Oxidative Stress Biomarkers
3.12.1. Plasma Antioxidant Capacity
3.12.2. Antioxidant Enzymes, Glutathione and Xanthine Oxidase
3.12.3. Lipid Peroxidation and Protein Carbonylation
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Li, Y.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Willett, W.C.; Hu, F.B.; Sun, Q. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: Two prospective longitudinal cohort studies. BMJ 2016, 355, i5796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 5, CD011737. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Micha, R.; Wu, J.H.Y.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: A systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016, 13, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging mechanisms of cardiovascular protection for the Omega-3 fatty acid eicosapentaenoic acid. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1135–1147. [Google Scholar] [CrossRef]
- Tai, C.C.; Ding, S.T. N-3 polyunsaturated fatty acids regulate lipid metabolism through several inflammation mediators: Mechanisms and implications for obesity prevention. J. Nutr. Biochem. 2010, 21, 357–363. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhong, W.F.; Liu, S.; Kraus, V.B.; Zhang, Y.J.; Gao, X.; Lv, Y.B.; Shen, D.; Zhang, X.R.; Zhang, P.D.; et al. Associations of habitual fish oil supplementation with cardiovascular outcomes and all cause mortality: Evidence from a large population based cohort study. BMJ 2020, 368, m456. [Google Scholar] [CrossRef] [Green Version]
- Dasilva, G.; Pazos, M.; García-Egido, E.; Gallardo, J.M.; Rodríguez, I.; Cela, R.; Medina, I. Healthy effect of different proportions of marine ω-3 PUFAs EPA and DHA supplementation in Wistar rats: Lipidomic biomarkers of oxidative stress and inflammation. J. Nutr. Biochem. 2015, 26, 1385–1392. [Google Scholar] [CrossRef] [Green Version]
- Dasilva, G.; Pazos, M.; García-Egido, E.; Gallardo, J.M.; Ramos-Romero, S.; Torres, J.L.; Romeu, M.; Nogués, M.R.; Medina, I. A lipidomic study on the regulation of inflammation and oxidative stress targeted by marine ω-3 PUFA and polyphenols in high-fat high-sucrose diets. J. Nutr. Biochem. 2017, 43, 53–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.B.; Huang, L.L.; Rong, R.; Tan, R.; Wang, J.; Kang, J.X. Endogenously decreasing tissue n-6/n-3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein E-deficient mice by inhibiting systemic and vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2487–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez, L.; Muñoz, S.; Miralles-Pérez, B.; Rosa Nogués, M.; Ramos-Romero, S.; Torres, J.L.; Medina, I. Modulation of the liver protein carbonylome by the combined effect of marine omega-3 PUFAs and grape polyphenols supplementation in rats fed an obesogenic high fat and high sucrose diet. Mar. Drugs 2020, 18, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez, L.; Ciordia, S.; Fernández, M.S.; Juárez, S.; Ramos, A.; Pazos, M.; Gallardo, J.M.; Torres, J.L.; Nogués, M.R.; Medina, I. Changes in liver proteins of rats fed standard and high-fat and sucrose diets induced by fish omega-3 PUFAs and their combination with grape polyphenols according to quantitative proteomics. J. Nutr. Biochem. 2017, 41, 84–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taltavull, N.; Muñoz-Cortés, M.; Lluís, L.; Jové, M.; Fortuño, À.; Molinar-Toribio, E.; Torres, J.L.; Pazos, M.; Medina, I.; Nogués, M.R. Eicosapentaenoic acid/docosahexaenoic acid 1:1 ratio improves histological alterations in obese rats with metabolic syndrome. Lipids Health Dis. 2014, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lluís, L.; Taltavull, N.; Muñoz-Cortés, M.; Sánchez-Martos, V.; Romeu, M.; Giralt, M.; Molinar-Toribio, E.; Torres, J.L.; Pérez-Jiménez, J.; Pazos, M.; et al. Protective effect of the omega-3 polyunsaturated fatty acids: Eicosapentaenoic acid/Docosahexaenoic acid 1:1 ratio on cardiovascular disease risk markers in rats. Lipids Health Dis. 2013, 12, 140. [Google Scholar] [CrossRef] [Green Version]
