Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits
Abstract
1. Introduction
2. Marine-Based Beneficial Molecules
2.1. Chitin and Chitosan
2.1.1. Antioxidant Properties
2.1.2. Antimicrobial Properties
2.1.3. Anti-Hypertensive Activity
2.1.4. Anti-Allergy and Anti-Inflammatory Activity
2.1.5. Anti-Obesity and Anti-Diabetic Activity
2.1.6. Anti-Cancer and Anti-Tumor Activity
2.2. Beneficial Molecules from Marine Macroalgae
2.2.1. Pigments
2.2.2. Polysaccharides
2.2.3. Phenolic Compounds
2.3. Fish Oil
2.4. EAA in Protein Supplement Systems
| Fish Oils | Functional Substances | Beneficial Effects in Human Health and Pharmaceutical Properties | References | |
|---|---|---|---|---|
| Krill oil | Fatty acids |
|
| [107] |
| Vitamins |
|
| [108] | |
| Pigments |
|
| [108] | |
| Tuna oil | Fatty acids |
|
| [108,109,110,111] |
| Vitamins |
|
| [110,112] | |
| Mackerel oil | Fatty acids |
|
| [110,112,113,114] |
| Vitamins |
|
| [110,112] | |
| Salmon oil | Fatty acids |
|
| [115,116,117,118,119] |
| Vitamins |
|
| [108,110] | |
| Pigments |
|
| [120] | |
| Sardine oil | Fatty acids |
|
| [121,122,123] |
| Vitamins |
|
| [110] | |
| Herring oil | Fatty acids |
|
| [112,119,124,125] |
| Menhaden oil | Fatty acids |
|
| [112,126,127,128] |
| Vitamins |
|
| [108,129] | |
| Essential Amino Acid (EAA) | Examples of Marine Sources | Beneficial Effects in Human Health and Pharmaceutical Properties | References |
|---|---|---|---|
| Arginine (Arg) |
|
| [130,131,132,133] |
| Histidine (His) |
|
| [131,134,135,136] |
| Isoleucine (Iso) |
|
| [131,137] |
| Leucine (Leu) |
|
| [130,131,137,138] |
| Lysine (Lys) |
|
| [130,131,134,138,139,140] |
| Methionine (Met) |
|
| [130,131] |
| Phenylalanine (Phe) |
|
| [130,131,141] |
| Tryptophan (Trp) | Fish: Sardina spp., K. pelamis, Thunnus sp., T. putitora |
| [130,137] |
| Valine (Val) |
|
| [130,137] |
| Proline (Pro) |
|
| [130,137] |
| Glycine (Gly) |
|
| [130,137,142,143] |
2.5. Minerals in Seafood for Human Diet
2.6. Marine-Based Vitamin Sources
| Vitamin | Source | Key Findings | Reference |
|---|---|---|---|
| E | Crude oil from farmed tuna liver Crude oil from farmed tuna gill and gut Crude oil from sardine heads, gut and fins Crude oil from whole sardine Crude oil from farmed seabass and seabream heads and gut | Significantly lower α-tocopherol in all crude oils then in cod liver oil. Oil from tuna by-products had similar α-tocopherol as tuna liver oil Crude oil from sardine by-products had significantly higher α-tocopherol then crude oil from whole sardines No correlation found between higher α-tocopherol content and crude oil stability | [177] |
| Cod liver oil | Refining of crude oil resulted in 31–45% decrease in α-tocopherol | [94] | |
| Oil from rainbow trout heads, bones and tails Oil from rainbow trout intestines | The oil extraction temperature did not affect α-tocopherol of different oils The α-tocopherol level in oils ranged from ~90–160 µg/g of oil | ||
| Fresh Caulerpa sp. leaves | Vitamin E content of 2.2 mg/kg | [178] | |
| Rainbow trout flesh | Out of 5 extraction methods of α-tocopherol, solid-liquid extraction with n-hexane showed the best performance | [179] | |
| K | Meat of Atlantic salmon fed a diet with high vitamin D3 and K1 | Improvement in several bone formation and resorption markers after consuming salmon fed with high vitamin D and K. The results were obtained despite using vitamin K1 for supplementation | [180] |
| D | Anchovy filleting wastes | Oil extracted using d-limonene as biosolvent contained 81 µg of vitamin D3/kg of oil | [181] |
| Wakame and combu leaves | Vitamin D <0.05 µg/100 g in both fresh and dried leaves | [182] | |
| A | Pangasius catfish filleting wastes | Fish oil obtained as part of a zero-waste procedure, contained 334 µg of retinol/kg of oil | [183] |
| Fresh Caulerpa sp. leaves | High vitamin A reaching 4810 mg/kg | [178] | |
| Dried Ulva lactuca | Vitamin A below detection limit | [184] |
2.7. Dopamine in Seafood as Drug and Supplement
2.8. Bioactive Peptides from Marine Sources
| Marine Source | Biological Activity | Amino Acid Sequence | Reference |
|---|---|---|---|
| Cuttlefish (Sepia officinalis) | ACE inhibitory | Val-Glu-Leu-Tyr-Pro | [244] |
| Flounder fish (Paralichthys olivaceus) | ACE inhibitory | Met-Glu-Val-Phe-Val-Pro | [245] |
| Lizard fish | ACE inhibitory | Gly-Met-Lys-Cys-Ala-Phe | [246] |
| Pacific cod (Gadus macrocephalus) | ACE inhibitory | Gly-Ala-Ser-Ser-Gly-Met-Pro-Gly and Leu-Ala-Tyr-Ala | [247] |
| Shrimp paste | ACE inhibitory | Ser-Val and Ile-Phe | [248] |
| Jellyfish (Rhopilemae sculentum) | ACE inhibitory | Gln-Pro-Gly-Pro-Thr and Gly-Asp-Ile-Gly-Tyr | [249] |
| Marine snail (Cenchritis muricatus) | Antifungal activity | Ser-Arg-Ser-Glu-Leu-Ile-Val-His-Gln-Arg | [250] |
| Spirulina maxima | Anti-atherosclerotic activity | Leu-Asp-Ala-Val-Asn-Arg and Met-Met-Leu-Asp-Phe | [251] |
| Pyropia yezoensis | Anti-inflammatory activity | Lys-Ala-Gln-Ala-Asp | [252] |
| Skate (Okamejei kenojei) | ACE inhibitory | Leu-Gly-Pro-Leu-Gly-His-Gln and Met-Val-Gly-Ser-Ala-Pro-Gly-Val-Leu | [253] |
| Dulse (Palmaria palmata) | Renin inhibitory, Antihypertensive effect | Ile-Arg-Leu-Ile-Ile-Val-Leu-Met-Pro-Ile-Leu-Met-Ala | [254] |
| Half-fin anchovy (Setipinna taty) | Pro-apoptotic on PC-3 cells | Tyr-Ala-Leu-Arg-Ala-His | [255] |
| Greater pipefish (Syngnathus acus) | Pro-apoptotic on A549 and CCRF-CEM cells | Lys-Arg-Asp-Leu-Gly-Phe-Val-Asp-Glu-Ile-Ser-Ala-His-Tyr | [256] |
| Japanese flounder (Palatichtys olivaceus) | Antioxidative activity | Gly-Gly-Phe-Asp-Met-Gly | [257] |
| Nori (Porphyra yezoensis) | Anticoagulant activity | NH2-Asn-Met-Glu-Lys-Gly-Ser-Ser-Ser-Val-Val-Ser-Ser-Arg-Met-Lys-Gln-COOH | [258] |
| Porphyra haitanesis | Anti-proliferation activity | Val-Pro-Gly-Thr-Pro-Lys-Asn-Leu-Asp-Ser-Pro-Arg and Met-Pro-Ala-Pro-Ser-Cys-Ala-Leu-Pro-Arg-Ser-Val-Val-Pro-Pro-Arg | [259] |
| Dulse (Palmaria palmata) | Antioxidant activity | Ser-Asp-Ile-Thr-Arg-Pro-Gly-Gly-Asn-Met | [260] |
| Laver (Porphyra spp) | α-Amylase inhibitory activity | Gly-Gly-Ser-Lys and Glu-Leu-Ser | [261] |
| Atlantic salmon (Salmo salar) | Anti-allergic activity | Thr-Pro-Glu-Val-His-Ile-Ala-Val-Asp-Lys-Phe | [262] |
| Fermented anchovies (Ilisha melastoma) sauce (Budu) | Antioxidant activity | Lue-Asp-Asp-ProVal-Phe-Ile-His | [263] |
| Blood cockle (Tegillar cagranosa) | Antioxidant activity | Met-Asp-Leu-Phe-Thr-Glu and Trp-Pro-Pro-Asp | [264] |
| Mackerel (Scomber japonicus) | Antioxidant activity | ALSTWTLQLGSTSFSASPM | [243] |
| Oyster (Crassostrea gigas) | Antioxidant activity | Leu-Lys-Gln-Glu-Leu-Glu-Asp-Leu-Leu-Glu-Lys-Gln-Glu | [234] |
| Marine crab (Charybdis natator) | Anti-inflammatory effect | G-L-G-A-A-V-L | [135] |
| Red scorpionfish (Scorpaena notata) | ACE inhibitory and antioxidant activity | Gln-Gln- Pro-His-Ser-Arg-Ser-Lys-Gly-Phe-Pro-Gly-Pro, Gly-Gln-Lys-Ser-Val-Pro-Glu-Val- Arg and Val-Glu-Gly-Lys-Ser-Pro-Asn-Val | [265] |
| Pearl oyster (Pinctada fucata martensii) | ACE inhibitory | His-Leu-His-Thr, and Gly-Trp-Ala | [266] |
| Spotless smoothhound (Mustelus griseus) | Antioxidant activity | Gly-Ala-Glu-Arg-Pro, Gly-Glu-Arg-Glu-Ala-Asn-Val-Met and Ala-Glu-Val-Gly | [267] |
| Tetradesmus obliquus microalgae | Antioxidant and ACE-inhibitory activity | Trp-Pro-Arg-Gly-Tyr-Phe-Leu, Gly-Pro-Asp-Arg-Pro-Lys-Phe-Leu-Gly-Pro-Phe, Trp-Tyr-Gly-Pro-Asp-Arg-Pro-Lys-Phe-Leu and Ser-Asp-Trp-Asp-Arg-Phe | [268] |
| Nile tilapia (Oreochromis niloticus) | Antimicrobial activity | Phe-Ile-His-His-Ile-Ile-Gly-Gly-Leu-Phe-Ser-Ala-Gly-Lys-Ala-Ile-His-Arg-Leu-Ile-Arg-Arg-Arg-Arg-Arg | [269] |
| Sea cucumber (Stichopus japonicus) | ACE inhibitory | Asn-Ala-Pro-His-Met-Arg | [270] |
| Sponge (Xestospongia testudinaria) | Cytotoxic to cancerous HeLa cells | Lys-Glu-Asn-Pro-Val-Leu-Ser-Leu-Val-Asn-Gly-Met-Phe | [271] |
Gelatin from Marine Sources
3. Health Benefit of Nano-Based Materials for Bioactive Compounds from Marine-Based Sources
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suleria, H.A.R.; Gobe, G.; Masci, P.; Osborne, S.A. Marine Bioactive Compounds and Health Promoting Perspectives; Innovation Pathways for Drug Discovery. Trends Food Sci. Technol. 2016, 50, 44–55. [Google Scholar] [CrossRef]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Ande, M.P.; Syamala, K.; SrinivasaRao, P.; MuraliMohan, K.; Lingam, S.S. Marine Nutraceuticals. Mar. Omi. Princ. Appl. 2016, 329–345. [Google Scholar] [CrossRef]
- Marine-Derived Drugs Market Growing at a CAGR of 11.20% and Expected to Reach $21,955.6 Million by 2025—Exclusive Report by Infinium Global Research. Infinium Global Research. 2019. Available online: https://www.medgadget.com/2019/07/marine-derived-drugs-market-growing-at-a-cagr-of-11-20-and-expected-to-reach-21955-6-million-by-2025-exclusive-report-by-infinium-global-research.html (accessed on 15 October 2020).
