(Semi)-Synthetic Fucosylated Chondroitin Sulfate Oligo- and Polysaccharides
Abstract
1. Introduction
2. Total Synthetic Approaches
3. Semi-Synthetic Strategies
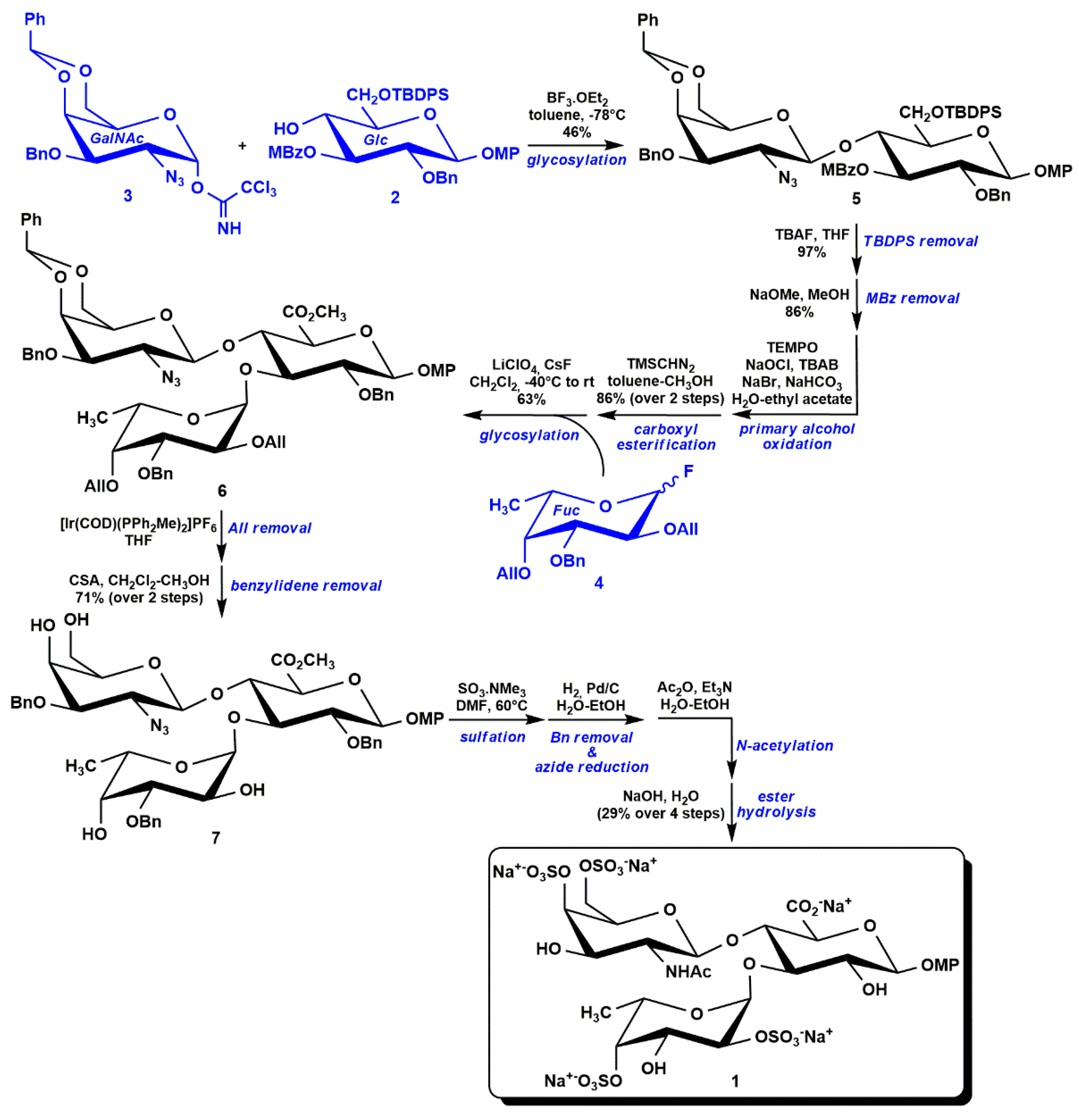
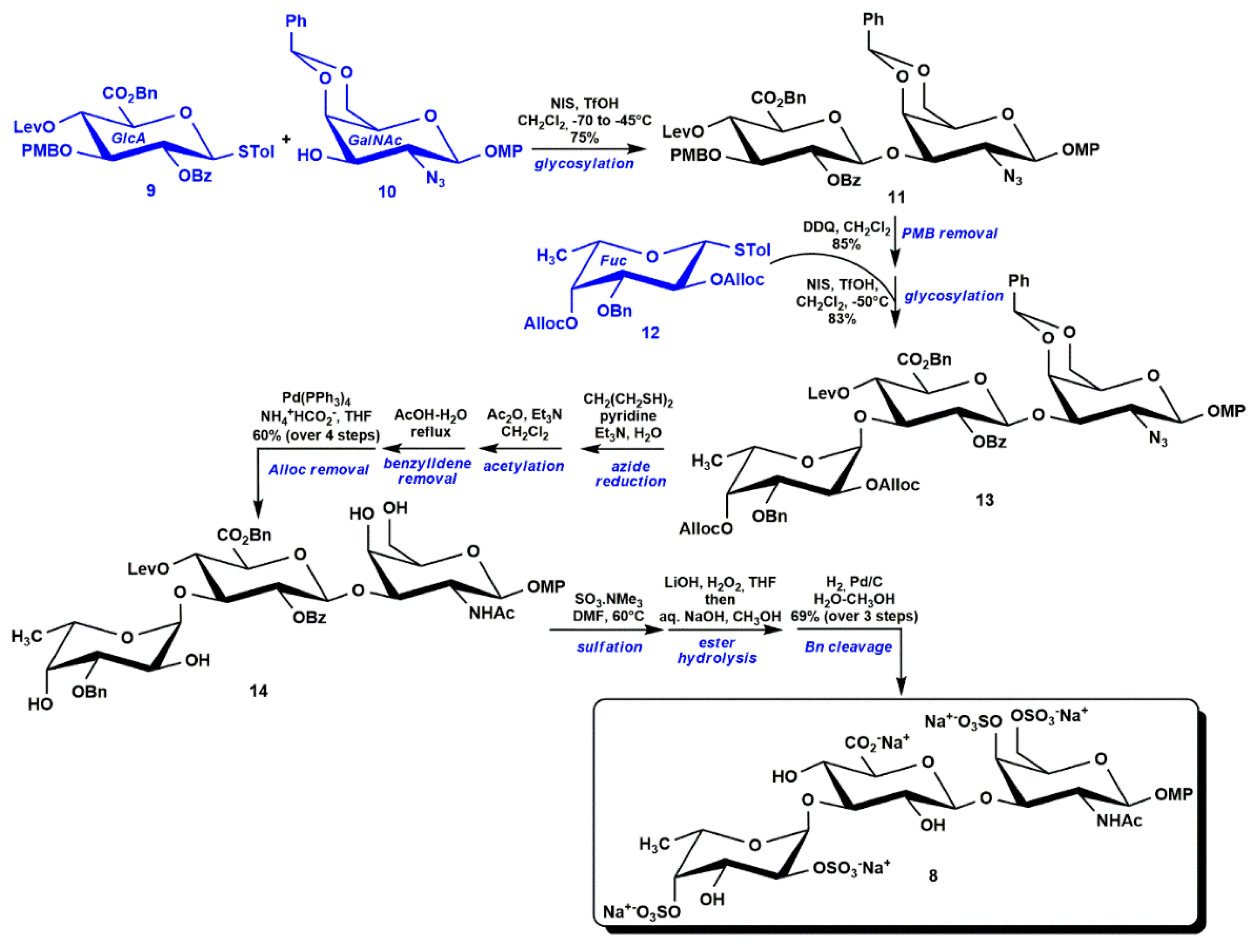
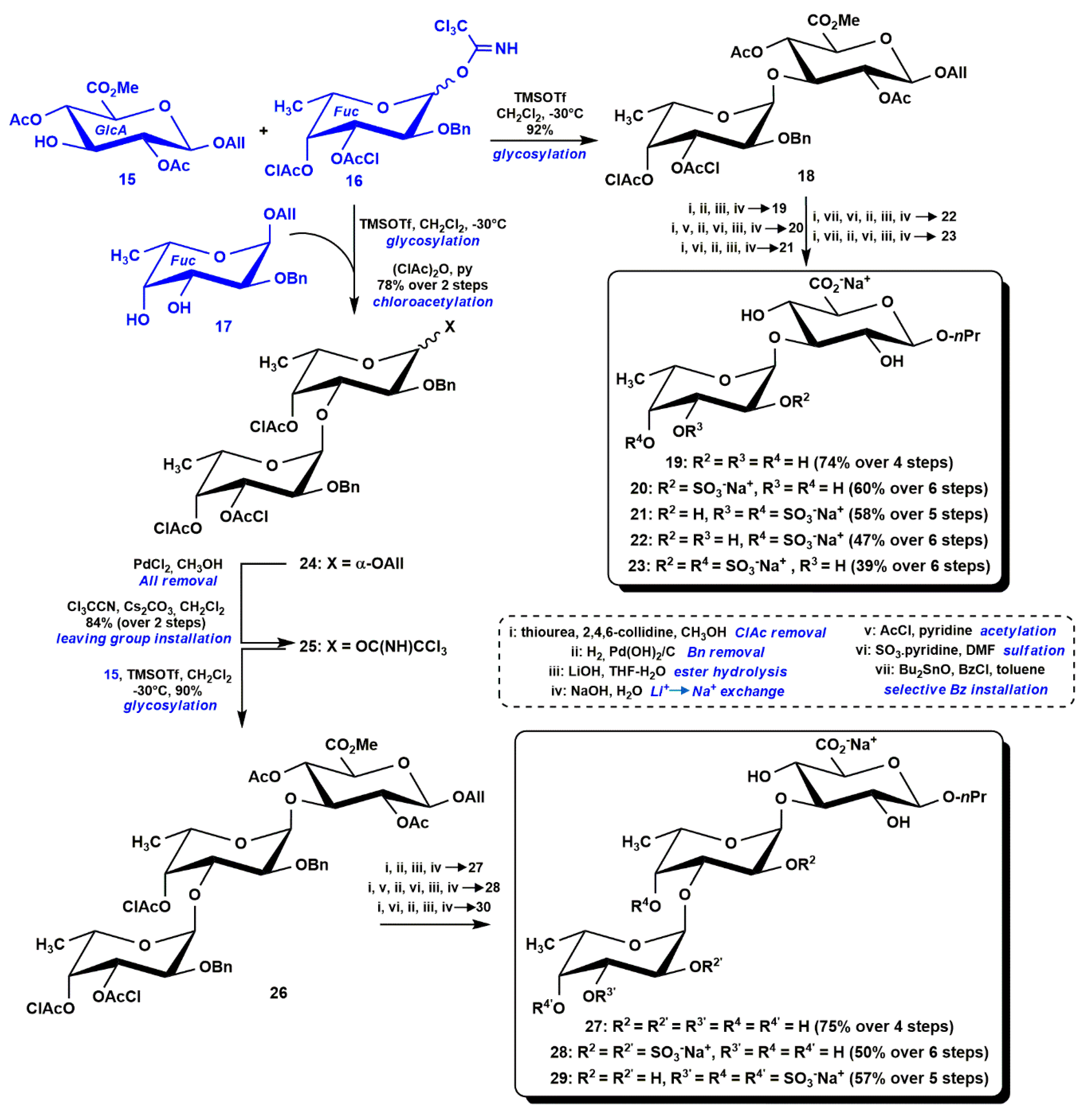
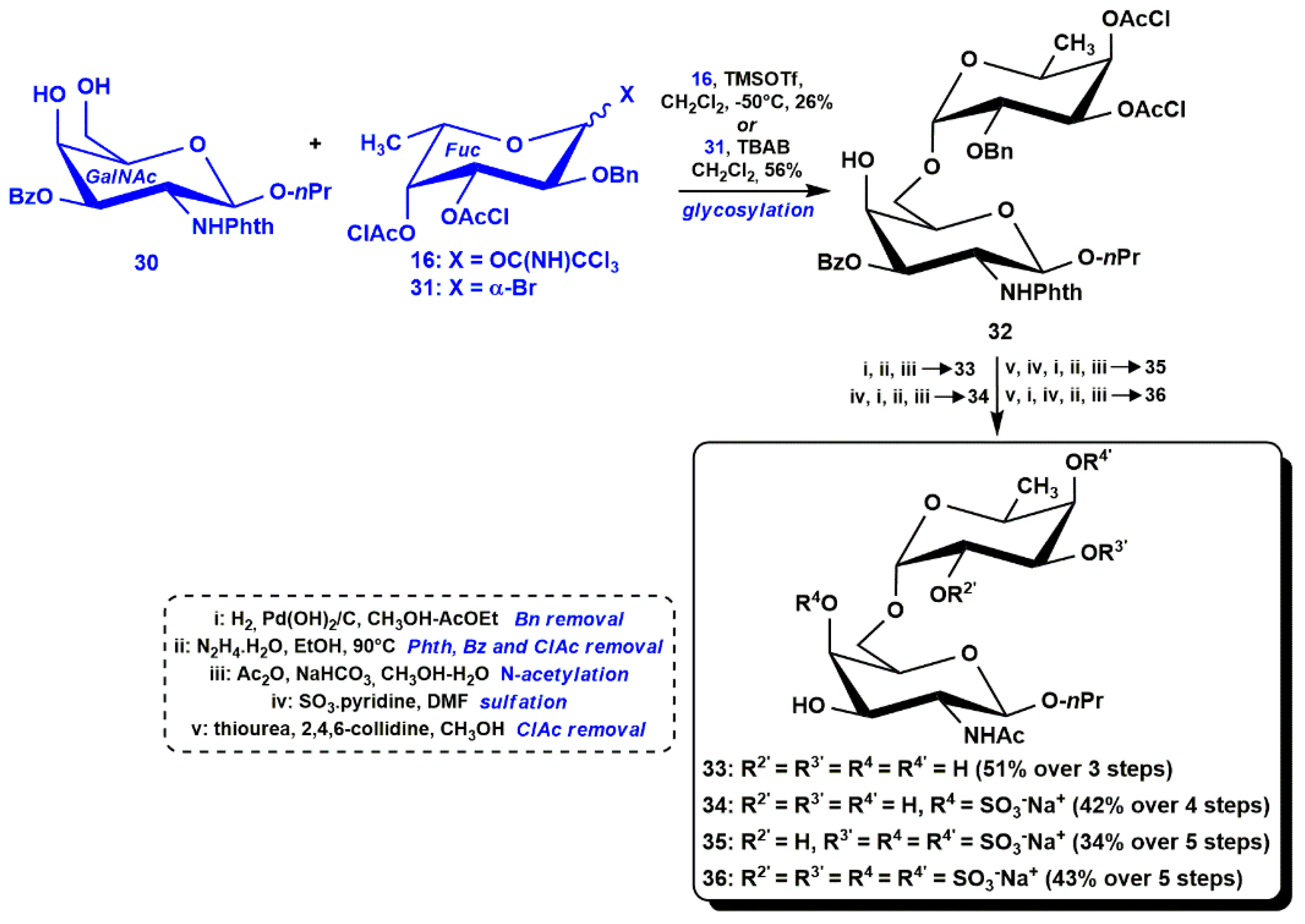
3.1. Semi-Synthesis of fCS Oligosaccharides
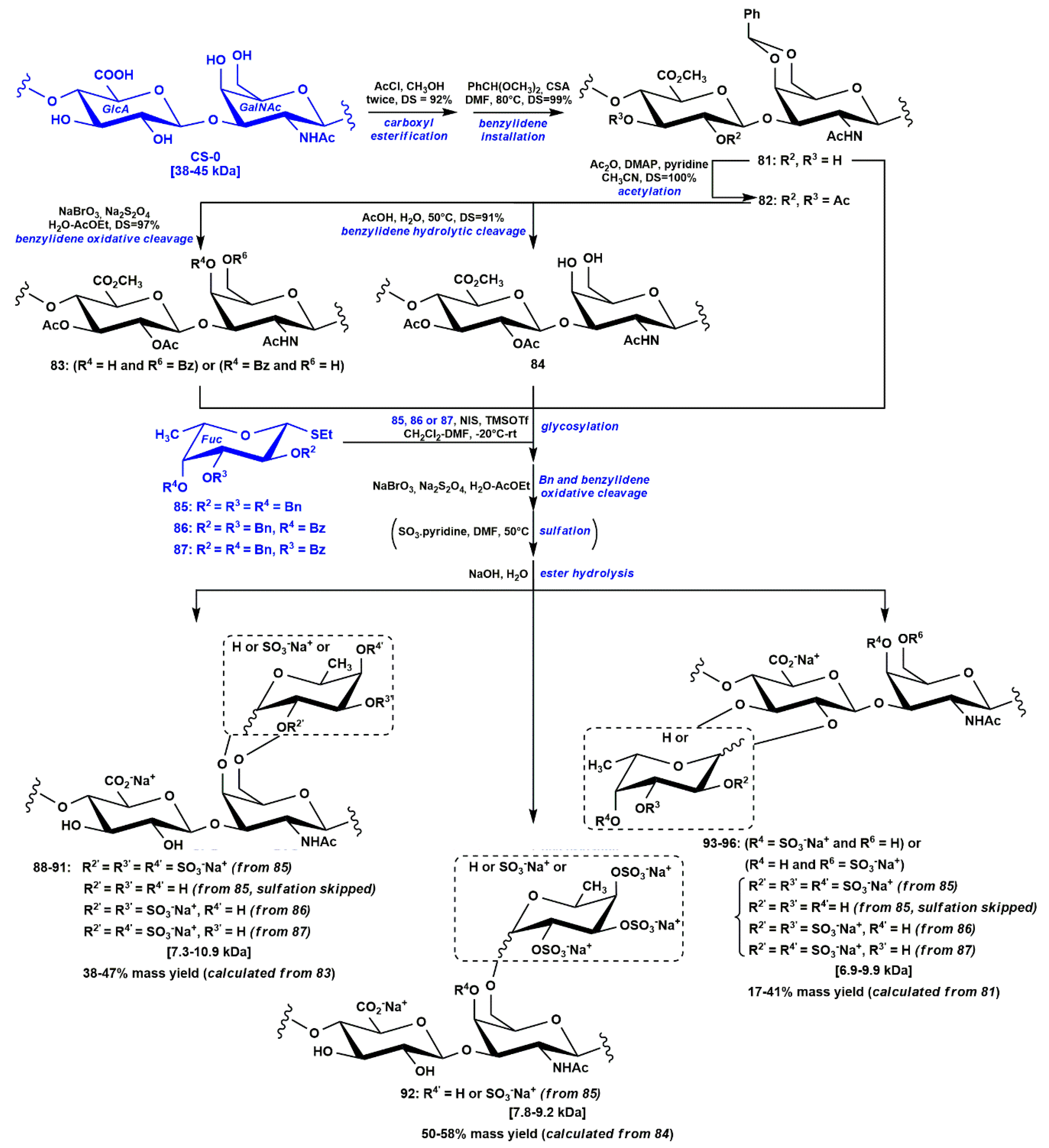
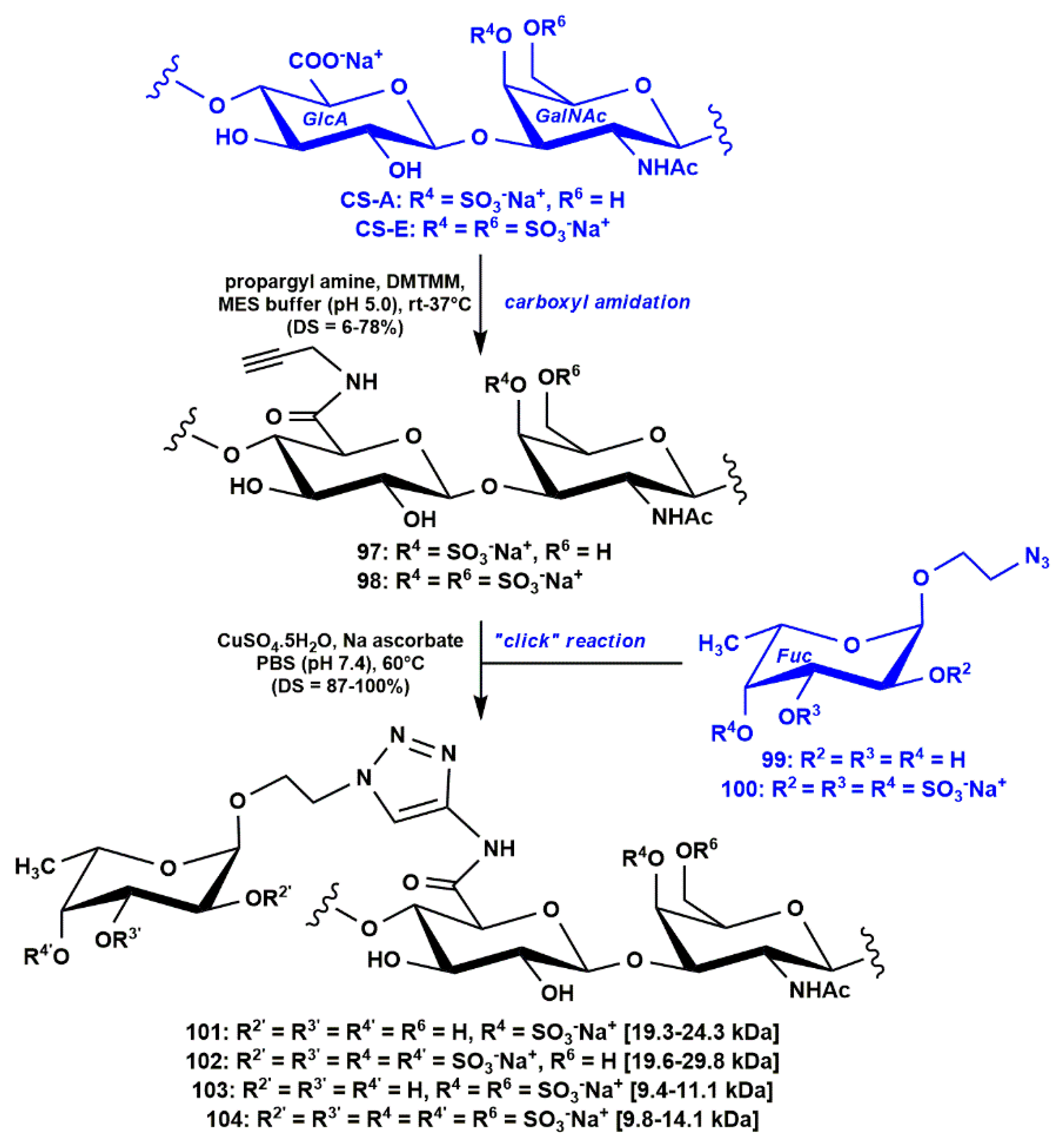
3.2. Semi-Synthesis of Low Molecular Weight fCS Polysaccharides
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lindahl, U.; Couchman, J.; Kimata, K.; Esko, J.D. Proteoglycans and sulfated glycosaminoglycans. In Essential of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017; Chapter 17. [Google Scholar]
- Kinoshita-Toyoda, A.; Yamada, S.; Haslam, S.M.; Khoo, K.H.; Sugiura, M.; Morris, H.R.; Dell, A.; Sugahara, K. Structural determination of five novel tetrasaccharides containing 3-O-sulfated d-glucuronic acid and two rare oligosaccharides containing a β-d-glucose branch isolated from squid cartilage chondroitin sulfate. Biochemistry 2004, 43, 11063–11074. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Takeda, K.; Mukuno, A.; Okamoto, Y.; Masuko, S.; Linhardt, R.J.; Toida, T. Identification of keratan sulfate disaccharide at C-3 position of glucuronate of chondroitin sulfate from Mactra chinensis. Biochem. J. 2016, 473, 4145–4148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pomin, V.H. Holothurian fucosylated chondroitin sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.S. Perspective on the use of sulfated polysaccharides from marine organisms as a source of new antithrombotic drugs. Mar. Drugs 2015, 13, 2770–2784. [Google Scholar] [CrossRef] [PubMed]
- Glauser, B.F.; Pereira, M.S.; Monteiro, R.Q.; Mourão, P.A.S. Serpin-independent anticoagulant activity of a fucosylated chondroitin sulfate. Thromb. Haemost. 2018, 100, 420–428. [Google Scholar] [CrossRef]
- Buyue, Y.; Sheehan, J.P. Fucosylated chondroitin sulfate inhibits plasma thrombin generation via targeting of the factor IXa heparin-binding exosite. Blood 2009, 114, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.J.C.; Sucupira, I.D.; Oliveira, S.-N.M.C.G.; Santos, G.R.C.; Mourão, P.A.S. Improved anticoagulant effect of fucosylated chondroitin sulfate orally administered as gastroresistant tablets. Thromb. Haemost. 2017, 117, 662–670. [Google Scholar] [PubMed]
- Myron, P.; Siddiquee, S.; Al Azad, S. Fucosylated chondroitin sulfate diversity in sea cucumbers: A review. Carbohydr. Polym. 2014, 112, 173–178. [Google Scholar] [CrossRef]
