Bioactive Indolyl Diketopiperazines from the Marine Derived Endophytic Aspergillus versicolor DY180635
Abstract
1. Introduction
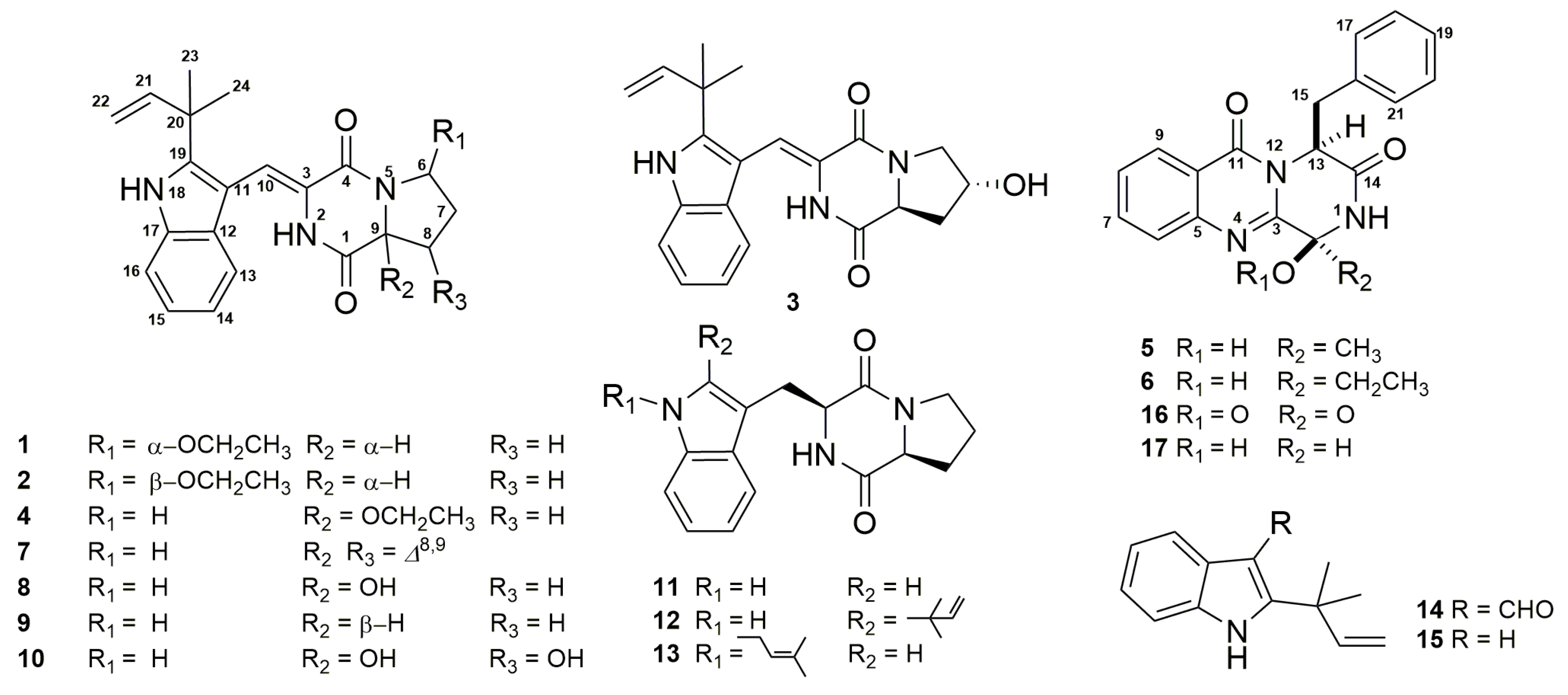
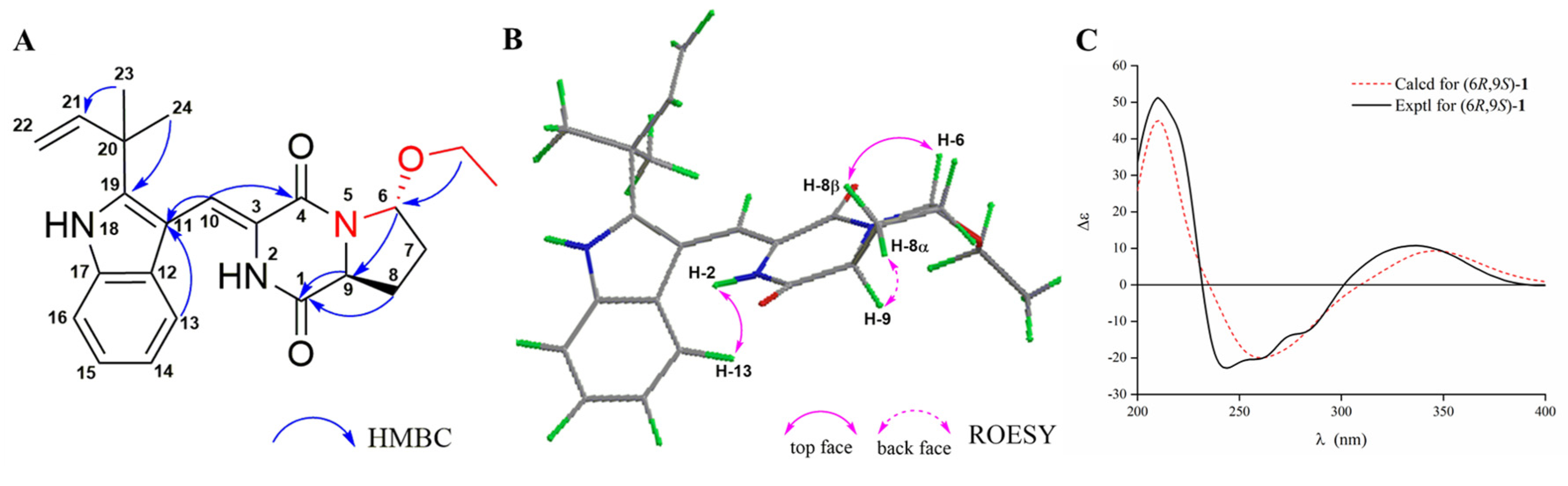
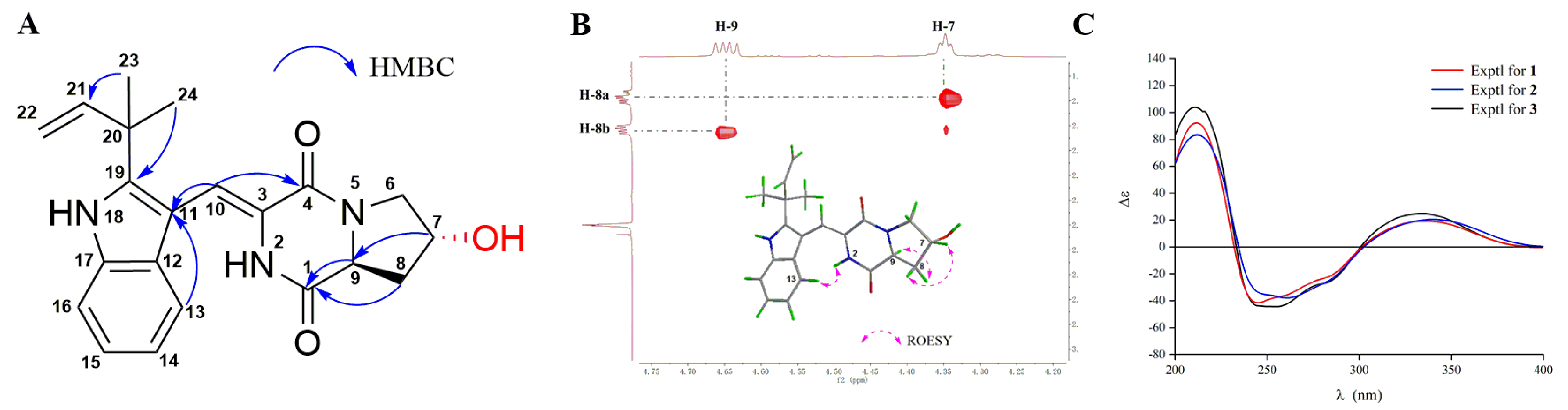
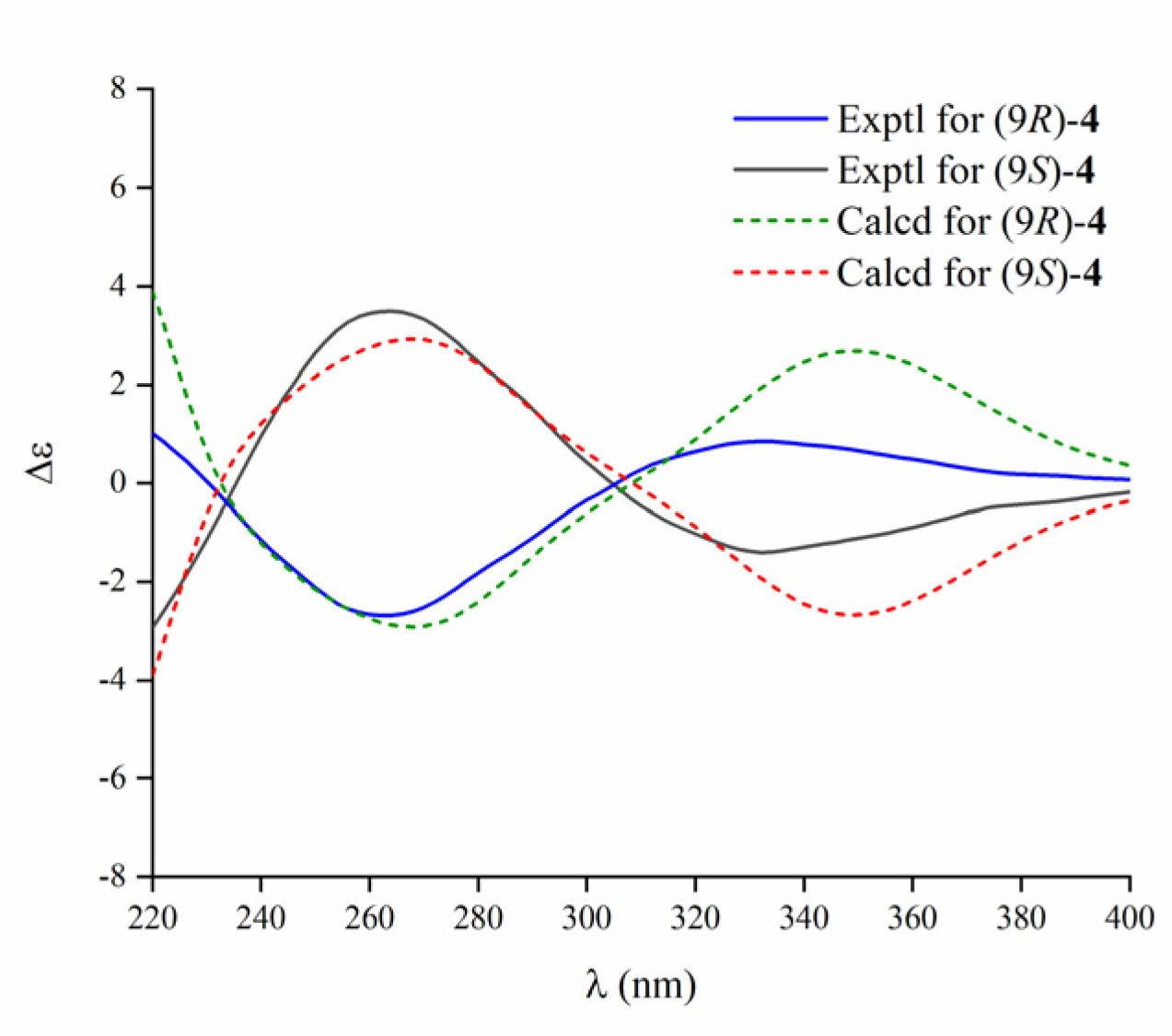
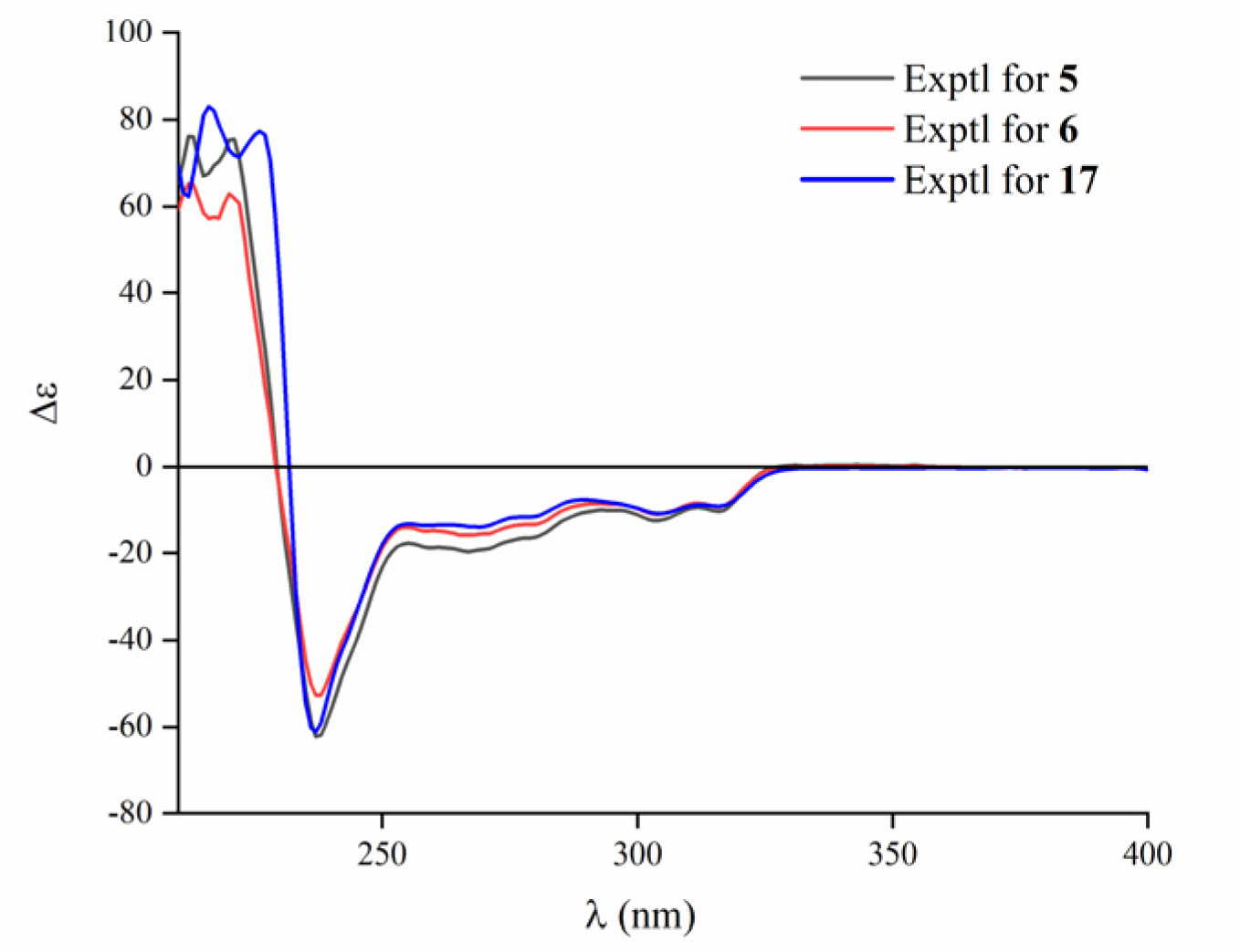
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.4. ECD Calculation
3.5. Virtual Screening Against COVID-19 Main Protease
3.5.1. Protein and Ligand Preparation
3.5.2. Virtual Screening
3.6. Cell Culture and Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Bugni, T.S.; Ireland, C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef]
- Ma, Y.M.; Liang, X.A.; Kong, Y.; Jia, B. Structural diversity and biological activities of indole diketopiperazine alkaloids from fungi. J. Agric. Food Chem. 2016, 64, 6659–6671. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Lu, Z.Y.; Tao, H.W.; Zhu, T.J.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Isoechinulin-type alkaloids, variecolorins A-L, from halotolerant Aspergillus variecolor. J. Nat. Prod. 2007, 70, 1558–1564. [Google Scholar] [CrossRef]
- Cai, S.X.; Sun, S.W.; Peng, J.X.; Kong, X.L.; Zhou, H.N.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Okaramines S-U, three new indole diketopiperazine alkaloids from Aspergillus taichungensis ZHN-7-07. Tetrahedron 2015, 71, 3715–3719. [Google Scholar] [CrossRef]
- Wang, F.Z.; Fang, Y.C.; Zhu, T.J.; Zhang, M.; Lin, A.Q.; Gu, Q.Q.; Zhu, W.M. Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron 2008, 64, 7986–7991. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kato, H.; Samizo, M.; Nojiri, Y.; Onuki, H.; Hirota, H.; Ohta, T. Notoamides F−K, prenylated indole alkaloids isolated from a marine-derived Aspergillus sp. J. Nat. Prod. 2008, 71, 2064–2067. [Google Scholar] [CrossRef]