- Sharanagat, V.S.; Singla, V.; Singh, L. Bioactive Compounds from Marine Sources. In Technological Processes for Marine Foods-from Water to Fork: Bioactive Compounds, Industrial Applications and Genomics; Goyal, M.R., Rasul Suleria, H.A., Kirubanandan, S., Eds.; Apple Academic Press, Inc.: Oakville, ON, Canada, 2020. [Google Scholar]
- Nalini, S.; Sandy Richard, D.; Mohammed Riyaz, S.U.; Kavitha, G.; Inbakandan, D. Antibacterial Macro Molecules from Marine Organisms. Int. J. Biol. Macromol. 2018, 115, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Biologically Active Macromolecules: Extraction Strategies, Therapeutic Potential and Biomedical Perspective. Int. J. Biol. Macromol. 2020, 151, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sudatta, B.P.; Sugumar, V.; Varma, R.; Nigariga, P. Extraction, Characterization and Antimicrobial Activity of Chitosan from Pen Shell, Pinna Bicolor. Int. J. Biol. Macromol. 2020, 163, 423–430. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Kang, K.H.; Je, J.Y.; Pham, H.N.D.; Byun, H.G.; Kim, S.K. Biological Effects of Chitosan and Its Derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Anraku, M.; Fujii, T.; Kondo, Y.; Kojima, E.; Hata, T.; Tabuchi, N.; Tsuchiya, D.; Goromaru, T.; Tsutsumi, H.; Kadowaki, D.; et al. Antioxidant Properties of High Molecular Weight Dietary Chitosan in Vitro and in Vivo. Carbohydr. Polym. 2011, 83, 501–505. [Google Scholar] [CrossRef]
- Je, J.Y.; Park, P.J.; Kim, S.K. Radical Scavenging Activity of Hetero-Chitooligosaccharides. Eur. Food Res. Technol. 2004, 219, 60–65. [Google Scholar] [CrossRef]
- Anraku, M.; Fujii, T.; Furutani, N.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Tomida, H. Antioxidant Effects of a Dietary Supplement: Reduction of Indices of Oxidative Stress in Normal Subjects by Water-Soluble Chitosan. Food Chem. Toxicol. 2009, 47, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Iohara, D.; Michihara, A.; Ifuku, S.; Azuma, K.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Hirayama, F.; Anraku, M. Effects of Surface-Deacetylated Chitin Nanofibers on Non-Alcoholic Steatohepatitis Model Rats and Their Gut Microbiota. Int. J. Biol. Macromol. 2020, 164, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, Mode of Action, and in Vivo Activity of Chitosan and Its Micro- and Nanoparticles as Antimicrobial Agents: A Review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the Mode of Action of Chitosan as an Antibacterial Compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and Its Derivatives: Structural Properties and Biomedical Applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef]
- Chien, R.C.; Yen, M.T.; Mau, J.L. Antimicrobial and Antitumor Activities of Chitosan from Shiitake Stipes, Compared to Commercial Chitosan from Crab Shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Hamed, A.A.; Abdelhamid, I.A.; Saad, G.R.; Elkady, N.A.; Elsabee, M.Z. Synthesis, Characterization and Antimicrobial Activity of a Novel Chitosan Schiff Bases Based on Heterocyclic Moieties. Int. J. Biol. Macromol. 2020, 153, 492–501. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Comparative Study on Antimicrobial Activity and Biocompatibility of N-Selective Chitosan Derivatives. React. Funct. Polym. 2018, 124, 149–155. [Google Scholar] [CrossRef]
- Salama, H.E.; Abdel Aziz, M.S.; Sabaa, M.W. Development of Antibacterial Carboxymethyl Cellulose/Chitosan Biguanidine Hydrochloride Edible Films Activated with Frankincense Essential Oil. Int. J. Biol. Macromol. 2019, 139, 1162–1167. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan Oligosaccharide: Biological Activities and Potential Therapeutic Applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Huang, R.; Mendis, E.; Kim, S.K. Improvement of ACE Inhibitory Activity of Chitooligosaccharides (COS) by Carboxyl Modification. Bioorg. Med. Chem. 2005, 13, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Park, P.J.; Ahn, C.B.; Jeon, Y.J.; Je, J.Y. Renin Inhibition Activity by Chitooligosaccharides. Bioorganic Med. Chem. Lett. 2008, 18, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Kong, C.S.; Kim, S.K. Inhibitory Effects of Chitooligosaccharides on Degranulation and Cytokine Generation in Rat Basophilic Leukemia RBL-2H3 Cells. Carbohydr. Polym. 2011, 84, 649–655. [Google Scholar] [CrossRef]
- Chung, M.J.; Park, J.K.; Park, Y.I. Anti-Inflammatory Effects of Low-Molecular Weight Chitosan Oligosaccharides in IgE-Antigen Complex-Stimulated RBL-2H3 Cells and Asthma Model Mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tao, N.; Wang, X.; Xiao, J.; Wang, M. Marine-Derived Bioactive Compounds with Anti-Obesity Effect: A Review. J. Funct. Foods 2016, 21, 372–387. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Chang, T.C.; Liu, S.H.; Chiang, M.T. The Regulatory Effects of Fish Oil and Chitosan on Hepatic Lipogenic Signals in High-Fat Diet-Induced Obese Rats. J. Food Drug Anal. 2017, 25, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Inanli, A.G.; Tümerkan, E.T.A.; El Abed, N.; Regenstein, J.M.; Özogul, F. The Impact of Chitosan on Seafood Quality and Human Health: A Review. Trends Food Sci. Technol. 2020, 97, 404–416. [Google Scholar] [CrossRef]
- Panith, N.; Wichaphon, J.; Lertsiri, S.; Niamsiri, N. Effect of Physical and Physicochemical Characteristics of Chitosan on Fat-Binding Capacities under in Vitro Gastrointestinal Conditions. LWT Food Sci. Technol. 2016, 71, 25–32. [Google Scholar] [CrossRef]
- Wydro, P.; Krajewska, B.; Ha, K.; Wydro, P. Chitosan as a Lipid Binder: A Langmuir Monolayer Study of Chitosan−Lipid Interactions Chitosan as a Lipid Binder: A Langmuir Monolayer Study of Chitosan-Lipid Interactions. Am. Chem. Soc. 2007, 8, 2611–2617. [Google Scholar]
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant Activities of Chitosans and Its Derivatives in in Vitro and in Vivo Studies. Carbohydr. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and Biomedical Applications of Chitin and Chitosan Nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Bondiolotti, G.; Bareggi, S.R.; Frega, N.G.; Strabioli, S.; Cornelli, U. Activity of Two Different Polyglucosamines, L112® and FF45®, on Body Weight in Male Rats. Eur. J. Pharmacol. 2007, 567, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Kaats, G.R.; Michalek, J.E.; Preuss, H.G. Evaluating Efficacy of a Chitosan Product Using a Double-Blinded, Placebo-Controlled Protocol. J. Am. Coll. Nutr. 2006, 25, 389–394. [Google Scholar] [CrossRef]
- Gades, M.D.; Stern, J.S. Chitosan Supplementation and Fecal Fat Excretion in Men. Obes. Res. 2003, 11, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ito, M. Antidiabetic Action of Low Molecular Weight Chitosan in Genetically Obese Diabetic KK-Ay Mice. Biol. Pharm. Bull. 2002, 25, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Gorzelanny, C.; Pöppelmann, B.; Strozyk, E.; Moerschbacher, B.M.; Schneider, S.W. Specific Interaction between Chitosan and Matrix Metalloprotease 2 Decreases the Invasive Activity of Human Melanoma Cells. Biomacromolecules 2007, 8, 3035–3040. [Google Scholar] [CrossRef] [PubMed]
- Sayari, N.; Sila, A.; Abdelmalek, B.E.; Abdallah, R.B.; Ellouz-Chaabouni, S.; Bougatef, A.; Balti, R. Chitin and Chitosan from the Norway Lobster By-Products: Antimicrobial and Anti-Proliferative Activities. Int. J. Biol. Macromol. 2016, 87, 163–171. [Google Scholar] [CrossRef]
- Resmi, R.; Yoonus, J.; Beena, B. Anticancer and Antibacterial Activity of Chitosan Extracted from Shrimp Shell Waste. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- El-Naggar, M.M.; Haneen, D.S.A.; Mehany, A.B.M.; Khalil, M.T. New Synthetic Chitosan Hybrids Bearing Some Heterocyclic Moieties with Potential Activity as Anticancer and Apoptosis Inducers. Int. J. Biol. Macromol. 2020, 150, 1323–1330. [Google Scholar] [CrossRef]
- Sedghi, R.; Gholami, M.; Shaabani, A.; Saber, M.; Niknejad, H. Preparation of Novel Chitosan Derivative Nanofibers for Prevention of Breast Cancer Recurrence. Eur. Polym. J. 2020, 123, 109421. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential Biomedical Applications of Marine Algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, C.; Liu, B.; Lin, L.; Sarker, S.D.; Nahar, L.; Yu, H.; Cao, H.; Xiao, J. Bioactive Compounds from Marine Macroalgae and Their Hypoglycemic Benefits. Trends Food Sci. Technol. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-Based Metallic Nanoparticles: Synthesis, Characterization and Applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef] [PubMed]
