Cytoprotective Peptides from Blue Mussel Protein Hydrolysates: Identification and Mechanism Investigation in Human Umbilical Vein Endothelial Cells Injury
Abstract
:1. Introduction
2. Results
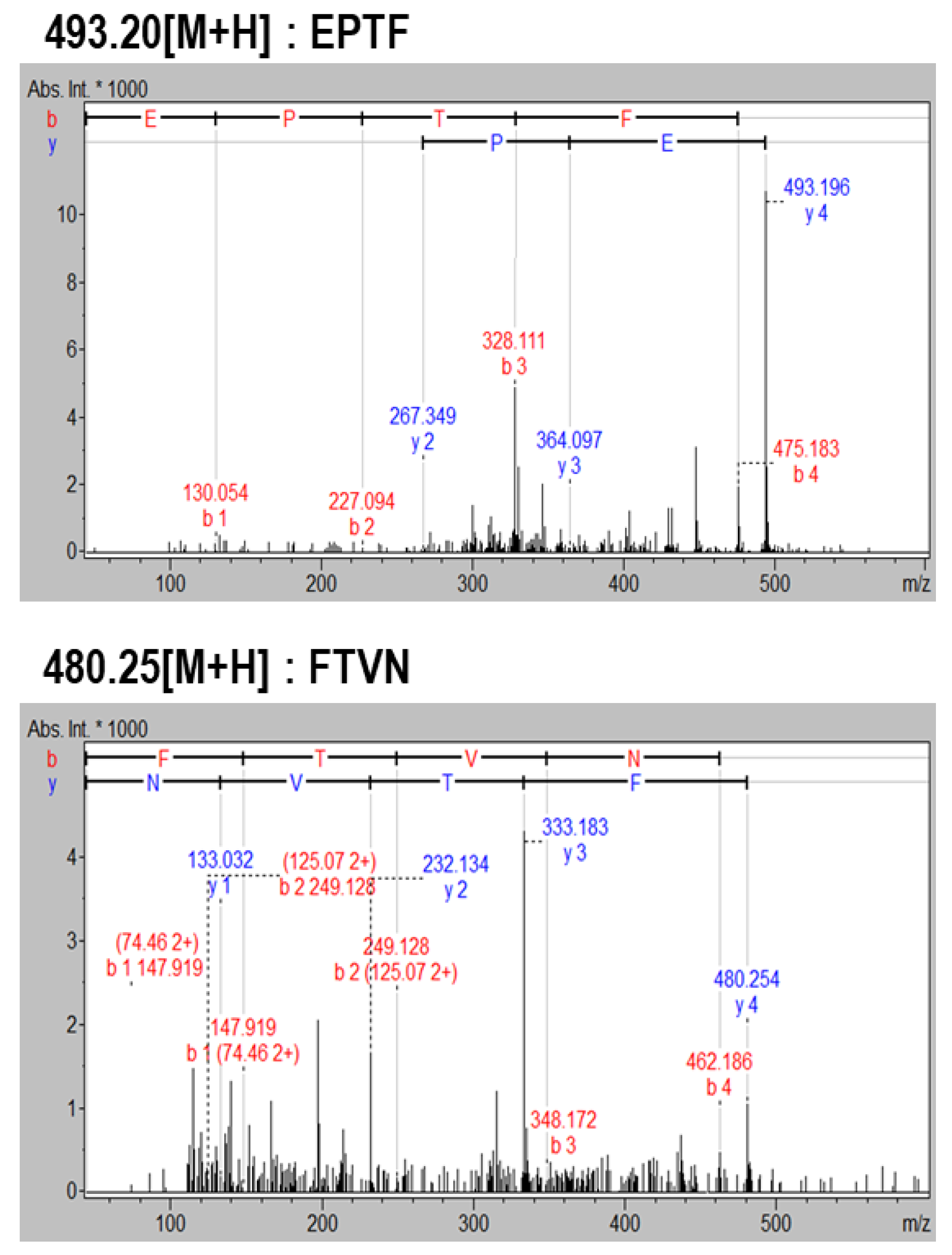
2.1. Purification and Identification of Cytoprotective Peptides
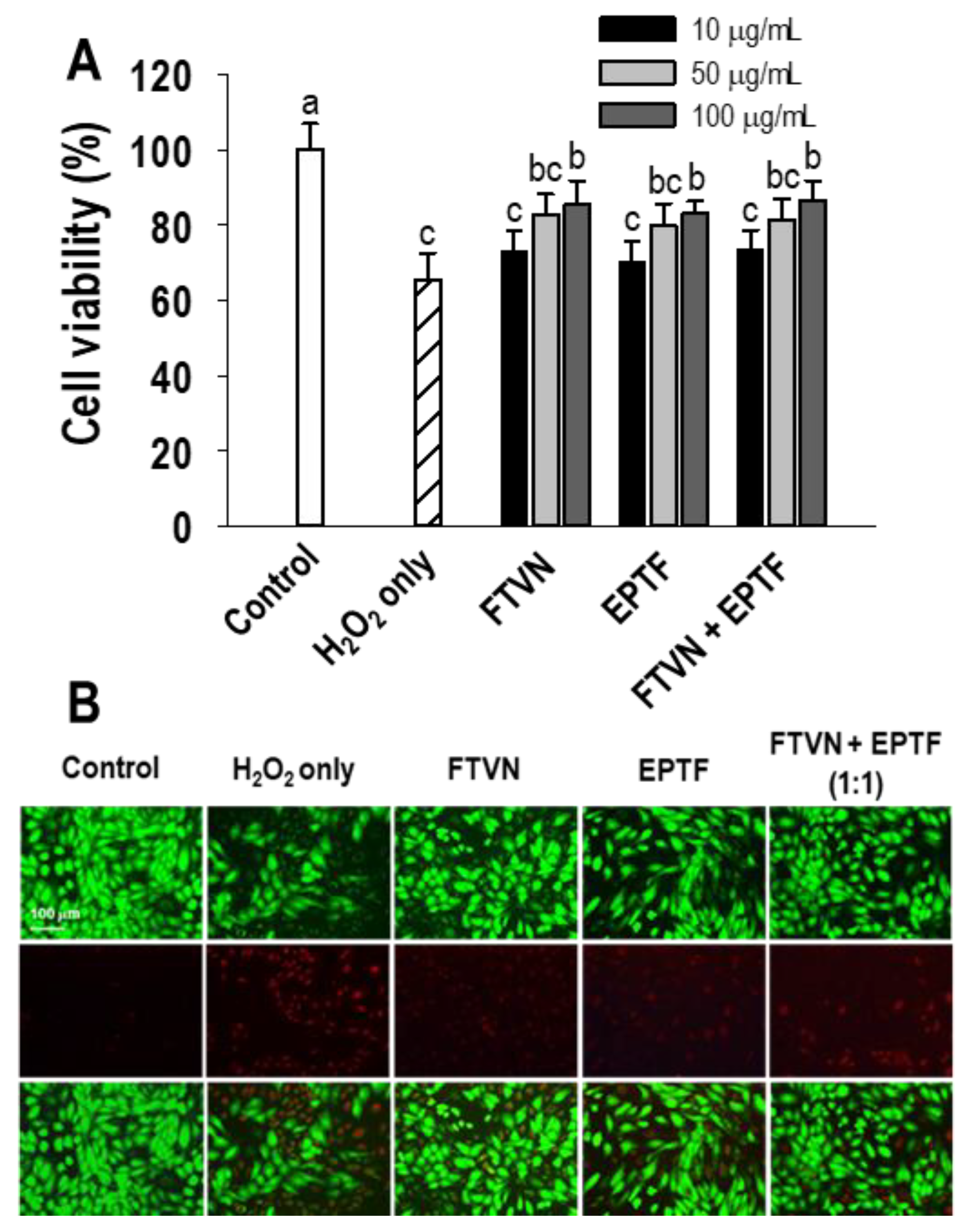
2.2. Cytoprotective Activity in H2O2-Mediated HUVECs Injury
2.3. Inhibition of Intracellular ROS Generation
2.4. HO-1/Nrf2 Pathway Activation by Cytoprotective Peptides
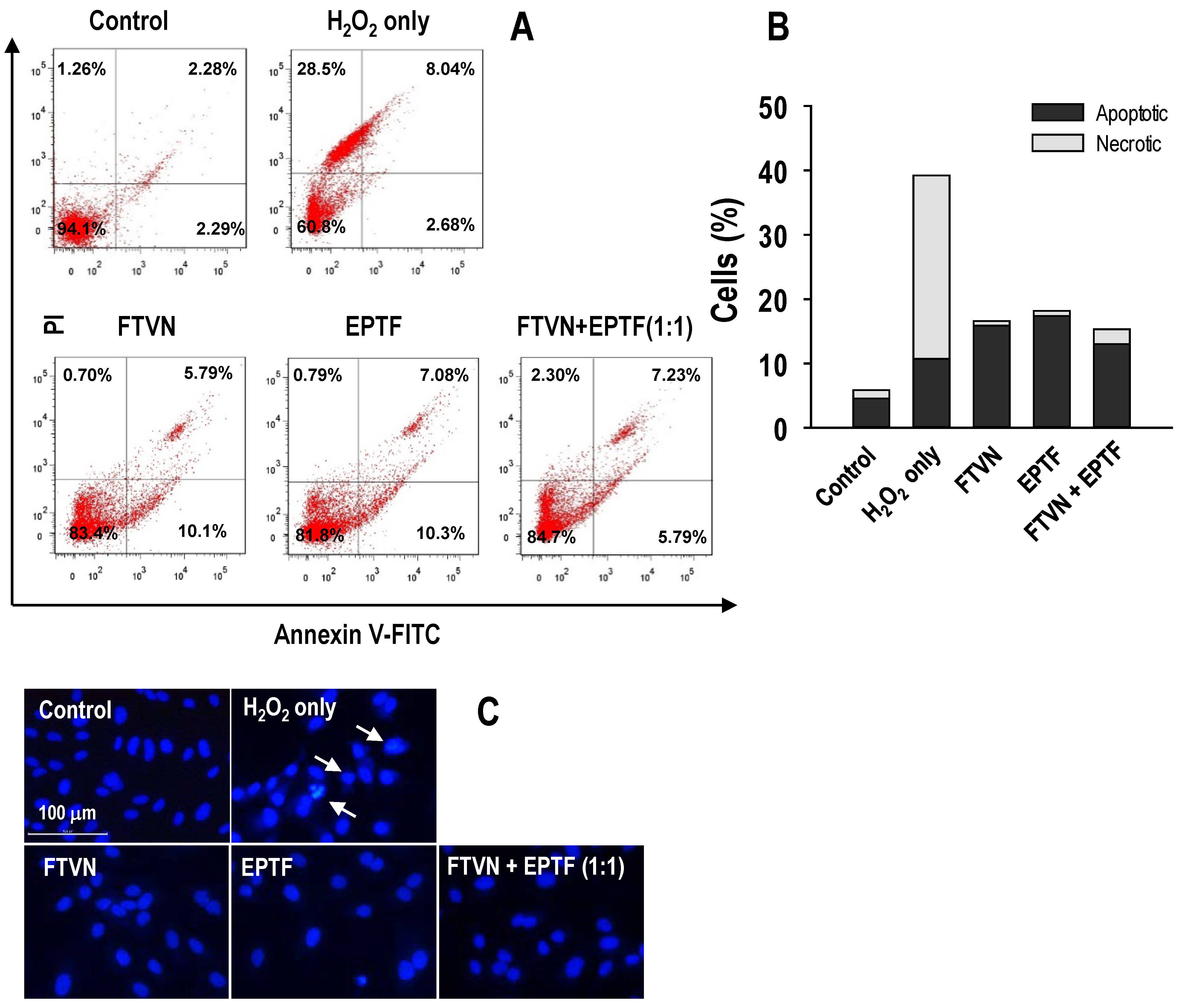
2.5. Anti-Apoptotic Activity against H2O2-Induced HUVECs Damage
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Blue Mussel Protein Hydrolysate by α-Chymotrypsin-Assisted Hydrolysis
4.3. Purification and Identification of Cytoprotective Peptides
4.4. HUVECs Culture and Treatment
4.5. Cell Viability Assessment
4.6. Determination of Intracellular ROS
4.7. Nrf2 Nuclear Translocation Assessment
4.8. Annexin V-FITC/PI for Apoptotic Cells
4.9. Hoechst 33342 Staining Analysis
4.10. Western Blot Analysis
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oh, Y.; Ahn, C.-B.; Nam, K.-H.; Kim, Y.-K.; Yoon, N.Y.; Je, J.-Y. Amino acid composition, antioxidant, and cytoprotective effect of blue mussel (Mytilus edulis) hydrolysate through the inhibition of caspase-3 activation in oxidative stress-mediated endothelial cell injury. Mar. Drugs 2019, 17, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Toan, S.; Zhou, H. New insights into the role of mitochondria in cardiac microvascular ischemia/reperfusion injury. Angiogenesis 2020, 23, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Paone, S.; Baxter, A.A.; Hulett, M.D.; Poon, I.K. Endothelial cell apoptosis and the role of endothelial cell-derived extracellular vesicles in the progression of atherosclerosis. Cell. Mol. Life Sci. 2019, 76, 1093–1106. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jun, H.