Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment
Abstract
1. Introduction
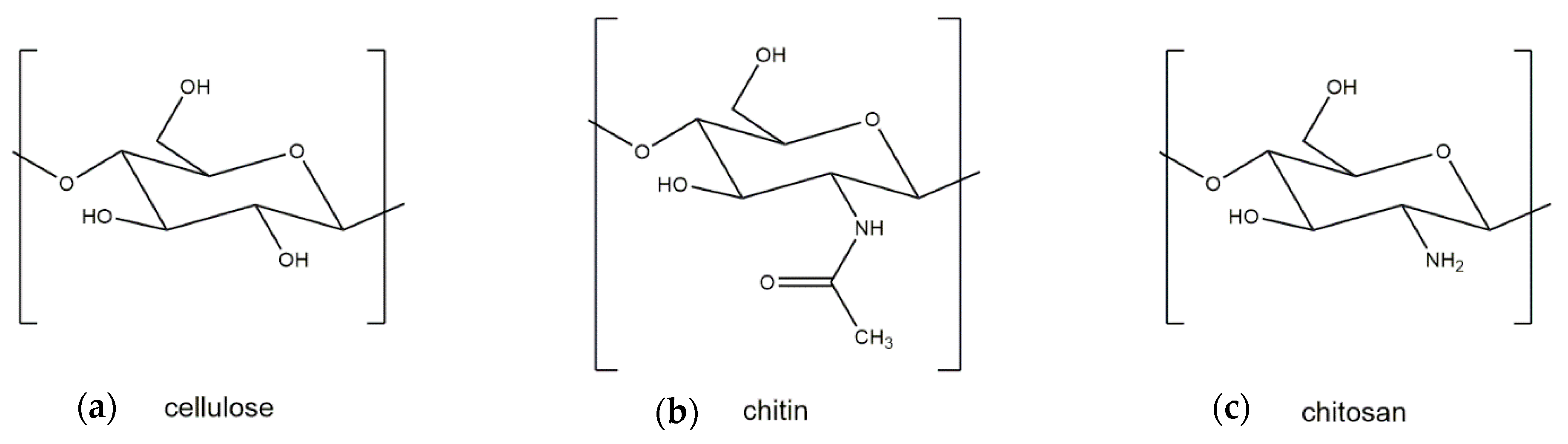
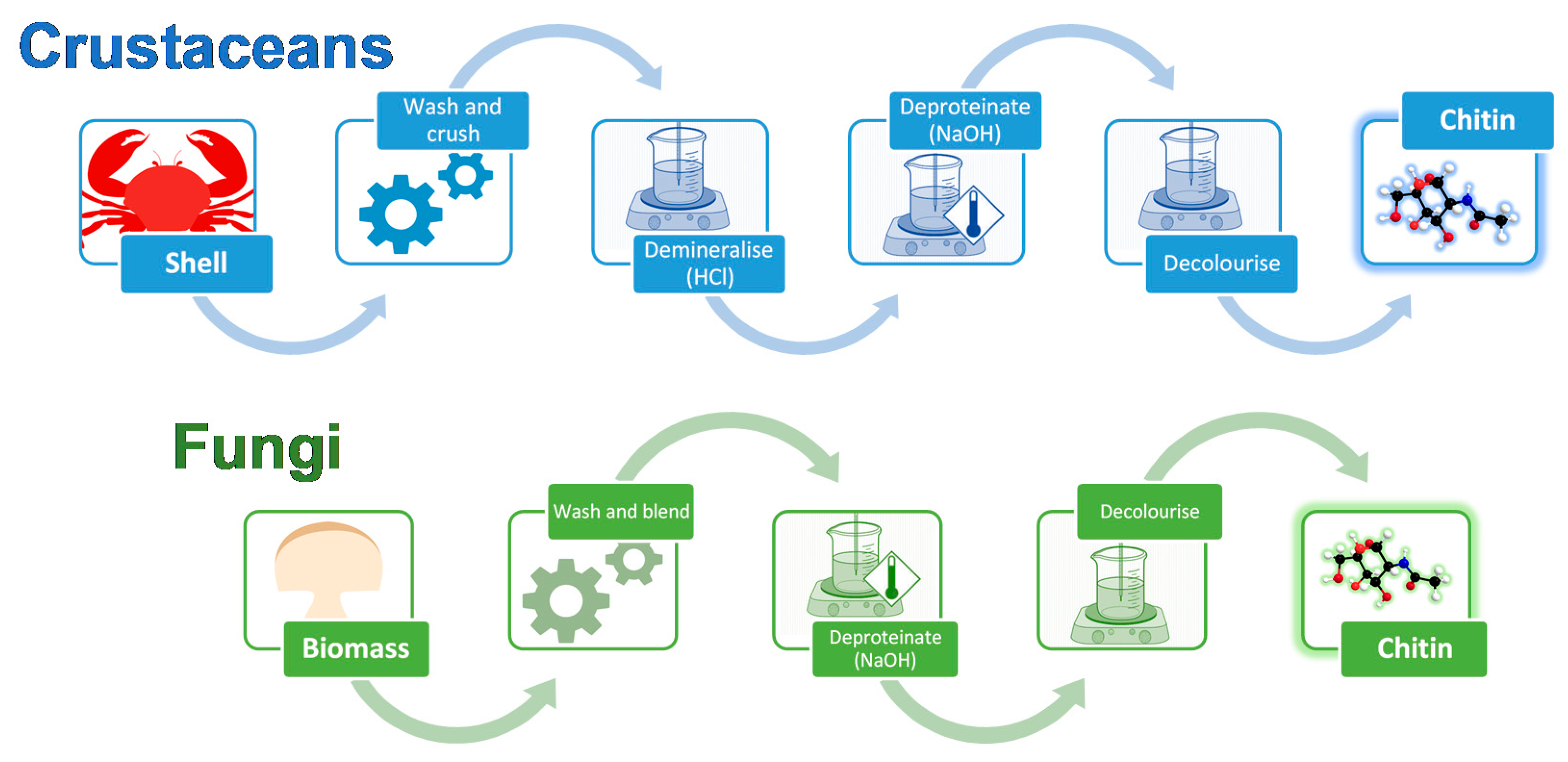
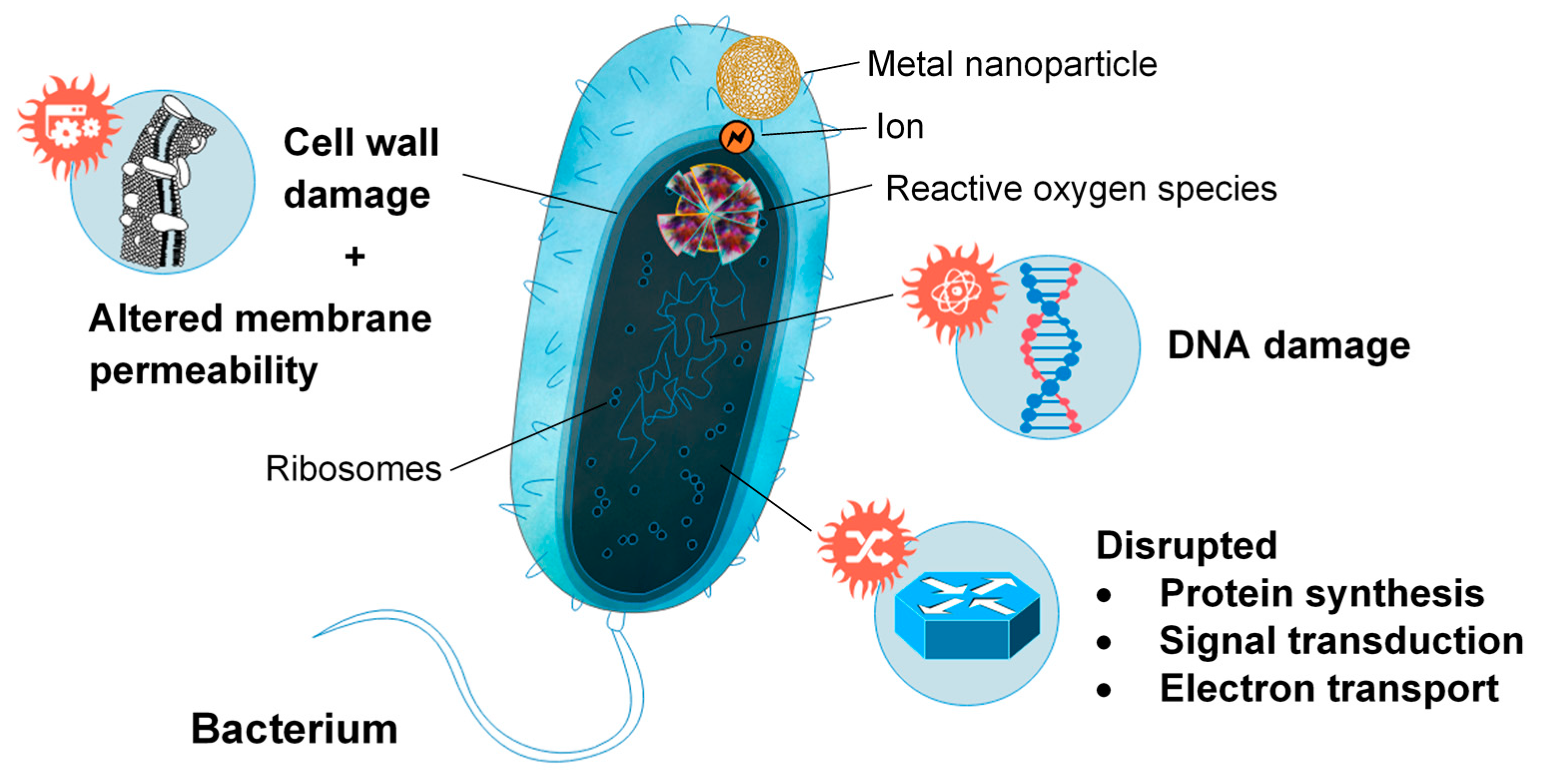
2. Differences between Crustacean and Fungal Chitin
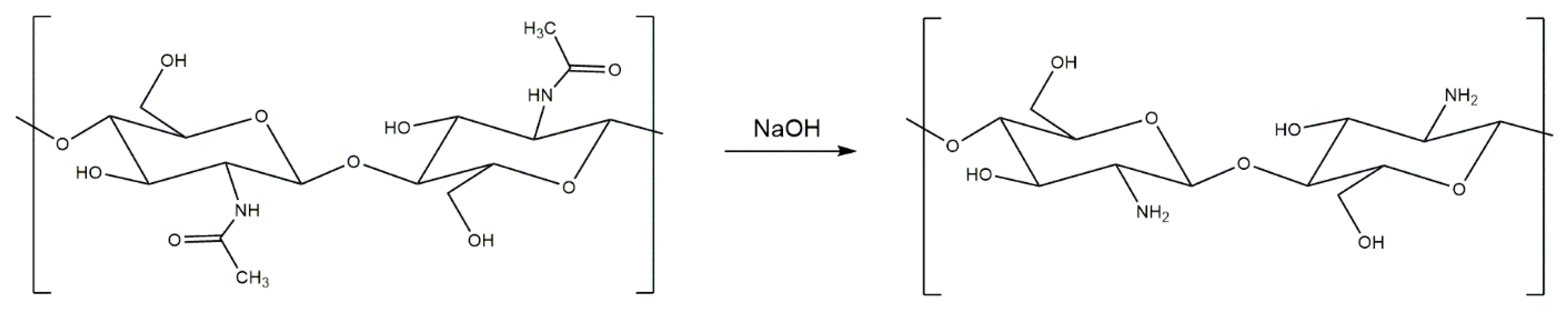
3. Generation and Properties of Chitosan
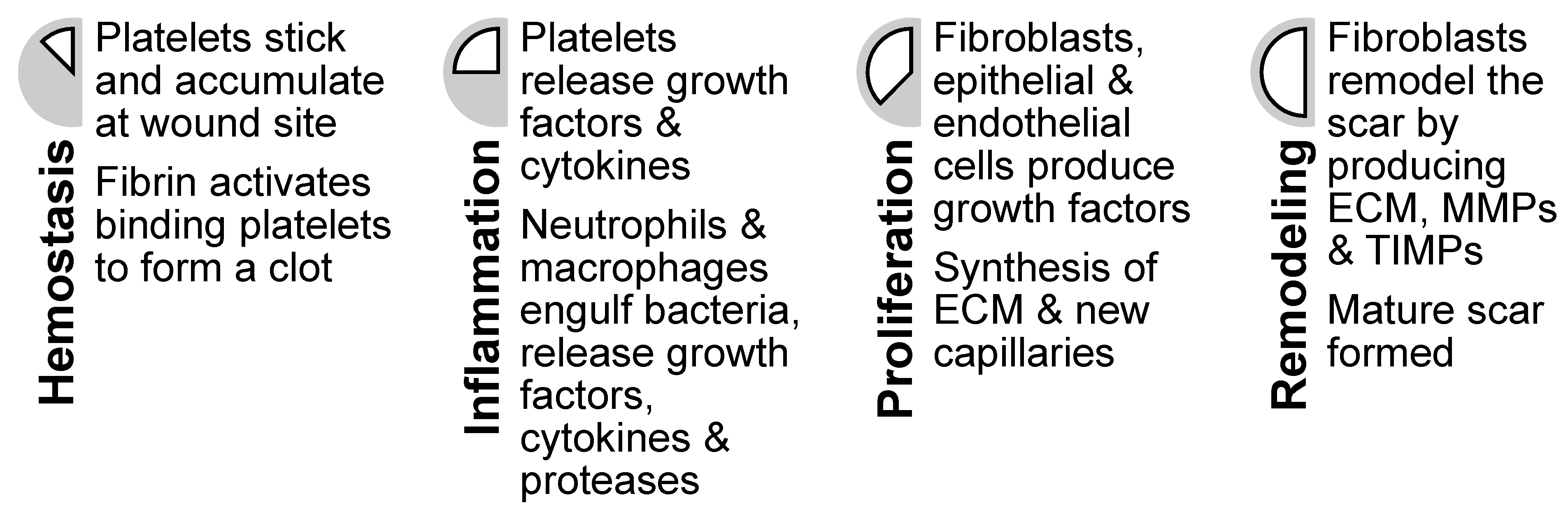
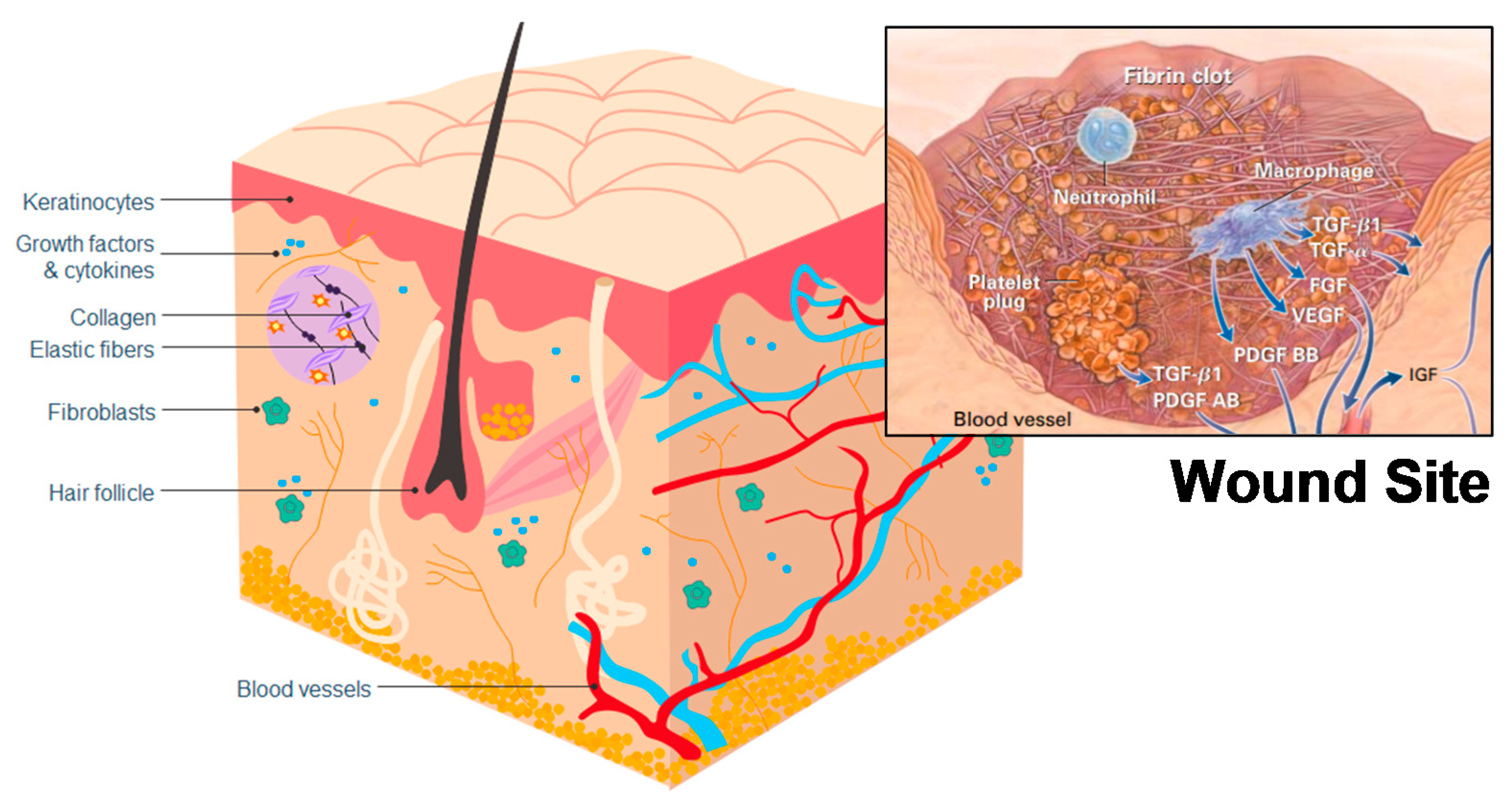
4. Healing Mechanisms of Chitin and Chitosan
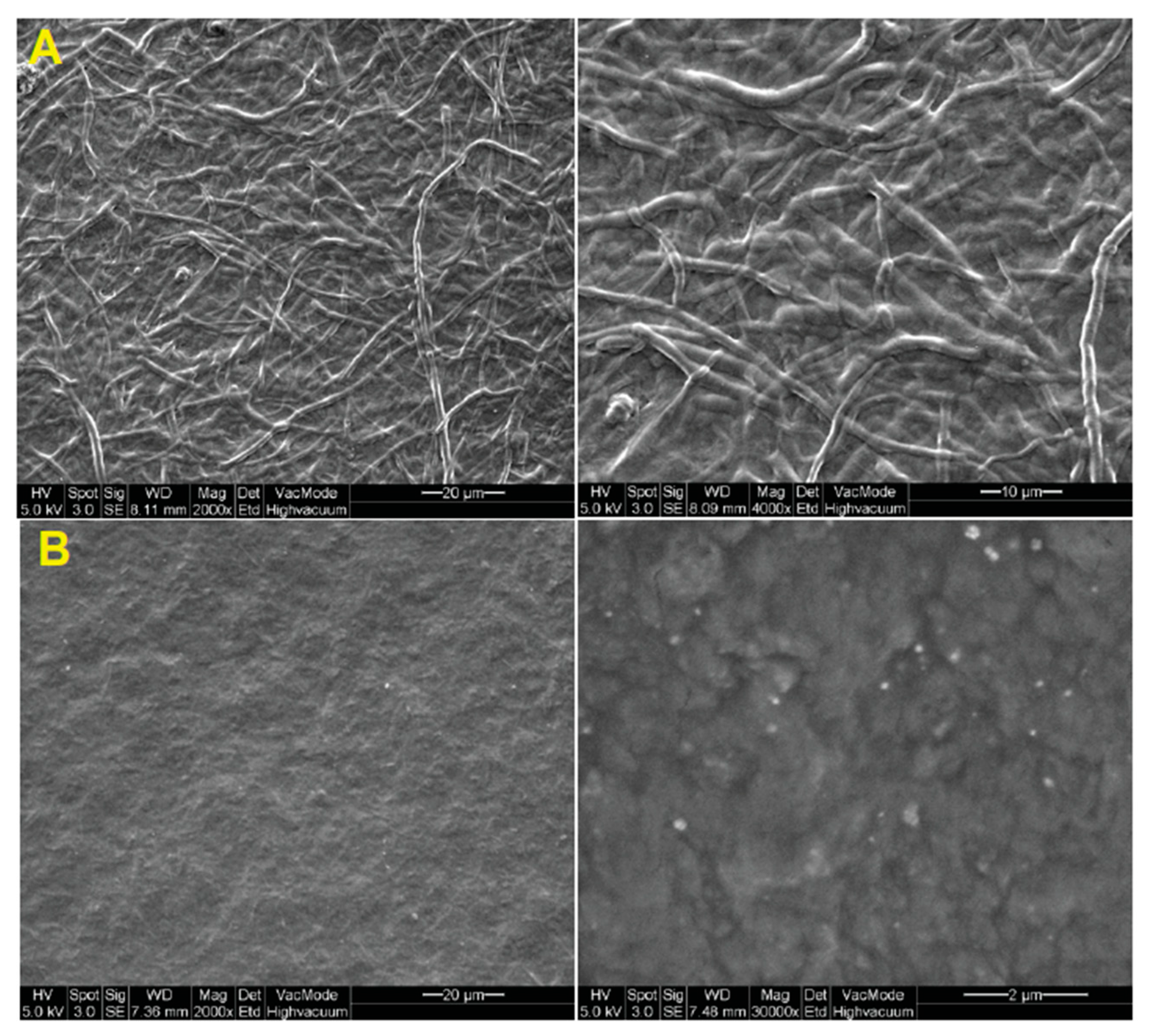
5. Fungi-Derived Chitin and Chitosan Wound Dressings
6. Crustacean-Derived Chitin and Chitosan Wound Dressings
6.1. Derivatization of Chitin and Chitosan to Improve Solubility and Biomedical Properties
6.2. Chitin and Chitosan Nanocomposite Architectures as Wound Dressings
7. Human Clinical Trials Utilizing Chitin and Chitosan for Wound Treatment
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morganti, P.; Morganti, G.; Coltelli, M.B. Chitin Nanomaterials and Nanocomposites for Tissue Repair. In Marine-Derived Biomaterials for Tissue Engineering Applications; Springer: Berlin, Germany, 2019; pp. 523–544. [Google Scholar]
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Algan, C.; Jacobs, V.; John, M.; Oksman, K.; Mathew, A.P. Electrospun chitosan-based nanocomposite mats reinforced with chitin nanocrystals for wound dressing. Carbohydr. Polym. 2014, 109, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Buabeid, M.A.; Arafa, E.-S.A.; Hussain, I.; Li, L.; Murtaza, G. The wound healing and antibacterial potential of triple-component nanocomposite (chitosan-silver-sericin) films loaded with moxifloxacin. Int. J. Pharm. 2019, 564, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.; Kumar, S.; Koh, J.; Seena, S.; Singh, P.; Ramalho, A.; Sobral, A.J. Bio-based chitosan/gelatin/Ag@ ZnO bionanocomposites: Synthesis and mechanical and antibacterial properties. Cellulose 2019, 26, 1–15. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Nawawi, W.; Jones, M.; Murphy, R.J.; Lee, K.-Y.; Kontturi, E.; Bismarck, A. Nanomaterials derived from fungal sources—Is it the new hype? Biomacromolecules 2019, 21, 30–55. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Atkinson, A.; Siegel, V.; Pakhomov, E.; Jessopp, M.; Loeb, V. A re-appraisal of the total biomass and annual production of Antarctic krill. Deep Sea Res. Part I Oceanogr. Res. Pap. 2009, 56, 727–740. [Google Scholar] [CrossRef]
- Jeuniaux, C.; Voss-Foucart, M.F. Chitin biomass and production in the marine environment. Biochem. Syst. Ecol. 1991, 19, 347–356. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203. [Google Scholar] [CrossRef]
