Marine-Inspired Bis-indoles Possessing Antiproliferative Activity against Breast Cancer; Design, Synthesis, and Biological Evaluation
Abstract
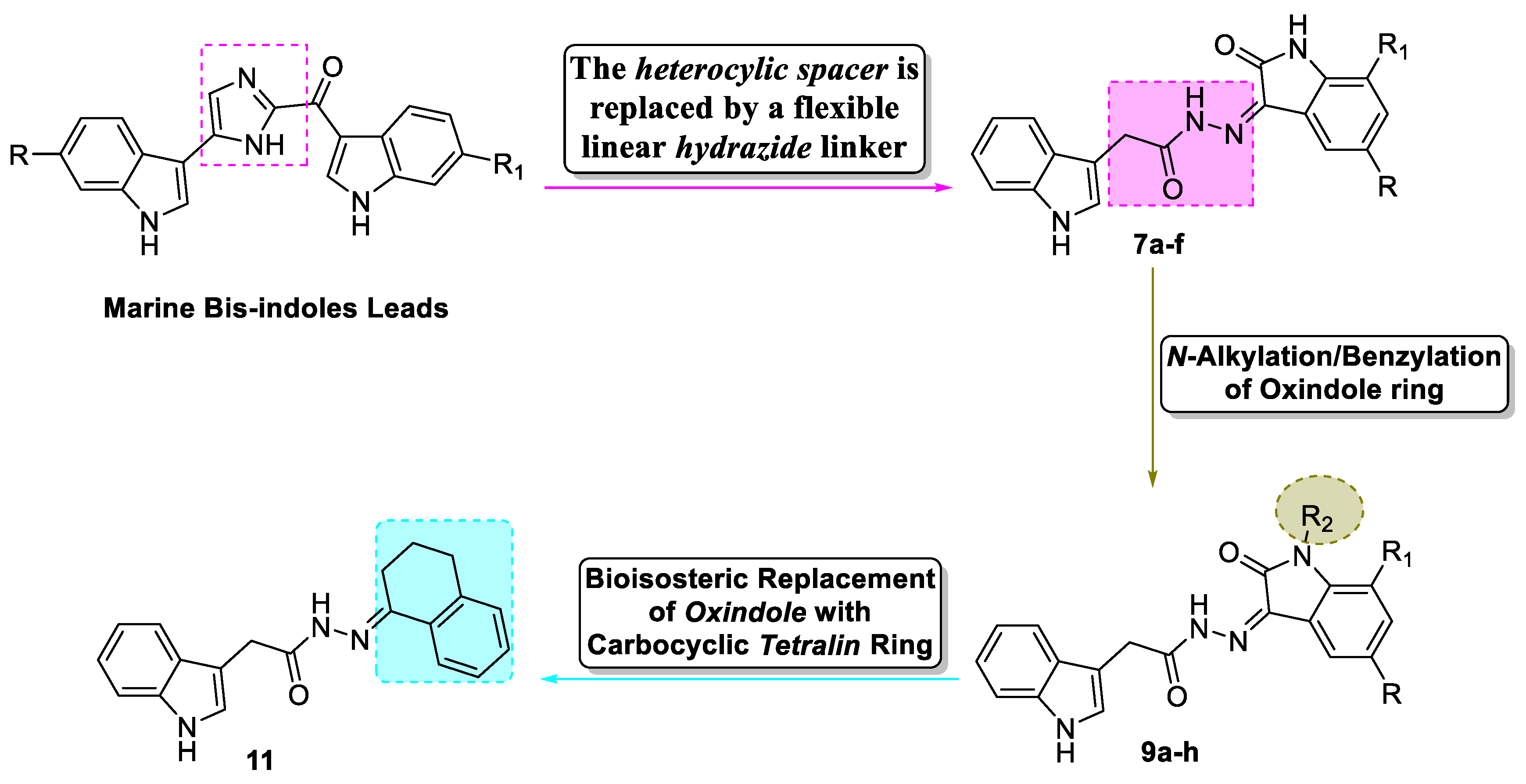
1. Introduction
2. Results
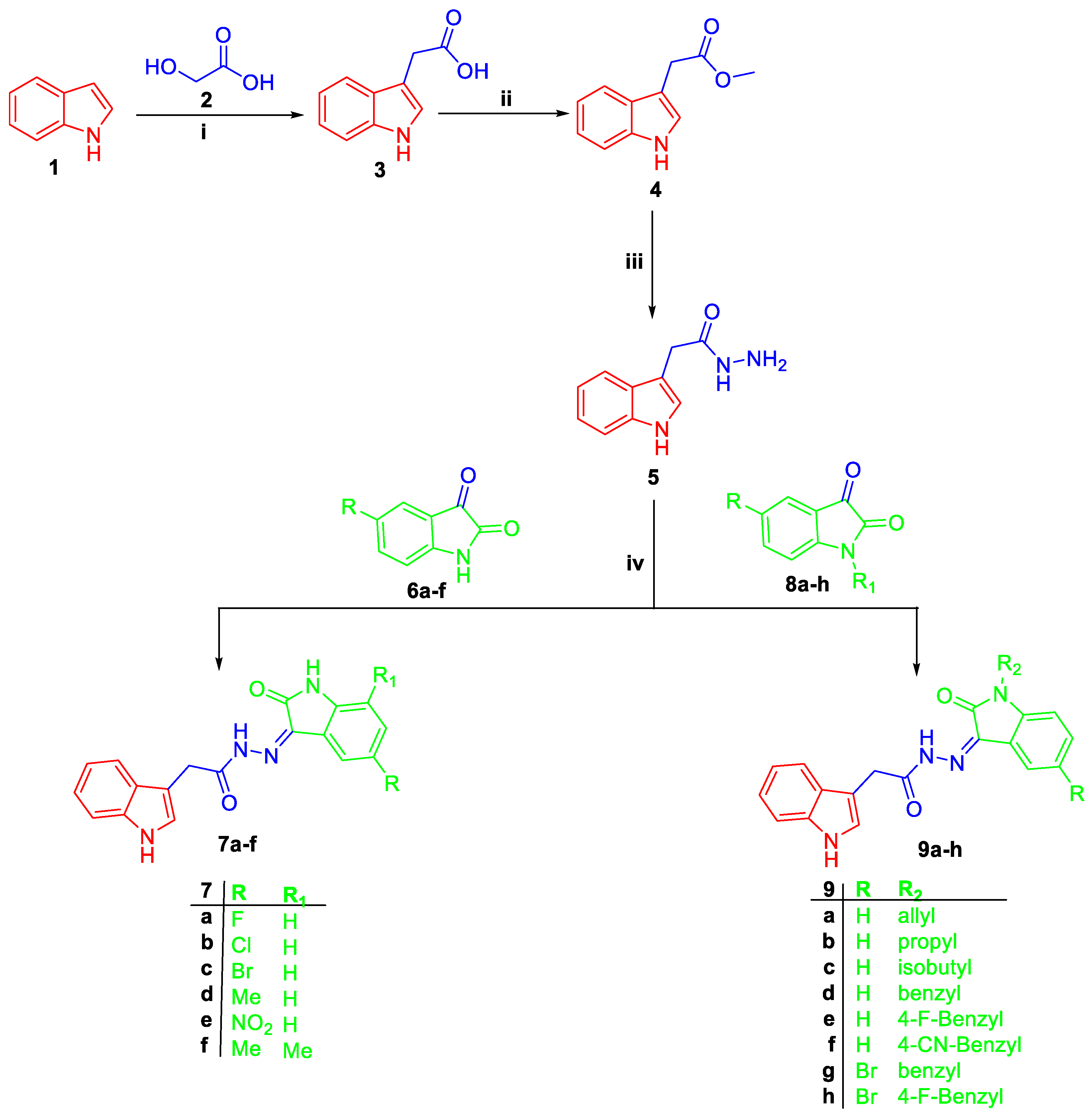
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Antiproliferative Activity against Breast Cancer MCF-7 and MDA-MB-231
2.2.2. In Vitro Cytotoxic Activity against Non-Tumorigenic Human Breast Cell Line
2.2.3. Cell Cycle Analysis
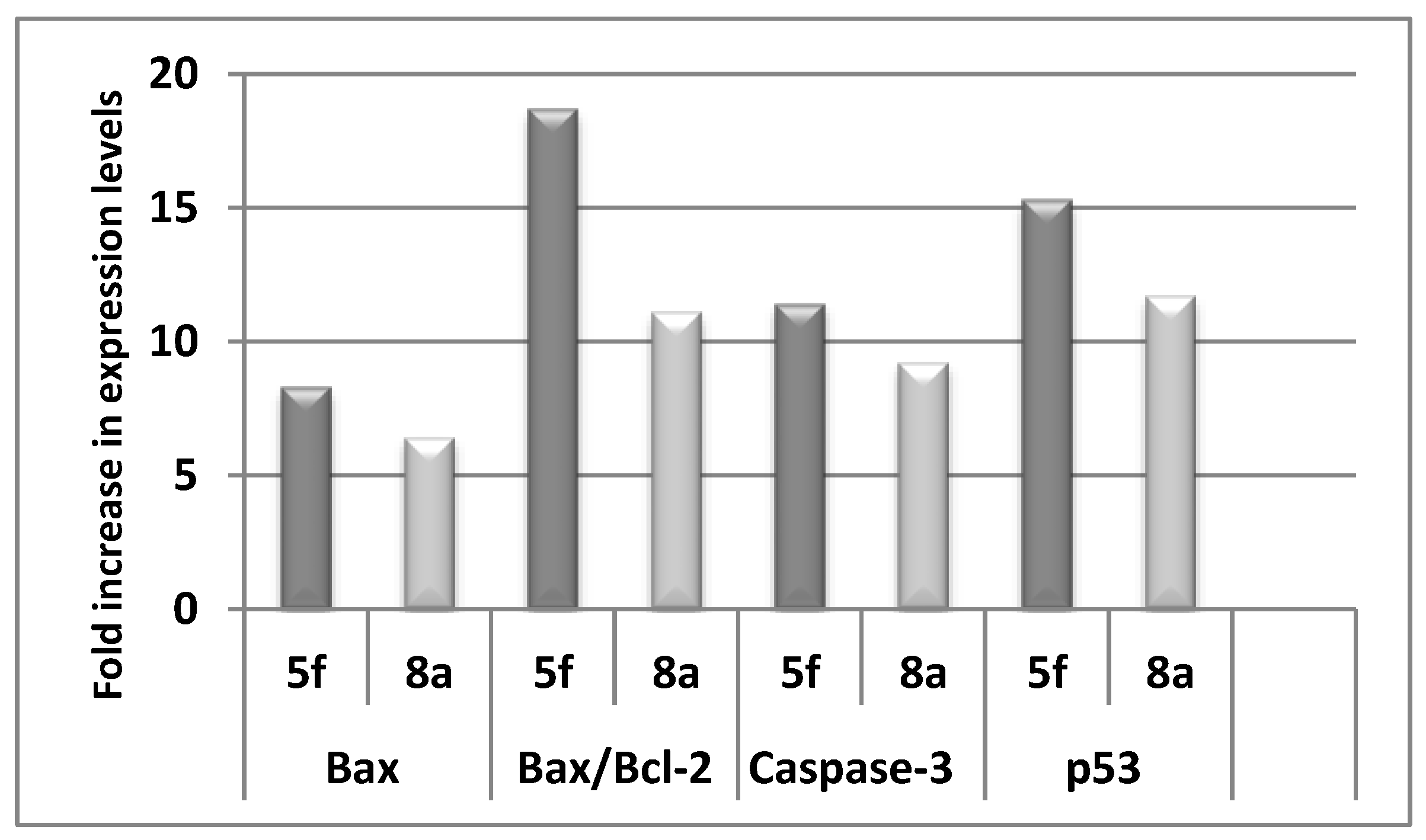
2.2.4. Effect of 5e, 5f, and 8a on the Level of the Apoptotic Markers (Bax, Bcl-2, caspase-3, and p53)
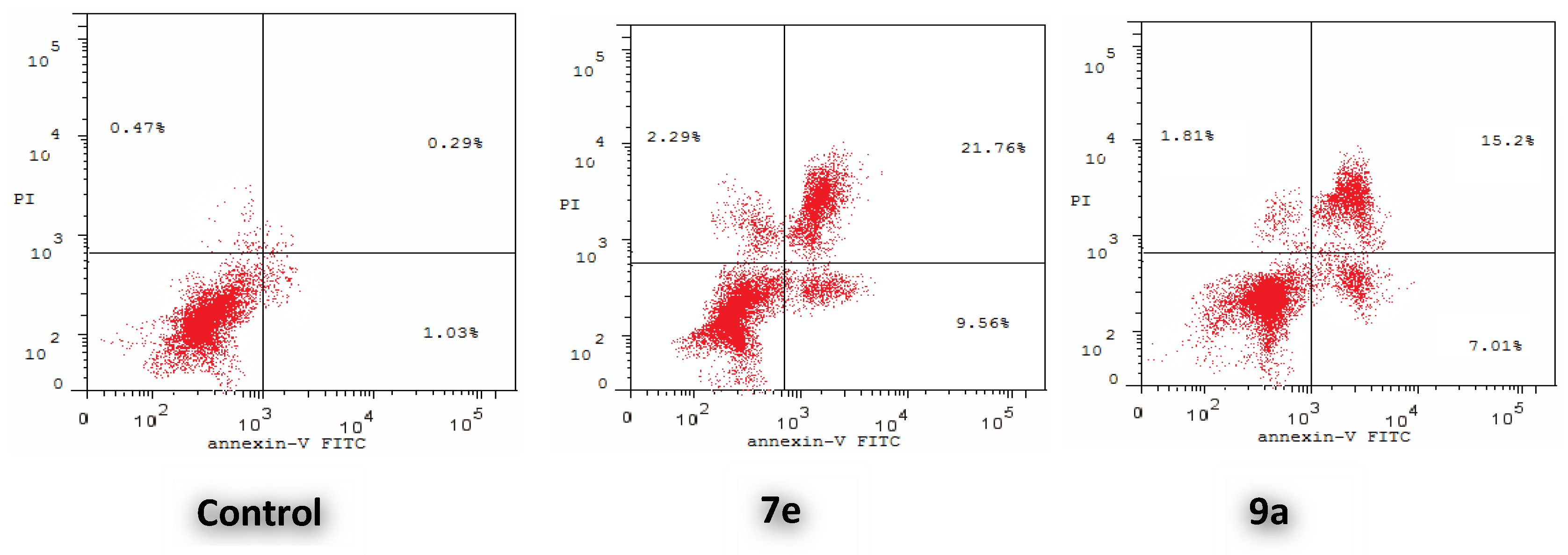
2.2.5. Annexin V-FITC Apoptosis Assay
2.2.6. CDK2 Inhibitory Activity
3. Experimental
3.1. Chemistry
3.1.1. General
3.1.2. General Procedure for Synthesis of the Target Bis-indoles (7a–f and 9a–h), and 11
N′-(5-Fluoro-2-oxoindolin-3-ylidene)-2-(1H-indol-3-yl)acetohydrazide (7a)
N′-(5-Chloro-2-oxoindolin-3-ylidene)-2-(1H-indol-3-yl)acetohydrazide (7b)
N′-(5-Bromo-2-oxoindolin-3-ylidene)-2-(1H-indol-3-yl)acetohydrazide (7c)
2-(1H-Indol-3-yl)-N′-(5-methyl-2-oxoindolin-3-ylidene)acetohydrazide (7d)
2-(1H-Indol-3-yl)-N′-(5-nitro-2-oxoindolin-3-ylidene)acetohydrazide (7e)
N′-(5,7-Dimethyl-2-oxoindolin-3-ylidene)-2-(1H-indol-3-yl)acetohydrazide (7f)
N′-(1-Allyl-2-oxoindolin-3-ylidene)-2-(1H-indol-3-yl)acetohydrazide (9a)
2-(1H-Indol-3-yl)-N′-(2-oxo-1-propylindolin-3-ylidene)acetohydrazide (9b)