-S. Effects of glucagon-like peptide-1 on oxidative stress and Nrf2 signaling. Int. J. Mol. Sci. 2018, 19, 26. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Zhang, J.; Wang, Z.; Yao, Y.; Atungulu, G.G.; Ju, X.; Wang, L. Absorption and metabolism of peptide WDHHAPQLR derived from rapeseed protein and inhibition of HUVEC apoptosis under oxidative stress. J. Agric. Food Chem. 2018, 66, 5178–5189. [Google Scholar] [CrossRef] [PubMed]
- Togliatto, G.; Lombardo, G.; Brizzi, M.F. The future challenge of reactive oxygen species (ROS) in hypertension: From bench to bed side. Int. J. Mol. Sci. 2017, 18, 1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Dong, Y.; Liu, J.; Qian, L.; Wang, T.; Gao, X.; Wang, K.; Zhou, L. Effects of REDOX in regulating and treatment of metabolic and inflammatory cardiovascular diseases. Oxidative Med. Cell. Longev. 2020, 2020, 5860356. [Google Scholar] [CrossRef]
- Jin, J.-E.; Ahn, C.-B.; Je, J.-Y. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed ark shell (Scapharca subcrenata). Process Biochem. 2018, 72, 170–176. [Google Scholar] [CrossRef]
- Hyung, J.-H.; Ahn, C.-B.; Kim, B.I.; Kim, K.; Je, J.-Y. Involvement of Nrf2-mediated heme oxygenase-1 expression in anti-inflammatory action of chitosan oligosaccharides through MAPK activation in murine macrophages. Eur. J. Pharmacol. 2016, 793, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Ahn, C.-B.; Je, J.-Y. Anti-inflammatory action of high molecular weight Mytilus edulis hydrolysates fraction in LPS-induced RAW264. 7 macrophage via NF-κB and MAPK pathways. Food Chem. 2016, 202, 9–14. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, S.Y.; Hwang, J.-Y.; Ahn, C.-B. Amino acid composition and in vitro antioxidant and cytoprotective activity of abalone viscera hydrolysate. J. Funct. Foods 2015, 16, 94–103. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Cho, Y.-S.; Je, J.-Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Shim, K.-B.; Ahn, C.-B.; Kim, S.S.; Je, J.-Y. Sea squirt (Halocynthia roretzi) hydrolysates induce apoptosis in human colon cancer HT-29 cells through activation of reactive oxygen species. Nutr. Cancer 2019, 71, 118–127. [Google Scholar] [CrossRef]