- Charoenvuttitham, P.; Shi, J.; Mittal, G.S. Chitin extraction from black tiger shrimp (Penaeus monodon) waste using organic acids. Sep. Sci. Technol. 2006, 41, 1135–1153. [Google Scholar] [CrossRef]
- Gopalan Nair, K.; Dufresne, A. Crab shell chitin whisker reinforced natural rubber nanocomposites. 1. Processing and swelling behavior. Biomacromolecules 2003, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Weiland, K.; Kujundzic, M.; Theiner, J.; Kahlig, H.; Kontturi, E.; John, S.; Bismarck, A.; Mautner, A. Waste-Derived Low-Cost Mycelium Nanopapers with Tunable Mechanical and Surface Properties. Biomacromolecules 2019, 20, 3513–3523. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, F.; Rapana, P.; Tomati, U.; Galli, E. Chitin and chitosan from Basidiomycetes. Int. J. Biol. Macromol. 2008, 43, 8–12. [Google Scholar] [CrossRef]
- Hassainia, A.; Satha, H.; Boufi, S. Chitin from Agaricus bisporus: Extraction and characterization. Int. J. Biol. Macromol. 2018, 117, 1334–1342. [Google Scholar] [CrossRef]
- Nawawi, W.; Lee, K.-Y.; Kontturi, E.; Murphy, R.; Bismarck, A. Chitin nanopaper from mushroom extract: Natural composite of nanofibres and glucan from a single bio-based source. ACS Sustain. Chem. Eng. 2019, 7, 6492–6496. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Barber, A.R.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioresour. Bioprocess. 2017, 4, 27. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Cuong, H.N.; Minh, N.C.; Van Hoa, N.; Trung, T.S. Preparation and characterization of high purity β-chitin from squid pens (Loligo chenisis). Int. J. Biol. Macromol. 2016, 93, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Fesel, P.H.; Zuccaro, A. β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Gaill, F.; Shillito, B.; Menard, F.; Goffinet, G.; Childress, J.J. Rate and process of tube production by the deep-sea hydrothermal vent tubeworm Riftia pachyptila. Mar. Ecol. Prog. Ser. 1997, 148, 135–143. [Google Scholar] [CrossRef]
- Kaya, M.; Bitim, B.; Mujtaba, M.; Koyuncu, T. Surface morphology of chitin highly related with the isolated body part of butterfly (Argynnis pandora). Int. J. Biol. Macromol. 2015, 81, 443–449. [Google Scholar] [CrossRef]
- Naczk, M.; Synowiecki, J.; Sikorski, Z.E. The gross chemical composition of Antarctic krill shell waste. Food Chem. 1981, 7, 175–179. [Google Scholar] [CrossRef]
- Pandharipande, S.; Bhagat, P.H. Synthesis of chitin from crab shells and its utilization in preparation of nanostructured film. Synthesis 2016, 5, 1378–1383. [Google Scholar]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Tauber, O.E. The distribution of chitin in an insect. J. Morphol. 1934, 56, 51–58. [Google Scholar] [CrossRef]