N′-(1-(sec-Butyl)-2-oxoindolin-3-ylidene)-2-(1H-indol-3-yl)acetohydrazide (9c)
N′-(1-Benzyl-2-oxoindolin-3-ylidene)-2-(1H-indol-3-yl)acetohydrazide (9d)
N′-(1-(4-Fluorobenzyl)-2-oxoindolin-3-ylidene)-2-(1H-indol-3-yl)acetohydrazide (9e)
N′-(1-(4-Cyanobenzyl)-2-oxoindolin-3-ylidene)-2-(1H-indol-3-yl)acetohydrazide (9f)
N′-(1-Benzyl-5-bromo-2-oxoindolin-3-ylidene)-2-(1H-indol-3-yl)acetohydrazide (9g)
N′-(5-Bromo-1-(4-fluorobenzyl)-2-oxoindolin-3-ylidene)-2-(1H-indol-3-yl)acetohydrazide (9h)
N′-(3,4-Dihydronaphthalen-1(2H)-ylidene)-2-(1H-indol-3-yl)acetohydrazide (11)
3.2. Biological Evaluation
3.2.1. Cytotoxic Activity against Human Breast Cancer and Non-Tumorigenic Cell Lines
3.2.2. Cell Cycle Analysis
3.2.3. ELISA Immunoassay
3.2.4. Annexin V-FITC/PI Apoptosis Assay
3.2.5. CDK2 Kinase Inhibitory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lunagariya, J.; Bhadja, P.; Zhong, S.; Vekariya, R.; Xu, S. Marine Natural Product Bis-indole Alkaloid Caulerpin: Chemistry and Biology. Mini Mini-Rev. Med. Chem. 2019, 19, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Talwar, A.; Chauhan, P.M.S. Bis and Tris Indole Alkaloids from Marine Organisms: New Leads for Drug Discovery. Front. Med. Chem. 2012, 6, 361–385. [Google Scholar]
- Netz, N.; Opatz, T. Marine indole alkaloids. Mar. Drugs 2015, 13, 4814–4914. [Google Scholar] [CrossRef] [PubMed]