- Mekinić, I.G.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic Content of Brown Algae (Pheophyceae) Species: Extraction, Identification, and Quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Biological Activities and Health Benefit Effects of Natural Pigments Derived from Marine Algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; Chacón-Garza, L.E.; Valdivia-Najár, G.; Arredondo-Valdés, R.; Castro-López, C.; Ventura-Sobrevilla, J.M.; Aguilar-Gonzáles, C.N.; Boone-Villa, D. Nanosystems of Plant-Based Pigments and Its Relationship with Oxidative Stress. Food Chem. Toxicol. 2020, 143. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive Compounds in Seaweeds: An Overview of Their Biological Properties and Safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- Chakdar, H.; Pabbi, S. Algal Pigments for Human Health and Cosmeceuticals; Elsevier B.V.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Martins, N.; Ferreira, I.C.F.R. Wastes and By-Products: Upcoming Sources of Carotenoids for Biotechnological Purposes and Health-Related Applications. Trends Food Sci. Technol. 2017, 62, 33–48. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial Potential of Carotenoid Pigments from Microalgae: Current Trends and Future Prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S. Carotenoids. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Nagappan, H.; Pee, P.P.; Kee, S.H.Y.; Ow, J.T.; Yan, S.W.; Chew, L.Y.; Kong, K.W. Malaysian Brown Seaweeds Sargassum Siliquosum and Sargassum Polycystum: Low Density Lipoprotein (LDL) Oxidation, Angiotensin Converting Enzyme (ACE), α-Amylase, and α-Glucosidase Inhibition Activities. Food Res. Int. 2017, 99, 950–958. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed Nutraceuticals and Their Therapeutic Role in Disease Prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Kraan, S. Pigments and Minor Compounds in Algae; Woodhead Publishing Limited: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Biris-Dorhoi, E.S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M. Marine Bioactive Components: Sources, Health Benefits, and Future Prospects. In Technological Processes for Marine Foods-from Water to Fork: Bioactive Compounds, Industrial Applications and Genomics; Apple Academic Press: New York, NY, USA, 2020; pp. 61–72. [Google Scholar]
- Sanjeewa, K.K.A.; Kang, N.; Ahn, G.; Jee, Y.; Kim, Y.T.; Jeon, Y.J. Bioactive Potentials of Sulfated Polysaccharides Isolated from Brown Seaweed Sargassum Spp in Related to Human Health Applications: A Review. Food Hydrocoll. 2018, 81, 200–208. [Google Scholar] [CrossRef]
- Udayangani, R.M.A.C.; Somasiri, G.D.P.; Wickramasinghe, I.; Kim, S. Potential Health Benefits of Sulfated Polysaccharides from Marine Algae. Encycl. Mar. Biotechnol. 2020, 629–635. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Lee, J.S.; Kim, W.S.; Jeon, Y.J. The Potential of Brown-Algae Polysaccharides for the Development of Anticancer Agents: An Update on Anticancer Effects Reported for Fucoidan and Laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A Fucoidan Fraction Purified from Chnoospora Minima; a Potential Inhibitor of LPS-Induced Inflammatory Responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Stringer, D.N.; Park, A.Y.; Karpiniec, S.S. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef]
- Han, M.H.; Lee, D.S.; Jeong, J.W.; Hong, S.H.; Choi, I.W.; Cha, H.J.; Kim, S.; Kim, H.S.; Park, C.; Kim, G.Y.; et al. Fucoidan Induces ROS-Dependent Apoptosis in 5637 Human Bladder Cancer Cells by Downregulating Telomerase Activity via Inactivation of the PI3K/Akt Signaling Pathway. Drug Dev. Res. 2017, 78, 37–48. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Manikandakrishnan, M.; Anjali, R.; Rajasekar, P.; Marudhupandi, T.; Manikandan, R.; Vaseeharan, B.; Prabhu, N.M. Investigation of Antioxidant and Anticancer Potential of Fucoidan from Sargassum Polycystum. Int. J. Biol. Macromol. 2018, 116, 151–161. [Google Scholar] [CrossRef]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.S.; Chitra, J. In Vitro Anticancer Activity of Fucoidan Extracted from Sargassum Cinereum against Caco-2 Cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from Brown Seaweeds Sargassum Hornery, Eclonia Cava, Costaria Costata: Structural Characteristics and Anticancer Activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, Structure and Biofunctional Activities of Laminarin from Brown Algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical Applications of Laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, Antibacterial and in Vivo Wound Healing Properties of Laminaran Purified from Cystoseira Barbata Seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Custódio, C.A.; Reis, R.L.; Mano, J.F. Photo-Cross-Linked Laminarin-Based Hydrogels for Biomedical Applications. Biomacromolecules 2016, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and Characterization of Sodium Alginate from Moroccan Laminaria Digitata Brown Seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, Physical and Biological Properties of Alginates and Their Biomedical Implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Emerton, V.; Choi, E. Essential Guide to Food Additives; Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Adrian, G.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers (Basel) 2019, 11, 1837. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N.; Kuznetsova, T.A.; Zaporozhets, T.S.; Ermakova, S.P.; Zvyagintseva, T.N.; Chingizova, E.A.; Gazha, A.K.; Smolina, T.P. Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects. Biomedicines 2020, 8, 301. [Google Scholar] [CrossRef]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Kim, D.; Banerjee, I.; Pal, K. Carrageenan: A Wonder Polymer from Marine Algae for Potential Drug Delivery Applications. Curr. Pharm. Des. 2019, 25, 1172–1186. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan Based Hydrogels for Drug Delivery, Tissue Engineering and Wound Healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Besednova, N.N.; Zaporozhets, T.S.; Kuznetsova, T.A.; Makarenkova, I.D.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Ermakova, S.P. Extracts and Marine Algae Polysaccharides in Therapy and Prevention of Inflammatory Diseases of the Intestine. Mar. Drugs 2020, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Robic, A. Structure and Function Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A Systematic Review of Extraction, Composition and Function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Abd-Ellatef, G.E.F.; Ahmed, O.M.; Abdel-Reheim, E.S.; Abdel-Hamid, A.H.Z. Ulva Lactuca Polysaccharides Prevent Wistar Rat Breast Carcinogenesis through the Augmentation of Apoptosis, Enhancement of Antioxidant Defense System, and Suppression of Inflammation. Breast Cancer Targets Ther. 2017, 9, 67–83. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Kirke, D.A.; Rai, D.K.; Smyth, T.J.; Stengel, D.B. An Assessment of Temporal Variation in the Low Molecular Weight Phlorotannin Profiles in Four Intertidal Brown Macroalgae. Algal Res. 2019, 41, 101550. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Li, Z.; Mou, Q. The Anti-Allergic Activity of Polyphenol Extracted from Five Marine Algae. J. Ocean Univ. China 2015, 14, 681–684. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective Effects of Marine Algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential Role of Seaweed Polyphenols in Cardiovascular-Associated Disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-Proliferative and Potential Anti-Diabetic Effects of Phenolic-Rich Extracts from Edible Marine Algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Liu, H.C.; Chang, C.J.; Yang, T.H.; Chiang, M.T. Long-Term Feeding of Red Algae (Gelidium Amansii) Ameliorates Glucose and Lipid Metabolism in a High Fructose Diet-Impaired Glucose Tolerance Rat Model. J. Food Drug Anal. 2017, 25, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Nakai, Y.; Izumi, H.; Nagaosa, K.; Ishijima, T.; Nakano, T.; Abe, K. Oral Administration of Edible Seaweed Undaria Pinnatifida (Wakame) Modifies Glucose and Lipid Metabolism in Rats: A DNA Microarray Analysis. Mol. Nutr. Food Res. 2018, 62, 1–6. [Google Scholar] [CrossRef]
- Vestland, T.L.; Jacobsen, Ø.; Sande, S.A.; Myrset, A.H.; Klaveness, J. Characterization of Omega-3 Tablets. Food Chem. 2016, 197, 496–502. [Google Scholar] [CrossRef]
- Schmidt, N.; Møller, G.; Bæksgaard, L.; Østerlind, K.; Stark, K.D.; Lauritzen, L.; Andersen, J.R. Fish Oil Supplementation in Cancer Patients. Capsules or Nutritional Drink Supplements? A Controlled Study of Compliance. Clin. Nutr. ESPEN 2020, 35, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Soldo, B.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. Production and Refinement of Omega-3 Rich Oils from Processing By-Products of Farmed Fish Species. Foods 2019, 3, 125. [Google Scholar] [CrossRef]
- Jamshidi, A.; Cao, H.; Xiao, J.; Simal-Gandara, J. Advantages of Techniques to Fortify Food Products with the Benefits of Fish Oil. Food Res. Int. 2020, 137, 109353. [Google Scholar] [CrossRef]
- Naqshbandi, A.; Khan, M.W.; Rizwan, S.; ur Rehman, S.; Khan, F. Studies on the Protective Effect of Dietary Fish Oil on Cisplatin Induced Nephrotoxicity in Rats. Food Chem. Toxicol. 2012, 50, 265–273. [Google Scholar] [CrossRef]
- Das, S.; Paul, B.; Sengupta, J.; Datta, A. Beneficial Effects of Fish Oil to Human Health: A Review. Agric. Rev. 2009, 30, 199–205. [Google Scholar]
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Bioactive Packaging: Combining Nanotechnologies with Packaging for Improved Food Functionality; Elsevier Inc.: Philadelphia, PA, USA, 2018. [Google Scholar] [CrossRef]
- Farooqui, A.A. Beneficial Effects of Fish Oil on Human Brain; Springer: New York, NY, USA, 2009. [Google Scholar]
- Pipingas, A.; Sinclair, A.; Croft, K.D.; Januszewski, A.S.; Jenkins, A.J.; Mori, T.A.; Cockerell, R.; Grima, N.A.; Stough, C.; Scholey, A.; et al. Fish Oil and Multivitamin Supplementation Reduces Oxidative Stress but Not Inflammation in Healthy Older Adults: A Randomised Controlled Trial. J. Funct. Foods 2015, 19, 949–957. [Google Scholar] [CrossRef]