- Kozlovsky, A.G.; Vinokurova, N.G.; Adanin, V.M. Diketopiperazine alkaloids from the fungus Penicillium piscarium westling. Appl. Biochem. Microbiol. 2000, 36, 271–275. [Google Scholar] [CrossRef]
- Ravikanth, V.; Niranjan Reddy, V.L.; Ramesh, P.; Prabhakar Rao, T.; Diwan, P.V.; Khar, A.; Venkateswarlu, Y. An immunosuppressive tryptophan-derived alkaloid from Lepidagathis cristata. Phytochemistry 2001, 58, 1263–1266. [Google Scholar] [CrossRef]
- Fujimoto, H.; Sumino, M.; Okuyama, E.; Ishibashi, M. Immunomodulatory constituents from an Ascomycete. J. Nat. Prod. 2004, 67, 98–102. [Google Scholar] [CrossRef]
- Kuramochi, K.; Ohnishi, K.; Fujieda, S.; Nakajima, M.; Saitoh, Y.; Watanabe, N.; Takeuchi, T.; Nakazaki, A.; Sugawara, F.; Arai, T.; et al. Synthesis and biological activities of neoechinulin A derivatives: New aspects of structure-activity relationships for neoechinulin A. Chem. Pharm. Bull. 2008, 56, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Sun, Z.H.; Liu, Z.; Chen, Y.C.; Liu, H.X.; Li, H.H.; Zhang, W.M. Dichotocejpins A-C: New diketopiperazines from a deep-sea-derived fungus Dichotomomyces cejpii FS110. Mar. Drugs 2016, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mohanlal, R.W.; Lloyd, K.; Huang, L. Plinabulin, a novel small molecule clinical stage IO agent with anti-cancer activity, to prevent chemo-induced neutropenia and immune related AEs. J. Clin. Oncol. 2018, 36, 126. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Lefranc, F.; Kijjoa, A.; Kiss, R. Can some marine-ferived fungal metabolites become actual anticancer agents? Mar. Drugs 2015, 13, 3950–3991. [Google Scholar] [CrossRef]