- Gul, W.; Hamann, M.T. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005, 78, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Edwards, V.; Benkendorff, K.; Young, F. Marine compounds selectively induce apoptosis in female reproductive cancer cells but not in primary-derived human reproductive granulosa cells. Mar. Drugs 2012, 10, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Esmaeelian, B.; Abbott, C.A.; Le Leu, R.K.; Benkendorff, K. 6-bromoisatin found in muricid mollusc extracts inhibits colon cancer cell proliferation and induces apoptosis, preventing early stage tumor formation in a colorectal cancer rodent model. Mar. Drugs 2014, 12, 17–35. [Google Scholar] [CrossRef]
- Shimizu, S.; Yamamoto, Y.; Inagaki, L.; Koshimura, S. Antitumor effect and structure-activity relationship of asterriquinone analogs. Gann = Gan 1982, 73, 642–648. [Google Scholar]
- Kohmoto, S.; Kashman, Y.; McConnell, O.J.; Rinehart, K.L., Jr.; Wright, A.; Koehn, F. Dragmacidin, a new cytotoxic bis(indole)alkaloid from a deep water marine sponge, Dragmacidon sp. J. Org. Chem. 1988, 53, 3116–3118. [Google Scholar] [CrossRef]
- Morris, S.A.; Andersen, R.J. Brominated bis(indole)alkaloids from the marine sponge Hexadella sp. Tetrahedron 1990, 46, 715–720. [Google Scholar] [CrossRef]
- Fahy, E.; Potts, B.C.M.; Faulkner, D.J.; Smith, K. 6-Bromotryptamine derivatives from the Gulf of California tunicate Didemnum candidum. J. Nat. Prod. 1991, 54, 564–569. [Google Scholar] [CrossRef]