- Monteiro-Machado, M.; Tomaz, M.A.; Fonseca, R.J.C.; Strauch, M.A.; Cons, B.L.; Borges, P.A.; Patrão-Neto, F.C.; Tavares-Henriques, M.S.; Teixeira-Cruz, J.M.; Calil-Elias, S.; et al. Occurrence of sulfated fucose branches in fucosylated chondroitin sulfate are essential for the polysaccharide effect preventing muscle damage induced by toxins and crude venom from Bothrops jararacussu snake. Toxicon 2015, 98, 20–33. [Google Scholar] [CrossRef]
- Mourão, P.A.S.; Guimarães, M.A.M.; Mulloy, B.; Thomas, S.; Gray, E. Antithrombotic activity of a fucosylated chondroitin sulphate from echinoderm: Sulphated fucose branches on the polysaccharide account for its antithrombotic action. Br. J. Haematol. 1998, 101, 647–652. [Google Scholar] [CrossRef]
- Mourão, P.A.S.; Pereira, M.S.; Pavão, M.S.; Mulloy, B.; Tollefsen, D.M.; Mowinckel, M.C.; Abildgaard, U. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm. Sulfated fucose branches on the polysaccharide account for its high anticoagulant action. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cai, C.; Chang, Y.; Zhang, F.; Linhardt, R.J.; Xue, C.; Li, G.; Yu, G. A novel structural fucosylated chondroitin sulfate from Holothuria mexicana and its effects on growth factors binding and anticoagulation. Carbohydr. Polym. 2018, 181, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Nifantiev, N.E.; Usov, A.I. The structure of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria frondosa. Carbohydr. Polym. 2017, 165, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Shashkov, A.S.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Structural characterization of fucosylated chondroitin sulfates from sea cucumbers Apostichopus japonicus and Actinopyga mauritiana. Carbohydr. Polym. 2016, 153, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Yang, S.; Lv, Z. Separation, purification, structures and anticoagulant activities of fucosylated chondroitin sulfates from Holothuria scabra. Int. J. Biol. Macromol. 2018, 108, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.S.; Vilanova, E.; Soares, P.A.G. Unveiling the structure of sulfated fucose-rich polysaccharides via nuclear magnetic resonance spectroscopy. Curr. Opin. Struct. Biol. 2018, 50, 33–41. [Google Scholar] [CrossRef]
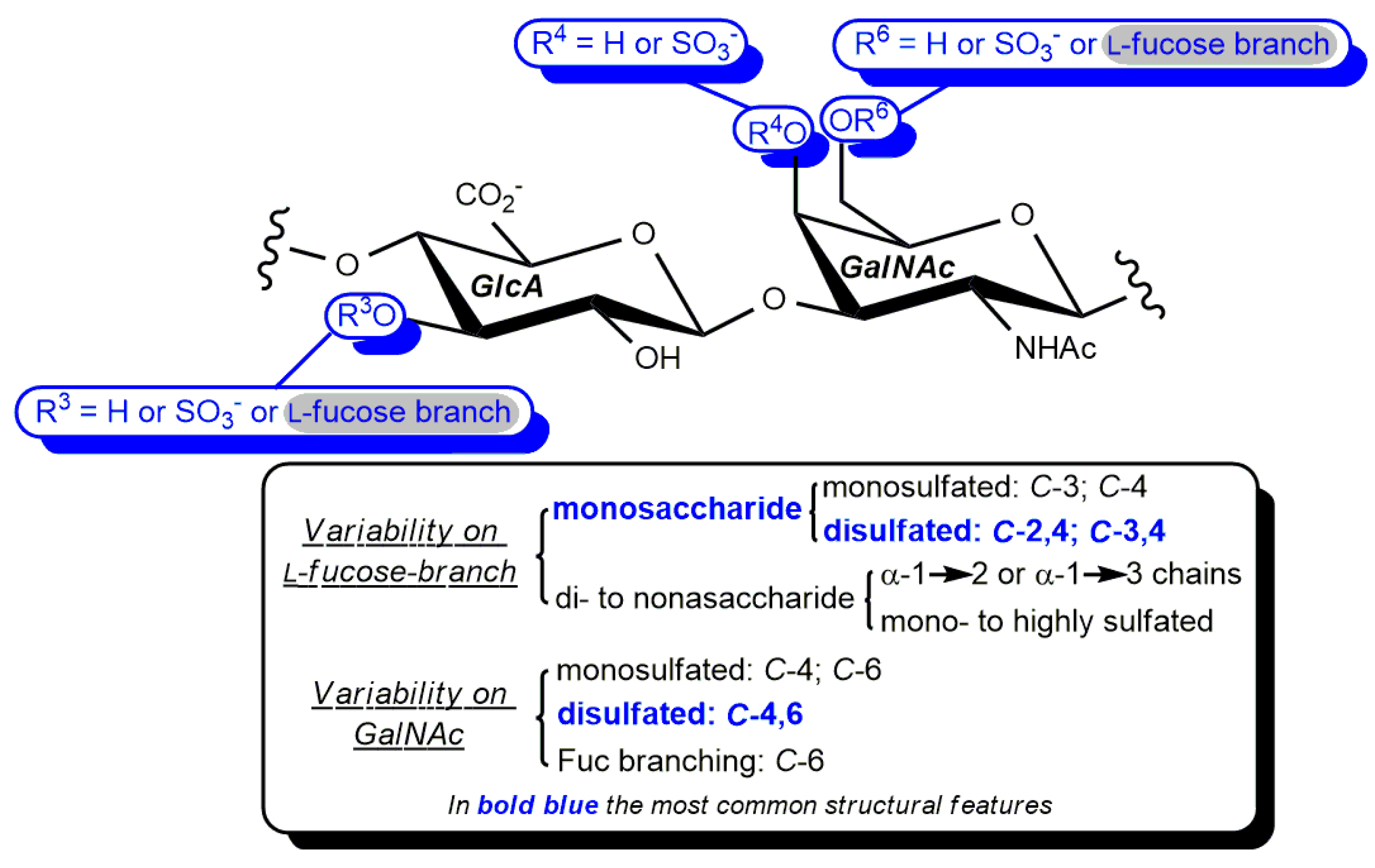
- Ustyuzhanina, N.E.; Bilan, M.I.; Nifantiev, N.E.; Usov, A.I. New insight on the structural diversity of holothurian fucosylated chondroitin sulfates. Pure Appl. Chem. 2019, 91, 1065–1071. [Google Scholar] [CrossRef]
- Khotimchenko, Y. Pharmacological potential of sea cucumbers. Int. J. Mol. Sci. 2018, 19, 1342. [Google Scholar] [CrossRef]
- Chahed, L.; Balti, R.; Elhiss, S.; Bouchemal, N.; Ajzenberg, N.; Ollivier, V.; Chaubet, F.; Mounir Maaroufi, R.; Ben Mansour, M. Anticoagulant activity of fucosylated chondroitin sulfate isolated from Cucumaria syracusana. Process Biochem. 2020, 91, 149–157. [Google Scholar] [CrossRef]