- Curado Borges, M.; de Miranda Moura dos Santos, F.; Weiss Telles, R.; Melo de Andrade, M.V.; Toulson Davisson Correia, M.I.; Lanna, C.C.D. Omega-3 Fatty Acids, Inflammatory Status and Biochemical Markers of Patients with Systemic Lupus Erythematosus: A Pilot Study. Rev. Bras. Reumatol. 2017, 57, 526–534. [Google Scholar] [CrossRef] [PubMed]
- De Souza, D.R.; da Silva Pieri, B.L.; Comim, V.H.; de Oliveira Marques, S.; Luciano, T.F.; Rodrigues, M.S.; De Souza, C.T. Fish Oil Reduces Subclinical Inflammation, Insulin Resistance, and Atherogenic Factors in Overweight/Obese Type 2 Diabetes Mellitus Patients: A Pre-Post Pilot Study. J. Diabetes Complicat. 2020, 34, 107553. [Google Scholar] [CrossRef] [PubMed]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine Omega-3 Phospholipids: Metabolism and Biological Activities. Int. J. Mol. Sci. 2012, 13, 15401–15419. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef]
- Suseno, S.H.; Saraswati, S.; Hayati, S.; Izaki, A.F. Fatty Acid Composition of Some Potential Fish Oil from Production Centers in Indonesia. Orient. J. Chem. 2014, 30, 975–980. [Google Scholar] [CrossRef][Green Version]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Good Fats versus Bad Fats: A Comparison of Fatty Acids in the Promotion of Insulin Resistance, Inflammation, and Obesity. Mo. Med. 2017, 114, 303–307. [Google Scholar]
- Huang, T.H.; Wang, P.W.; Yang, S.C.; Chou, W.L.; Fang, J.Y. Cosmetic and Therapeutic Applications of Fish Oil’s Fatty Acids on the Skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef]
- Alaswad, K.; Lavie, C.J.; Milani, R.V.; O’Keefe, J.H. Fish Oil in Cardiovascular Prevention. Ochsner J. 2002, 4, 83–91. [Google Scholar]
- Kromhout, D.; Yasuda, S.; Geleijnse, J.M.; Shimokawa, H. Fish Oil and Omega-3 Fatty Acids in Cardiovascular Disease: Do They Really Work? Eur. Heart J. 2012, 33, 436–443. [Google Scholar] [CrossRef]
- Connor, W.E.; Cefrancesco, C.A.; Connor, S. N-3 Fatty Acids from Fish Oil Effects on Plasma Lipoproteins and Hypertriglyceridemic Patients. Ann. N. Y. Acad. Sci. 1993, 683, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xu, Z.; Prinyawiwatkul, W. FA Composition of the Oil Extracted from Farmed Atlantic Salmon (Salmo Salar L.) Viscera. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 615–619. [Google Scholar] [CrossRef]
- Choulis, N.H. Miscellaneous Drugs Materials, Medical Devices, and Techniques. In Side Effects of Drugs Annual; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 33, pp. 1009–1029. [Google Scholar] [CrossRef]
- Kahveci, D.; Falkeborg, M.; Gregersen, S.; Xu, X. Upgrading of Farmed Salmon Oil Through Lipase-Catalyzed Hydrolysis. Open Biotechnol. J. 2014, 4, 47–55. [Google Scholar] [CrossRef]
- Uçak, I.; Oz, M.; Maqsood, S. Products Based on Omega-3 Polyunsaturated Fatty Acids and Health Effects. In The Role of Alternative and Innovative Food Ingredients and Products in Consumer Wellness; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Haq, M.; Park, S.K.; Kim, M.J.; Cho, Y.J.; Chun, B.S. Modifications of Atlantic Salmon By-Product Oil for Obtaining Different ω-3 Polyunsaturated Fatty Acids Concentrates: An Approach to Comparative Analysis. J. Food Drug Anal. 2018, 26, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, K.; Noguchi, R.; Hosokawa, M.; Fukunaga, K.; Nishiyama, T.; Takahashi, R.; Miyashita, K. Separation of Sardine Oil without Heating from Surimi Waste and Its Effect on Lipid Metabolism in Rats. J. Agric. Food Chem. 2004, 52, 2372–2375. [Google Scholar] [CrossRef]
- Solaesa, Á.G.; Bucio, S.L.; Sanz, M.T.; Beltrán, S.; Rebolleda, S. Characterization of Triacylglycerol Composition of Fish Oils by Using Chromatographic Techniques. J. Oleo Sci. 2014, 63, 449–460. [Google Scholar] [CrossRef]
- Sharma, R.; Katz, J. Fish Proteins in Coronary Artery Disease Prevention: Amino Acid–Fatty Acid Concept. In Bioactive Food as Dietary Interventions for Cardiovascular Disease; Academic Press: New York, NY, USA, 2013; pp. 525–549. [Google Scholar]
- Aidos, I.; Van Der Padt, A.; Boom, R.M.; Luten, J.B. Quality of Crude Fish Oil Extracted from Herring Byproducts of Varying States of Freshness. J. Food Sci. 2003, 68, 458–465. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Raorane, C.J.; Oh, S.T.; Park, J.G.; Lee, J. Herring Oil and Omega Fatty Acids Inhibit Staphylococcus Aureus Biofilm Formation and Virulence. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Shireman, R. Essential Fatty Acids. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Elsevier Science Ltd.: London, UK, 2003; pp. 2169–2176. [Google Scholar]
- Alexa-Stratulat, T.; Luca, A.; Badescu, M.; Bohotin, C.R.; Alexa, I.D. Nutritional Modulators in Chemotherapy-Induced Neuropathic Pain. In Nutritional Modulators of Pain in the Aging Population; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 9–33. [Google Scholar] [CrossRef]
- Hahn, B.H.; Kono, D.H. Animal Models in Lupus. In Dubois’ Lupus Erythematosus and Related Syndromes; Wallace, D.J., Hahn, B.H., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 164–215. [Google Scholar]
- Pigott, G.M.; Tucker, B.W. Production Composition and Properties Dietary Importance Production. In Encyclopedia of Food Sciences and Nutrition; Elsevier Inc.: Amsterdam, The Netherlands, 2003; pp. 2169–2176. [Google Scholar]
- Shah, M.A.; Niaz, K.; Aslam, N.; Vargas-de la Cruz, C.; Kabir, A.; Khan, A.H.; Khan, F.; Panichayupakaranant, P. Analysis of Proteins, Peptides, and Amino Acids. In Recent Advances in Natural Products Analysis; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 723–747. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive Peptides from Marine Processing Waste and Shellfish: A Review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Rogers, M.; Bare, R.; Gray, A.; Scott-Moelder, T.; Heintz, R. Assessment of Two Feeds on Survival, Proximate Composition, and Amino Acid Carbon Isotope Discrimination in Hatchery-Reared Chinook Salmon. Fish. Res. 2019, 219, 105303. [Google Scholar] [CrossRef]
- Özogul, F.; Hamed, I.; Özogul, Y.; Regenstein, J.M. Crustacean By-Products. In Encyclopedia of Food Chemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 33–38. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Teixeira, N.; Andrade, P.B. Amino Acids, Fatty Acids and Sterols Profile of Some Marine Organisms from Portuguese Waters. Food Chem. 2013, 141, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Chandika, P.; Ko, S.C.; Jung, W.K. Marine-Derived Biological Macromolecule-Based Biomaterials for Wound Healing and Skin Tissue Regeneration. Int. J. Biol. Macromol. 2015, 77, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Shiels, K.; Murray, P.; Saha, S.K. Marine Cyanobacteria as Potential Alternative Source for GABA Production. Bioresour. Technol. Rep. 2019, 8, 100342. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Antihypertensive Peptides from Animal Products, Marine Organisms, and Plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Mahanty, A.; Ganguly, S.; Sankar, T.V.; Chakraborty, K.; Rangasamy, A.; Paul, B.; Sarma, D.; Mathew, S.; Asha, K.K.; et al. Amino Acid Compositions of 27 Food Fishes and Their Importance in Clinical Nutrition. J. Amino Acids 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Qi, C.; Wang, X.; Han, F.; Jia, Y.; Lin, Z.; Wang, C.; Lu, J.; Yang, L.; Wang, X.; Li, E.; et al. Arginine Supplementation Improves Growth, Antioxidant Capacity, Immunity and Disease Resistance of Juvenile Chinese Mitten Crab, Eriocheir Sinensis. Fish Shellfish Immunol. 2019, 93, 463–473. [Google Scholar] [CrossRef]
- Flores, E.; Arévalo, S.; Burnat, M. Cyanophycin and Arginine Metabolism in Cyanobacteria. Algal Res. 2019, 42, 101577. [Google Scholar] [CrossRef]
- Pyz-Łukasik, R.; Paszkiewicz, W. Species Variations in the Proximate Composition, Amino Acid Profile, and Protein Quality of the Muscle Tissue of Grass Carp, Bighead Carp, Siberian Sturgeon, and Wels Catfish. J. Food Qual. 2018, 2018. [Google Scholar] [CrossRef]
- Narayanasamy, A.; Balde, A.; Raghavender, P.; Shashanth, D.; Abraham, J.; Joshi, I.; Nazeer, R.A. Isolation of Marine Crab (Charybdis Natator) Leg Muscle Peptide and Its Anti-Inflammatory Effects on Macrophage Cells. Biocatal. Agric. Biotechnol. 2020, 25, 101577. [Google Scholar] [CrossRef]
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef]
- Wu, G. Functional Amino Acids in Nutrition and Health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Tabakaeva, O.V.; Tabakaev, A.V.; Piekoszewski, W. Nutritional Composition and Total Collagen Content of Two Commercially Important Edible Bivalve Molluscs from the Sea of Japan Coast. J. Food Sci. Technol. 2018, 55, 4877–4886. [Google Scholar] [CrossRef] [PubMed]
- Cutrona, K.J.; Kaufman, B.A.; Figueroa, D.M.; Elmore, D.E. Role of Arginine and Lysine in the Antimicrobial Mechanism of Histone-Derived Antimicrobial Peptides. FEBS Lett. 2015, 589, 3915–3920. [Google Scholar] [CrossRef] [PubMed]
- Olin-Sandoval, V.; Yu, J.S.L.; Miller-Fleming, L.; Alam, M.T.; Kamrad, S.; Correia-Melo, C.; Haas, R.; Segal, J.; Peña Navarro, D.A.; Herrera-Dominguez, L.; et al. Lysine Harvesting Is an Antioxidant Strategy and Triggers Underground Polyamine Metabolism. Nature 2019, 572, 249–253. [Google Scholar] [CrossRef]
