Characterization of Regulatory and Transporter Genes in the Biosynthesis of Anti-Tuberculosis Ilamycins and Production in a Heterologous Host
Abstract
1. Introduction
2. Results
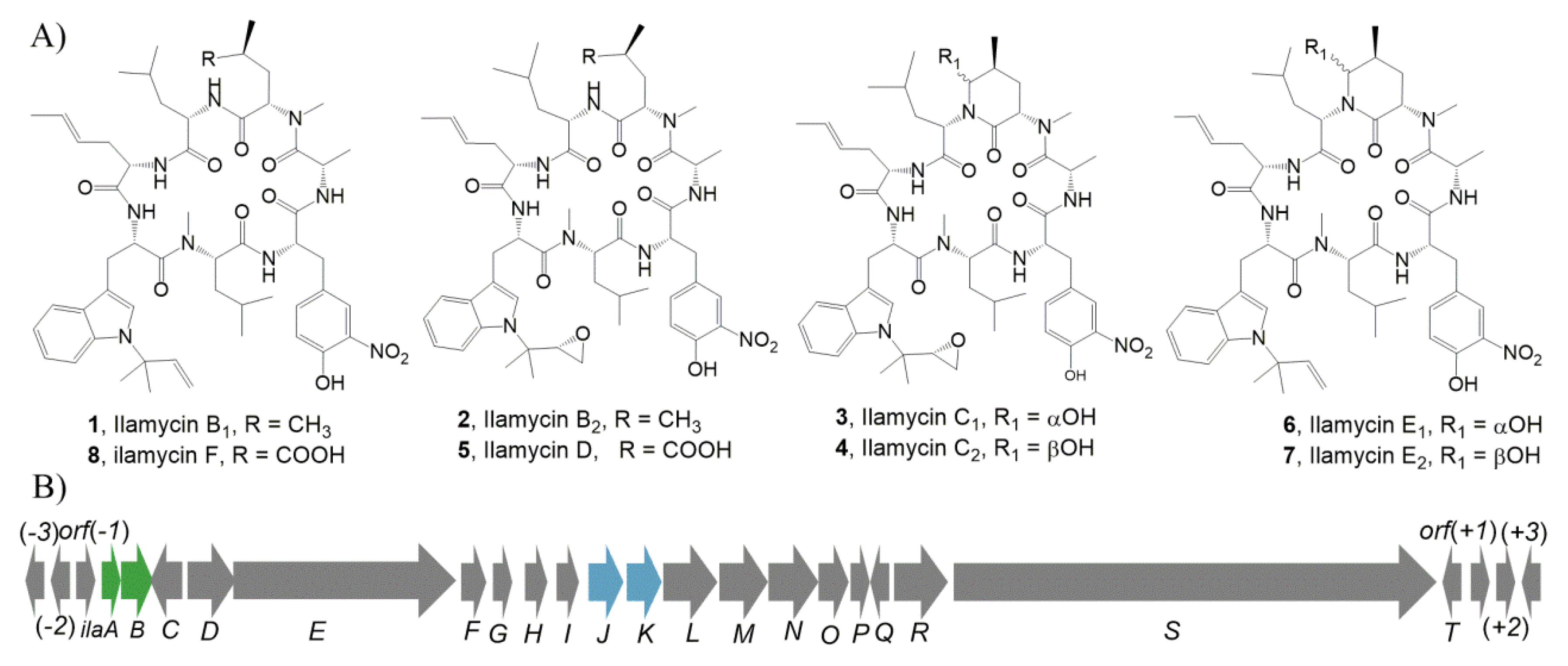
2.1. Identification of IlaA as a LysR Family Regulator and IlaB as a Streptomycin Biosynthesis Operon Regulator by Bioinformatics Analysis
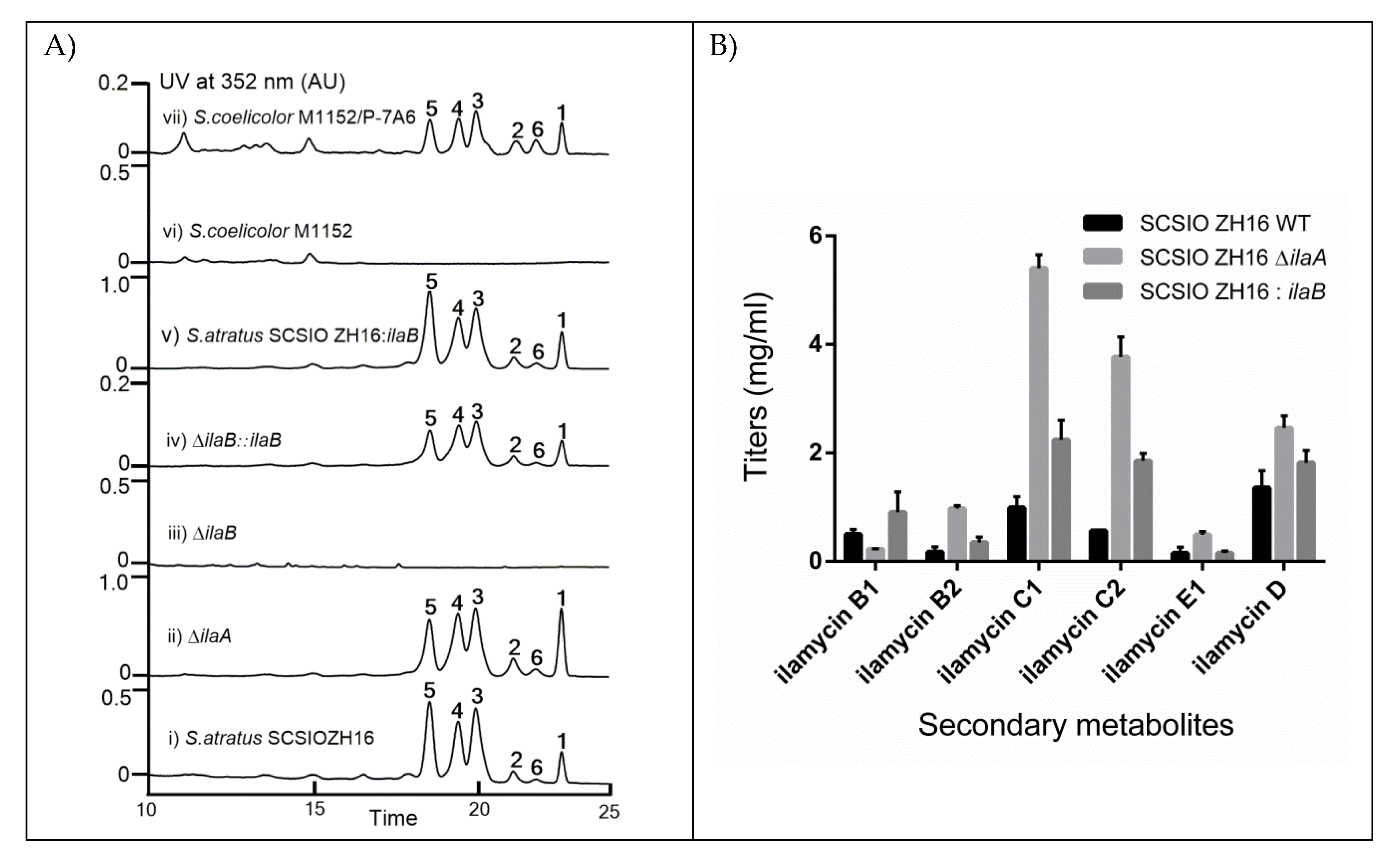
2.2. Identification of IlaA and IlaB as Negative and Positive Regulators Regulating the Production of Ilamycins
2.3. Overexpression of IlaB Enhanced the Production of Ilamycins
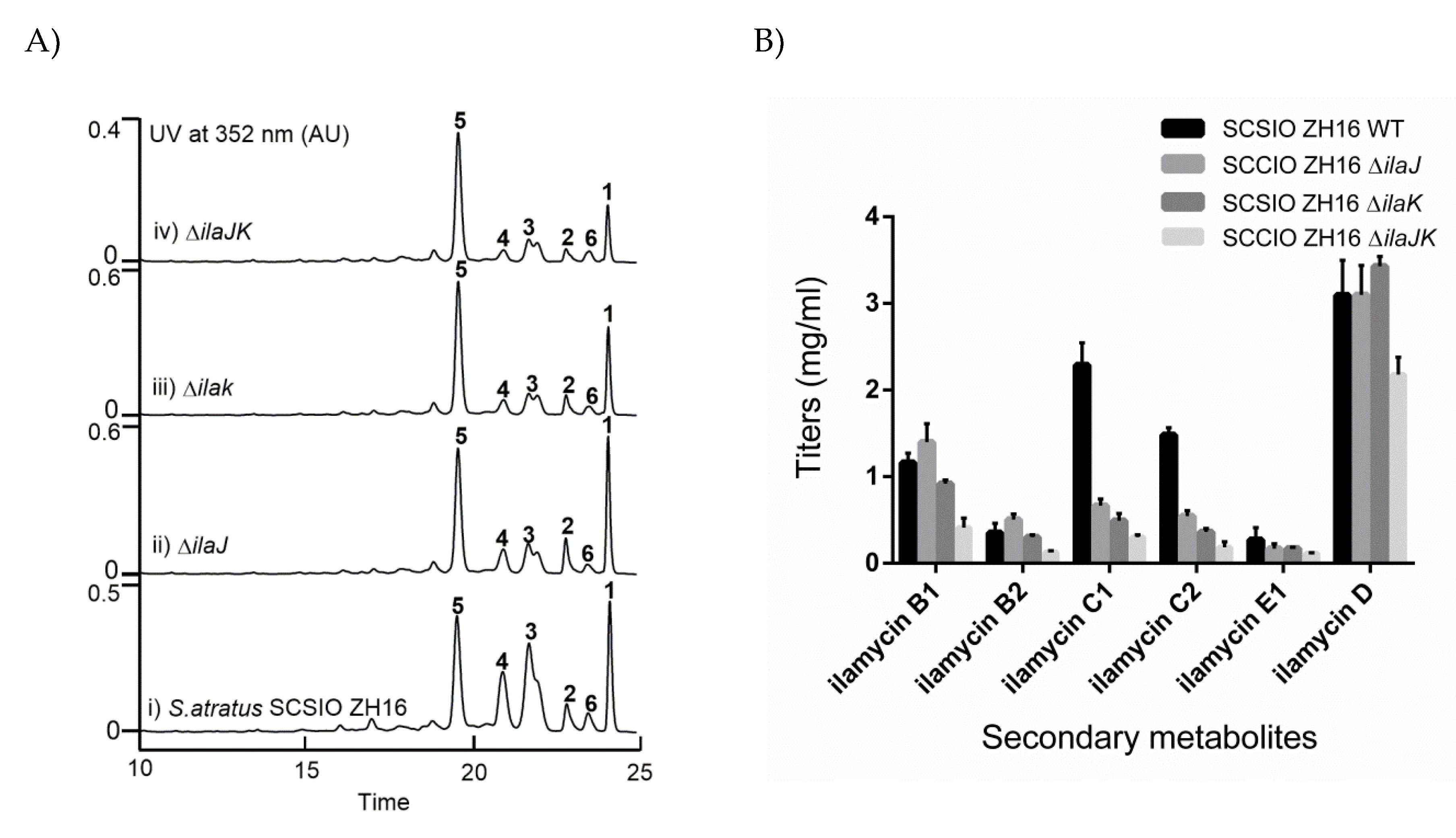
2.4. Identification of IlaJ/K as Type I ABC-Exporter Transporters Mediating the Secretion of Ilamycins
2.5. Heterologous Expression of the Ila Biosynthetic Gene Cluster (BGC) in Streptomyces Coelicolor M1152
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Bacterial Strains, Plasmids, and Culture Conditions
5.2. General Genetic Manipulations and Reagents
5.3. Genomic Library Screening
5.4. Construction of Genetic Mutants
5.5. Complementation of the ΔilaB Mutant