- Janesch, J.; Jones, M.; Bacher, M.; Kontturi, E.; Bismarck, A.; Mautner, A. Mushroom-derived chitosan-glucan nanopaper filters for the treatment of water. React. Funct. Polym. 2019, 146, 104428. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitin nanostructures in living organisms. In Chitin; Springer: Berlin, Germany, 2011; pp. 1–34. [Google Scholar]
- Hackman, R. Studies on chitin IV. The occurrence of complexes in which chitin and protein are covalently linked. Aust. J. Biol. Sci. 1960, 13, 568–577. [Google Scholar] [CrossRef]
- Attwood, M.M.; Zola, H. The association between chitin and protein in some chitinous tissues. Comp. Biochem. Physiol. 1967, 20, 993–998. [Google Scholar] [CrossRef]
- Kramer, K.J.; Hopkins, T.L.; Schaefer, J. Applications of solids NMR to the analysis of insect sclerotized structures. Insect Biochem. Mol. Biol. 1995, 25, 1067–1080. [Google Scholar] [CrossRef]
- Percot, A.; Viton, C.; Domard, A. Characterization of shrimp shell deproteinization. Biomacromolecules 2003, 4, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Sietsma, J.; Wessels, J. Evidence for covalent linkages between chitin and β-glucan in a fungal wall. Microbiology 1979, 114, 99–108. [Google Scholar] [CrossRef]
- Kollár, R.; Reinhold, B.B.; Petráková, E.; Yeh, H.J.; Ashwell, G.; Drgonová, J.; Kapteyn, J.C.; Klis, F.M.; Cabib, E. Architecture of the yeast cell wall β (1→6)-glucan interconnects mannoprotein, β (1→3)-glucan, and chitin. J. Biol. Chem. 1997, 272, 17762–17775. [Google Scholar] [CrossRef] [PubMed]
- Heux, L.; Brugnerotto, J.; Desbrieres, J.; Versali, M.-F.; Rinaudo, M. Solid state NMR for determination of degree of acetylation of chitin and chitosan. Biomacromolecules 2000, 1, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Latge, J.P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef]
- Bartnicki-Garcia, S.; Nickerson, W.J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim. Biophys. Acta 1962, 58, 102–119. [Google Scholar] [CrossRef]
- Karimi, K.; Zamani, A. Mucor indicus: Biology and industrial application perspectives: A review. Biotechnol. Adv. 2013, 31, 466–481. [Google Scholar] [CrossRef]
- Rice, M. Get an Old Blender and Make Your Own Deckle and Mould. Mushroom J. Wild Mushrooming 1992, 10, 22–26. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Sietsma, J.; Wessels, J. Chemical analysis of the hyphal walls of Schizophyllum commune. Biochim. Biophys. Acta Gen. Subj. 1977, 496, 225–239. [Google Scholar] [CrossRef]
- Peniston, Q.P.; Johnson, E.L. Process for the Manufacture of Chitosan. U.S. Patent No. 4,195,175, 25 March 1980. [Google Scholar]
- Thanou, M.; Verhoef, J.; Junginger, H. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Deliv. Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.R.; Islam, M. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Bamba, Y.; Ogawa, Y.; Saito, T.; Berglund, L.A.; Isogai, A. Estimating the Strength of Single Chitin Nanofibrils via Sonication-Induced Fragmentation. Biomacromolecules 2017, 18, 4405–4410. [Google Scholar] [CrossRef]