- Bemani, E.; Ghanati, F.; Rezaei, A.; Jamshidi, M. Effect of Phenylalanine on Taxol Production and Antioxidant Activity of Extracts of Suspension-Cultured Hazel (Corylus Avellana L.) Cells. J. Nat. Med. 2013, 67, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Okada, S.; Wakiguchi, H.; Kudo, K.; Kittaka, S.; Hara, M.; Furukawa, S. Cysteine, Histidine and Glycine Exhibit Anti-Inflammatory Effects in Human Coronary Arterial Endothelial Cells. Clin. Exp. Immunol. 2012, 167, 269–274. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine Metabolism in Animals and Humans: Implications for Nutrition and Health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Sampels, S. Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- Pal, J.; Shukla, B.N.; Maurya, A.K.; Verma, H.O. A Review on Role of Fish in Human Nutrition with Special Emphasis to Essential Fatty Acid. Int. J. Fish. Acquat. Stud. 2018, 6, 427–430. [Google Scholar]
- Bruno, S.F.; Ekorong, F.J.A.A.; Karkal, S.S.; Cathrine, M.S.B.; Kudre, T.G. Green and Innovative Techniques for Recovery of Valuable Compounds from Seafood By-Products and Discards: A Review. Trends Food Sci. Technol. 2019, 85, 10–22. [Google Scholar] [CrossRef]
- Owuamanam, S.; Cree, D. Progress of Bio-Calcium Carbonate Waste Eggshell and Seashell Fillers in Polymer Composites: A Review. J. Compos. Sci. 2020, 4, 70. [Google Scholar] [CrossRef]
- Menon, V.V.; Lele, S.S. Nutraceuticals and Bioactive Compounds from Seafood Processing Waste. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1405–1425. [Google Scholar]
- Paradelo, R.; Conde-Cid, M.; Cutillas-Barreiro, L.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. Phosphorus Removal from Wastewater Using Mussel Shell: Investigation on Retention Mechanisms. Ecol. Eng. 2016, 97, 558–566. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Zhang, R.; Jiang, D.; Zhu, Q.; Wang, S. Extraction and Characterization of HA/β-TCP Biphasic Calcium Phosphate from Marine Fish. Mater. Lett. 2019, 236, 680–682. [Google Scholar] [CrossRef]
- Miranda, G.; Sousa, F.; Costa, M.M.; Bartolomeu, F.; Silva, F.S.; Carvalho, O. Surface Design Using Laser Technology for Ti6Al4V-Hydroxyapatite Implants. Opt. Laser Technol. 2019, 109, 488–495. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Filipescu, M.; Barbaro, K.; Bonciu, A.; Birjega, R.; Cotrut, C.M.; Galvano, E.; Fosca, M.; Fadeeva, I.V.; Vadalà, G.; et al. Iron Ion-Doped Tricalcium Phosphate Coatings Improve the Properties of Biodegradable Magnesium Alloys for Biomedical Implant Application. Adv. Mater. Interfaces 2020, 7, 2000531. [Google Scholar] [CrossRef]
- Castelo-Branco, C.; Cancelo Hidalgo, M.J.; Palacios, S.; Ciria-Recasens, M.; Fernández-Pareja, A.; Carbonell-Abella, C.; Manasanch, J.; Haya-Palazuelos, J. Efficacy and Safety of Ossein-Hydroxyapatite Complex versus Calcium Carbonate to Prevent Bone Loss. Climacteric 2020, 23, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Hanh, N.T.; Bich, P.T.N.; Thao, H.T.T. Acute and Subchronic Oral Toxicity Assessment of Calcium Hydroxyapatite-Alginate in Animals. Vietnam J. Chem. 2019, 57, 16–20. [Google Scholar] [CrossRef]
- Remya, N.S.; Syama, S.; Sabareeswaran, A.; Mohanan, P.V. Investigation of Chronic Toxicity of Hydroxyapatite Nanoparticles Administered Orally for One Year in Wistar Rats.E. Mater. Sci. Eng. C 2017, 76, 518–527. [Google Scholar] [CrossRef]
- Suresh, P.V.; Kudre, T.G.; Johny, L.C. Sustainable Valorization of Seafood Processing By-Product/Discard. In Waste to Wealth; Springer: Singapore, 2018; pp. 111–140. [Google Scholar] [CrossRef]
- Bubel, F.; Dobrzański, Z.; Bykowski, P.J.; Chojnacka, K.; Opaliński, S.; Trziszka, T. Production of Calcium Preparations by Technology of Saltwater Fish by Product Processing. Open Chem. 2015, 13, 1333–1340. [Google Scholar] [CrossRef]
- Nemati, M.; Huda, N.; Ariffin, F. Development of Calcium Supplement from Fish Bone Wastes of Yellowfin Tuna (Thunnus Albacares) and Characterization of Nutritional Quality. Int. Food Res. J. 2017, 24, 2419–2426. [Google Scholar]
- Flammini, L.; Martuzzi, F.; Vivo, V.; Ghirri, A.; Salomi, E.; Bignetti, E.; Barocelli, E. Hake Fish Bone as a Calcium Source for Efficient Bone Mineralization. Int. J. Food Sci. Nutr. 2016, 67, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Du, H.; Zhang, J.; Xiong, S. Preparation and Characterization of Ultrafine Fish Bone Powder. J. Aquat. Food Prod. Technol. 2016, 25, 1045–1055. [Google Scholar] [CrossRef]
- Guéguen, L.; Pointillart, A. The Bioavailability of Dietary Calcium. J. Am. Coll. Nutr. 2000, 19, 119S–136S. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, T.; Xiong, S.; Huang, Q.; You, J.; Hu, Y.; Liu, R.; Li, Y.J. Mechanism on Releasing and Solubilizing of Fish Bone Calcium during Nano-Milling. J. Food Process Eng. 2020, 43. [Google Scholar] [CrossRef]
- Yin, T.; Park, J.W.; Xiong, S. Physicochemical Properties of Nano Fish Bone Prepared by Wet Media Milling. LWT Food Sci. Technol. 2015, 64, 367–373. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.C.; Hsu, C.W.; Chang, W.H. Effects of Nano Calcium Carbonate and Nano Calcium Citrate on Toxicity in ICR Mice and on Bone Mineral Density in an Ovariectomized Mice Model. Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- Javeed, A.; Mahendrakar, N.S. Effect of Different Levels of Molasses and Salt on Acid Production and Volume of Fermenting Mass During Ensiling of Tropical Freshwater Fish Viscera. J. Food Sci. Technol. 1995, 32, 115–118. [Google Scholar]
- Giri, S.S.; Sahoo, S.K.; Sahu, A.K.; Mukhopadhyay, P.K. Nutrient Digestibility and Intestinal Enzyme Activity of Clarias Batrachus (Linn.) Juveniles Fed on Dried Fish and Chicken Viscera Incorporated Diets. Bioresour. Technol. 2000, 71, 97–101. [Google Scholar] [CrossRef]
- Kandyliari, A.; Mallouchos, A.; Papandroulakis, N.; Golla, J.P.; Lam, T.K.T.; Sakellari, A.; Karavoltsos, S.; Vasiliou, V.; Kapsokefalou, M. Nutrient Composition and Fatty Acid and Protein Profiles of Selected Fish By-Products. Foods 2020, 9, 190. [Google Scholar] [CrossRef]
- Afonso, C.; Bandarra, N.M.; Nunes, L.; Cardoso, C. Tocopherols in Seafood and Aquaculture Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 128–140. [Google Scholar] [CrossRef]
- Laskowski, W.; Górska-Warsewicz, H.; Kulykovets, O. Meat, Meat Products and Seafood as Sources of Energy and Nutrients in the Average Polish Diet. Nutrients 2018, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, R.; Yoshida, M.; Fukunaga, K. Seafood Consumption and Components for Health. Glob. J. Health Sci. 2012, 4, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.K. Health Benefits of Seafood; Is It Just the Fatty Acids? Food Chem. 2013, 140, 413–420. [Google Scholar] [CrossRef]
- Nadeeshani, H.; Rajapakse, N.; Kim, S. Traditional and Novel Seafood Processing Techniques Targeting Human Health Promotion. In Encyclopedia of Marine Biotechnology; John Wiley & Sons: New York, NY, USA, 2020; pp. 3041–3084. [Google Scholar]
- Šimat, V.; Vlahović, J.; Soldo, B.; Generalić Mekinić, I.; Čagalj, M.; Hamed, I.; Skroza, D. Production and Characterization of Crude Oils from Seafood Processing By-Products. Food Biosci. 2020, 33, 100484. [Google Scholar] [CrossRef]
- Nurjanah; Nurilmala, M.; Hidayat, T.; Sudirdjo, F. Characteristics of Seaweed as Raw Materials for Cosmetics. Aquat. Procedia 2016, 7, 177–180. [Google Scholar] [CrossRef]
- Araújo, M.; Alves, R.C.; Pimentel, F.B.; Costa, A.S.G.; Fernandes, T.J.R.; Valente, L.M.P.; Rema, P.; Oliveira, M.B.P.P. New Approach for Vitamin E Extraction in Rainbow Trout Flesh: Application in Fish Fed Commercial and Red Seaweed-Supplemented Diets. Eur. J. Lipid Sci. Technol. 2015, 117, 1398–1405. [Google Scholar] [CrossRef]
- Graff, I.E.; Øyen, J.; Kjellevold, M.; Frøyland, L.; Gjesdal, C.G.; Almås, B.; Rosenlund, G.; Lie, Ø. Reduced Bone Resorption by Intake of Dietary Vitamin D and K from Tailor-Made Atlantic Salmon: A Randomized Intervention Trial. Oncotarget 2016, 7, 69200–69215. [Google Scholar] [CrossRef]
- Scurria, A.; Lino, C.; Pitonzo, R.; Pagliaro, M.; Avellone, G.; Ciriminna, R. Vitamin D3 in Fish Oil Extracted with Limonene from Anchovy Leftovers. Chem. Data Collect. 2020, 25, 100311. [Google Scholar] [CrossRef]
- Hughes, L.; Black, L.; Sherriff, J.; Dunlop, E.; Strobel, N.; Lucas, R.; Bornman, J. Vitamin D Content of Australian Native Food Plants and Australian-Grown Edible Seaweed. Nutrients 2018, 10, 876. [Google Scholar] [CrossRef]
- Nam, P.V.; Van Hoa, N.; Anh, T.T.L.; Trung, T.S. Towards Zero-Waste Recovery of Bioactive Compounds from Catfish (Pangasius Hypophthalmus) By-Products Using an Enzymatic Method. Waste Biomass Valorization 2020, 11, 4195–4206. [Google Scholar] [CrossRef]
- Rasyid, A. Evaluation of Nutritional Composition of The Dried Seaweed Ulva Lactuca from Pameungpeuk Waters, Indonesia. Trop. Life Sci. Res. 2017, 28, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Aparna, P.; Muthathal, S.; Nongkynrih, B.; Gupta, S. Vitamin D Deficiency in India. J. Fam. Med. Prim. Care 2018, 7, 324. [Google Scholar] [CrossRef]
- Holick, M.F. The Vitamin D Deficiency Pandemic: Approaches for Diagnosis, Treatment and Prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Midtbø, L.K.; Nygaard, L.B.; Markhus, M.W.; Kjellevold, M.; Lie, Ø.; Dahl, L.; Kvestad, I.; Frøyland, L.; Graff, I.E.; Øyen, J. Vitamin D Status in Preschool Children and Its Relations to Vitamin D Sources and Body Mass Index—Fish Intervention Studies-KIDS (FINS-KIDS). Nutrition 2020, 70, 110595. [Google Scholar] [CrossRef]