- Soares, P.A.G.; Ribeiro, K.A.; Valente, A.P.; Capillé, N.V.; Oliveira, S.-N.M.C.G.; Tovar, A.M.F.; Pereira, M.S.; Vilanova, E.; Mourão, P.A.S. A unique fucosylated chondroitin sulfate type II with strikingly homogeneous and neatly distributed α-fucose branches. Glycobiology 2018, 28, 565–579. [Google Scholar] [CrossRef]
- Fonseca, R.J.C.; Oliveira, S.-N.M.C.G.; Pomin, V.H.; Mecawi, A.S.; Araujo, I.G.; Mourão, P.A.S. Effects of oversulfated and fucosylated chondroitin sulfates on coagulation. Thromb. Haemost. 2010, 103, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanhina, N.E.; Bilan, M.I.; Nifantiev, N.E.; Usov, A.I. Structural analysis of holothurian fucosylated chondroitin sulfates: Degradation versus non-degradative approach. Carbohydr. Res. 2019, 476, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Borodina, E.Y.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. A highly regular fucosylated chondroitin sulfate from the sea cucumber Massinium magnum: Structure and effects on coagulation. Carbohydr. Polym. 2017, 167, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Gao, N.; Yin, R.; Lin, L.; Xiao, C.; Zhou, L.; Li, Z.; Purcell, S.W.; Wu, M.; Zhao, J. Precise structures of fucosylated glycosaminoglycan and its oligosaccharides as novel intrinsic factor Xase inhibitors. Eur. J. Med. Chem. 2018, 148, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, W.; Li, X.; Zhou, L.; Wang, Z.; Lin, L.; Chen, D.; Zhao, L.; Li, Z.; Liu, S.; et al. Precise structure and anti-intrinsic tenase complex activity of three fucosylated glycosaminoglycans and their fragments. Carbohydr. Polym. 2019, 246, 115146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, L.; Huang, H.; Linhardt, R.J. Chemoenzymatic synthesis of glycosaminoglycans. Acc. Chem. Res. 2020, 53, 335–346. [Google Scholar] [CrossRef]
- Mende, M.; Bednarek, C.; Wawryszyn, M.; Sauter, P.; Biskup, M.B.; Schepers, U.; Bräse, S. Chemical synthesis of glycosaminoglycans. Chem. Rev. 2016, 116, 8193–8255. [Google Scholar] [CrossRef]
- Bedini, E.; Laezza, A.; Iadonisi, A. Chemical derivatization of sulfated glycosaminoglycans. Eur. J. Org. Chem. 2016, 3018–3042. [Google Scholar] [CrossRef]
- Tamura, J.; Tanaka, H.; Nakamura, A.; Takeda, N. Synthesis of β-d-GalNAc(4,6-diS)(1–4)[α-l-Fuc(2,4-diS)(1–3)]-β-d-GlcA, a novel trisaccharide unit of chondroitin sulfate with a fucose branch. Tetrahedron Lett. 2013, 54, 3940–3943. [Google Scholar] [CrossRef]
- He, H.; Chen, D.; Li, X.; Li, C.; Zhao, J.-H.; Qin, H.-B. Synthesis of trisaccharide repeating unit of fucosylated chondroitin sulfate. Org. Biomol. Chem. 2019, 17, 2877–2882. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Fomitskaya, P.A.; Gerbst, A.G.; Dmitrenok, A.S.; Nifantiev, N.E. Synthesis of the oligosaccharides related to branching sites of fucosylated chondroitin sulfates from sea cucumbers. Mar. Drugs 2015, 13, 770–787. [Google Scholar] [CrossRef] [PubMed]