- Webster, J.; Weber, R. Introduction to Fungi; Cambridge University Press: Cambrige, UK, 2007. [Google Scholar]
- Cui, J.; Yu, Z.; Lau, D. Effect of Acetyl Group on Mechanical Properties of Chitin/Chitosan Nanocrystal: A Molecular Dynamics Study. Int. J. Mol. Sci. 2016, 17, 61. [Google Scholar] [CrossRef]
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Martin, P. Wound healing-aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Su, C.-H.; Liu, S.-H.; Yu, S.-Y.; Hsieh, Y.-L.; Ho, H.-O.; Hu, C.-H.; Sheu, M.-T. Development of fungal mycelia as a skin substitute: Characterization of keratinocyte proliferation and matrix metalloproteinase expression during improvement in the wound-healing process. J. Biomed. Mater. Res. Part A 2005, 72, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-H.; Sun, C.-S.; Juan, S.-W.; Hu, C.-H.; Ke, W.-T.; Sheu, M.-T. Fungal mycelia as the source of chitin and polysaccharides and their applications as skin substitutes. Biomaterials 1997, 18, 1169–1174. [Google Scholar] [CrossRef]
- Ohshima, Y.; Nishino, K.; Yonekura, Y.; Kishimoto, S.; Wakabayashi, S. Clinical application of chitin non-woven fabric as wound dressing. Eur. J. Plast. Surg. 1987, 10, 66–69. [Google Scholar] [CrossRef]
- Malette, W.; Quigley, H.; Adickes, E. Chitosan effect in vascular surgery, tissue culture and tissue regeneration. In Nature and Technology; Springer: Berlin, Germany, 1986; pp. 435–442. [Google Scholar]
- Andrews, D.A.; Low, P.S. Role of red blood cells in thrombosis. Curr. Opin. Hematol. 1999, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A. Biochemical significance of exogenous chitins and chitosans in animals and patients. Carbohydr. Polym. 1993, 20, 7–16. [Google Scholar] [CrossRef]
- Klokkevold, P.R.; Lew, D.S.; Ellis, D.G.; Bertolami, C.N. Effect of chitosan on lingual hemostasis in rabbits. J. Oral Maxillofac. Surg. 1991, 49, 858–863. [Google Scholar] [CrossRef]
- Brandenberg, G.; Leibrock, L.G.; Shuman, R.; Malette, W.G.; Quigley, H. Chitosan: A New Topical Hemostatic Agent for Diffuse Capillary Bleeding in Brain Tissue. Neurosurgery 1984, 15, 9–13. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Dunn, J.D.; Lindblad, W.J.; Cohen, I.K. Analysis of the effects of chitosan on inflammation, angiogenesis, fibroplasia, and collagen deposition in polyvinyl alcohol sponge implants in rat wounds. Wound Repair Regen. 1996, 4, 48–52. [Google Scholar] [CrossRef]