- Aakre, I.; Næss, S.; Kjellevold, M.; Markhus, M.W.; Alvheim, A.R.; Dalane, J.Ø.; Kielland, E.; Dahl, L. New Data on Nutrient Composition in Large Selection of Commercially Available Seafood Products and Its Impact on Micronutrient Intake. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Jääskeläinen, T.; Itkonen, S.T.; Lundqvist, A.; Erkkola, M.; Koskela, T.; Lakkala, K.; Dowling, K.G.; Hull, G.L.; Kröger, H.; Karppinen, J.; et al. The Positive Impact of General Vitamin D Food Fortification Policy on Vitamin D Status in a Representative Adult Finnish Population: Evidence from an 11-y Follow-up Based on Standardized 25-Hydroxyvitamin D Data. Am. J. Clin. Nutr. 2017, 105, 1512–1520. [Google Scholar] [CrossRef]
- Al Khalifah, R.; Alsheikh, R.; Alnasser, Y.; Alsheikh, R.; Alhelali, N.; Naji, A.; Al Backer, N. The Impact of Vitamin D Food Fortification and Health Outcomes in Children: A Systematic Review and Meta-Regression. Syst. Rev. 2020, 9, 144. [Google Scholar] [CrossRef]
- Emadzadeh, M.; Sahebi, R.; Khedmatgozar, H.; Sadeghi, R.; Farjami, M.; Sharifan, P.; Ravanshad, Y.; Ferns, G.A.; Ghayour-Mobarhan, M. A Systematic Review and Meta-analysis of the Effect of Vitamin D-fortified Food on Glycemic Indices. BioFactors 2020, 46, 502–513. [Google Scholar] [CrossRef]
- Jahn, S.; Tsalis, G.; Lähteenmäki, L. How Attitude towards Food Fortification Can Lead to Purchase Intention. Appetite 2019, 133, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Enhancing and Improving the Extraction of Omega-3 from Fish Oil. Sustain. Chem. Pharm. 2017, 5, 54–59. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Avellone, G.; Pagliaro, M. A Circular Economy Approach to Fish Oil Extraction. ChemistrySelect 2019, 4, 5106–5109. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Bioaccessibility of Marine Carotenoids. Mar. Drugs 2018, 16, 397. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Gieysztor, R.; Maziarczyk, I.; Hodurek, P.; Rój, E.; Skalicka-Woźniak, K. Supercritical Fluid Chromatography with Photodiode Array Detection in the Determination of Fat-Soluble Vitamins in Hemp Seed Oil and Waste Fish Oil. Molecules 2018, 23, 1131. [Google Scholar] [CrossRef]
- Azzi, A. Tocopherols, Tocotrienols and Tocomonoenols: Many Similar Molecules but Only One Vitamin E. Redox Biol. 2019, 26, 101259. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Calvo, M.M.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Characterization and Storage Stability of Astaxanthin Esters, Fatty Acid Profile and α-Tocopherol of Lipid Extract from Shrimp (L. Vannamei) Waste with Potential Applications as Food Ingredient. Food Chem. 2017, 216, 37–44. [Google Scholar] [CrossRef]
- Feng, X.; Tjia, J.Y.Y.; Zhou, Y.; Liu, Q.; Fu, C.; Yang, H. Effects of Tocopherol Nanoemulsion Addition on Fish Sausage Properties and Fatty Acid Oxidation. LWT 2020, 118, 108737. [Google Scholar] [CrossRef]
- Honold, P.J.; Nouard, M.L.; Jacobsen, C. Fish Oil Extracted from Fish-Fillet by-Products Is Weakly Linked to the Extraction Temperatures but Strongly Linked to the Omega-3 Content of the Raw Material. Eur. J. Lipid Sci. Technol. 2016, 118, 874–884. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef]
- Vermeer, C.; Raes, J.; van’t Hoofd, C.; Knapen, M.; Xanthoulea, S. Menaquinone Content of Cheese. Nutrients 2018, 10, 446. [Google Scholar] [CrossRef]
- Kamao, M.; Suhara, Y.; Tsugawa, N.; Uwano, M.; Yamaguchi, N.; Uenishi, K.; Ishida, H.; Sasaki, S.; Okano, T. Vitamin K Content of Foods and Dietary Vitamin K Intake in Japanese Young Women. J. Nutr. Sci. Vitaminol. (Tokyo) 2007, 53, 464–470. [Google Scholar] [CrossRef]
- Tarento, T.D.C.; McClure, D.D.; Vasiljevski, E.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. Microalgae as a Source of Vitamin K1. Algal Res. 2018, 36, 77–87. [Google Scholar] [CrossRef]
- Tarento, T.D.C.; McClure, D.D.; Talbot, A.M.; Regtop, H.L.; Biffin, J.R.; Valtchev, P.; Dehghani, F.; Kavanagh, J.M. A Potential Biotechnological Process for the Sustainable Production of Vitamin K 1. Crit. Rev. Biotechnol. 2019, 39, 1–19. [Google Scholar] [CrossRef]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a Diet Supplement with Impact in Human Health: Current Evidence in Age-Related Diseases. Nutrients 2020, 12, 138. [Google Scholar] [CrossRef]
- Rizzo, G.; Laganà, A.; Rapisarda, A.; La Ferrera, G.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Hwang, J.H.; Cho, Y.-O. One-Half of Korean Adults Studied Had Marginal Vitamin B 12 Status Assessed by Plasma Vitamin B 12. Nutr. Res. 2018, 50, 37–43. [Google Scholar] [CrossRef]
- Marushka, L.; Kenny, T.-A.; Batal, M.; Cheung, W.W.L.; Fediuk, K.; Golden, C.D.; Salomon, A.K.; Sadik, T.; Weatherdon, L.V.; Chan, H.M. Potential Impacts of Climate-Related Decline of Seafood Harvest on Nutritional Status of Coastal First Nations in British Columbia, Canada. PLoS ONE 2019, 14, e0211473. [Google Scholar] [CrossRef]
- Bito, T.; Tanioka, Y.; Watanabe, F. Characterization of Vitamin B12 Compounds from Marine Foods. Fish. Sci. 2018, 84, 747–755. [Google Scholar] [CrossRef]
- Iemolo, A.; De Risi, M.; De Leonibus, E. Role of Dopamine in Memory Consolidation. In Memory Consolidation; Nova Science Publishers, Inc.: New York, NY, USA, 2015. [Google Scholar]
- Pace-Schott, E.F. The Neurobiology of Dreaming. In Principles and Practice of Sleep Medicine; Elsevier: Amsterdam, The Netherlands, 2011; pp. 563–575. [Google Scholar] [CrossRef]
- Perogamvros, L.; Dang-Vu, T.T.; Desseilles, M.; Schwartz, S. Sleep and Dreaming Are for Important Matters. Front. Psychol. 2013, 4. [Google Scholar] [CrossRef]
- Tokunaga, N.; Choudhury, M.E.; Nishikawa, N.; Nagai, M.; Tujii, T.; Iwaki, H.; Kaneta, M.; Nomoto, M. Pramipexole Upregulates Dopamine Receptor D2 and D3 Expression in Rat Striatum. J. Pharmacol. Sci. 2012, 120, 133–137. [Google Scholar] [CrossRef]
- Hondebrink, L.; Tan, S.; Hermans, E.; van Kleef, R.G.D.M.; Meulenbelt, J.; Westerink, R.H.S. Additive Inhibition of Human A1β2γ2 GABAA Receptors by Mixtures of Commonly Used Drugs of Abuse. Neurotoxicology 2013, 35, 23–29. [Google Scholar] [CrossRef]
- Szyrwiel, L.; Pap, J.S.; Malinka, W.; Szewczuk, Z.; Kotynia, A.; Brasun, J. Interactions of Anti-Parkinson Drug Benserazide with Zn(II), Cu(II), Fe(II) Ions. J. Pharm. Biomed. Anal. 2013, 76, 36–43. [Google Scholar] [CrossRef]
- Tarazi, F.I.; Neill, J.C. The Preclinical Profile of Asenapine: Clinical Relevance for the Treatment of Schizophrenia and Bipolar Mania. Expert Opin. Drug Discov. 2013, 8, 93–103. [Google Scholar] [CrossRef]
- Saikia, A.; Bhattacharya, P.; Sudip, P. Importance of Dopamine in Parkinson’s Disease. Adv. Tissue Eng. Regen. Med. Open Access 2018, 4. [Google Scholar] [CrossRef]
- Pacifici, G.M. Clinical Pharmacology of Dobutamine and Dopamine in Preterm Neonates. Med. Express 2014, 1. [Google Scholar] [CrossRef]
- Dilli, D.; Soylu, H.; Tekin, N. Turkish Neonatal Society Guideline on the Neonatal Hemodynamics and Management of Hypotension in Newborns. Türk Pediatr. Arşivi 2019, 53 (Suppl. 1), 65–75. [Google Scholar] [CrossRef]
- Derby, C.D.; Kicklighter, C.E.; Johnson, P.M.; Zhang, X. Chemical Composition of Inks of Diverse Marine Molluscs Suggests Convergent Chemical Defenses. J. Chem. Ecol. 2007, 33, 1105–1113. [Google Scholar] [CrossRef]
- Gleadall, I.G.; Guerrero-Kommritz, J.; Hochberg, F.G.; Laptikhovsky, V.V. The Inkless Octopuses (Cephalopoda: Octopodidae) of the Southwest Atlantic. Zoolog. Sci. 2010, 27, 528. [Google Scholar] [CrossRef]
- Derby, C. Cephalopod Ink: Production, Chemistry, Functions and Applications. Mar. Drugs 2014, 12, 2700–2730. [Google Scholar] [CrossRef]
- Fahmy, S.R.; Soliman, A.M.; Ali, E.M. Antifungal and Antihepatotoxic Effects of Sepia Ink Extract against Oxidative Stress as a Risk Factor of Invasive Pulmonary Aspergillosis in Neutropenic Mice. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 148–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jismi, J.; Krishnakumar, K.; Dineshkumar, B. Squid Ink and Its Pharmacological Activities. GSC Biol. Pharm. Sci. 2018, 2, 017–022. [Google Scholar] [CrossRef]
- Palumbo, A.; Di Cosmo, A.; Gesualdo, I.; Hearing, V.J. Subcellular Localization and Function of Melanogenic Enzymes in the Ink Gland of Sepia Officinalis. Biochem. J. 1997, 323, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Lucero, M.T.; Farrington, H.; Gilly, W.F. Quantification of L-Dopa and Dopamine in Squid Ink: Implications for Chemoreception. Biol. Bull. 1994, 187, 55–63. [Google Scholar] [CrossRef]
- Fiore, G.; Poli, A.; Di Cosmo, A.; D’ischia, M.; Palumbo, A. Dopamine in the Ink Defence System of Sepia Officinalis: Biosynthesis, Vesicular Compartmentation in Mature Ink Gland Cells, Nitric Oxide (NO)/CGMP-Induced Depletion and Fate in Secreted Ink1. Biochem. J. 2004, 378, 785–791. [Google Scholar] [CrossRef]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of Biogenic Amines in Food-Existing and Emerging Approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef]
- Bales, J.W.; Kline, A.E.; Wagner, A.K.; Dixon, C.E. Targeting Dopamine in Acute Traumatic Brain Injury. Open Drug Discov. J. 2010, 2, 119–128. [Google Scholar] [CrossRef]
- Khalid, S.; Abbas, M.; Bader-Ul-Ain, H.; Hafiz Ansar Rasul, H. Pharmacological Applications Of Marine-Derived Compounds: A Preventive Approach. In Technological Processes for Marine Foods-from Water to Fork: Bioactive Compounds, Industrial Applications and Genomics; Goyal, M.R., Suleria, H.A.R., Kirubanandan, S., Eds.; Apple Academic Press: New York, NY, USA, 2019; pp. 3–21. [Google Scholar]