- Méndez, L.; Pazos, M.; Gallardo, J.M.; Torres, J.L.; Pérez-Jiménez, J.; Nogués, R.; Romeu, M.; Medina, I. Reduced protein oxidation in Wistar rats supplemented with marine ω3 PUFAs. Free Radic. Biol. Med. 2013, 55, 8–20. [Google Scholar] [CrossRef]
- Allaire, J.; Couture, P.; Leclerc, M.; Charest, A.; Marin, J.; Lépine, M.C.; Talbot, D.; Tchernof, A.; Lamarche, B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED) Study. Am. J. Clin. Nutr. 2016, 104, 280–287. [Google Scholar] [CrossRef]
- Kim, E.H.; Bae, J.S.; Hahm, K.B.; Cha, J.Y. Endogenously synthesized n-3 polyunsaturated fatty acids in fat-1 mice ameliorate high-fat diet-induced non-alcoholic fatty liver disease. Biochem. Pharmacol. 2012, 84, 1359–1365. [Google Scholar] [CrossRef]
- Neschen, S.; Morino, K.; Dong, J.; Wang-Fischer, Y.; Cline, G.W.; Romanelli, A.J.; Rossbacher, J.C.; Moore, I.K.; Regittnig, W.; Munoz, D.S.; et al. N-3 fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-α-dependent manner. Diabetes 2007, 56, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Vanden Heuvel, J.P.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem. Biophys. Res. Commun. 2005, 336, 909–917. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Feng, X.; Zhang, J.; Zhang, Y.; Xia, F.; Liu, L.; Jin, Z.; Lu, C.; Xia, Y.; Papadimos, T.J.; et al. ω-3 Fish oil fat emulsion preconditioning mitigates myocardial oxidative damage in rats through aldehydes stress. Biomed. Pharmacother. 2019, 118, 109198. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- De Castro, G.S.; Deminice, R.; Simões-Ambrosio, L.M.C.; Calder, P.C.; Jordão, A.A.; Vannucchi, H. Dietary docosahexaenoic acid and eicosapentaenoic acid influence liver triacylglycerol and insulin resistance in rats fed a high-fructose diet. Mar. Drugs 2015, 13, 1864–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladine, C.; Roy, N.C.; Rigaudière, J.P.; Laillet, B.; Da Silva, G.; Joly, C.; Pujos-Guillot, E.; Morio, B.; Feillet-Coudray, C.; McNabb, W.C.; et al. Increasing intake of long-chain n-3 PUFA enhances lipoperoxidation and modulates hepatic gene expression in a dose-dependent manner. Br. J. Nutr. 2012, 107, 1254–1273. [Google Scholar] [CrossRef] [Green Version]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.F.; Jacob, R.F.; Bjork, R.E.; Jeffers, B.; Buch, J.; Mizuno, Y.; Mason, R.P. Circulating lipid hydroperoxides predict cardiovascular events in patients with stable coronary artery disease. The PREVENT Study. J. Am. Coll. Cardiol. 2008, 51, 1196–1202. [Google Scholar] [CrossRef] [Green Version]
- Bargut, T.C.L.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. A high-fish-oil diet prevents adiposity and modulates white adipose tissue inflammation pathways in mice. J. Nutr. Biochem. 2015, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.-M.M.; Holm, J.B.; Sjøberg, K.A.; Bojsen-Møller, K.N.; Myrmel, L.S.; Fjære, E.; Jensen, B.A.H.; Nicolaisen, T.S.; Hingst, J.R.; Hansen, S.L.; et al. Mechanisms preserving insulin action during high dietary fat intake. Cell Metab. 2019, 29, 50–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durašević, S.; Jasnić, N.; Prokić, M.; Grigorov, I.; Martinović, V.; Dordević, J.; Pavlović, S. The protective role of virgin coconut oil on the alloxan-induced oxidative stress in the liver, kidneys and heart of diabetic rats. Food Funct. 2019, 10, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Mu, H.; Shen, H.; Deng, K.; Liu, D.; Yang, M.; Zhang, Y.; Zhang, W.; Mai, K. High level of dietary soybean oil affects the glucose and lipid metabolism in large yellow croaker Larimichthys crocea through the insulin-mediated PI3K/AKT signaling pathway. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 231, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, T.; Pellizzon, M.A.; Aihara, M.; Stylianou, I.M.; Billheimer, J.T.; Rothblat, G.; Rader, D.J. Fish oil promotes macrophage reverse cholesterol transport in mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1502–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Morvan, V.; Dumon, M.F.; Palos-Pinto, A.; Bérard, A.M. n-3 FA increase liver uptake of HDL-cholesterol in mice. Lipids 2002, 37, 767–772. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Q.; Wu, H.; Xue, Y.; Cai, L.; Fang, M.; Liu, Z.; Yao, P.; Wu, Y.; Gong, Z. Protective role of n6/n3 PUFA supplementation with varying DHA/EPA ratios against atherosclerosis in mice. J. Nutr. Biochem. 2016, 32, 171–180. [Google Scholar] [CrossRef]
- Milte, C.M.; Coates, A.M.; Buckley, J.D.; Hill, A.M.; Howe, P.R.C. Dose-dependent effects of docosahexaenoic acid-rich fish oil on erythrocyte docosahexaenoic acid and blood lipid levels. Br. J. Nutr. 2008, 99, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small dense low-density lipoprotein as biomarker for Atherosclerotic diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef]
- Yang, Z.H.; Amar, M.; Sampson, M.; Courville, A.B.; Sorokin, A.V.; Gordon, S.M.; Aponte, A.M.; Stagliano, M.; Playford, M.P.; Fu, Y.P.; et al. Comparison of omega-3 eicosapentaenoic acid versus docosahexaenoic acid-rich fish oil supplementation on plasma lipids and lipoproteins in normolipidemic adults. Nutrients 2020, 12, 749. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Ma, J.; Campos, H.; Hankinson, S.E.; Hu, F.B. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 2007, 86, 74–81. [Google Scholar] [CrossRef]
- Calzada, C.; Colas, R.; Guillot, N.; Guichardant, M.; Laville, M.; Véricel, E.; Lagarde, M. Subgram daily supplementation with docosahexaenoic acid protects low-density lipoproteins from oxidation in healthy men. Atherosclerosis 2010, 208, 467–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fumagalli, M.; Moltke, I.; Grarup, N.; Racimo, F.; Bjerregaard, P.; Jørgensen, M.E.; Korneliussen, T.S.; Gerbault, P.; Skotte, L.; Linneberg, A.; et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science 2015, 349, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, H.; Kang, S.B.; Park, W.J. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Strable, M.S.; Ntambi, J.M. Stearoyl CoA desaturase 1: Role in cellular inflammation and stress. Adv. Nutr. 2011, 2, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Petursdottir, D.H.; Olafsdottir, I.; Hardardottir, I. Dietary fish oil increases tumor necrosis factor secretion but decreases interleukin-10 secretion by murine peritoneal macrophages. J. Nutr. 2002, 132, 3740–3743. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.C.; Kuo, W.H.; Chen, C.Y.; Lin, H.Y.; Wu, H.T.; Liu, B.H.; Chen, C.H.; Mersmann, H.J.; Chang, K.J.; Ding, S.T. Docosahexaenoic acid regulates serum amyloid A protein to promote lipolysis through down regulation of perilipin. J. Nutr. Biochem. 2010, 21, 317–324. [Google Scholar] [CrossRef]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell Proteomics 2002, 1, 845–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Vovk, M.A.; Batalova, A.A.; Jenkins, R.O.; Goncharov, N.V. The universal soldier: Enzymatic and non-enzymatic antioxidant functions of serum albumin. Antioxidants 2020, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Carini, M.; Vistoli, G.; Gamberoni, L.; Giustarini, D.; Colombo, R.; Maffei Facino, R.; Rossi, R.; Milzani, A.; Aldini, G. Actin Cys374 as a nucleophilic target of α,β-unsaturated aldehydes. Free Radic. Biol. Med. 2007, 42, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Gao, X.; Pang, X.; Wang, Y.; Wu, X.; Deng, X.; Zhang, Q.; Sun, C.; Li, Y. Elevated serum xanthine oxidase activity is associated with the development of type 2 diabetes: A prospective cohort study. Diabetes Care 2018, 41, 884–890. [Google Scholar] [CrossRef] [Green Version]