- Usami, Y.; Minami, S.; Okamoto, Y.; Matsuhashi, A.; Shigemasa, Y. Influence of chain length of N-acetyl-D-glucosamine and D-glucosamine residues on direct and complement-mediated chemotactic activities for canine polymorphonuclear cells. Carbohydr. Polym. 1997, 32, 115–122. [Google Scholar] [CrossRef]
- Usami, Y.; Okamoto, Y.; Minami, S.; Matsuhashi, A.; Kumazawa, N.H.; Tanioka, S.-I.; Shigemasa, Y. Migration of canine neutrophils to chitin and chitosan. J. Vet. Med. Sci. 1994, 56, 1215–1216. [Google Scholar] [CrossRef]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Ueno, H.; Yamada, H.; Tanaka, I.; Kaba, N.; Matsuura, M.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 1999, 20, 1407–1414. [Google Scholar] [CrossRef]
- Okamoto, Y.; Shibazaki, K.; Minami, S.; Matsuhashi, A.; Tanioka, S.-I.; Shigemasa, Y. Evaluation of chitin and chitosan on open wound healing in dogs. J. Vet. Med. Sci. 1995, 57, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Nakamura, F.; Murakami, M.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Evaluation effects of chitosan for the extracellular matrix production by fibroblasts and the growth factors production by macrophages. Biomaterials 2001, 22, 2125–2130. [Google Scholar] [CrossRef]
- Nishimura, K.; Ishihara, C.; Ukei, S.; Tokura, S.; Azuma, I. Stimulation of cytokine production in mice using deacetylated chitin. Vaccine 1986, 4, 151–156. [Google Scholar] [CrossRef]
- Nishimura, K.; Nishimura, S.; Nishi, N.; Saiki, I.; Tokura, S.; Azuma, I. Immunological activity of chitin and its derivatives. Vaccine 1984, 2, 93–99. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
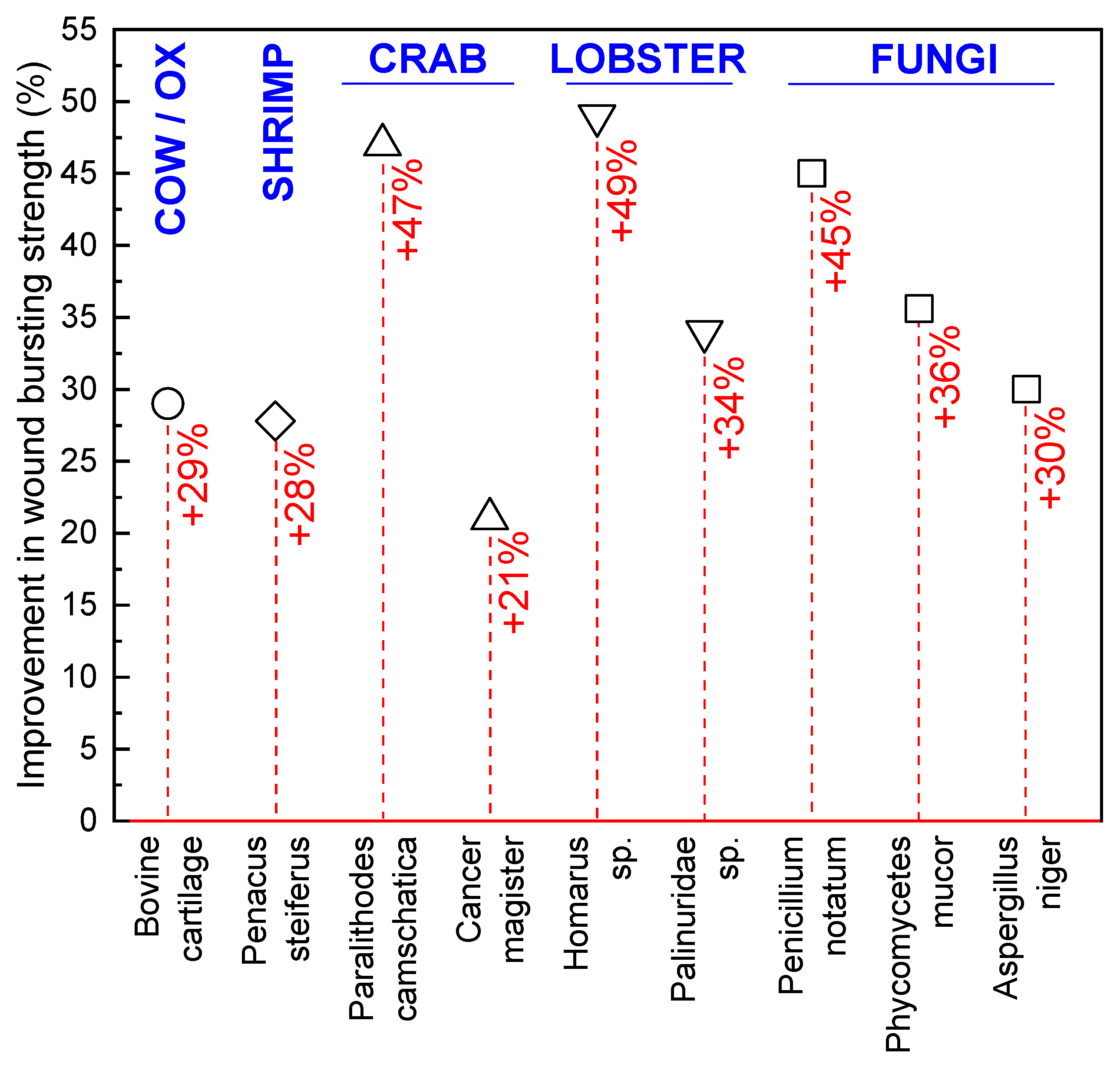
- Minagawa, T.; Okamura, Y.; Shigemasa, Y.; Minami, S.; Okamoto, Y. Effects of molecular weight and deacetylation degree of chitin/chitosan on wound healing. Carbohydr. Polym. 2007, 67, 640–644. [Google Scholar] [CrossRef]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef]
- Pillai, C.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Alsarra, I.A. Chitosan topical gel formulation in the management of burn wounds. Int. J. Biol. Macromol. 2009, 45, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.J.; Chen, S.Q. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 131–137. [Google Scholar] [CrossRef]
- Wainwright, M.; Rally, L.; Ali, T.A. The scientific basis of mould therapy. Mycologist 1992, 6, 108–110. [Google Scholar] [CrossRef]
- Wainwright, M. Moulds in folk medicine. Folklore 1989, 100, 162–166. [Google Scholar] [CrossRef]
- Baker, T. Fungal styptics. Mycologist 1989, 3, 19–20. [Google Scholar] [CrossRef]
- Wainwright, M. Moulds in ancient and more recent medicine. Mycologist 1989, 3, 21–23. [Google Scholar] [CrossRef]
- Prudden, J.F.; Migel, P.; Hanson, P.; Friedrich, L.; Balassa, L. The discovery of a potent pure chemical wound-healing accelerator. Am. J. Surg. 1970, 119, 560–564. [Google Scholar] [CrossRef]