- Gerbst, A.G.; Dmitrenok, A.S.; Ustyuzhanina, N.E.; Nifantiev, N.E. Conformational analysis of the oligosaccharides related to side chains of holothurian fucosylated chondroitin sulfates. Mar. Drugs 2015, 13, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Vinnitskiy, D.Z.; Ustyuzhanina, N.E.; Dmitrenok, A.S.; Shashkov, A.S.; Nifantiev, N.E. Synthesis and NMR analysis of model compounds related to fucosylated chondroitin sulfates: GalNAc and Fuc(1→6)GalNAc derivatives. Carbohydr. Res. 2017, 438, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Nifantiev, N.E.; Usov, A.I. Two fucosylated chondroitin sulfates from the sea cucumber Eupentacta fraudatrix. Carbohydr. Polym. 2017, 164, 8–12. [Google Scholar] [CrossRef]
- Santos, G.R.C.; Glauser, B.F.; Parreiras, L.A.; Vilanova, E.; Mourão, P.A.S. Distinct structures of the α-fucose branches in fucosylated chondroitin sulfates do not affect their anticoagulant activity. Glycobiology 2015, 25, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Lopin-Bon, C.; Jacquinet, J.-C. From polymer to size-defined oligomers: An expeditious route for the preparation of chondroitin oligosaccharides. Angew. Chem. Int. Ed. 2006, 45, 2574–2578. [Google Scholar] [CrossRef] [PubMed]
- Vibert, A.; Lopin-Bon, C.; Jacquinet, J.-C. From polymer to size-defined oligomers: A step economy process for the efficient and stereocontrolled construction of chondroitin oligosaccharides and biotinylated conjugates thereof: Part 1. Chem. Eur. J. 2009, 15, 9561–9578. [Google Scholar] [CrossRef] [PubMed]
- Jacquinet, J.-C.; Lopin-Bon, C.; Vibert, A. A Highly Divergent and stereocontrolled construction of chondroitin sulfate A, C, D, E, K, L, and M oligomers from a single precursor: Part 2. Chem. Eur. J. 2009, 15, 9579–9595. [Google Scholar] [CrossRef] [PubMed]
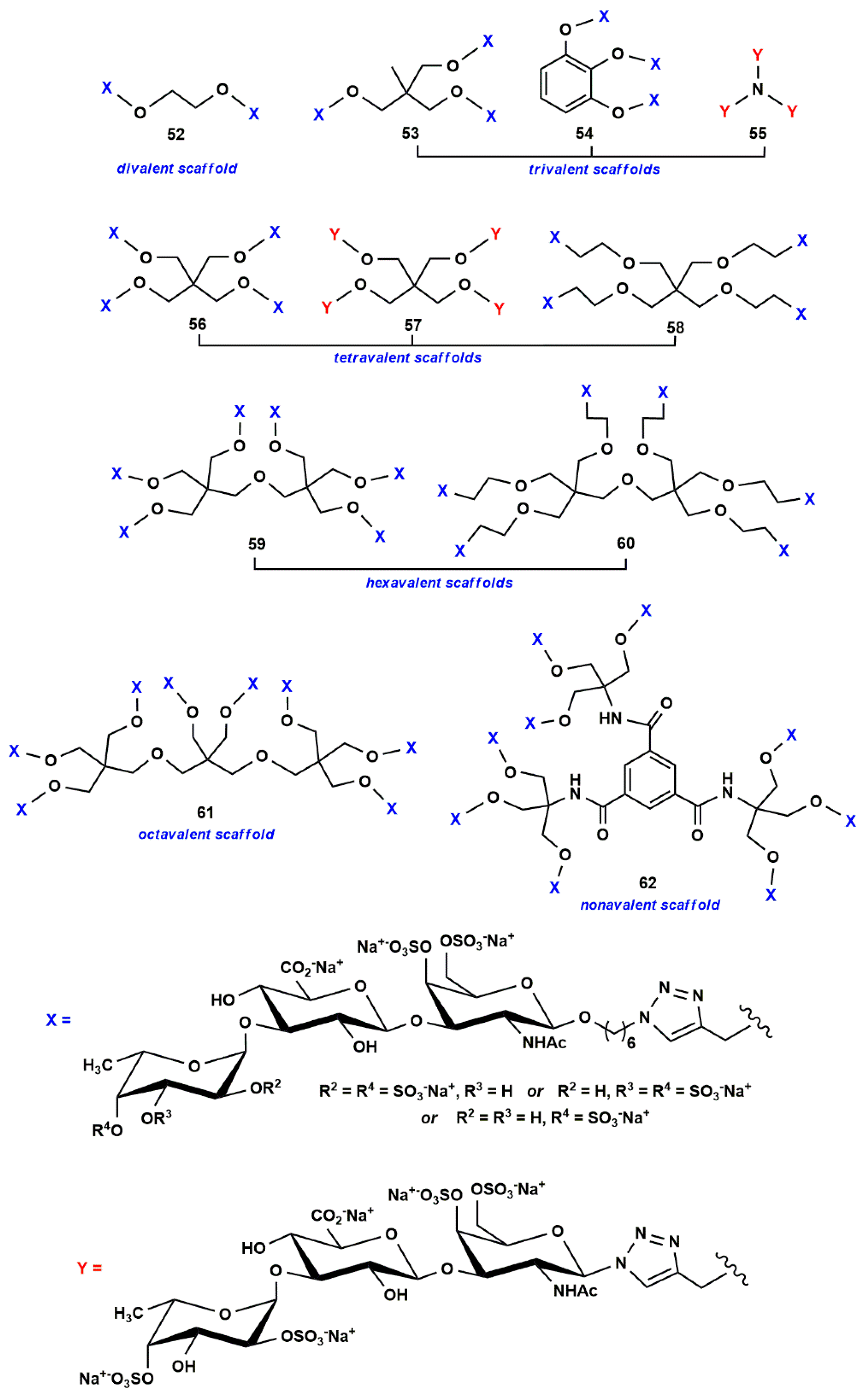
- Zhang, X.; Yao, W.; Xu, X.; Sun, H.; Zhao, J.; Meng, X.; Wu, M.; Li, Z. Synthesis of fucosylated chondroitin sulfate glycoclusters: A robust route to new anticoagulant agents. Chem. Eur. J. 2018, 24, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Wu, M.; Li, Z. Synthesis and anticoagulation studies of “short-armed” fucosylated chondroitin sulfate glycoclusters. Carbohydr. Res. 2018, 467, 45–51. [Google Scholar] [CrossRef]
- Kornilov, A.V.; Sukhova, E.V.; Nifantiev, N.E. Preparative route to glucuronyl donors bearing temporary protecting group at O-3 via 6,3-lactonisation by Bz2O or Piv2O. Carbohydr. Res. 2001, 336, 309–313. [Google Scholar] [CrossRef]