- Je, J.-Y.; Qian, Z.-J.; Lee, S.-H.; Byun, H.-G.; Kim, S.-K. Purification and antioxidant properties of bigeye tuna (Thunnus obesus) dark muscle peptide on free radical-mediated oxidative systems. J. Med. Food 2008, 11, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Park, S.Y.; Han, E.J.; Kim, H.-S.; Kang, D.-S.; Je, J.-Y.; Ahn, C.-B.; Ahn, G. Isolation of an antioxidant peptide from krill protein hydrolysates as a novel agent with potential hepatoprotective effects. J. Funct. Foods 2020, 67, 103889. [Google Scholar] [CrossRef]
- Kim, S.S.; Ahn, C.-B.; Moon, S.W.; Je, J.-Y. Purification and antioxidant activities of peptides from sea squirt (Halocynthia roretzi) protein hydrolysates using pepsin hydrolysis. Food Biosci. 2018, 25, 128–133. [Google Scholar] [CrossRef]
- Park, S.Y.; Ahn, C.B.; Je, J.Y. Antioxidant and anti-inflammatory activities of protein hydrolysates from Mytilus edulis and ultrafiltration membrane fractions. J. Food Biochem. 2014, 38, 460–468. [Google Scholar] [CrossRef]
- Oh, Y.; Ahn, C.-B.; Je, J.-Y. Low molecular weight blue mussel hydrolysates inhibit adipogenesis in mouse mesenchymal stem cells through upregulating HO-1/Nrf2 pathway. Food Res. Int. 2020, 136, 109603. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.-S.; Ahn, C.-B.; Je, J.-Y. Partial purification and identification of three antioxidant peptides with hepatoprotective effects from blue mussel (Mytilus edulis) hydrolysate by peptic hydrolysis. J. Funct. Foods 2016, 20, 88–95. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Byun, H.-G.; Jung, W.-K.; Kim, S.