5.6. Heterologous Expression of the Biosynthetic Gene Cluster of Ilamycins
5.7. Fermentation, Extraction, and Quantitative Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jensen, P.R.; Moore, B.S.; Fenicala, W. The marine actinomycete genus Salinispora: A model organism for secondary metabolite discovery. Nat. Prod. Rep. 2015, 32, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Amoutzias, G.D.; Chaliotis, A.; Mossialos, D. Discovery strategies of bioactive compounds synthesized by nonribosomal peptide synthetases and type-I polyketide synthases derived from marine microbiomes. Mar. Drugs 2016, 14, 80. [Google Scholar] [CrossRef]
- Agrawal, S.; Acharya, D.; Adholeya, A.; Barrow, C.J.; Deshmukh, S.K. Nonribosomal peptides from marine microbes and their antimicrobial and anticancer potential. Front. Pharmacol. 2017, 8, 828. [Google Scholar] [CrossRef] [PubMed]
- Smolewski, P.; Robak, T. The discovery and development of romidepsin for the treatment of T-cell lymphoma. Expert Opin. Drug Discov. 2017, 12, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, D.; Tobinai, K.; Ogura, M.; Uchida, T.; Hatake, K.; Taniwaki, M.; Ando, K.; Tsukasaki, K.; Ishida, T.; Kobayashi, N.; et al. Romidepsin in Japanese patients with relapsed or refractory peripheral T-cell lymphoma: A phase I/II and pharmacokinetics study. Int. J. Hematol. 2017, 106, 655–665. [Google Scholar] [CrossRef]
- Sun, W.J.; Huang, H.; He, B.; Hu, D.H.; Li, P.H.; Yu, Y.J.; Zhou, X.H.; Lv, Z.; Zhou, L.; Hu, T.Y.; et al. Romidepsin induces G2/M phase arrest via Erk/cdc25C/cdc2/cyclinB pathway and apoptosis induction through JNK/c-Jun/caspase3 pathway in hepatocellular carcinoma cells. Biochem. Pharmacol. 2017, 127, 90–100. [Google Scholar] [CrossRef]
- Eliopoulos, G.M.; Willey, S.; Reiszner, E.; Spitzer, P.G.; Caputo, G.; Moellering, R.C., Jr. In vitro and in vivo activity of LY 146032, a new cyclic lipopeptide antibiotic. Antimicrob. Agents Chemother. 1986, 30, 532–535. [Google Scholar] [CrossRef]
- Wanger, A.R.; Murray, B.E. Activity of LY146032 against Enterococci with and without high-level aminoglycoside resistance, including two penicillinase-producing strains. Antimicrob. Agents Chemother. 1987, 31, 1779–1781. [Google Scholar] [CrossRef][Green Version]