- Balassa, L.; Prudden, J. In Applications of chitin and chitosan in wound-healing acceleration. In Proceedings of the 1st International Conference on Chitin/Chitosan, Springfield, VA, USA, 11–13 April 1977; National Technical Information. pp. 296–305. [Google Scholar]
- Chung, L.Y.; Schmidt, R.J.; Hamlyn, P.F.; Sagar, B.F.; Andrew, A.M.; Turner, T.D. Biocompatibility of potential wound management products: Fungal mycelia as a source of chitin/chitosan and their effect on the proliferation of human F1000 fibroblasts in culture. J. Biomed. Mater. Res. 1994, 28, 463–469. [Google Scholar] [CrossRef]
- Chung, L.Y.; Schmidt, R.; Hamlyn, P.; Sagar, B.F.; Andrews, A.; Turner, T. Biocompatibility of potential wound management products: Hydrogen peroxide generation by fungal chitin/chitosans and their effects on the proliferation of murine L929 fibroblasts in culture. J. Biomed. Mater. Res. 1998, 39, 300–307. [Google Scholar] [CrossRef]
- Chen, R.-N.; Lee, L.-W.; Chen, L.-C.; Ho, H.-O.; Lui, S.-C.; Sheu, M.-T.; Su, C.-H. Wound-healing effect of micronized sacchachitin (mSC) nanogel on corneal epithelium. Int. J. Nanomed. 2012, 7, 4697. [Google Scholar]
- Mahapatra, S.; Banerjee, D. Fungal exopolysaccharide: Production, composition and applications. Microbiol. Insights 2013, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Madla, S.; Methacanon, P.; Prasitsil, M.; Kirtikara, K. Characterization of biocompatible fungi-derived polymers that induce IL-8 production. Carbohydr. Polym. 2005, 59, 275–280. [Google Scholar] [CrossRef]
- Üzere, Y.Ö.M.O.K.; Polimer, A.A.Y.F. Antibacterial agent loaded fungal polymer for use as a wound dressing. J. Biol. Chem. 2011, 39, 297–303. [Google Scholar]
- Stalhberger, T.; Simenel, C.; Clavaud, C.; Eijsink, V.G.; Jourdain, R.; Delepierre, M.; Latgé, J.-P.; Breton, L.; Fontaine, T. Chemical organization of the cell wall polysaccharide core of Malassezia restricta. J. Biol. Chem. 2014, 289, 12647–12656. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Novák, M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. β-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Blackwell, M. The Fungi: 1, 2, 3 … 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef]
- Jones, M.; Huynh, T.; Dekiwadia, C.; Daver, F.; John, S. Mycelium Composites: A Review of Engineering Characteristics and Growth Kinetics. J. Bionanoscience 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Wösten, H.; Krijgsheld, P.; Montalti, M.; Läkk, H.; Summerer, L. Growing Fungi Structures in Space. Available online: http://www.esa.int/gsp/ACT/doc/ARI/ARI%20Study%20Report/ACT-RPT-HAB-ARI-16-6101-Fungi_structures.pdf (accessed on 26 November 2019).