-K. Angiotensin I converting enzyme (ACE) inhibitory peptide derived from the sauce of fermented blue mussel, Mytilus edulis. Bioresour. Technol. 2005, 96, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Magtaan, J.K.; Fitzpatrick, B.; Murphy, R. Elucidating the Biological Activity of Fish-Derived Collagen and Gelatine Hydrolysates using Animal Cell Culture-A Review. Curr. Pharm. Des. 2021, 27, 1365–1381. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Ahn, C.-B.; Yoon, N.Y.; Nam, K.H.; Kim, Y.-K.; Je, J.-Y. Protective effect of enzymatic hydrolysates from seahorse (Hippocampus abdominalis) against H2O2-mediated human umbilical vein endothelial cell injury. Biomed. Pharmacother. 2018, 108, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.-S. Regulation of Nrf2-mediated phase II detoxification and anti-oxidant genes. Biomol. Ther. 2012, 20, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, Y.; Ahn, C.-B.; Je, J.-Y. Cytoprotective Role of Edible Seahorse (Hippocampus abdominalis)-Derived Peptides in H2O2-Induced Oxidative Stress in Human Umbilical Vein Endothelial Cells. Mar. Drugs 2021, 19, 86. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Liu, X.-F.; Zheng, P.-S. Grape seed proanthocyanidins (GSPs) inhibit the growth of cervical cancer by inducing apoptosis mediated by the mitochondrial pathway. PLoS ONE 2014, 9, e107045. [Google Scholar] [CrossRef] [Green Version]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, M.; Tu, M.; Wang, Z.; Mao, F.; Chen, H.; Qin, L.; Du, M. Identification and antithrombotic activity of peptides from blue mussel (Mytilus edulis) protein. Int. J. Mol. Sci. 2018, 19, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, Y.; Ahn, C.-B.; Je, J.-Y. Blue Mussel-Derived Peptides PIISVYWK and FSVVPSPK Trigger Wnt/β-Catenin Signaling-Mediated Osteogenesis in Human Bone Marrow Mesenchymal Stem Cells. Mar. Drugs 2020, 18, 510. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Ahn, C.-B.; Cho, W.H.; Yoon, N.Y.; Je, J.