- Hwang, D.; Kang, M.; Jo, M.; Seo, Y.; Park, N.; Kim, G.-D. Anti-Inflammatory Activity of β-Thymosin Peptide Derived from Pacific Oyster (Crassostrea Gigas) on NO and PGE2 Production by Down-Regulating NF-ΚB in LPS-Induced RAW264.7 Macrophage Cells. Mar. Drugs 2019, 17, 129. [Google Scholar] [CrossRef]
- Scarfì, S.; Pozzolini, M.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Fenoglio, D.; Altosole, T.; Ilan, M.; Bertolino, M.; et al. Identification, Purification and Molecular Characterization of Chondrosin, a New Protein with Anti-Tumoral Activity from the Marine Sponge Chondrosia Reniformis Nardo 1847. Mar. Drugs 2020, 18, 409. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant Peptides from Marine By-Products: Isolation, Identification and Application in Food Systems. A Review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and Functional Properties of Food Protein-Derived Antioxidant Peptides. J. Food Biochem. 2019, 1–13. [Google Scholar] [CrossRef]
- Aluko, R.E. Antihypertensive Peptides from Food Proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and Angiotensin-i-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Mar. Drugs 2019, 17, 613. [Google Scholar] [CrossRef] [PubMed]
- Pujiastuti, D.Y.; Ghoyatul Amin, M.N.; Alamsjah, M.A.; Hsu, J.L. Marine Organisms as Potential Sources of Bioactive Peptides That Inhibit the Activity of Angiotensin I-Converting Enzyme: A Review. Molecules 2019, 24, 2541. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Wijesekara, I. Development and Biological Activities of Marine-Derived Bioactive Peptides: A Review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Byun, H.-G.; Lee, J.K.; Park, H.G.; Jeon, J.-K.; Kim, S.-K. Antioxidant Peptides Isolated from the Marine Rotifer, Brachionus Rotundiformis. Process Biochem. 2009, 44, 842–846. [Google Scholar] [CrossRef]
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and Identification of Novel Antioxidant Peptides from Enzymatic Hydrolysates of Sardinelle (Sardinella Aurita) by-Products Proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Identification and Characterization of Novel Antioxidant Peptides from Mackerel (Scomber Japonicus) Muscle Protein Hydrolysates. Food Chem. 2020, 323, 126809. [Google Scholar] [CrossRef]
- Balti, R.; Bougatef, A.; Sila, A.; Guillochon, D.; Dhulster, P.; Nedjar-Arroume, N. Nine Novel Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Cuttlefish (Sepia Officinalis) Muscle Protein Hydrolysates and Antihypertensive Effect of the Potent Active Peptide in Spontaneously Hypertensive Rats. Food Chem. 2015, 170, 519–525. [Google Scholar] [CrossRef]
- Ko, J.Y.; Kang, N.; Lee, J.H.; Kim, J.S.; Kim, W.S.; Park, S.J.; Kim, Y.T.; Jeon, Y.J. Angiotensin I-Converting Enzyme Inhibitory Peptides from an Enzymatic Hydrolysate of Flounder Fish (Paralichthys Olivaceus) Muscle as a Potent Anti-Hypertensive Agent. Process Biochem. 2016, 51, 535–541. [Google Scholar] [CrossRef]
- Lan, X.; Liao, D.; Wu, S.; Wang, F.; Sun, J.; Tong, Z. Rapid Purification and Characterization of Angiotensin Converting Enzyme Inhibitory Peptides from Lizard Fish Protein Hydrolysates with Magnetic Affinity Separation. Food Chem. 2015, 182, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Vo, T.-S.; Ryu, B.; Kim, S.-K. Angiotensin-I-Converting Enzyme (ACE) Inhibitory Peptides from Pacific Cod Skin Gelatin Using Ultrafiltration Membranes. Process Biochem. 2016, 51, 1622–1628. [Google Scholar] [CrossRef]
- Kleekayai, T.; Harnedy, P.A.; O’Keeffe, M.B.; Poyarkov, A.A.; Cunhaneves, A.; Suntornsuk, W.; Fitzgerald, R.J. Extraction of Antioxidant and ACE Inhibitory Peptides from Thai Traditional Fermented Shrimp Pastes. Food Chem. 2015, 176, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Jia, A.; Zhang, Y.; Zhu, H.; Zhang, C.; Sun, Z.; Liu, C. Purification and Characterization of Angiotensin I Converting Enzyme Inhibitory Peptides from Jellyfish Rhopilema Esculentum. Food Res. Int. 2013, 50, 339–343. [Google Scholar] [CrossRef]
- López-Abarrategui, C.; Alba, A.; Silva, O.N.; Reyes-Acosta, O.; Vasconcelos, I.M.; Oliveira, J.T.A.; Migliolo, L.; Costa, M.P.; Costa, C.R.; Silva, M.R.R.; et al. Functional Characterization of a Synthetic Hydrophilic Antifungal Peptide Derived from the Marine Snail Cenchritis Muricatus. Biochimie 2012, 94, 968–974. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.K. Down-Regulation of Histamine-Induced Endothelial Cell Activation as Potential Anti-Atherosclerotic Activity of Peptides from Spirulina Maxima. Eur. J. Pharm. Sci. 2013, 50, 198–207. [Google Scholar] [CrossRef]
- Lee, H.A.; Kim, I.H.; Nam, T.J. Bioactive Peptide from Pyropia Yezoensis and Its Anti-Inflammatory Activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kang, K.H.; Ryu, B.; Vo, T.S.; Jung, W.K.; Byun, H.G.; Kim, S.K. Angiotensin-I Converting Enzyme Inhibitory Peptides from Antihypertensive Skate (Okamejei Kenojei) Skin Gelatin Hydrolysate in Spontaneously Hypertensive Rats. Food Chem. 2014, 174, 37–43. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Aluko, R.E.; Hossain, M.; Rai, D.K.; Hayes, M. Potential of a Renin Inhibitory Peptide from the Red Seaweed Palmaria Palmata as a Functional Food Ingredient Following Confirmation and Characterization of a Hypotensive Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2014, 62, 8352–8356. [Google Scholar] [CrossRef]
- Song, R.; Wei, R.B.; Luo, H.Y.; Yang, Z.S. Isolation and Identification of an Antiproliferative Peptide Derived from Heated Products of Peptic Hydrolysates of Half-Fin Anchovy (Setipinna Taty). J. Funct. Foods 2014, 10, 104–111. [Google Scholar] [CrossRef]
- Wang, M.; Nie, Y.; Peng, Y.; He, F.; Yang, J.; Wu, C.; Li, X. Purification, Characterization and Antitumor Activities of a New Protein from Syngnathus Acus, an Officinal Marine Fish. Mar. Drugs 2012, 10, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Himaya, S.W.A.; Ryu, B.; Ngo, D.H.; Kim, S.K. Peptide Isolated from Japanese Flounder Skin Gelatin Protects against Cellular Oxidative Damage. J. Agric. Food Chem. 2012, 60, 9112–9119. [Google Scholar] [CrossRef] [PubMed]
- Indumathi, P.; Mehta, A. A Novel Anticoagulant Peptide from the Nori Hydrolysate. J. Funct. Foods 2016, 20, 606–617. [Google Scholar] [CrossRef]
- Fan, X.; Bai, L.; Mao, X.; Zhang, X. Novel Peptides with Anti-Proliferation Activity from the Porphyra Haitanesis Hydrolysate. Process Biochem. 2017, 60, 98–107. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and Identification of Antioxidant Peptides from an Enzymatically Hydrolysed Palmaria Palmata Protein Isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Identification of Bioactive Peptides with α-Amylase Inhibitory Potential from Enzymatic Protein Hydrolysates of Red Seaweed (Porphyra Spp). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef]
- Wang, K.; Siddanakoppalu, P.N.; Ahmed, I.; Pavase, T.R.; Lin, H.; Li, Z. Purification and Identification of Anti-Allergic Peptide from Atlantic Salmon (Salmo Salar) Byproduct Enzymatic Hydrolysates. J. Funct. Foods 2020, 72, 104084. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. Purification and Identification of Antioxidant Peptides from Fermented Fish Sauce (Budu) Purification and Identification of Antioxidant Peptides From. J. Aquat. Food Prod. Technol. 2018, 28, 1–11. [Google Scholar] [CrossRef]
- Yang, X.R.; Qiu, Y.T.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Purification and Characterization of Antioxidant Peptides Derived from Protein Hydrolysate of the Marine Bivalve Mollusk Tergillarca Granosa. Mar. Drugs 2019, 17, 251. [Google Scholar] [CrossRef]
- Aissaoui, N.; Abidi, F.; Hardouin, J.; Abdelkafi, Z.; Marrakchi, N.; Jouenne, T.; Marzouki, M.N. ACE Inhibitory and Antioxidant Activities of Novel Peptides from Scorpaena Notata By-Product Protein Hydrolysate. Int. J. Pept. Res. Ther. 2017, 23, 13–23. [Google Scholar] [CrossRef]
- Liu, P.; Lan, X.; Yaseen, M.; Wu, S.; Feng, X.; Zhou, L.; Sun, J.; Liao, A.; Liao, D.; Sun, L. Purification, Characterization and Evaluation of Inhibitory Mechanism of ACE Inhibitory Peptides from Pearl Oyster (Pinctada Fucata Martensii) Meat Protein Hydrolysate. Mar. Drugs 2019, 17, 463. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Bioactive Peptides from Cartilage Protein Hydrolysate of Spotless Smoothhound and Their Antioxidant Activity In Vitro. Mar. Drugs 2018, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Peptidomic Strategy for Purification and Identification of Potential ACE-Inhibitory and Antioxidant Peptides in Tetradesmus Obliquus Microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586. [Google Scholar] [CrossRef]
- Narayana, J.L.; Huang, H.N.; Wu, C.J.; Chen, J.Y. Efficacy of the Antimicrobial Peptide TP4 against Helicobacter Pylori Infection: In Vitro Membrane Perturbation via Micellization and in Vivo Suppression of Host Immune Responses in a Mouse Model. Oncotarget 2015, 6, 12936–12954. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Sun, L.C.; Yan, L.J.; Lin, Y.C.; Liu, G.M.; Cao, M.J. Production, Optimisation and Characterisation of Angiotensin Converting Enzyme Inhibitory Peptides from Sea Cucumber (: Stichopus Japonicus) Gonad. Food Funct. 2018, 9, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Quah, Y.; Mohd Ismail, N.I.; Ooi, J.L.S.; Affendi, Y.A.; Abd Manan, F.; Wong, F.C.; Chai, T.T. Identification of Novel Cytotoxic Peptide KENPVLSLVNGMF from Marine Sponge Xestospongia Testudinaria, with Characterization of Stability in Human Serum. Int. J. Pept. Res. Ther. 2018, 24, 189–199. [Google Scholar] [CrossRef]