- Geiger, W.B.; Conn, J.E.; Waksman, S.A. Chaetomin, a new antibiotic substance produced by Chaetomium cochliodes: II. Isolation and Concentration. J. Bacteriol. 1944, 48, 531–536. [Google Scholar] [CrossRef]
- Tian, W.; Sun, C.; Zheng, M.; Harmer, J.R.; Yu, M.; Zhang, Y.; Peng, H.; Zhu, D.; Deng, Z.; Chen, S.L.; et al. Efficient biosynthesis of heterodimeric C3-aryl pyrroloindoline alkaloids. Nat. Commun. 2018, 9, 4428. [Google Scholar] [CrossRef]
- Ye, Y.; Du, L.; Zhang, X.W.; Newmister, S.A.; McCauley, M.; Alegre-Requena, J.V.; Zhang, W.; Mu, S.; Minami, A.; Fraley, A.E.; et al. Fungal-derived brevianamide assembly by a stereoselective semipinacolase. Nat. Catal. 2020. [Google Scholar] [CrossRef]
- Song, F.; Liu, X.; Guo, H.; Ren, B.; Chen, C.; Piggott, A.M.; Yu, K.; Gao, H.; Wang, Q.; Liu, M.; et al. Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Org. Lett. 2012, 14, 4770–4773. [Google Scholar] [CrossRef]
- James, E.D.; Knuckley, B.; Alqahtani, N.; Porwal, S.; Ban, J.; Karty, J.A.; Viswanathan, R.; Lane, A.L. Two distinct cyclodipeptide synthases from a marine Actinomycete catalyze biosynthesis of the same diketopiperazine natural product. ACS Synth. Biol. 2016, 5, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Haines, B.E.; Nelson, B.M.; Grandner, J.M.; Kim, J.; Houk, K.N.; Movassaghi, M.; Musaev, D.G. Mechanism of permanganate-promoted dihydroxylation of complex diketopiperazines: Critical roles of counter-cation and ion-pairing. J. Am. Chem. Soc. 2018, 140, 13375–13386. [Google Scholar] [CrossRef]