- Abdukeyum, G.; Owen, A.; Larkin, T.; McLennan, P. Up-regulation of mitochondrial antioxidant superoxide dismutase underpins persistent cardiac nutritional-preconditioning by long chain n-3 polyunsaturated fatty acids in the rat. J. Clin. Med. 2016, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Tsuboyama-Kasaoka, N.; Nakatani, T.; Ishii, M.; Tsutsumi, S.; Aburatani, H.; Ezaki, O. Fish oil feeding alters liver gene expressions to defend against PPARα activation and ROS production. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, 338–348. [Google Scholar] [CrossRef] [Green Version]
- Varady, J.; Eder, K.; Ringseis, R. Dietary oxidized fat activates the oxidative stress-responsive transcription factors NF-κB and Nrf2 in intestinal mucosa of mice. Eur. J. Nutr. 2011, 50, 601–609. [Google Scholar] [CrossRef]
- Awada, M.; Soulage, C.O.; Meynier, A.; Debard, C.; Plaisancié, P.; Benoit, B.; Picard, G.; Loizon, E.; Chauvin, M.A.; Estienne, M.; et al. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: Role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J. Lipid Res. 2012, 53, 2069–2080. [Google Scholar] [CrossRef] [Green Version]
- Arunima, S.; Rajamohan, T. Effect of virgin coconut oil enriched diet on the antioxidant status and paraoxonase 1 activity in ameliorating the oxidative stress in rats-a comparative study. Food Funct. 2013, 4, 1402–1409. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012, 10, 2815. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Medina, I.; Aubourg, S.; Gallardo, J.M.; Pérez-Martín, R. Comparison of six methylation methods for analysis of the fatty acid composition of albacore lipid. Int. J. Food Sci. Technol. 1992, 27, 597–601. [Google Scholar] [CrossRef]
- Lal, J.J.; Sreeranjit Kumar, C.V.; Indira, M. Coconut palm. In Encyclopedia of Food Sciences and Nutrition; Trugo, L., Finglas, P.M., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2003; Volume 1, pp. 1464–1475. [Google Scholar]
- Chapman, R.A.; Mackay, K. The estimation of peroxides in fats and oils by ferric thiocyanate method. J. Am. Oil Chem. Soc. 1949, 26, 360–363. [Google Scholar] [CrossRef]
- Hernáez, Á.; Fernández-Castillejo, S.; Farràs, M.; Catalán, Ú.; Subirana, I.; Montes, R.; Solà, R.; Muñoz-Aguayo, D.; Gelabert-Gorgues, A.; Díaz-Gil, Ó.; et al. Olive oil polyphenols enhance high-density lipoprotein function in humans: A randomized controlled trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119. [Google Scholar] [CrossRef] [Green Version]
- Drabkin, D.L.; Austin, J.H. Spectrophotometric studies: II. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. J. Biol. Chem. 1935, 112, 51–65. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Fortuño-Mar, À.; Pasquali, P. Cryobiopsy, cryoanesthesia, and cryoanalgesia. In Cryosurgery. A Practica Manual; Pasquali, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 85–91. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of ‘“antioxidant power”’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Cohen, G.; Dembiec, D.M.J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970, 34, 30–38. [Google Scholar] [CrossRef]
- Wheeler, C.R.; Salzman, J.A.; Elsayed, N.M.; Omaye, S.T.; Korte, D.W., Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 1990, 184, 193–199. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Maia, L.; Mira, L. Xanthine oxidase and aldehyde oxidase: A simple procedure for the simultaneous purification from rat liver. Arch. Biochem. Biophys. 2002, 400, 48–53. [Google Scholar] [CrossRef]
- Muñoz, S.; Méndez, L.; Dasilva, G.; Torres, J.L.; Ramos-Romero, S.; Romeu, M.; Nogués, M.R.; Medina, I. Targeting hepatic protein carbonylation and oxidative stress occurring on diet-induced metabolic diseases through the supplementation with fish oils. Mar. Drugs 2018, 16, 353. [Google Scholar] [CrossRef] [Green Version]