-Y. Anti-Osteoporotic Effects of Antioxidant Peptides PIISVYWK and FSVVPSPK from Mytilus edulis on Ovariectomized Mice. Antioxidants 2020, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, L.; Chi, C.-F.; Ma, J.-H.; Luo, H.-Y.; Xu, Y.-f. Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chem. 2013, 138, 1713–1719. [Google Scholar] [CrossRef]
- Shah, D.; Das, P.; Alam, M.A.; Mahajan, N.; Romero, F.; Shahid, M.; Singh, H.; Bhandari, V. MicroRNA-34a promotes endothelial dysfunction and mitochondrial-mediated apoptosis in murine models of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2019, 60, 465–477. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, R.M.; Rodrigues, D.; Pereira, C.A.; Silva, J.F.; Alves, J.V.; Lobato, N.S.; Tostes, R.C. Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front. Pharmacol. 2019, 10, 382. [Google Scholar] [CrossRef] [Green Version]
- Ryter, S.W.; Choi, A.M. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. 2016, 167, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of coptisine against oxidative stress-induced DNA damage and apoptosis in HaCaT keratinocytes. Gen. Physiol. Biophys. 2019, 38, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Lu, Y.; Wang, H.; Feng, Y.; Jiang, S.; Gao, X.-H.; Qi, R.; Wu, Y.; Chen, H.-D. Paeoniflorin resists H2O2-induced oxidative stress in melanocytes by JNK/Nrf2/HO-1 pathway. Front. Pharmacol. 2020, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Peker, E.; Amponsah, P.S.; Hoehne, M.N.; Riemer, T.; Mai, M.; Bienert, G.P.; Deponte, M.; Morgan, B.; Riemer, J. Hyperoxidation of mitochondrial peroxiredoxin limits H2O2-induced cell death in yeast. Embo J. 2019, 38, e101552. [Google Scholar] [CrossRef] [PubMed]