- Wu, M.; Wen, D.; Gao, N.; Xiao, L.; Yang, L.; Xu, W.; Lian, W.; Peng, J.; Jiang, J.; Zhao, J. Anticoagulant and antithrombotic evaluation of native fucosylated chondroitin sulfates and their derivatives as selective inhibitors of intrinsic factor Xase. Eur. J. Med. Chem. 2015, 92, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, H.; Lin, L.; Yao, W.; Zhao, J.; Wu, M.; Li, Z. Synthesis of fucosylated chondroitin sulfate nonasaccharide as a novel anticoagulant targeting intrinsic factor Xase complex. Angew. Chem. Int. Ed. 2018, 57, 12880–12885. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, I.; Koizumi, H.; Chen, F.; Endo, M. Inhibitory effect of chondroitin sulfate oligosaccharides on bovine testicular hyaluronidase. Carbohydr. Polym. 2015, 121, 362–371. [Google Scholar] [CrossRef]
- Tanaka, T.; Nagai, H.; Noguchi, M.; Kobayashi, A.; Shoda, S.-I. One-step conversion of unprotected sugars to β-glycosyl azides using 2-chloroimidazolinium salt in aqueous solution. Chem. Commun. 2009, 23, 3378–3379. [Google Scholar] [CrossRef]
- Cheng, A.; Hendel, J.L.; Colangelo, K.; Bonin, M.; Auzanneau, F.I. Convenient temporary methyl imidate protection of N-acetylglucosamine and glycosylation at O-4. J. Org. Chem. 2008, 73, 7574–7579. [Google Scholar] [CrossRef]
- Yin, R.; Zhou, L.; Gao, N.; Li, Z.; Zhao, L.; Shang, F.; Wu, M.; Zhao, J. Oligosaccharides from depolymerized fucosylated glycosaminoglycan: Structures and minimum size for intrinsic factor Xase complex inhibition. J. Biol. Chem. 2018, 293, 14089–14099. [Google Scholar] [CrossRef]
- Santos, G.R.C.; Porto, A.C.O.; Soares, P.A.G.; Vilanova, E.; Mourão, P.A.S. Exploring the structure of fucosylated chondroitin sulfate through bottom-up nuclear magnetic resonance and electrospray ionization-high-resolution mass spectrometry approaches. Glycobiology 2017, 625–634. [Google Scholar] [CrossRef]
- Xu, L.; Gao, N.; Xiao, C.; Lin, L.; Purcell, S.W.; Wu, M.; Zhao, J. Modulating the degree of fucosylation of fucosylated chondroitin sulfate enhances heparin cofactor II-dependent thrombin inhibition. Eur. J. Med. Chem. 2018, 154, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Wu, F.; Yi, L.; Chen, L.; Jin, Y.; Ding, X.; Ouyang, Y.; Yao, Y.; Jiang, Y.; Zhang, Z. Structure characterization of a heavily fucosylated chondroitin sulfate from sea cucumber (H. leucospilota) with bottom-up strategies. Carbohydr. Polym. 2020, 240, 116337. [Google Scholar] [CrossRef]
- Shi, D.; Qi, J.; Zhang, H.; Yang, H.; Yang, Y.; Zhao, X. Comparison of hydrothermal depolymerization and oligosaccharide profile of fucoidan and fucosylated chondroitin sulfate from Holothuria floridana. Int. J. Biol. Macromol. 2019, 132, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Ye, X.; Guo, X.; Liao, N.; Yin, X.; Hu, Y.; Sun, Y.; Liu, D.; Chen, S. Depolymerization of fucosylated chondroitin sulfate from sea cucumber, Pearsonothuria graeffei, via 60Co irradiation. Carbohydr. Polym. 2013, 93, 604–614. [Google Scholar] [CrossRef]