- Buege, J.; Aust, S. Microsomal lipid peroxidation. Methods Enzym. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Richard, M.J.; Portal, B.; Meo, J.; Coudray, C.; Hadjian, A.; Favier, A. Malondialdehyde kit evaluated for determining plasma and lipoprotein fractions that react with thiobarbituric acid. Clin. Chem. 1992, 38, 704–709. [Google Scholar] [CrossRef]

| Coconut | Soybean | EPA/DHA 1:1 | 80% DHA | p-Value | |
|---|---|---|---|---|---|
| Glucose metabolism | |||||
| Blood glucose (mmol/L) | 5.1 ± 0.3 | 5.5 ± 0.4 a | 5.0 ± 0.2 b | 5.0 ± 0.2 b | 0.008 † |
| Blood HbA1c (%) | 2.9 ± 0.1 | 3.0 ± 0.3 | 3.2 ± 0.2 | 3.2 ± 0.3 | 0.035 * |
| Plasma lipid profile | |||||
| Fat content (%) | 0.52 ± 0.04 | 0.45 ± 0.03 | 0.44 ± 0.03 a | 0.37 ± 0.04 a,b | 0.033 * |
| TAG (mmol/L) | 0.7 ± 0.1 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | NS * |
| TC (mmol/L) | 2.7 ± 0.3 | 2.5 ± 0.4 | 2.4 ± 0.2 | 2.1 ± 0.2 a,b | 0.001 * |
| HDL (mmol/L) | 1.80 ± 0.21 | 1.69 ± 0.21 | 1.62 ± 0.12 a | 1.47 ± 0.15 a,b,c | 0.004 † |
| LDL (mmol/L) | 0.45 ± 0.08 | 0.35 ± 0.13 a | 0.45 ± 0.05 b | 0.39 ± 0.09 | 0.027 † |
| LDL/HDL ratio | 0.25 ± 0.03 | 0.20 ± 0.06 a | 0.28 ± 0.03 a,b | 0.27 ± 0.06 b | 0.003 † |
| Ectopic fat | |||||
| Liver fat content (%) | 5.36 ± 0.19 | 5.13 ± 0.35 | 4.92 ± 0.31 a | 5.12 ± 0.32 c | 0.019 * |
| Liver TAG (µmol/g tissue) | 11.2 ± 1.1 | 10.9 ± 1.7 | 11.0 ± 1.3 | 10.9 ± 1.3 | NS † |
| Liver TC (µmol/g tissue) | 4.7 ± 0.9 | 4.8 ± 1.1 | 5.8 ± 0.6 | 5.6 ± 0.6 | 0.014 * |
| Muscle fat content (%) | 2.13 ± 0.67 | 2.27 ± 0.53 | 2.17 ± 0.43 | 1.64 ± 0.17 a,b | 0.014 * |
| Transaminases | |||||
| Plasma AST (U/L) | 91.2 ± 29.4 | 85.8 ± 31.7 | 110.2 ± 15.5 | 120.7 ± 21.0 | 0.023 * |
| Plasma ALT (U/L) | 43.9 ± 15.9 | 43.8 ± 11.3 | 49.0 ± 12.2 | 53.8 ± 23.8 | NS † |
| Plasma AST/ALT ratio | 2.13 ± 0.55 | 1.92 ± 0.34 | 2.39 ± 0.71 | 2.49 ± 0.88 | NS † |
| Erythrocyte membrane properties | |||||
| Fat content (%) | 2.05 ± 0.19 | 2.04 ± 0.20 | 1.98 ± 0.26 | 1.98 ± 0.23 | NS * |
| Fluidity (AU) | 4.6 ± 0.9 | 5.1 ± 0.9 | 4.9 ± 0.9 | 4.9 ± 0.8 | NS † |
| Inflammatory biomarkers | |||||
| Plasma TNFα (pg/mL) | 21.6 ± 5.7 | 18.3 ± 8.8 | 27.9 ± 6.8 a,b | 30.6 ± 10.2 a,b | 0.004 † |
| Plasma CRP (μg/mL) | 390 ± 30 | 431 ± 56 | 380 ± 40 | 388 ± 47 | NS * |
| FA | Coconut | Soybean | EPA/DHA 1:1 | 80% DHA | p-Value |
|---|---|---|---|---|---|
| 14:0 | 0.31 ± 0.08 | 0.26 ± 0.05 | 0.34 ± 0.22 | 0.20 ± 0.02 | NS |
| 15:0 | 0.37 ± 0.03 | 0.32 ± 0.06 | 0.37 ± 0.04 | 0.34 ± 0.03 | 0.021 |
| 16:0 | 30.02 ± 0.70 | 29.59 ± 0.86 | 30.89 ± 1.02 b | 30.79 ± 0.78 b | 0.003 |
| 16:1 ω-7 | 0.51 ± 0.08 | 0.52 ± 0.11 | 0.53 ± 0.16 | 0.42 ± 0.06 | NS |
| 17:0 | 0.47 ± 0.05 | 0.46 ± 0.07 | 0.51 ± 0.04 | 0.39 ± 0.14 c | 0.033 |
| 18:0 | 11.85 ± 0.34 | 12.08 ± 0.37 | 11.58 ± 0.28 b | 12.11 ± 0.33 c | 0.003 |
| 18:1 ω-9 | 5.68 ± 0.30 | 5.79 ± 0.19 | 5.44 ± 0.77 | 5.60 ± 0.24 | NS |
| 18:1 ω-7 | 3.47 ± 0.16 | 3.30 ± 0.14 | 3.67 ± 1.02 | 2.95 ± 0.14 c | 0.034 |
| 18:2 ω-6 | 8.91 ± 0.46 | 9.24 ± 0.19 | 10.22 ± 0.4 a,b | 10.94 ± 0.43 a,b,c | <0.001 |
| 18:3 ω-6 | 0.05 ± 0.04 | 0.06 ± 0.04 | 0.01 ± 0.03 b | ND a,b | <0.001 |
| 18:3 ω-3 | 0.04 ± 0.05 | 0.07 ± 0.04 | 0.07 ± 0.04 | ND b,c | <0.001 |
| 20:1 ω-9 | 0.17 ± 0.03 | 0.18 ± 0.04 | 0.18 ± 0.07 | 0.15 ± 0.02 | NS |
| 20:2 ω-6 | 0.33 ± 0.04 | 0.34 ± 0.04 | 0.33 ± 0.05 | 0.33 ± 0.04 | NS |
| 20:3 ω-6 | 0.41 ± 0.05 | 0.39 ± 0.06 | 0.47 ± 0.05 b | 0.54 ± 0.06 a,b | 0.015 |
| 20:4 ω-6 | 22.71 ± 0.45 | 22.47 ± 0.74 | 19.69 ± 0.87 a,b | 18.55 ± 0.85 a,b,c | <0.001 |
| 22:1 ω-9 | 0.33 ± 0.10 | 0.34 ± 0.09 | 0.26 ± 0.06 | 0.33 ± 0.09 | NS |
| 20:5 ω-3 | 0.21 ± 0.04 | 0.22 ± 0.03 | 0.86 ± 0.33 a,b | 0.87 ± 0.21 a,b | <0.001 |
| 24:0 | 1.06 ± 0.11 | 1.23 ± 0.14 a | 1.16 ± 0.05 | 1.19 ± 0.12 | 0.013 |
| 22:4 ω-6 | 2.68 ± 0.22 | 2.67 ± 0.15 | 1.40 ± 0.18 a,b | 1.07 ± 0.15 a,b,c | <0.001 |
| 24:1 ω-9 | 0.55 ± 0.04 | 0.68 ± 0.14 a | 0.60 ± 0.07 | 0.55 ± 0.06 b | 0.006 |
| 22:5 ω-6 | 1.09 ± 0.11 | 1.04 ± 0.08 | 0.61 ± 0.05 a,b | 0.63 ± 0.06 a,b | <0.001 |