- Wright, A.E.; Pomponi, S.A.; Cross, S.S.; McCarthy, P. A new bis-(indole)alkaloids from a deep-water marine sponge of the genus Spongosorites. J. Org. Chem. 1992, 57, 4772–4775. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2008, 36, 35–94. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Zhang, P.; Lee, Y.; Hong, J.; Lee, C.; Jung, J.H. Monoindole Alkaloids from a Marine SpongeSpongosorites sp. Mar. Drugs 2007, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bartik, K.; Braekman, J.; Daloze, D.; Stoller, C. Topsentins, new toxic bis-indole alkaloids from the marinesponge Topsentia genitrix. Can. J. Chem. 1987, 65, 2118–2121. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Eldehna, W.M.; Ghabbour, H.; Al-Ansary, G.H.; Assaf, A.M.; Al-Dhfyan, A. Synthesis, crystal study, and anti-proliferative activity of some 2-benzimidazolylthioacetophenones towards triple-negative breast cancer MDA-MB-468 cells as apoptosis-inducing agents. Int. J. Mol. Sci. 2016, 17, 1221. [Google Scholar] [CrossRef]
- Eldehna, W.M.; El-Naggar, D.H.; Hamed, A.R.; Ibrahim, H.S.; Ghabbour, H.A.; Abdel-Aziz, H.A.; Ghabbour, H.A. Abdel-Aziz One-pot three-component synthesis of novel spirooxindoles with potential cytotoxic activity against triple-negative breast cancer MDAMB-231 cells. J. Enzym. Inhib. Med. Chem. 2018, 33, 309–318. [Google Scholar] [CrossRef]
- Ismail, R.S.; Abou-Seri, S.M.; Eldehna, W.M.; Ismail, N.S.; Elgazwi, S.M.; Ghabbour, H.A.; Ahmed, M.S.; Halaweish, F.T.; El Ella, D.A.A. Novel series of 6-(2-substitutedacetamido)-4-anilinoquinazolines as EGFR-ERK signal transduction inhibitors in MCF-7 breast cancer cells. Eur. J. Med. Chem. 2018, 155, 782–796. [Google Scholar] [CrossRef]