- Wu, M.; Xu, S.; Zhao, J.; Kang, H.; Ding, H. Free-radical depolymerization of glycosaminoglycan from sea cucumber Thelenata ananas by hydrogen peroxide and copper ions. Carbohydr. Polym. 2010, 80, 1116–1124. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Jiang, T.; Lv, L.; Zhang, B.; Lv, Z. Depolymerized glycosaminoglycan and its anticoagulant activities from sea cucumber Apostichopus japonicus. Int. J. Biol. Macromol. 2015, 72, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, J.; Shan, X.; Zhang, X.; Zhao, X.; Li, Q.; Wang, X.; Cai, C.; Li, G.; Yu, G. Antithrombotic activities of fucosylated chondroitin sulfates and their depolymerized fragments from two sea cucumbers. Carbohydr. Polym. 2016, 152, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Li, G.; Li, C.; Li, Q.; Li, J.; Liu, C.; Pan, L.; Li, S.; Cai, C.; Hao, J.; et al. Two different fucosylated chondroitin sulfates: Structural elucidation, stimulating hematopoiesis and immune-enhancing effects. Carbohydr. Polym. 2020, 230, 115698. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, S.; Zhi, Z.; Yan, L.; Ye, X.; Ding, T.; Yan, L.; Linhardt, R.J.; Chen, S. Depolymerization of fucosylated chondroitin sulfate with a modified Fenton-system and anticoagulant activity of the resulting fragments. Mar. Drugs 2016, 14, 170. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Wu, L.; Yang, H.; Wei, C.; Ding, T.; Linhardt, R.J.; Zheng, X.; Ye, X.; Chen, S. Ultrasound-assisted fast preparation of low molecular weight fucosylated chondroitin sulfate with antitumor activity. Carbohydr. Polym. 2019, 209, 82–91. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Yan, L.; Ding, T.; Linhardt, R.J.; Yu, Y.; Liu, X.; Liu, D.; Ye, X.; Chen, S. Fucosylated chondroitin sulfate oligosaccharides exert anticoagulant activity by targeting at intrinsic tenase complex with low FXII activation: Importance of sulfation pattern and molecular size. Eur. J. Med. Chem. 2017, 139, 191–200. [Google Scholar] [CrossRef]
- Guo, Y.; Conrad, H.E. The disaccharide composition of heparins and heparan sulfates. Anal. Biochem. 1989, 176, 96–104. [Google Scholar] [CrossRef]