- Lv, L.C.; Huang, Q.Y.; Ding, W.; Xiao, X.H.; Zhang, H.Y.; Xiong, L.X. Fish Gelatin: The Novel Potential Applications. J. Funct. Foods 2019, 63. [Google Scholar] [CrossRef]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of Marine By-Products for the Recovery of Value-Added Products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Morawska, M.; Kulawik, P.; Zając, M. Characterization of Carp (Cyprinus Carpio) Skin Gelatin Extracted Using Different Pretreatments Method. Food Hydrocoll. 2018, 81, 169–179. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L. Food Waste Valorization; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Xu, M.; Wei, L.; Xiao, Y.; Bi, H.; Yang, H.; Du, Y. Physicochemical and Functional Properties of Gelatin Extracted from Yak Skin. Int. J. Biol. Macromol. 2017, 95, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Karim, A.A. Ultraviolet Radiation Improves Gel Strength of Fish Gelatin. Food Chem. 2009, 113, 1160–1164. [Google Scholar] [CrossRef]
- Kwak, H.W.; Shin, M.; Lee, J.Y.; Yun, H.; Song, D.W.; Yang, Y.; Shin, B.-S.; Park, Y.H.; Lee, K.H. Fabrication of an Ultrafine Fish Gelatin Nanofibrous Web from an Aqueous Solution by Electrospinning. Int. J. Biol. Macromol. 2017, 102, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Aksun Tumerkan, E.T.; Cansu, U.; Boran, G.; Regenstein, J.M.; Ozogul, F. Physiochemical and Functional Properties of Gelatin Obtained from Tuna, Frog and Chicken Skins. Food Chem. 2019, 287, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R. Gelatin; Switch Back to Halal: A Mini-Review. PSM Biol. Res. 2019, 4, 63–73. [Google Scholar]
- Karaman, S.; Cengiz, E.; Kayacier, A.; Dogan, M. Exposure to Air Accelerates the Gelation of Gelatin: Steady and Dynamic Shear Rheological Characterization to See the Effect of Air on the Strength of Gelatin Gel. Int. J. Food Prop. 2016, 19, 721–730. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Wang, H.; Liu, W.; Zhang, L.; Zhang, Y.; ShangGuan, X.C. Comparison of Rheological Behaviors and Nanostructure of Bighead Carp Scales Gelatin Modified by Different Modification Methods. J. Food Sci. Technol. 2017, 54, 1256–1265. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and Identification of Antioxidative Peptides from Loach (Misgurnus Anguillicaudatus) Protein Hydrolysate by Consecutive Chromatography and Electrospray Ionization-Mass Spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Koli, J.M.; Basu, S.; Nayak, B.B.; Nagalakshmi, K.; Venkateshwarlu, G. Improvement of Gel Strength and Melting Point of Fish Gelatin by Addition of Coenhancers Using Response Surface Methodology. J. Food Sci. 2011, 76, E503–E509. [Google Scholar] [CrossRef]
- Jridi, M.; Souissi, N.; Mbarek, A.; Chadeyron, G.; Kammoun, M.; Nasri, M. Comparative Study of Physico-Mechanical and Antioxidant Properties of Edible Gelatin Films from the Skin of Cuttlefish. Int. J. Biol. Macromol. 2013, 61, 17–25. [Google Scholar] [CrossRef]
- Jeevithan, E.; Qingbo, Z.; Bao, B.; Wu, W. Biomedical and Pharmaceutical Application of Fish Collagen and Gelatin: A Review. J. Nutr. Ther. 2013. [Google Scholar] [CrossRef]
- Loo, C.P.Y.; Sarbon, N.M. Chicken Skin Gelatin Films with Tapioca Starch. Food Biosci. 2020, 35, 100589. [Google Scholar] [CrossRef]
- Manikandan, A.; Thirupathi Kumara Raja, S.; Thiruselvi, T.; Gnanamani, A. Engineered Fish Scale Gelatin: An Alternative and Suitable Biomaterial for Tissue Engineering. J. Bioact. Compat. Polym. 2018, 33, 332–346. [Google Scholar] [CrossRef]
- Mahmoudi Saber, M. Strategies for Surface Modification of Gelatin-Based Nanoparticles. Colloids Surfaces B Biointerfaces 2019, 183, 110407. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Nayak, S.K.; Anis, A.; Ray, S.S.; Kim, D.; Hanh Nguyen, T.T.; Pal, K. Introduction of Biopolymers; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Du, C.; Abdullah, J.J.; Greetham, D.; Fu, D.; Yu, M.; Ren, L.; Li, S.; Lu, D. Valorization of Food Waste into Biofertiliser and Its Field Application. J. Clean. Prod. 2018, 187, 273–284. [Google Scholar] [CrossRef]
- Weiss, A.V.; Fischer, T.; Iturri, J.; Benitez, R.; Toca-Herrera, J.L.; Schneider, M. Mechanical Properties of Gelatin Nanoparticles in Dependency of Crosslinking Time and Storage. Colloids Surfaces B Biointerfaces 2019, 175, 713–720. [Google Scholar] [CrossRef]
- Ceylan, Z.; Unal SengOr, G.F.; Yilmaz, M.T. Amino Acid Composition of Gilthead Sea Bream Fillets (Sparus Aurata) Coated with Thymol-Loaded Chitosan Nanofibers during Cold Storage. J. Biotechnol. 2017, 256, S28. [Google Scholar] [CrossRef]
- Ceylan, Z.; Meral, R.; Cavidoglu, I.; Yagmur Karakas, C.; Tahsin Yilmaz, M. A New Application on Fatty Acid Stability of Fish Fillets: Coating with Probiotic Bacteria-loaded Polymer-based Characterized Nanofibers. J. Food Saf. 2018, 38. [Google Scholar] [CrossRef]
- Ceylan, Z.; Yaman, M.; Sağdıç, O.; Karabulut, E.; Yilmaz, M.T. Effect of Electrospun Thymol-Loaded Nanofiber Coating on Vitamin B Profile of Gilthead Sea Bream Fillets (Sparus Aurata). LWT 2018, 98, 162–169. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Ceylan, Z.; Meral, R.; Karakaş, C.Y.; Dertli, E.; Yilmaz, M.T. A Novel Strategy for Probiotic Bacteria: Ensuring Microbial Stability of Fish Fillets Using Characterized Probiotic Bacteria-Loaded Nanofibers. Innov. Food Sci. Emerg. Technol. 2018, 48, 212–218. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino Acids, Fatty Acids, and Dietary Fibre in Edible Seaweed Products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Venkatraman, A.; Yahoob, S.A.M.; Nagarajan, Y.; Harikrishnan, S.; Vasudevan, S.; Murugasamy, T. Pharmacological Activity of Biosynthesized Gold Nanoparticles from Brown Algae-Seaweed Turbinaria Conoides. NanoWorld J. 2018, 4. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, R.; Cao, X.; Xiong Liu, X.; Qin, X. Improved Physicochemical Properties of Yogurt Fortified with Fish Oil/γ-Oryzanol by Nanoemulsion Technology. Molecules 2018, 23, 56. [Google Scholar] [CrossRef]
- Chanthini, A.B.; Balasubramani, G.; Ramkumar, R.; Sowmiya, R.; Balakumaran, M.D.; Kalaichelvan, P.T.; Perumal, P. Structural Characterization, Antioxidant and in Vitro Cytotoxic Properties of Seagrass, Cymodocea Serrulata (R.Br.) Asch. & Magnus Mediated Silver Nanoparticles. J. Photochem. Photobiol. B Biol. 2015, 153, 145–152. [Google Scholar] [CrossRef]
- Anand, B.G.; Thomas, C.K.N.; Prakash, S.; Kumar, C.S. Biosynthesis of Silver Nano-Particles by Marine Sediment Fungi for a Dose Dependent Cytotoxicity against HEp2 Cell Lines. Biocatal. Agric. Biotechnol. 2015, 4, 150–157. [Google Scholar] [CrossRef]
- Muthuirulappan, S.; Francis, S.P. Anti-Cancer Mechanism and Possibility of Nano-Suspension Formulation for a Marine Algae Product Fucoxanthin. Asian Pacific J. Cancer Prev. 2013, 14, 2213–2216. [Google Scholar] [CrossRef]
- Phleger, C.F.; Nelson, M.M.; Mooney, B.D.; Nichols, P.D. Interannual and between Species Comparison of the Lipids, Fatty Acids and Sterols of Antarctic Krill from the US AMLR Elephant Island Survey Area. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 131, 733–747. [Google Scholar] [CrossRef]
- Tou, J.C.; Jaczynski, J.; Chen, Y.-C. Krill for Human Consumption: Nutritional Value and Potential Health Benefits. Nutr. Rev. 2008, 65, 63–77. [Google Scholar] [CrossRef]
- Racine, R.A.; Deckelbaum, R.J. Sources of the Very-Long-Chain Unsaturated Omega-3 Fatty Acids: Eicosapentaenoic Acid and Docosahexaenoic Acid. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 123–128. [Google Scholar] [CrossRef]
- Haider, J.; Majeed, H.; Williams, P.A.; Safdar, W.; Zhong, F. Formation of Chitosan Nanoparticles to Encapsulate Krill Oil (Euphausia superba) for Application as a Dietary Supplement. Food Hydrocoll. 2017, 63, 27–34. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, F.; Zhang, M.; Liu, J. A Green Enzymatic Extraction Optimization and Oxidative Stability of Krill Oil from Euphausia superba. Mar. Drugs 2020, 18, 82. [Google Scholar] [CrossRef] [PubMed]
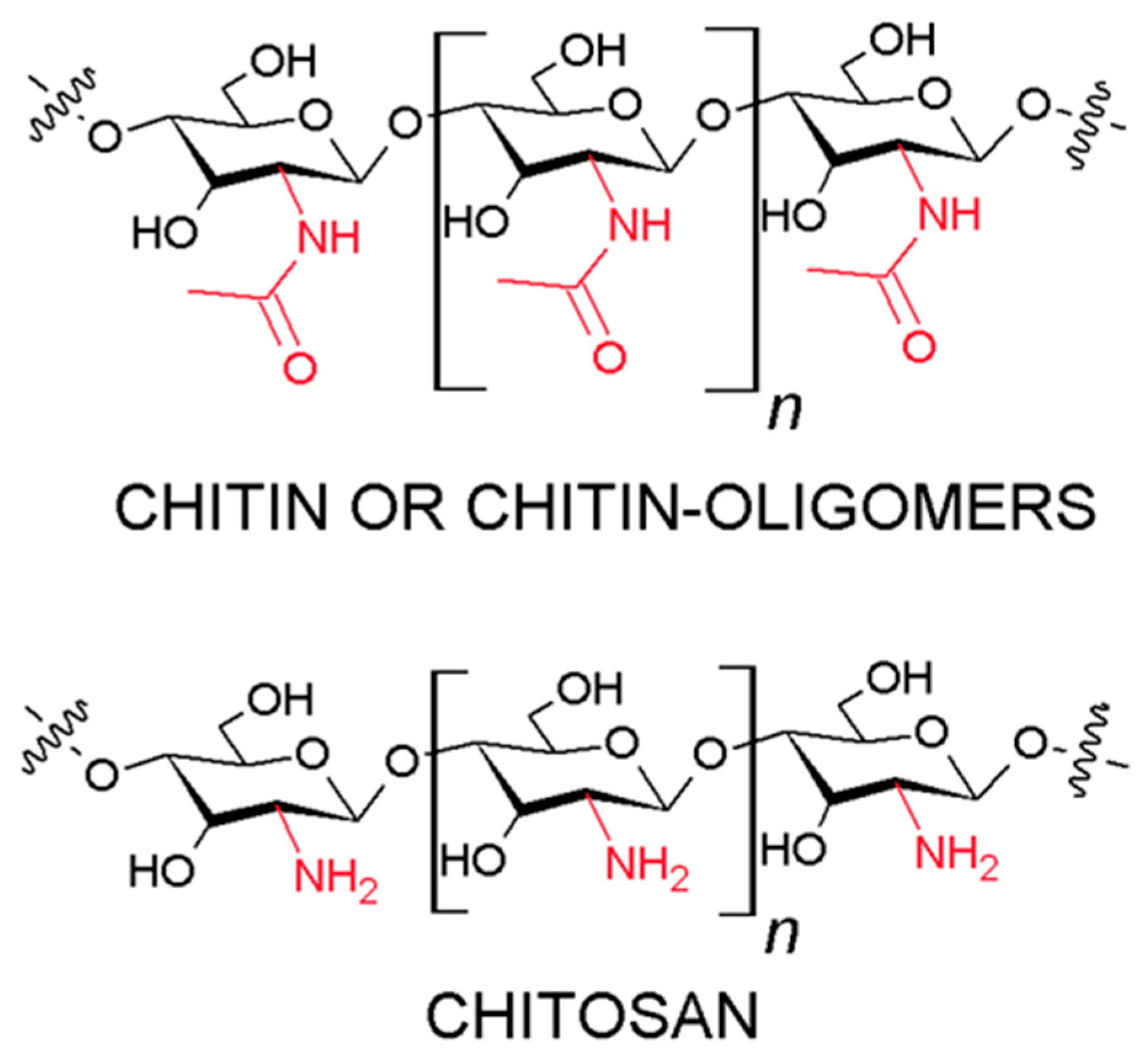
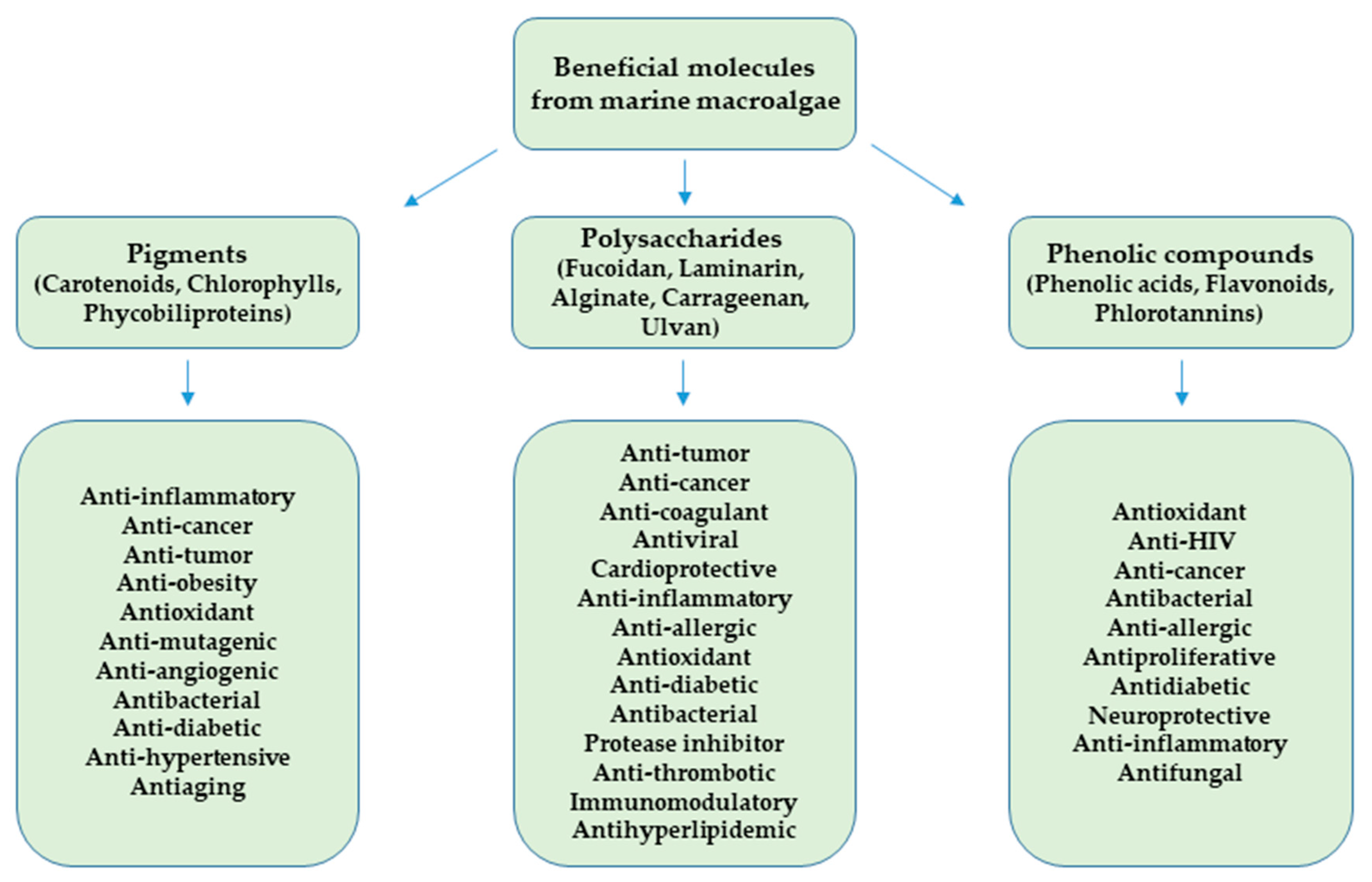
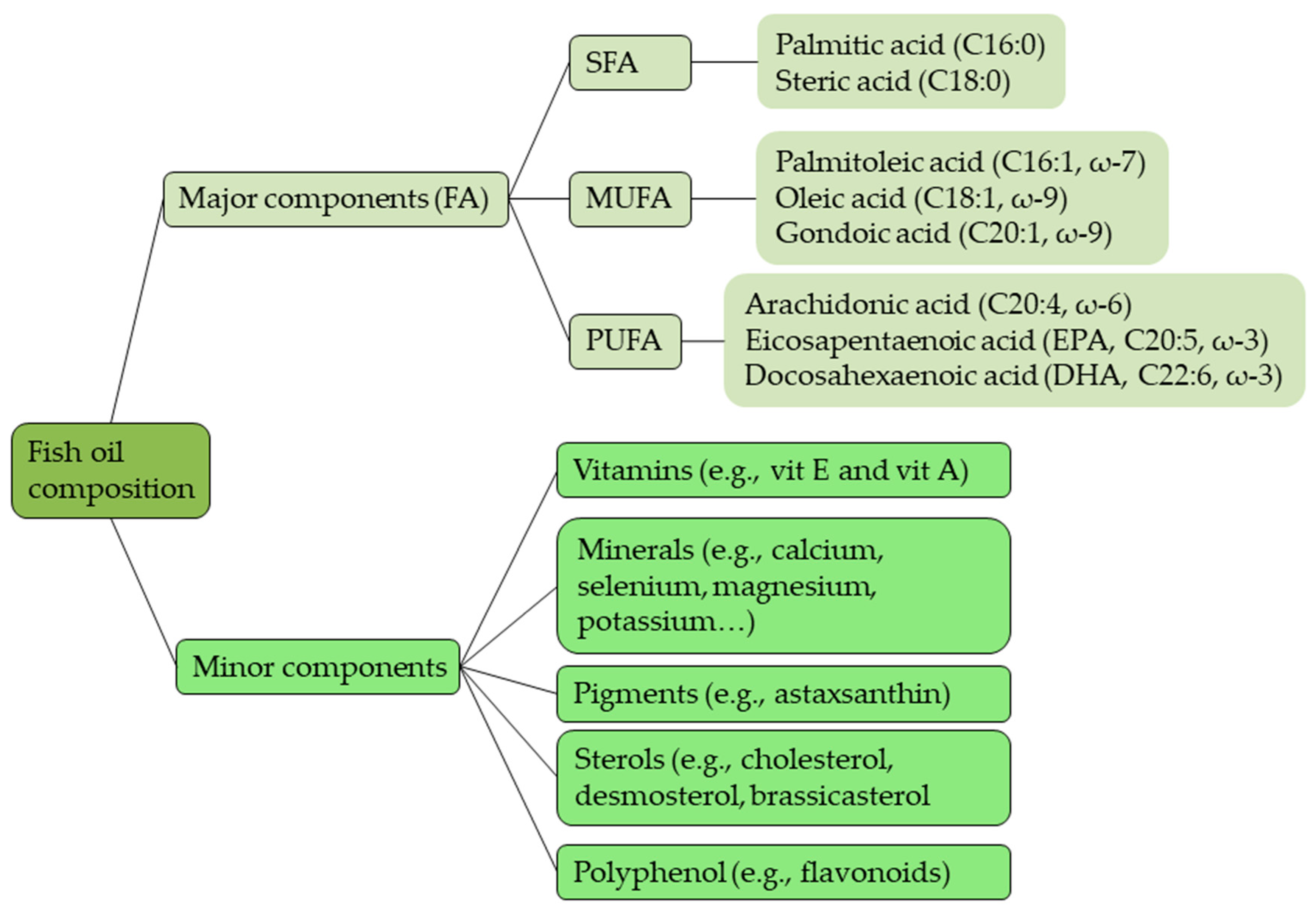
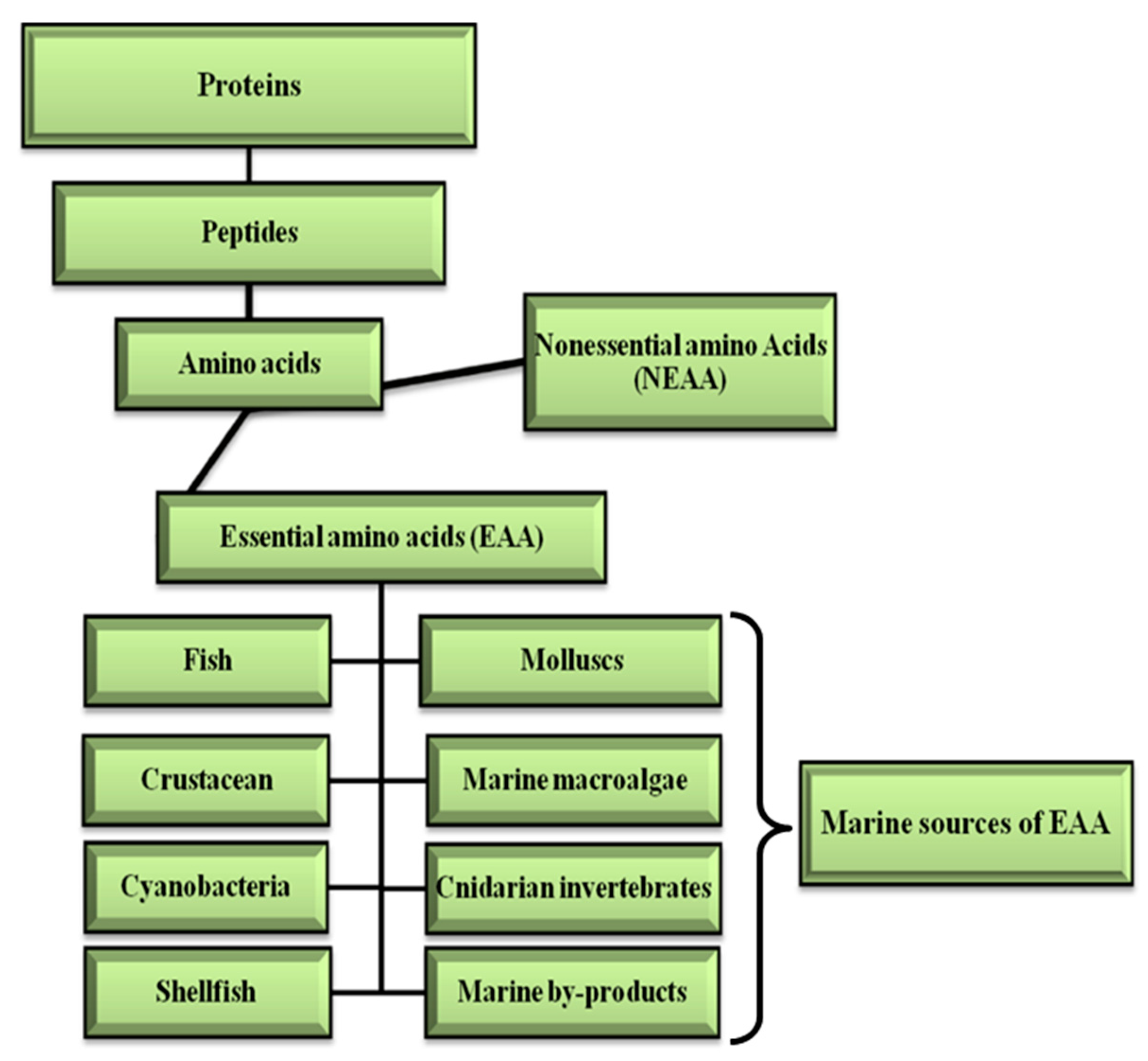
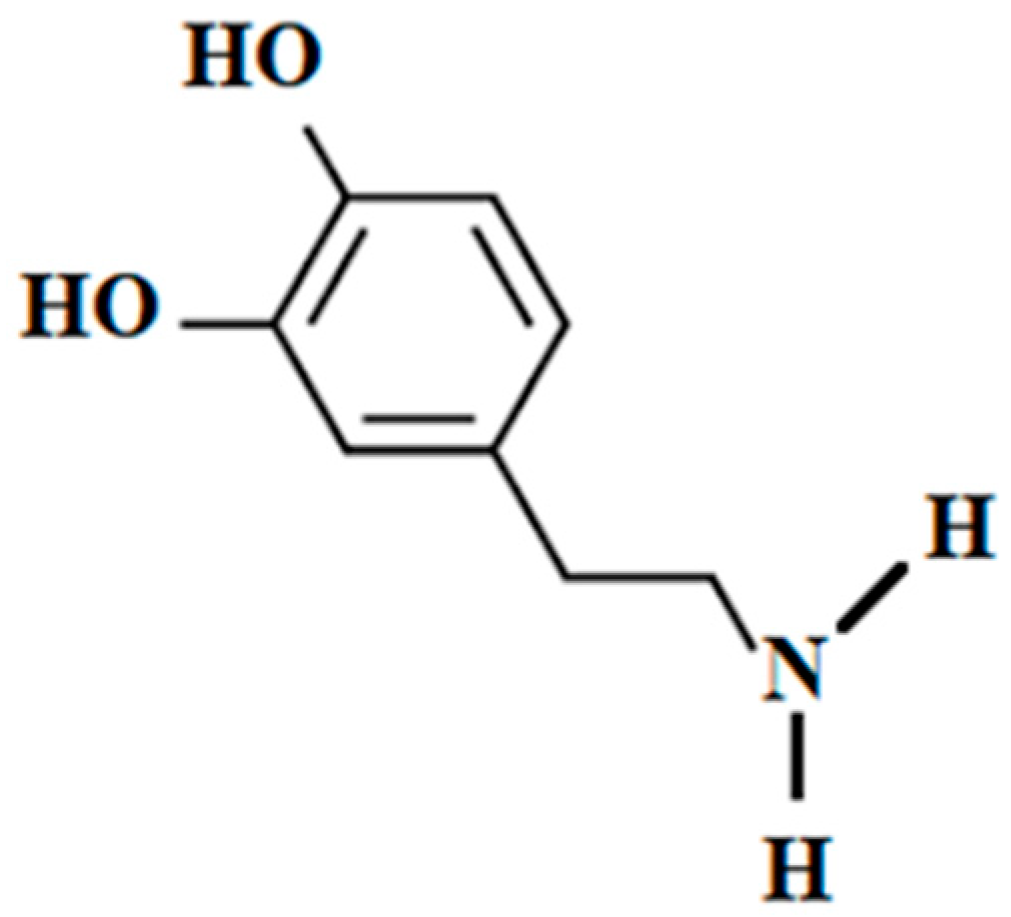
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimat, V.; Elabed, N.; Kulawik, P.; Ceylan, Z.; Jamroz, E.; Yazgan, H.; Čagalj, M.; Regenstein, J.M.; Özogul, F. Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Mar. Drugs 2020, 18, 627. https://doi.org/10.3390/md18120627
Šimat V, Elabed N, Kulawik P, Ceylan Z, Jamroz E, Yazgan H, Čagalj M, Regenstein JM, Özogul F. Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Marine Drugs. 2020; 18(12):627. https://doi.org/10.3390/md18120627
Chicago/Turabian StyleŠimat, Vida, Nariman Elabed, Piotr Kulawik, Zafer Ceylan, Ewelina Jamroz, Hatice Yazgan, Martina Čagalj, Joe M. Regenstein, and Fatih Özogul. 2020. "Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits" Marine Drugs 18, no. 12: 627. https://doi.org/10.3390/md18120627
APA StyleŠimat, V., Elabed, N., Kulawik, P., Ceylan, Z., Jamroz, E., Yazgan, H., Čagalj, M., Regenstein, J. M., & Özogul, F. (2020). Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Marine Drugs, 18(12), 627. https://doi.org/10.3390/md18120627