- Mccormick, M.H.; Mcguire, J.M.; Pittenger, G.E.; Pittenger, R.C.; Stark, W.M. Vancomycin, a new antibiotic. I. Chemical and biologic properties. Antibiot. Annu. 1955, 3, 606–611. [Google Scholar] [PubMed]
- Mcguire, J.M.; Wolfe, R.N.; Ziegler, D.W. Vancomycin, a new antibiotic. II. In vitro antibacterial studies. Antibiot. Annu. 1955, 3, 612–618. [Google Scholar]
- Griffith, R.S.; Peck, F.B., Jr. Vancomycin, a new antibiotic. III. Preliminary clinical and laboratory studies. Antibiot. Annu. 1955, 3, 619–622. [Google Scholar]
- Dijkstra, J.A.; van der Laan, T.; Akkerman, O.W.; Bolhuis, M.S.; de Lange, W.C.M.; Kosterink, J.G.W.; van der Werf, T.S.; Alffenaar, J.W.C.; van Soolingen, D. In Vitro Susceptibility of Mycobacterium tuberculosis to Amikacin, Kanamycin, and Capreomycin. Antimicrob. Agents Chemother. 2018, 62, e01724-17. [Google Scholar] [CrossRef] [PubMed]
- Takeda Chemical Industries, Ltd. Ruformycin. U.S. Patent 923938, 7 February 1961.