- Petreni, A.; Bonardi, A.; Lomelino, C.; Osman, S.M.; ALOthman, Z.A.; Eldehna, W.M.; El-Haggar, R.; McKenna, R.; Nocentini, A.; Supuran, C.T. Inclusion of a 5-fluorouracil moiety in nitrogenous bases derivatives as human carbonic anhydrase IX and XII inhibitors produced a targeted action against MDA-MB-231 and T47D breast cancer cells. Eur. J. Med. Chem. 2020, 190, 112112. [Google Scholar] [CrossRef]
- Johnson, H.E.; Crosby, D.G. 3-Indoleacetic Acid. J. Org. Chem. 1963, 28, 1246–1248. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Lopez, J.; Tait, S.W.G. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Kavanagh, J.J. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003, 4, 721–729. [Google Scholar] [CrossRef]
- Delbridge, A.R.; Grabow, S.; Strasser, A.; Vaux, D.L. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer 2016, 16, 99. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, P.J.; Su, D.; Feng, J.; Ma, S.L. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol. Med. Rep. 2014, 9, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Sen-Gupta, A.k.; Gupta, A.A. Synthesis of some new indolinone derived hydrazones as possible antibacterial agents. Eur. J. Med. Chem. 1983, 18, 181–184. [Google Scholar]
- Eldehna, W.M.; El Kerdawy, A.M.; Al-Ansary, G.H.; Al-Rashood, S.T.; Ali, M.M.; Mahmoud, A.E. Type IIA-Type IIB protein tyrosine kinase inhibitors hybridization as an efficient approach for potent multikinase inhibitor development: Design, synthesis, anti-proliferative activity, multikinase inhibitory activity and molecular modeling of novel indolinone-based ureides and amides. Eur. J. Med. Chem. 2019, 163, 37–53. [Google Scholar] [PubMed]