| 22:5 ω-3 | 1.54 ± 0.08 | 1.69 ± 0.17 | 2.56 ± 0.14 a,b | 1.86 ± 0.12 a,c | <0.001 |
| 22:6 ω-3 | 1.83 ± 0.19 | 1.86 ± 0.1 | 3.30 ± 0.2 a,b | 5.21 ± 0.56 a,b,c | <0.001 |
| SAT | 48.12 ± 0.85 | 47.87 ± 0.83 | 48.59 ± 1.09 | 48.87 ± 0.99 | NS |
| MUFA | 11.51 ± 0.37 | 11.4 ± 0.34 | 11.29 ± 0.42 | 10.6 ± 0.3 a,b,c | <0.001 |
| PUFA | 40.36 ± 0.9 | 40.72 ± 0.87 | 40.13 ± 1.35 | 40.53 ± 1.09 | NS |
| ω-3 | 3.63 ± 0.22 | 3.85 ± 0.24 | 6.79 ± 0.51 a,b | 7.93 ± 0.8 a,b,c | <0.001 |
| ω-6 | 36.18 ± 0.81 | 36.2 ± 0.73 | 32.73 ± 1.08 a,b | 32.05 ± 0.97 a,b | <0.001 |
| ω-6/ω-3 ratio | 10.01 ± 0.59 | 9.44 ± 0.58 | 4.84 ± 0.34 a,b | 4.08 ± 0.48 a,b,c | <0.001 |
| SCD-16 = [16:1 ω-7/16:0] | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.017 |
| SCD-18 = [18:1 ω-9/18:0] | 0.48 ± 0.02 | 0.48 ± 0.02 | 0.47 ± 0.07 | 0.46 ± 0.02 | NS |
| Δ5D = [20:4 ω-6/20:3 ω-6] | 56.14 ± 6.69 | 59.32 ± 9.01 | 42.35 ± 3.58 a,b | 35.16 ± 5.36 a,b | <0.001 |
| Δ6D = [20:3 ω-6/18:2 ω-6] | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.00 | 0.05 ± 0.00 | NS |
| Δ5/6D = [20:5 ω-3/18:3 ω-3] | 2.40 ± 0.78 | 2.62 ± 0.66 | 11.01 ± 4.65 | ND |
| FA | Coconut | Soybean | EPA/DHA 1:1 | 80% DHA | p-Value |
|---|---|---|---|---|---|
| 14:0 | 1.15 ± 0.17 | 0.94 ± 0.11 a | 0.98 ± 0.11 a | 0.87 ± 0.09 a | <0.001 |
| 15:0 | 0.33 ± 0.04 | 0.30 ± 0.03 | 0.33 ± 0.03 | 0.32 ± 0.04 | NS |
| 16:0 | 19.22 ± 1.78 | 19.25 ± 1.79 | 19.41 ± 1.75 | 18.52 ± 1.62 | NS |
| 16:1 ω-9 | 0.59 ± 0.05 | 0.59 ± 0.04 | 0.57 ± 0.03 | 0.62 ± 0.06 | NS |
| 16:1 ω-7 | 3.86 ± 1.21 | 3.41 ± 0.73 | 3.95 ± 1.52 | 3.28 ± 1.17 | NS |
| 17:0 | 0.24 ± 0.03 | 0.24 ± 0.02 | 0.25 ± 0.03 | 0.25 ± 0.03 | NS |
| 18:0 | 2.38 ± 0.30 | 2.45 ± 0.24 | 2.43 ± 0.38 | 2.47 ± 0.32 | NS |
| 18:1 ω-9 | 23.21 ± 0.40 | 23.44 ± 0.4 | 23.01 ± 0.46 | 22.88 ± 0.44 | 0.033 |
| 18:1 ω-7 | 3.88 ± 0.32 | 3.77 ± 0.22 | 3.87 ± 0.19 | 3.6 ± 0.22 | NS |
| 18:2 ω-6 | 40.37 ± 2.02 | 41.15 ± 1.71 | 39.99 ± 1.5 | 41.38 ± 1.44 | NS |
| 18:3 ω-6 | 0.11 ± 0.01 | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.09 ± 0.01 a | 0.022 |
| 20:0 | 0.07 ± 0.02 | 0.08 ± 0.02 | 0.10 ± 0.03 | 0.08 ± 0.01 | NS |
| 18:3 ω-3 | 1.99 ± 0.32 | 2.01 ± 0.27 | 1.93 ± 0.30 | 1.98 ± 0.18 | NS |
| 20:1 ω-9 | 0.31 ± 0.08 | 0.29 ± 0.04 | 0.32 ± 0.07 | 0.29 ± 0.05 | NS |
| 20:1 ω-7 | 0.37 ± 0.11 | 0.36 ± 0.07 | 0.42 ± 0.12 | 0.36 ± 0.08 | NS |
| 20:2 ω-6 | 0.31 ± 0.09 | 0.28 ± 0.08 | 0.29 ± 0.09 | 0.32 ± 0.1 | NS |
| 20:3 ω-6 | 0.18 ± 0.05 | 0.14 ± 0.05 | 0.16 ± 0.05 | 0.19 ± 0.07 | NS |
| 20:4 ω-6 | 0.80 ± 0.21 | 0.69 ± 0.20 | 0.69 ± 0.29 | 0.67 ± 0.24 | NS |
| 20:5 ω-3 | 0.02 ± 0.03 | ND | 0.2 ± 0.04 a,b | 0.12 ± 0.03 a,b,c | <0.001 |
| 22:4 ω-6 | 0.24 ± 0.06 | 0.20 ± 0.05 | 0.18 ± 0.07 | 0.19 ± 0.07 | NS |
| 22:5 ω-3 | 0.20 ± 0.08 | 0.16 ± 0.06 | 0.33 ± 0.08 a,b | 0.34 ± 0.09 a,b | <0.001 |
| 22:6 ω-3 | 0.19 ± 0.07 | 0.17 ± 0.06 | 0.5 ± 0.12 a,b | 1.18 ± 0.25 a,b,c | <0.001 |
| SAT | 23.38 ± 2.09 | 23.26 ± 2.11 | 23.49 ± 2.18 | 22.5 ± 2 | NS |
| MUFA | 32.22 ± 1.18 | 31.85 ± 0.73 | 32.13 ± 1.42 | 31.04 ± 1.41 | NS |
| PUFA | 44.40 ± 2.44 | 44.89 ± 2.30 | 44.37 ± 1.88 | 46.46 ± 1.65 | NS |
| ω-3 | 2.39 ± 0.48 | 2.33 ± 0.37 | 2.96 ± 0.50 b | 3.63 ± 0.43 a,b,c | 0.003 |
| ω-6 | 42 ± 2.23 | 42.56 ± 1.98 | 41.42 ± 1.58 | 42.83 ± 1.58 | NS |
| ω-6/ω-3 ratio | 19.47 ± 3.43 | 19.94 ± 2.30 | 15.61 ± 2.28 a,b | 13.20 ± 1.66 a,b | 0.006 |
| SCD-16 = [16:1 ω-7/16:0] | 0.20 ± 0.07 | 0.18 ± 0.05 | 0.21 ± 0.10 | 0.18 ± 0.07 | NS |
| SCD-18 = [18:1 ω-9/18:0] | 9.98 ± 1.24 | 9.73 ± 0.87 | 9.80 ± 1.84 | 9.51 ± 1.46 | NS |
| Δ5D = [20:4 ω-6/20:3 ω-6] | 4.66 ± 0.79 | 4.99 ± 0.57 | 4.28 ± 0.51 | 3.73 ± 0.60 a,b | <0.001 |
| Δ6D = [20:3 ω-6/18:2 ω-6] | 0.004 ± 0.001 | 0.003 ± 0.001 | 0.004 ± 0.001 | 0.004 ± 0.001 | NS |
| Δ5/6D = [20:5 ω-3/18:3 ω-3] | 0.008 ± 0.013 | 0.000 ± 0.000 | 0.103 ± 0.026 a,b | 0.062 ± 0.014 a,b,c | <0.001 |
| FA | Coconut | Soybean | EPA/DHA 1:1 | 80% DHA | p-Value |
|---|---|---|---|---|---|