- Ding, Y.; An, F.L.; Zhu, X.J.; Yu, H.Y.; Hao, L.L.; Lu, Y.H. Curdepsidones B-G, six depsidones with anti-inflammatory activities from the marine-derived fungus Curvularia sp. IFB-Z10. Mar. Drugs 2019, 17, 266. [Google Scholar] [CrossRef]
- Liu, W.H.; Ding, Y.; Ji, X.; An, F.L.; Lu, Y.H. Curvulaide A, a bicyclic polyketide with anti-anaerobic bacteria activity from marine-derived Curvularia sp. J. Antibiot. 2019, 72, 111–113. [Google Scholar] [CrossRef]
- Guo, M.M.; Lu, Y.; Yang, J.P.; Zhao, X.; Lu, Y.H. Inhibitory effects of Schisandra chinensis extract on acne-related inflammation and UVB-induced photoageing. Pharm. Biol. 2016, 54, 2987–2994. [Google Scholar] [CrossRef] [PubMed]
- Dillman, R.L.; Cardellina, J.H. Aromatic secondary metabolites from the sponge Tedania ignis. J. Nat. Prod. 1991, 54, 1056–1061. [Google Scholar] [CrossRef]
- Jin, Z.M.; Du, X.Y.; Xu, Y.C.; Deng, Y.Q.; Liu, M.Q.; Zhao, Y.; Zhang, B.; Li, X.F.; Zhang, L.K.; Peng, C.F.; et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Yang, T.; Luo, Y.G.; Chen, X.Z.; Fang, D.M.; Zhang, G.L. Brevianamide J, a new indole alkaloid dimer from fungus Aspergillus versicolor. Org. Lett. 2009, 11, 3714–3717. [Google Scholar] [CrossRef]
- Li, G.Y.; Li, L.M.; Yang, T.; Chen, X.Z.; Fang, D.M.; Zhang, G.L. Four new alkaloids, brevianamides O-R, from the fungus Aspergillus versicolor. Helv. Chim. Acta 2010, 93, 2075–2080. [Google Scholar] [CrossRef]