- Zhao, L.; Lai, S.; Huang, R.; Wu, M.; Gao, N.; Xu, L.; Qin, H.; Peng, W.; Zhao, J. Structure and anticoagulant activity of fucosylated glycosaminoglycan degraded by deaminative cleavage. Carbohydr. Polym. 2013, 98, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, M.; Xiao, C.; Yang, L.; Zhou, L.; Gao, N.; Li, Z.; Chen, J.; Chen, J.; Liu, J.; et al. Discovery of an intrinsic tenase complex inhibitor: Pure nonasaccharide from fucosylated glycosaminoglycan. Proc. Natl. Acad. Sci. USA 2015, 112, 8284–8289. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Peng, Y.; Zhou, L.; Zheng, W.; Liu, X.; Wang, P.; Yuan, Q.; Gao, N.; Zhao, L.; Zhao, J. Precise structure and anticoagulant activity of fucosylated glycosaminoglycans from Apostichopus japonicas: Analysis of its depolymerized fragments. Mar. Drugs 2019, 17, 195. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, J.; Wang, D.; Ding, T.; Hu, Y.; Ye, X.; Linhardt, R.J.; Chen, S. Molecular size is important for safety and selective inhibition of intrinsic factor Xase for fucosylated chondroitin sulfate. Carbohydr. Polym. 2017, 178, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, L.; Li, J.; Yu, Y.; Liu, X.; Ye, X.; Linhardt, R.J.; Chen, S. Bottom-up analysis using liquid chromatography-Fourier transform mass spectrometry to characterize fucosylated chondroitin sulfates from sea cucumbers. Glycobiology 2019, 29, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J. β-Eliminative degradation of carbohydrates containing uronic acid residues. Adv. Carbohydr. Chem. Biochem. 1974, 29, 229–303. [Google Scholar]
- Gao, N.; Lu, F.; Xiao, C.; Yang, L.; Chen, J.; Zhou, K.; Wen, D.; Li, Z.; Wu, M.; Jiang, J.; et al. β-Eliminative depolymerization of the fucosylated chondroitin sulfate and anticoagulant activities of resulting fragments. Carbohydr. Polym. 2015, 127, 427–437. [Google Scholar] [CrossRef]
- Yang, W.; Chen, D.; He, Z.; Zhou, L.; Cai, Y.; Mao, H.; Gao, N.; Zuo, Z.; Yin, R.; Zhao, J. NMR characterization and anticoagulant activity of the oligosaccharides from the fucosylated glycosaminoglycan isolated from Holothuria coluber. Carbohydr. Polym. 2020, 233, 115844. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, Y.; Zong, C.; Lin, C.; Boons, G.J.; Zaia, J. Discovery of a heparan sulfate 3-O-sulfation specific peeling reaction. Anal. Chem. 2015, 87, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, C.; Cimini, D.; De Rosa, M. Production of chondroitin sulfate and chondroitin. Appl. Microbiol. Biotechnol. 2010, 87, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Laezza, A.; Iadonisi, A.; De Castro, C.; De Rosa, M.; Schiraldi, C.; Parrilli, M.; Bedini, E. Chemical fucosylation of a polysaccharide: A semisynthetic access to fucosylated chondroitin sulfate. Biomacromolecules 2015, 16, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Laezza, A.; Iadonisi, A.; Pirozzi, A.V.A.; Diana, P.; De Rosa, M.; Schiraldi, C.; Parrilli, M.; Bedini, E. A modular approach to a library of semi-synthetic fucosylated chondroitin sulfate polysaccharides with different sulfation and fucosylation patterns. Chem. Eur. J. 2016, 22, 18215–18226. [Google Scholar] [CrossRef] [PubMed]