- Takita, T.; Ohi, K.; Okami, Y.; Maeda, K.; Umezawa, H. New antibiotics, ilamycins. J. Antibiot. Ser. A 1962, 15, 46–48. [Google Scholar]
- Karl, P. Process for the isolation of ruformycin factors. Patent WO 00/78798 A1, 16 June 2000. [Google Scholar]
- Ma, J.; Huang, H.; Xie, Y.; Liu, Z.; Zhao, J.; Zhang, C.; Jia, Y.; Zhang, Y.; Zhang, H.; Zhang, T. Biosynthesis of ilamycins featuring unusual building blocks and engineered production of enhanced anti-tuberculosis agents. Nat. Commun. 2017, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Alaniappan, K.; Venkatraghavan, V. Rufomycin derivatives useful as antibiotics. Patent WO 00/78797 A1, 15 June 2000. [Google Scholar]
- Choules, M.P.; Wolf, N.M.; Lee, H.; Anderson, J.R.; Grzelak, E.M.; Wang, Y.; Ma, R.; Gao, W.; McAlpine, J.B.; Jin, Y.Y.; et al. Rufomycin Targets ClpC1 proteolysis in Mycobacterium tuberculosis and M. abscessus. Antimicrob. Agents Chemother. 2019, 63, e02204-18. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Yang, Z.; Huang, X.; Zhang, Z.; Li, J.; Ju, J.; Zhang, H.; Ma, J. Ilamycin C induces apoptosis and inhibits migration and invasion in triple-negative breast cancer by suppressing IL-6/STAT3 pathway. J. Hematol. Oncol. 2019, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fang, H.; Wu, Q.; Wang, X.; Liu, R.; Li, F.; Xiao, J.; Yuan, L.; Zhou, Z.; Ma, J.; et al. A natural product of marine actinomycete, inhibits triple-negative breast cancer partially through ER stress-CHOP-Bcl-2. Int. J. Biol. Sci. 2019, 15, 1723–1732. [Google Scholar] [CrossRef]
- Tomita, H.; Katsuyama, Y.; Minami, H.; Ohnishi, Y. Identification and characterization of a bacterial cytochrome P450 monooxygenase catalyzing the 3-nitration of tyrosine in rufomycin biosynthesis. J. Biol. Chem. 2017, 292, 15859–15869. [Google Scholar] [CrossRef]
- Huang, S.; Tong, M.H.; Qin, Z.; Deng, Z.; Deng, H.; Yu, Y. Identification and characterization of the biosynthetic gene cluster of thiolutin, a tumor angiogenesis inhibitor, in Saccharothrix algeriensis NRRL B-24137. Anticancer Agents Med. Chem. 2015, 15, 277–284. [Google Scholar] [CrossRef]
- Marshall, C.G.; Broadhead, G.; Leskiw, B.K.; Wright, G.D. D-Ala-D-Ala ligases from glycopeptide antibiotic-producing organisms are highly homologous to the enterococcal vancomycin-resistance ligases VanA and VanB. Proc. Natl. Acad. Sci. USA 1997, 94, 6480–6483. [Google Scholar] [CrossRef]
- Retzlaff, L.; Distler, J. The regulator of streptomycin gene expression, StrR, of Streptomyces griseus is a DNA binding activator protein with multiple recognition sites. Mol. Microbiol. 1995, 18, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Pootoolal, J.; Thomas, M.G.; Marshall, C.G.; Neu, J.M.; Hubbard, B.K.; Walsh, C.T.; Wright, G.D. Assembling the glycopeptide antibiotic scaffold: The biosynthesis of A47934 from Streptomyces toyocaensis NRRL15009. Proc. Natl. Acad. Sci. USA 2002, 99, 8962–8967. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019. [Google Scholar] [CrossRef]
- Menges, G.; Muth, W.; Wohelleben, W.; Stegmann, E. The ABC transporter Tba of Amycolatopsis balhimycina is required for efficient export of the glycopeptide antibiotic balhimycin. Appl. Microbiol. Biotechnol. 2007, 77, 125–134. [Google Scholar] [CrossRef]
- Kaur, P. Expression and characterization of DrrA and DrrB proteins of Streptomyces peucetius in Escherichia coli: DrrA is an ATP binding protein. J. Bacteriol. 1997, 179, 569–575. [Google Scholar] [CrossRef]
- Fernandez, E.; Lombo, F.; Mendez, C.; Salas, J.A. An ABC transporter is essential for resistance to the antitumor agent mithramycin in the producer Streptomyces argillaceus. Mol. Gen. Genet. 1996, 251, 692–698. [Google Scholar]