- Liu, Y.X.; Ma, S.G.; Wang, X.J.; Zhao, N.; Qu, J.; Yu, S.S.; Dai, J.G.; Wang, Y.H.; Si, Y.K. Diketopiperazine alkaloids produced by the endophytic fungus Aspergillus fumigatus from the Stem of Erythrophloeum fordii Oliv. Helv. Chim. Acta 2012, 95, 1401–1408. [Google Scholar] [CrossRef]
- Schkeryantz, J.M.; Woo, J.C.G.; Siliphaivanh, P.; Depew, K.M.; Danishefsky, S.J. Total synthesis of gypsetin, deoxybrevianamide E, brevianamide E, and tryprostatin B: Novel constructions of 2,3-disubstituted indoles. J. Am. Chem. Soc. 1999, 121, 11964–11975. [Google Scholar] [CrossRef]
- Sanz-Cervera, J.F.; Stocking, E.M.; Usui, T.; Osada, H.; Williams, R.M. Synthesis and evaluation of microtubule assembly inhibition and cytotoxicity of prenylated derivatives of cyclo-l-Trp-l-Pro. Bioorg. Med. Chem. 2000, 8, 2407–2415. [Google Scholar] [CrossRef]
- May Zin, W.W.; Buttachon, S.; Dethoup, T.; Pereira, J.A.; Gales, L.; Inácio, Â.; Costa, P.M.; Lee, M.; Sekeroglu, N.; Silva, A.M.S.; et al. Antibacterial and antibiofilm activities of the metabolites isolated from the culture of the mangrove-derived endophytic fungus Eurotium chevalieri KUFA 0006. Phytochemistry 2017, 141, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Pirrung, M.C.; Fujita, K.; Park, K. Organometallic routes to 2,5-dihydroxy-3-(indol-3-yl)-benzoquinones. Synthesis of demethylasterriquinone B4. J. Org. Chem. 2005, 70, 2537–2542. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.P.; Wang, B.; Xu, Y.C.; Zhu, G.L.; Lan, M.M.; Zhu, W.M.; Sun, K.L. Diketopiperazine and diphenylether derivatives from marine algae-derived Aspergillus versicolor OUCMDZ-2738 by epigenetic activation. Mar. Drugs 2019, 17, 6. [Google Scholar] [CrossRef]