| 14:0 | 0.34 ± 0.12 | 0.24 ± 0.03 | 0.24 ± 0.06 | 0.23 ± 0.07 | NS |
| 15:0 | 0.22 ± 0.06 | 0.20 ± 0.02 | 0.19 ± 0.02 | 0.20 ± 0.02 | NS |
| 16:0 | 22.29 ± 1.10 | 21.58 ± 0.78 | 22.66 ± 0.58 | 23.07 ± 1.18 b | 0.009 |
| 16:1 ω-7 | 1.19 ± 0.34 | 0.92 ± 0.16 | 0.87 ± 0.16 | 0.86 ± 0.29 a | 0.042 |
| 17:0 | 0.39 ± 0.05 | 0.40 ± 0.06 | 0.38 ± 0.03 | 0.37 ± 0.04 | NS |
| 18:0 | 13.08 ± 1.28 | 14.26 ± 1.19 | 13.79 ± 0.81 | 13.72 ± 1.54 | NS |
| 18:1 ω-9 | 5.74 ± 2.26 | 5.61 ± 0.56 | 5.07 ± 0.48 | 4.81 ± 1.31 | NS |
| 18:1 ω-7 | 3.82 ± 0.45 | 3.52 ± 0.25 | 3.19 ± 0.23 a | 2.91 ± 0.38 a,b | <0.001 |
| 18:2 ω-6 | 20.59 ± 1.17 | 20.16 ± 1.29 | 20.94 ± 1.02 | 22.37 ± 1.15 b | 0.014 |
| 18:3 ω-6 | 0.24 ± 0.05 | 0.25 ± 0.03 | 0.15 ± 0.03 a,b | 0.12 ± 0.02 a,b | <0.001 |
| 20:0 | 0.04 ± 0.04 | 0.08 ± 0.01 | 0.00 ± 0.00 a,b | 0.00 ± 0.00 a,b | <0.001 |
| 18:3 ω-3 | 0.38 ± 0.06 | 0.35 ± 0.05 | 0.31 ± 0.06 | 0.33 ± 0.11 | NS |
| 20:1 ω-9 | 0.12 ± 0.03 | 0.12 ± 0.02 | 0.10 ± 0.01 | 0.10 ± 0.04 | NS |
| 20:1 ω-7 | 0.25 ± 0.04 | 0.26 ± 0.04 | 0.26 ± 0.07 | 0.23 ± 0.04 | NS |
| 20:2 ω-6 | 0.35 ± 0.07 | 0.36 ± 0.07 | 0.29 ± 0.04 | 0.30 ± 0.03 | 0.021 |
| 20:3 ω-6 | 0.42 ± 0.09 | 0.41 ± 0.12 | 0.54 ± 0.08 b | 0.66 ± 0.09 a,b | <0.001 |
| 20:4 ω-6 | 22.01 ± 1.60 | 22.71 ± 0.94 | 19.26 ± 1.32 a,b | 16.15 ± 2.31 a,b | <0.001 |
| 22:1 ω-9 | 0.07 ± 0.04 | 0.1 ± 0.02 | 0.00 ± 0.00 a,b | 0.12 ± 0.02 c | 0.025 |
| 23:0 | 0.14 ± 0.02 | 0.14 ± 0.02 | 0.13 ± 0.03 | 0.13 ± 0.02 | NS |
| 20:5 ω-3 | 0.23 ± 0.08 | 0.21 ± 0.03 | 0.91 ± 0.2 a,b | 1.09 ± 0.23 a,b | <0.001 |
| 24:0 | 0.43 ± 0.04 | 0.42 ± 0.06 | 0.46 ± 0.03 | 0.45 ± 0.06 | NS |
| 22:4 ω-6 | 0.61 ± 0.06 | 0.58 ± 0.03 | 0.30 ± 0.02a,b | 0.21 ± 0.04 a,b,c | <0.001 |
| 24:1 ω-9 | 0.19 ± 0.17 | 0.13 ± 0.02 | 0.15 ± 0.01 | 0.15 ± 0.02 | NS |
| 22:5 ω-6 | 0.48 ± 0.15 | 0.41 ± 0.08 | 0.17 ± 0.02 a,b | 0.21 ± 0.06 a,b | 0.020 |
| 22:5 ω-3 | 1.23 ± 0.14 | 1.25 ± 0.14 | 1.53 ± 0.19 a,b | 1.02 ± 0.13 b,c | <0.001 |
| 22:6 ω-3 | 5.14 ± 0.70 | 5.35 ± 0.29 | 8.10 ± 0.51 a,b | 10.19 ± 1.35 a,b,c | <0.001 |
| SAT | 36.93 ± 1.25 | 37.33 ± 1.06 | 37.84 ± 0.63 | 38.17 ± 1.04 a | 0.032 |
| MUFA | 11.40 ± 2.60 | 10.65 ± 0.83 | 9.65 ± 0.75 | 9.18 ± 1.46 a,b | 0.012 |
| PUFA | 51.67 ± 1.40 | 52.02 ± 1.08 | 52.51 ± 0.58 | 52.64 ± 0.80 | NS |
| ω-3 | 6.97 ± 0.69 | 7.16 ± 0.32 | 10.86 ± 0.72 a,b | 12.63 ± 1.57 a,b,c | <0.001 |
| ω-6 | 44.69 ± 0.88 | 44.87 ± 1.11 | 41.65 ± 0.87 a,b | 40.01 ± 1.78 a,b,c | <0.001 |
| ω-6/ω-3 ratio | 6.42 ± 0.53 | 6.06 ± 0.29 | 3.79 ± 0.29 a,b | 3.07 ± 0.46 a,b,c | <0.001 |
| SCD-16 = [16:1 ω-7/16:0] | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 a | 0.012 |
| SCD-18 = [18:1 ω-9/18:0] | 0.50 ± 0.18 | 0.40 ± 0.07 | 0.37 ± 0.06 | 0.39 ± 0.13 | NS |
| Δ5D = [20:4 ω-6/20:3 ω-6] | 57.39 ± 13.78 | 59.20 ± 13.66 | 35.12 ± 6.98 a,b | 25.50 ± 5.64 a,b | 0.035 |
| Δ6D = [20:3 ω-6/18:2 ω-6] | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.00 | NS |
| Δ5/6D = [20:5 ω-3/18:3 ω-3] | 0.54 ± 0.17 | 0.63 ± 0.12 | 2.98 ± 0.81 a,b | 3.63 ± 0.83 a,b | <0.001 |
| Coconut | Soybean | EPA/DHA 1:1 | 80% DHA | p-Value | |
|---|---|---|---|---|---|
| Plasma | |||||
| ORAC (µmol TE/mL) | 14.6 ± 2.7 | 12.5 ± 2.8 | 16.3 ± 2.6 b | 17.0 ± 1.9 a,b | 0.004 † |
| FRAP (mmol TE/L) | 0.12 ± 0.02 | 0.12 ± 0.04 | 0.11 ± 0.02 | 0.11 ± 0.03 | NS * |
| GSH (nmol/mL) | 12.1 ± 3.0 | 10.8 ± 4.1 | 7.6 ± 3.0 | 9.7 ± 4.1 | NS * |
| GSSG (nmol/mL) | 37.2 ± 4.1 | 37.2 ± 4.3 | 40.5 ± 4.6 | 38.9 ± 3.0 | NS * |
| GSSG/GSH ratio | 3.24 ± 0.80 | 3.80 ± 1.08 | 5.25 ± 1.33 a,b | 4.72 ± 1.66 | 0.012 † |
| Albumin carbonylation index | 2.37 ± 0.29 | 2.14 ± 0.33 | 2.66 ± 0.65 | 2.93 ± 0.46 a,b | 0.002 * |
| Oxidized LDL (μg/mL) | 0.16 ± 0.03 | 0.15 ± 0.03 | 0.16 ± 0.03 | 0.18 ± 0.03 | NS † |
| TBARS (nmol MDA Eq/mL) | 0.77 ± 0.18 | 0.99 ± 0.11 | 0.69 ± 0.20 | 1.04 ± 0.40 c | 0.011 * |
| Erythrocytes | |||||
| SOD (U/g Hb) | 2631 ± 727 | 2731 ± 971 | 2415 ± 489 | 2470 ± 389 | NS * |