- Linton, K.J.; Cooper, H.N.; Hunter, I.S.; Leadlay, P.F. An ABC-transporter from Streptomyces longisporoflavus confers resistance to the polyetherionophore antibiotic tetronasin. Mol. Microbiol. 1994, 11, 777–785. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, X.; He, J.; Sun, C.; Ju, J.; Ma, J. Characterization of the noncanonical regulatory and transporter genes in atratumycin biosynthesis and production in a heterologous host. Mar. Drugs 2019, 17, 560. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Rao, D.K.; Gandlur, S.W. Biochemical characterization of domains in the membrane subunit DrrB that interact with the ABC subunit DrrA: Identification of a conserved motif. Biochemistry 2005, 44, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, Y.; Zhao, Z.; Gao, G.; Huang, S.X.; Kang, Q.; He, X.; Lin, S.; Pang, X.; Deng, Z.; et al. Functional Genome mining for metabolites encoded by large gene clusters through heterologous expression of a whole-genome bacterial artificial chromosome library in Streptomyces spp. Appl. Environ. Microbiol. 2016, 82, 5795–5805. [Google Scholar] [CrossRef]
- Tu, J.; Li, S.; Chen, J.; Song, Y.; Fu, S.; Ju, J.; Li, Q. Characterization and heterologous expression of the neoabyssomicin/abyssomicin biosynthetic gene cluster from Streptomyces koyangensis SCSIO 5802. Microb. Cell Fact. 2018, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Bibb, M.J. Regulation of secondary metabolism in Streptomycetes. Curr. Opin. Microbiol. 2005, 8, 208–215. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. Engineering of regulatory cascades and networks controlling antibiotic biosynthesis in Streptomyces. Curr. Opin. Microbiol. 2010, 13, 263–273. [Google Scholar] [CrossRef]
- Thamm, S.; Distler, J. Properties of C-terminal truncated derivatives of the activator, StrR, of the streptomycin biosynthesis in Streptomyces griseus. FEMS Microbiol. Lett. 1997, 149, 265–272. [Google Scholar] [CrossRef]
- Tomono, A.; Tsai, Y.; Yamazaki, H.; Ohnishi, Y.; Horinouchi, S. Transcriptional control by A-factor of StrR, the pathway-specific transcriptional activator for streptomycin biosynthesis in Streptomyces griseus. J. Bacteriol. 2005, 187, 5595–5604. [Google Scholar] [CrossRef]
- Lopez, D.; Vlamakis, H.; Kolter, R. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol. Rev. 2009, 33, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.L.; Dassa, E.; Orelle, C.; Chen, J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 2008, 72, 317–364. [Google Scholar] [CrossRef] [PubMed]
- Hollenstein, K.; Dawson, R.J.; Locher, K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007, 17, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chen, Q.; Song, Y.; Huang, H.; Li, J.; Ma, J.; Li, Q.; Ju, J. Deciphering the sugar biosynthetic pathway and tailoring steps of nucleoside antibiotic A201A unveils a GDP-l-galactose mutase. Proc. Natl. Acad. Sci. USA 2017, 114, 4948–4953. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Chen, Q.; Luo, M.; Sun, A.; Song, Y.; Ma, J.; Ju, J. Identification of the grincamycin gene cluster unveils divergent roles for GcnQ in different hosts, tailoring the l-rhodinose moiety. Org. Lett. 2013, 15, 3254–3257. [Google Scholar] [CrossRef] [PubMed]
- Gust, B.; Challis, G.L.; Fowler, K.; Kieser, T.; Chater, K.F. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA 2003, 100, 1541–1546. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Wei, X.; Yang, Z.; Li, Y.; Ju, J.; Ma, J. Characterization of Regulatory and Transporter Genes in the Biosynthesis of Anti-Tuberculosis Ilamycins and Production in a Heterologous Host. Mar. Drugs 2020, 18, 216. https://doi.org/10.3390/md18040216
He J, Wei X, Yang Z, Li Y, Ju J, Ma J. Characterization of Regulatory and Transporter Genes in the Biosynthesis of Anti-Tuberculosis Ilamycins and Production in a Heterologous Host. Marine Drugs. 2020; 18(4):216. https://doi.org/10.3390/md18040216
Chicago/Turabian StyleHe, Jianqiao, Xin Wei, Zhijie Yang, Yan Li, Jianhua Ju, and Junying Ma. 2020. "Characterization of Regulatory and Transporter Genes in the Biosynthesis of Anti-Tuberculosis Ilamycins and Production in a Heterologous Host" Marine Drugs 18, no. 4: 216. https://doi.org/10.3390/md18040216
APA StyleHe, J., Wei, X., Yang, Z., Li, Y., Ju, J., & Ma, J. (2020). Characterization of Regulatory and Transporter Genes in the Biosynthesis of Anti-Tuberculosis Ilamycins and Production in a Heterologous Host. Marine Drugs, 18(4), 216. https://doi.org/10.3390/md18040216