- Vessella, G.; Traboni, S.; Pirozzi, A.V.A.; Laezza, A.; Iadonisi, A.; Schiraldi, C.; Bedini, E. A study for the access to a semi-synthetic regioisomer of natural fucosylated chondroitin sulfate with fucosyl branches on N-acetyl-galactosamine units. Mar. Drugs 2019, 17, 655. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-R.; Lai, Y.-H.; Chen, J.-H.; Liu, C.-Y.; Mong, K.-K.T. Dimethylformamide: An unusual glycosylation modulator. Angew. Chem. Int. Ed. 2011, 50, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, M.; Barone, G.; Guariniello, L.; Iadonisi, A. Facile cleavage of carbohydrate benzyl ethers and benzylidene acetals using the NaBrO3-Na2S2O4 reagent under two-phase conditions. Tetrahedron Lett. 1999, 40, 8439–8441. [Google Scholar] [CrossRef]
- Comegna, D.; Bedini, E.; Di Nola, A.; Iadonisi, A.; Parrilli, M. The behaviour of deoxyhexose trihaloacetimidates in selected glycosylations. Carbohydr. Res. 2007, 342, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Vessella, G.; Traboni, S.; Iadonisi, A.; Schiraldi, C.; Bedini, E. manuscript in preparation.
- Vessella, G.; Traboni, S.; Cimini, D.; Iadonisi, A.; Schiraldi, C.; Bedini, E. Development of semisynthetic, regioselective pathways for accessing the missing sulfation patterns of chondroitin sulfate. Biomacromolecules 2019, 20, 3021–3030. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, P.; Wang, L.; Sun, T.; Cai, C.; Yu, G. Synthesis and properties of functional glycomimetics through click grafting of fucose onto chondroitin sulfates. Biomacromolecules 2019, 20, 3798–3808. [Google Scholar] [CrossRef]
- Gao, N.; Wu, M.; Liu, S.; Lian, W.; Li, Z.; Zhao, J. Preparation and characterization of O-acylated fucosylated chondroitin sulfate from sea cucumber. Mar. Drugs 2012, 10, 1647–1661. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vessella, G.; Traboni, S.; Laezza, A.; Iadonisi, A.; Bedini, E. (Semi)-Synthetic Fucosylated Chondroitin Sulfate Oligo- and Polysaccharides. Mar. Drugs 2020, 18, 293. https://doi.org/10.3390/md18060293
Vessella G, Traboni S, Laezza A, Iadonisi A, Bedini E. (Semi)-Synthetic Fucosylated Chondroitin Sulfate Oligo- and Polysaccharides. Marine Drugs. 2020; 18(6):293. https://doi.org/10.3390/md18060293
Chicago/Turabian StyleVessella, Giulia, Serena Traboni, Antonio Laezza, Alfonso Iadonisi, and Emiliano Bedini. 2020. "(Semi)-Synthetic Fucosylated Chondroitin Sulfate Oligo- and Polysaccharides" Marine Drugs 18, no. 6: 293. https://doi.org/10.3390/md18060293
APA StyleVessella, G., Traboni, S., Laezza, A., Iadonisi, A., & Bedini, E. (2020). (Semi)-Synthetic Fucosylated Chondroitin Sulfate Oligo- and Polysaccharides. Marine Drugs, 18(6), 293. https://doi.org/10.3390/md18060293