| CAT (mmol/g Hb) | 47.1 ± 11.5 | 43.0 ± 14.2 | 43.9 ± 7.2 | 52.5 ± 6.9 | NS * |
| GPx (U/g Hb) | 132 ± 31 | 116 ± 36 | 113 ± 22 | 164 ± 45 b,c | 0.011 * |
| GR (U/g Hb) | 0.27 ± 0.12 | 0.26 ± 0.09 | 0.24 ± 0.07 | 0.21 ± 0.07 | NS † |
| GSH (µmol/g Hb) | 1.85 ± 0.70 | 1.43 ± 0.67 | 1.50 ± 0.41 | 1.74 ± 0.63 | NS * |
| GSSG (µmol/g Hb) | 1.18 ± 0.40 | 1.06 ± 0.47 | 1.11 ± 0.21 | 1.20 ± 0.33 | NS * |
| GSSG/GSH ratio | 0.66 ± 0.19 | 0.78 ± 0.24 | 0.82 ± 0.34 | 0.74 ± 0.22 | NS * |
| TBARS (nmol MDA Eq/g Hb) | 0.24 ± 0.11 | 0.32 ± 0.27 | 0.52 ± 0.28 | 0.73 ± 0.19 a,b | <0.001 * |
| Coconut | Soybean | EPA/DHA 1:1 | 80% DHA | p-Value | |
|---|---|---|---|---|---|
| Perigonadal adipose tissue | |||||
| SOD (U/g tissue) | 226 ± 45 | 237 ± 44 | 236 ± 61 | 225 ± 36 | NS * |
| CAT (mmol/g tissue) | 0.11 ± 0.02 | 0.09 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.03 | NS † |
| GPx (U/g tissue) | 0.54 ± 0.20 | 0.58 ± 0.18 | 0.50 ± 0.16 | 0.41 ± 0.20 | NS * |
| GR (U/g tissue) | 0.40 ± 0.07 | 0.46 ± 0.10 | 0.39 ± 0.11 | 0.35 ± 0.08 b | 0.047 † |
| GSH (nmol/g tissue) | 0.93 ± 0.23 | 1.05 ± 0.15 | 1.03 ± 0.19 | 0.91 ± 0.14 | NS * |
| GSSG (nmol/g tissue) | 4.43 ± 0.58 | 4.56 ± 0.93 | 4.05 ± 0.52 | 4.53 ± 1.05 | NS * |
| GSSG/GSH ratio | 5.10 ± 1.78 | 4.46 ± 1.26 | 4.03 ± 0.67 | 5.11 ± 1.44 | NS † |
| Conjugated dienes (mmol hydroperoxides/kg lipid) | 9.87 ± 1.43 | 8.83 ± 2.03 | 10.33 ± 2.75 | 10.83 ± 2.86 | NS * |
| TBARS (nmol MDA Eq/g tissue) | 2.41 ± 2.16 | 3.18 ± 2.51 | 4.95 ± 2.47 a | 4.99 ± 2.64 a | 0.046 † |
| Liver | |||||
| SOD (U/g tissue) | 6411 ± 631 | 6879 ± 1046 | 6577 ± 872 | 6083 ± 829 | NS * |
| CAT (mmol/g tissue) | 10.8 ± 1.3 | 11.1 ± 0.5 | 11.4 ± 1.2 | 11.8 ± 0.7 | NS * |
| GPx (U/g tissue) | 29.5 ± 5.2 | 25.1 ± 2.9 | 26.8 ± 2.9 | 26.0 ± 3.2 | NS * |
| GR (U/g tissue) | 6.80 ± 1.19 | 7.62 ± 2.34 | 7.78 ± 2.00 | 7.53 ± 1.73 | NS * |
| GST (U/g tissue) | 113 ± 11 | 115 ± 22 | 104 ± 21 | 103 ± 16 | NS * |
| GSH (µmol/g tissue) | 2.22 ± 0.55 | 2.22 ± 0.59 | 2.09 ± 0.52 | 2.08 ± 0.60 | NS † |
| GSSG (µmol/g tissue) | 1.47 ± 0.20 | 1.38 ± 0.23 | 1.30 ± 0.16 | 1.23 ± 0.11 | 0.048 * |
| GSSG/GSH ratio | 0.71 ± 0.25 | 0.65 ± 0.20 | 0.66 ± 0.21 | 0.63 ± 0.16 | NS * |
| XO (mU/g tissue) | 96 ± 26 | 121 ± 36 | 89 ± 15 | 88 ± 20 | 0.022 * |
| Protein carbonylation index | 2.73 ± 0.68 | 2.11 ± 0.57 | 2.43 ± 0.67 | 3.05 ± 0.35 b | 0.007 * |
| Conjugated dienes (mmol hydroperoxides/kg lipid) | 20.7 ± 2.3 | 20.6 ± 1.3 | 21.9 ± 1.5 | 21.0 ± 2.8 | NS * |
| TBARS (nmol MDA Eq/g tissue) | 217 ± 72 | 229 ± 115 | 278 ± 90 | 320 ± 94 | NS * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miralles-Pérez, B.; Méndez, L.; Nogués, M.R.; Sánchez-Martos, V.; Fortuño-Mar, À.; Ramos-Romero, S.; Hereu, M.; Medina, I.; Romeu, M. Effects of a Fish Oil Rich in Docosahexaenoic Acid on Cardiometabolic Risk Factors and Oxidative Stress in Healthy Rats. Mar. Drugs 2021, 19, 555. https://doi.org/10.3390/md19100555
Miralles-Pérez B, Méndez L, Nogués MR, Sánchez-Martos V, Fortuño-Mar À, Ramos-Romero S, Hereu M, Medina I, Romeu M. Effects of a Fish Oil Rich in Docosahexaenoic Acid on Cardiometabolic Risk Factors and Oxidative Stress in Healthy Rats. Marine Drugs. 2021; 19(10):555. https://doi.org/10.3390/md19100555
Chicago/Turabian StyleMiralles-Pérez, Bernat, Lucía Méndez, Maria Rosa Nogués, Vanessa Sánchez-Martos, Àngels Fortuño-Mar, Sara Ramos-Romero, Mercè Hereu, Isabel Medina, and Marta Romeu. 2021. "Effects of a Fish Oil Rich in Docosahexaenoic Acid on Cardiometabolic Risk Factors and Oxidative Stress in Healthy Rats" Marine Drugs 19, no. 10: 555. https://doi.org/10.3390/md19100555
APA StyleMiralles-Pérez, B., Méndez, L., Nogués, M. R., Sánchez-Martos, V., Fortuño-Mar, À., Ramos-Romero, S., Hereu, M., Medina, I., & Romeu, M. (2021). Effects of a Fish Oil Rich in Docosahexaenoic Acid on Cardiometabolic Risk Factors and Oxidative Stress in Healthy Rats. Marine Drugs, 19(10), 555. https://doi.org/10.3390/md19100555