- El-Naggar, M.; Eldehna, W.M.; Almahli, H.; Elgez, A.; Fares, M.; Elaasser, M.M.; Abdel-Aziz, H.A. Novel thiazolidinone/thiazolo [3, 2-a] benzimidazolone-isatin conjugates as apoptotic anti-proliferative agents towards breast cancer: One-pot synthesis and in vitro biological evaluation. Molecules 2018, 23, 1420. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Almahli, H.; Al-Ansary, G.H.; Ghabbour, H.A.; Aly, M.H.; Ismael, O.E.; Al-Dhfyan, A.; Abdel-Aziz, H.A. Synthesis and in vitro antiproliferative activity of some novel isatins conjugated with quinazoline/phthalazine hydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosis-inducing agents. J. Enz. Inhib. Med. Chem. 2017, 32, 600–613. [Google Scholar] [CrossRef]


| Cpd. | R | R1 | R2 | IC50 (µM) a | |
|---|---|---|---|---|---|
| MCF-7 | MDA-MB-231 | ||||
| 7a | F | H | - | 1.53 ± 0.02 | 9.04 ± 0.32 |
| 7b | Cl | H | - | 8.87 ± 0.43 | 2.88 ± 0.08 |
| 7c | Br | H | - | 36.19 ± 2.78 | 36.57 ± 1.81 |
| 7d | CH3 | H | - | 10.95 ± 0.81 | 0.34 ± 0.02 |
| 7e | NO2 | H | - | 0.44 ± 0.01 | 1.32 ± 0.03 |
| 7f | CH3 | CH3 | - | NA b | 77.30 ± 6.21 |
| 9a | H | - | −CH2CH=CH2 | 1.28 ± 0.04 | 18.24 ± 0.62 |
| 9b | H | - | −CH2CH2CH3 | 28.24 ± 1.53 | 51.27 ± 3.59 |
| 9c | H | - | −CH2CH(CH3)2 | 47.10 ± 3.65 | NA b |