- Wösten, H.A. Filamentous fungi for the production of enzymes, chemicals and materials. Curr. Opin. Biotechnol. 2019, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Camere, S.; Karana, E. Fabricating materials from living organisms: An emerging design practice. J. Clean. Prod. 2018, 186, 570–584. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2019, 187, 108397. [Google Scholar] [CrossRef]
- Oh, B.H.L.; Bismarck, A.; Chan-Park, M.B. High Internal Phase Emulsion Templating with Self-Emulsifying and Thermoresponsive Chitosan-graft-PNIPAM-graft-Oligoproline. Biomacromolecules 2014, 15, 1777–1787. [Google Scholar] [CrossRef]
- Benhabiles, M.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Ribeiro, M.P.; Espiga, A.; Silva, D.; Baptista, P.; Henriques, J.; Ferreira, C.; Silva, J.C.; Borges, J.P.; Pires, E.; Chaves, P. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009, 17, 817–824. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
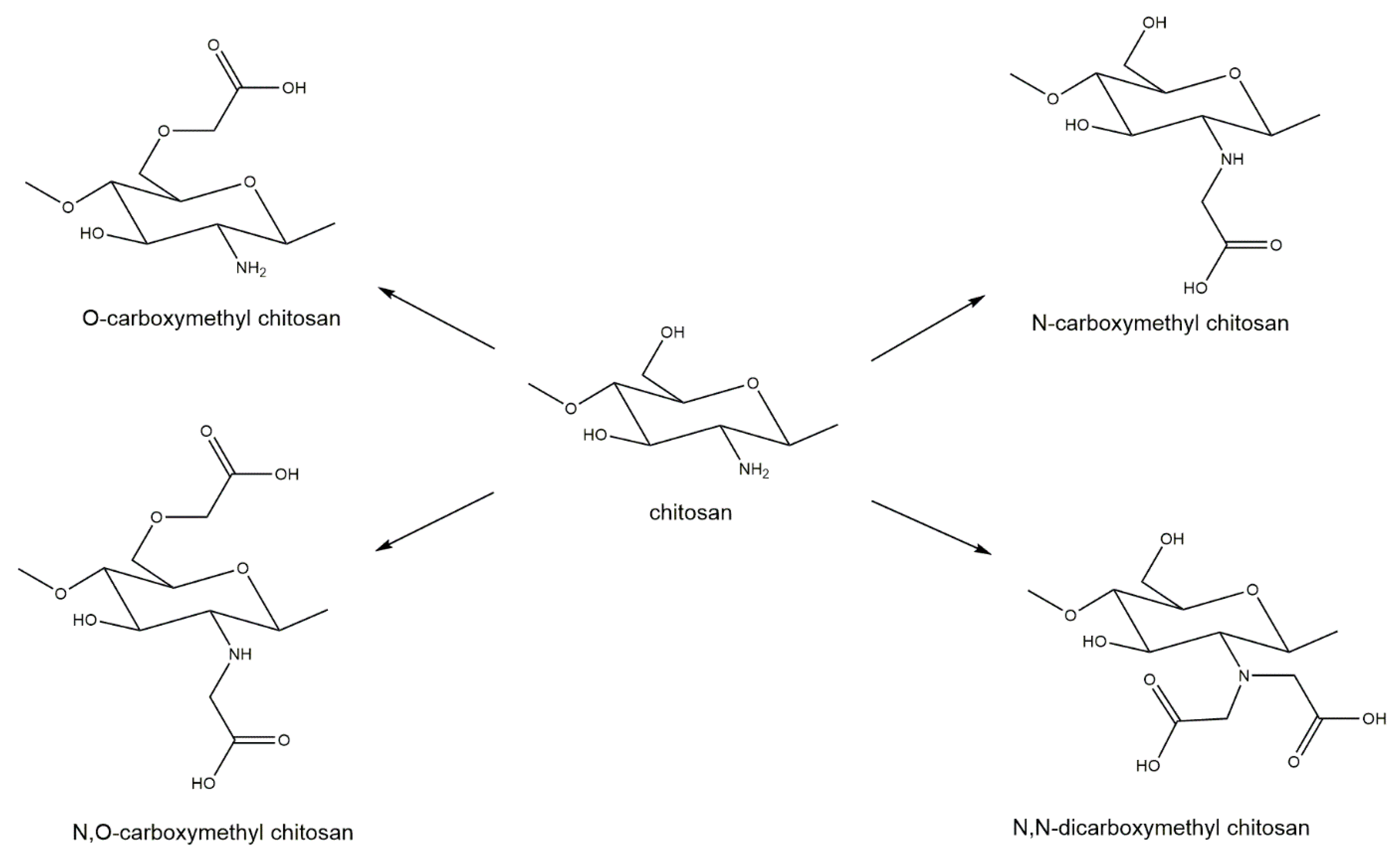
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 2013, 91, 452–466. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Qi, C.; Fang, Y.; Zhang, L.; Zhuo, R.; Jiang, X. Thermosensitive injectable in-situ forming carboxymethyl chitin hydrogel for three-dimensional cell culture. Acta Biomater. 2016, 35, 228–237. [Google Scholar] [CrossRef]
- Chang, J.; Liu, W.; Han, B.; Peng, S.; He, B.; Gu, Z. Investigation of the skin repair and healing mechanism of N-carboxymethyl chitosan in second-degree burn wounds. Wound Repair Regen. 2013, 21, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.L.; Yun, L.G.; Dong, Z.Y.; Zhi, L.; Kang, D.Y. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Anitha, A.; Rani, V.D.; Krishna, R.; Sreeja, V.; Selvamurugan, N.; Nair, S.; Tamura, H.; Jayakumar, R. Synthesis, characterization, cytotoxicity and antibacterial studies of chitosan, O-carboxymethyl and N, O-carboxymethyl chitosan nanoparticles. Carbohydr. Polym. 2009, 78, 672–677. [Google Scholar] [CrossRef]
- Mattioli-Belmonte, M.; Nicoli-Aldini, N.; De Benedittis, A.; Sgarbi, G.; Amati, S.; Fini, M.; Biagini, G.; Muzzarelli, R. Morphological study of bone regeneration in the presence of 6-oxychitin. Carbohydr. Polym. 1999, 40, 23–27. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Ramos, V.; Stanic, V.; Dubini, B.; Mattioli-Belmonte, M.; Tosi, G.; Giardino, R. Osteogenesis promoted by calcium phosphate N, N-dicarboxymethyl chitosan. Carbohydr. Polym. 1998, 36, 267–276. [Google Scholar] [CrossRef]
- Azuma, K.; Nishihara, M.; Shimizu, H.; Itoh, Y.; Takashima, O.; Osaki, T.; Itoh, N.; Imagawa, T.; Murahata, Y.; Tsuka, T. Biological adhesive based on carboxymethyl chitin derivatives and chitin nanofibers. Biomaterials 2015, 42, 20–29. [Google Scholar] [CrossRef]
- Dang, Q.; Liu, K.; Liu, C.; Xu, T.; Yan, J.; Yan, F.; Cha, D.; Zhang, Q.; Cao, Y. Preparation, characterization, and evaluation of 3, 6-ON-acetylethylenediamine modified chitosan as potential antimicrobial wound dressing material. Carbohydr. Polym. 2018, 180, 1–12. [Google Scholar] [CrossRef]
- Bi, B.; Liu, H.; Kang, W.; Zhuo, R.; Jiang, X. An injectable enzymatically crosslinked tyramine-modified carboxymethyl chitin hydrogel for biomedical applications. Colloids Surf. B Biointerfaces 2019, 175, 614–624. [Google Scholar] [CrossRef]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S. Synthesis, characteristic and antibacterial activity of N, N, N-trimethyl chitosan and its carboxymethyl derivatives. Carbohydr. Polym. 2010, 81, 931–936. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.-L.; Ng, I.-S.; Lin, G.-Y.; Chiu, C.-Y.; Chang, Y.-K. Antibacterial activity of quaternized chitosan modified nanofiber membrane. Int. J. Biol. Macromol. 2019, 126, 569–577. [Google Scholar] [CrossRef]
- Luan, F.; Wei, L.; Zhang, J.; Tan, W.; Chen, Y.; Wang, P.; Dong, F.; Li, Q.; Guo, Z. Synthesis, Characterization, and Antifungal Activity of N-Quaternized and N-Diquaternized Chitin Derivatives. Starch Stärke 2018, 70, 1800026. [Google Scholar] [CrossRef]
- Khattak, S.; Wahid, F.; Liu, L.-P.; Jia, S.-R.; Chu, L.-Q.; Xie, Y.-Y.; Li, Z.-X.; Zhong, C. Applications of cellulose and chitin/chitosan derivatives and composites as antibacterial materials: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Shi, X.; Li, X.; Cai, J.; Duan, B.; Du, Y. Homogeneous synthesis and characterization of quaternized chitin in NaOH/urea aqueous solution. Carbohydr. Polym. 2012, 87, 422–426. [Google Scholar] [CrossRef]
- Xu, H.; Fang, Z.; Tian, W.; Wang, Y.; Ye, Q.; Zhang, L.; Cai, J. Green Fabrication of Amphiphilic Quaternized β-Chitin Derivatives with Excellent Biocompatibility and Antibacterial Activities for Wound Healing. Adv. Mater. 2018, 30, 1801100. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.-R.S. Crosslinked poly (vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, Z.; Liu, Q.; Chen, X.; Ma, M. Effects of PVA, agar contents, and irradiation doses on properties of PVA/ws-chitosan/glycerol hydrogels made by γ-irradiation followed by freeze-thawing. Radiat. Phys. Chem. 2008, 77, 954–960. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Hasan, A.; Waibhaw, G.; Tiwari, S.; Dharmalingam, K.; Shukla, I.; Pandey, L.M. Fabrication and characterization of chitosan, polyvinylpyrrolidone, and cellulose nanowhiskers nanocomposite films for wound healing drug delivery application. J. Biomed. Mater. Res. Part A 2017, 105, 2391–2404. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef]