- Markowitz, M.; Saag, M.; Powderly, W.G.; Hurley, A.M.; Hsu, A.; Valdes, J.M.; Henry, D.; Sattler, F.; La Marca, A.; Leonard, J.M.; et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N. Engl. J. Med. 1995, 333, 1534–1539. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem 2004, 47, 1750–1759. [Google Scholar] [CrossRef]

| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH, Mult. (J in Hz) | δC | δH, Mult. (J in Hz) | δC | δH, Mult. (J in Hz) | |
| 1 | 165.9 | - | 166.8 | - | 166.2 | - |
| 2 | - | 9.01, s | - | 9.43, s | - | 8.93, s |
| 3 | 125.3 | - | 125.9 | - | 126.2 | - |
| 4 | 158.9 | - | 161.1 | - | 158.4 | - |
| 6a | 86.7 | 5.59, dd (5.7, 1.7) | 85.4 | 5.35, dd (2.8, 1.7) | 54.4 | 3.32, m |
| 6b | - | - | - | - | - | 3.75, dd (12.7, 4.5) |
| 7a | 29.6 | 1.75, dddd (13.4, 8.4, 5.0, 1.7) | 30.7 | 1.92, m | 66.6 | 4.35, t (4.5) |
| 7b | 1.97, m | - | ||||
| 8a | 25.9 | 2.13, dddd (12.2, 9.7, 6.7, 5.0) | 24.5 | 1.92, m | 37.6 | 1.98, ddd (12.7, 11.5, 4.5) |
| 8b | 2.27, m | 2.10, m | 2.12, dd (12.7, 6.0) | |||
| 9 | 56.5 | 4.57, dd (8.9, 6.7) | 58.7 | 4.44, m | 57.0 | 4.65, dd (11.5, 6.0) |
| 10 | 111.9 | 6.98, s | 113.2 | 7.05, s | 110.7 | 6.92, s |
| 11 | 103.9 | - | 104.3 | - | 103.8 | - |
| 12 | 125.9 | - | 125.8 | - | 126.0 | - |
| 13 | 119.6 | 7.29, d (8.0) | 119.7 | 7.23, d (8.0) | 119.4 | 7.29, d (8.0) |
| 14 | 119.4 | 6.99, m | 119.3 | 6.99, ddd (8.0, 7.0, 1.2) | 119.3 | 7.00, m |
| 15 | 120.7 | 7.08, ddd (8.0, 7.0, 1.2) | 120.7 | 7.07, ddd (8.0, 7.0, 1.2) | 120.7 | 7.08, ddd (8.0, 7.0, 1.2) |
| 16 | 111.5 | 7.41, d (8.0) | 111.6 | 7.41, m | 111.4 | 7.41, d (8.0) |