- Yaoxian, W.; Hui, Y.; Yunyan, Z.; Yanqin, L.; Xin, G.; Xiaoke, W. Emodin induces apoptosis of human cervical cancer hela cells via intrinsic mitochondrial and extrinsic death receptor pathway. Cancer Cell Int. 2013, 13, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The role of hydrogen peroxide in redox-dependent signaling: Homeostatic and pathological responses in mammalian cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (Bba)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Lin, Q.; Huang, P.; Wang, Y.; Li, J.; Zhang, L.; Cao, J. Rice bioactive peptide binding with TLR4 to overcome H2O2-induced injury in human umbilical vein endothelial cells through NF-κB signaling. J. Agric. Food Chem. 2018, 66, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, S.; Zeng, Y.; He, K.; Luo, Y.; Yu, F. Antioxidant peptides from Mytilus Coruscus on H2O2-induced human umbilical vein endothelial cell stress. Food Biosci. 2020, 38, 100762. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suryaningtyas, I.T.; Ahn, C.-B.; Je, J.-Y. Cytoprotective Peptides from Blue Mussel Protein Hydrolysates: Identification and Mechanism Investigation in Human Umbilical Vein Endothelial Cells Injury. Mar. Drugs 2021, 19, 609. https://doi.org/10.3390/md19110609
Suryaningtyas IT, Ahn C-B, Je J-Y. Cytoprotective Peptides from Blue Mussel Protein Hydrolysates: Identification and Mechanism Investigation in Human Umbilical Vein Endothelial Cells Injury. Marine Drugs. 2021; 19(11):609. https://doi.org/10.3390/md19110609
Chicago/Turabian StyleSuryaningtyas, Indyaswan Tegar, Chang-Bum Ahn, and Jae-Young Je. 2021. "Cytoprotective Peptides from Blue Mussel Protein Hydrolysates: Identification and Mechanism Investigation in Human Umbilical Vein Endothelial Cells Injury" Marine Drugs 19, no. 11: 609. https://doi.org/10.3390/md19110609
APA StyleSuryaningtyas, I. T., Ahn, C.-B., & Je, J.-Y. (2021). Cytoprotective Peptides from Blue Mussel Protein Hydrolysates: Identification and Mechanism Investigation in Human Umbilical Vein Endothelial Cells Injury. Marine Drugs, 19(11), 609. https://doi.org/10.3390/md19110609