| 9d | H | - | −CH2C6H5 | 1.51 ± 0.03 | 4.14 ± 0.19 |
| 9e | H | - | −CH2C6H4-4-F | 10.43 ± 0.81 | 17.66 ± 0.55 |
| 9f | H | - | −CH2C6H4-4-CN | 8.72 ± 0.39 | 25.41 ± 1.56 |
| 9g | Br | - | −CH2C6H5 | 2.76 ± 0.14 | 2.85 ± 0.07 |
| 9h | Br | - | −CH2C6H4-4F | 20.89 ± 0.04 | 2.29 ± 0.09 |
| 11 | - | - | - | 84.70 ± 4.02 | NA b |
| Staurosporine | - | - | - | 6.81 ± 0.22 | 10.29 ± 0.72 |
| Comp. | IC50 (µM) | Selectivity Index | |
|---|---|---|---|
| MCF-10A | MCF-7 | MCF-10A/MCF-7 | |
| 7a | 14.06 | 1.53 | 9.2 |
| 7b | 39.54 | 8.87 | 4.5 |
| 7d | 54.92 | 10.95 | 5.0 |
| 7e | 17.06 | 0.44 | 38.7 |
| 9a | 23.47 | 1.28 | 18.3 |
| 9d | 19.12 | 1.51 | 12.7 |
| 9e | 48.39 | 10.43 | 4.7 |
| 9f | 42.01 | 8.72 | 4.8 |
| 9g | 17.16 | 2.76 | 6.2 |
| 9h | 26.09 | 20.89 | 1.2 |
| Comp. | %G0-G1 | %S | %G2/M | %Sub-G1 |
|---|---|---|---|---|
| 7e | 31.66 | 25.44 | 42.9 | 33.61 |
| 9a | 43.82 | 24.91 | 31.27 | 24.02 |
| Control | 57.26 | 28.59 | 14.15 | 1.79 |
| Compound | Bax (pg/mg of Total Protein) | Bcl-2 (ng/mg of Total Protein) | Bax/Bcl-2 |
|---|---|---|---|
| 7e | 318.0 ± 10.5 | 2.07 ± 0.14 | 153.6 |
| 9a | 243.6 ± 12.4 | 2.67 ± 0.16 | 91.2 |
| Control | 38.3 ± 2.2 | 4.65 ± 0.23 | 8.2 |
| Compound | Caspase-3 (pg/mg) | p53 (pg/mg) |
|---|---|---|
| 7e | 409.2 ± 17.2 | 631.8 ± 35.8 |
| 9a | 331.0 ± 12.5 | 482.3 ± 27.4 |
| Control | 35.92 ± 1.8 | 41.26 ± 2.7 |
| Compound | Early Apoptosis (Lower Right %) | Late Apoptosis (Upper Right %) | Total (L.R % + U.R %) |
|---|---|---|---|
| 7e | 9.56 | 21.76 | 31.32 |