| 17 | 135.1 | - | 135.1 | - | 135.1 | - |
| 18 | 11.06, s | - | 11.06, s | - | 11.03, s | |
| 19 | 144.3 | - | 144.7 | - | 144.0 | - |
| 20 | 39.0 | - | 39.1 | - | 39.0 | - |
| 21 | 145.1 | 6.07, dd (17.4, 10.5) | 145.1 | 6.09, dd (17.1, 10.8) | 145.2 | 6.08, dd (17.3, 10.5) |
| 22 | 111.7 | 5.05, m | 111.7 | 5.06, m | 111.6 | 5.04, m |
| 23 | 27.4 | 1.45, s | 27.5 | 1.47, s | 27.4 | 1.46, s |
| 24 | 27.8 | 1.49, s | 27.9 | 1.51, s | 27.7 | 1.50, s |
| 6-OEt | 63.6 | 3.65, m | 64.3 | 3.60, m | - | - |
| 15.2 | 1.13, t (7.1) | 15.4 | 1.09, t (7.1) | - | - |
| No. | 4 | 5 | 6 | |||
|---|---|---|---|---|---|---|
| δ | δH, Mult. (J in Hz) | δC | δH, Mult. (J in Hz) | δC | δH, Mult. (J in Hz) | |
| 1 | 163.1 | - | - | - | - | - |
| 2 | - | 9.43, s | 83.9 | 5.27, d (4.7) | 78.3 | 6.40, d (4.3) |
| 3 | 125.5 | - | 146.9 | - | 151.1 | - |
| 4 | 159.4 | - | - | - | - | |
| 5 | - | - | 146.7 | - | 139.0 | - |
| 6 | 45.1 | 3.62, dd (8.6, 5.8) | 127.8 | 7.74, d (8.0) | 122.9 | 8.23, d, (8.0) |
| 7a | 19.5 | 1.92, m | 134.9 | 7.79, t (8.0) | 136.7 | 7.91, t (8.0) |
| 7b | 1.96, m | |||||
| 8a | 32.4 | 2.02, m | 128.0 | 7.53, t (8.0) | 129.7 | 7.66, t (8.0) |
| 8b | 2.33, m | |||||
| 9 | 91.0 | - | 127.0 | 8.24, d (8.0) | 127.9 | 8.23, d, (8.0) |
| 10 | 112.7 | 7.02, s | 120.8 | - | 119.1 | - |
| 11 | 103.9 | - | 160.3 | - | 157.4 | - |
| 12 | 126.3 | - | - | - | - | - |
| 13 | 119.1 | 7.21, d (8.0) | 57.4 | 5.53, dd (8.8, 6.5) | 58.1 | 5.54, t (7.8) |
| 14 | 119.0 | 7.00, m | 170.0 | - | 167.6 | - |
| 15 | 120.7 | 7.09, m | 40.0 | 3.43, m | 39.9 | 3.48, m |
| 16 | 111.6 | 7.43, d (8.0) | 135.9 | - | 134.9 | - |
| 17 | 135.1 | - | 129.8 | 7.29, m | 129.7 | 7.24, m |
| 18 | 11.10, s | 128.6 | 7.28, m | 128.8 | 7.24, m | |
| 19 | 144.4 | - | 127.0 | 7.24, m | 127.7 | 7.20, m |
| 20 | 39.0 | - | 128.6 | 7.28, m | 128.8 | 7.24, m |
| 21 | 145.2 | 6.09, ddd (17.1, 10.8, 1.6) | 129.8 | 7.29, m | 129.7 | 7.24, m |
| 22 | 111.7 | 5.06, m | - | - | - | - |
| 23 | 27.4 | 1.47, s | - | - | - | - |
| 24 | 27.8 | 1.50, s | - | - | - | - |
| 9-OEt | 59.2 | 3.53, qd (7.0, 3.9) | - | - | - | - |
| - | 15.2 | 1.22, td (7.0, 1.5) | - | - | - | - |
| 2-OMe/ 2-OEt | - | - | 56.0 | 3.53, s | 66.2 | 4.04, dt (9.0, 7.0) |
| 4.11, dt (9.0, 7.0) | ||||||
| - | - | - | - | 15.4 | 1.35, t (7.0) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Zhu, X.; Hao, L.; Zhao, M.; Hua, Q.; An, F. Bioactive Indolyl Diketopiperazines from the Marine Derived Endophytic Aspergillus versicolor DY180635. Mar. Drugs 2020, 18, 338. https://doi.org/10.3390/md18070338
Ding Y, Zhu X, Hao L, Zhao M, Hua Q, An F. Bioactive Indolyl Diketopiperazines from the Marine Derived Endophytic Aspergillus versicolor DY180635. Marine Drugs. 2020; 18(7):338. https://doi.org/10.3390/md18070338
Chicago/Turabian StyleDing, Yi, Xiaojing Zhu, Liling Hao, Mengyao Zhao, Qiang Hua, and Faliang An. 2020. "Bioactive Indolyl Diketopiperazines from the Marine Derived Endophytic Aspergillus versicolor DY180635" Marine Drugs 18, no. 7: 338. https://doi.org/10.3390/md18070338
APA StyleDing, Y., Zhu, X., Hao, L., Zhao, M., Hua, Q., & An, F. (2020). Bioactive Indolyl Diketopiperazines from the Marine Derived Endophytic Aspergillus versicolor DY180635. Marine Drugs, 18(7), 338. https://doi.org/10.3390/md18070338