| 9a | 7.01 | 15.20 | 22.21 |
| Control | 1.03 | 0.29 | 1.32 |
| Compound | % Enzyme Inhibitory Activity |
|---|---|
| 7a | 41 |
| 7b | 41 |
| 7d | 58 |
| 7e | 16 |
| 9a | 23 |
| Staurosporine | 99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eldehna, W.M.; Hassan, G.S.; Al-Rashood, S.T.; Alkahtani, H.M.; A. Almehizia, A.; Al-Ansary, G.H. Marine-Inspired Bis-indoles Possessing Antiproliferative Activity against Breast Cancer; Design, Synthesis, and Biological Evaluation. Mar. Drugs 2020, 18, 190. https://doi.org/10.3390/md18040190
Eldehna WM, Hassan GS, Al-Rashood ST, Alkahtani HM, A. Almehizia A, Al-Ansary GH. Marine-Inspired Bis-indoles Possessing Antiproliferative Activity against Breast Cancer; Design, Synthesis, and Biological Evaluation. Marine Drugs. 2020; 18(4):190. https://doi.org/10.3390/md18040190
Chicago/Turabian StyleEldehna, Wagdy M., Ghada S. Hassan, Sara T. Al-Rashood, Hamad M. Alkahtani, Abdulrahman A. Almehizia, and Ghada H. Al-Ansary. 2020. "Marine-Inspired Bis-indoles Possessing Antiproliferative Activity against Breast Cancer; Design, Synthesis, and Biological Evaluation" Marine Drugs 18, no. 4: 190. https://doi.org/10.3390/md18040190
APA StyleEldehna, W. M., Hassan, G. S., Al-Rashood, S. T., Alkahtani, H. M., A. Almehizia, A., & Al-Ansary, G. H. (2020). Marine-Inspired Bis-indoles Possessing Antiproliferative Activity against Breast Cancer; Design, Synthesis, and Biological Evaluation. Marine Drugs, 18(4), 190. https://doi.org/10.3390/md18040190