- Naseri, N.; Mathew, A.P.; Girandon, L.; Fröhlich, M.; Oksman, K. Porous electrospun nanocomposite mats based on chitosan–cellulose nanocrystals for wound dressing: Effect of surface characteristics of nanocrystals. Cellulose 2015, 22, 521–534. [Google Scholar] [CrossRef]
- Vongchan, P.; Sajomsang, W.; Subyen, D.; Kongtawelert, P. Anticoagulant activity of a sulfated chitosan. Carbohydr. Res. 2002, 337, 1239–1242. [Google Scholar] [CrossRef]
- Shanmugam, A.; Kathiresan, K.; Nayak, L. Preparation, characterization and antibacterial activity of chitosan and phosphorylated chitosan from cuttlebone of Sepia kobiensis (Hoyle, 1885). Biotechnol. Rep. 2016, 9, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Han, B.; Liu, W.; Xu, X. Preparation and antimicrobial activity of hydroxypropyl chitosan. Carbohydr. Res. 2005, 340, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wu, S.; Wei, P.; Xie, F.; Xu, X.; Cai, J.; Liu, Y. Versatile synthesis, characterization and properties of β-chitin derivatives from aqueous KOH/urea solution. Carbohydr. Polym. 2020, 227, 115345. [Google Scholar] [CrossRef]
- Shelma, R.; Paul, W.; Sharma, C.P. Chitin nanofibre reinforced thin chitosan films for wound healing application. Trends Biomater. Artif. Organs 2008, 22, 111–115. [Google Scholar]
- Zhang, H.; Neau, S.H. In Vitro degradation of chitosan by a commercial enzyme preparation: Effect of molecular weight and degree of deacetylation. Biomaterials 2001, 22, 1653–1658. [Google Scholar] [CrossRef]
- Vårum, K.M.; Myhr, M.M.; Hjerde, R.J.; Smidsrød, O. In Vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr. Res. 1997, 299, 99–101. [Google Scholar] [CrossRef]
- Cunha-Reis, C.; TuzlaKoglu, K.; Baas, E.; Yang, Y.; El Haj, A.; Reis, R. Influence of porosity and fibre diameter on the degradation of chitosan fibre-mesh scaffolds and cell adhesion. J. Mater. Sci. Mater. Med. 2007, 18, 195–200. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, J.; Zhang, L. Structure and properties of the nanocomposite films of chitosan reinforced with cellulose whiskers. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 1069–1077. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, S.; Chen, X.; Sheng, L. Nanocomposite films based on TEMPO-mediated oxidized bacterial cellulose and chitosan. Cellulose 2014, 21, 2757–2772. [Google Scholar] [CrossRef]
- Mehrabani, M.G.; Karimian, R.; Rakhshaei, R.; Pakdel, F.; Eslami, H.; Fakhrzadeh, V.; Rahimi, M.; Salehi, R.; Kafil, H.S. Chitin/silk fibroin/TiO2 bio-nanocomposite as a biocompatible wound dressing bandage with strong antimicrobial activity. Int. J. Biol. Macromol. 2018, 116, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, M.G.; Karimian, R.; Mehramouz, B.; Rahimi, M.; Kafil, H.S. Preparation of biocompatible and biodegradable silk fibroin/chitin/silver nanoparticles 3D scaffolds as a bandage for antimicrobial wound dressing. Int. J. Biol. Macromol. 2018, 114, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Niamsa, N.; Srisuwan, Y.; Baimark, Y.; Phinyocheep, P.; Kittipoom, S. Preparation of nanocomposite chitosan/silk fibroin blend films containing nanopore structures. Carbohydr. Polym. 2009, 78, 60–65. [Google Scholar] [CrossRef]
- Devi, M.P.; Sekar, M.; Chamundeswari, M.; Moorthy, A.; Krithiga, G.; Murugan, N.S.; Sastry, T. A novel wound dressing material-fibrin-chitosan-sodium alginate composite sheet. Bull. Mater. Sci. 2012, 35, 1157–1163. [Google Scholar] [CrossRef]
- Knill, C.; Kennedy, J.; Mistry, J.; Miraftab, M.; Smart, G.; Groocock, M.; Williams, H. Alginate fibres modified with unhydrolysed and hydrolysed chitosans for wound dressings. Carbohydr. Polym. 2004, 55, 65–76. [Google Scholar] [CrossRef]
- Dubey, P.; Gopinath, P. PEGylated graphene oxide-based nanocomposite-grafted chitosan/polyvinyl alcohol nanofiber as an advanced antibacterial wound dressing. RSC Adv. 2016, 6, 69103–69116. [Google Scholar] [CrossRef]
- Koosha, M.; Mirzadeh, H.; Shokrgozar, M.A.; Farokhi, M. Nanoclay-reinforced electrospun chitosan/PVA nanocomposite nanofibers for biomedical applications. RSC Adv. 2015, 5, 10479–10487. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, X.; Hanna, M.A. Chitosan/clay nanocomposite film preparation and characterization. J. Appl. Polym. Sci. 2006, 99, 1684–1691. [Google Scholar] [CrossRef]
- Sothornvit, R.; Rhim, J.-W.; Hong, S.-I. Effect of nano-clay type on the physical and antimicrobial properties of whey protein isolate/clay composite films. J. Food Eng. 2009, 91, 468–473. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef]
- Devi, N.; Dutta, J. Preparation and characterization of chitosan-bentonite nanocomposite films for wound healing application. Int. J. Biol. Macromol. 2017, 104, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Wu, C.; Xiong, S.; Zhou, C. Chitosan/halloysite nanotubes bionanocomposites: Structure, mechanical properties and biocompatibility. Int. J. Biol. Macromol. 2012, 51, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Aguzzi, C.; Rossi, S.; Bonferoni, M.C.; Bruni, G.; Boselli, C.; Cornaglia, A.I.; Riva, F.; Viseras, C.; Caramella, C. Halloysite and chitosan oligosaccharide nanocomposite for wound healing. Acta Biomater. 2017, 57, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Dutta, J. Development and in vitro characterization of chitosan/starch/halloysite nanotubes ternary nanocomposite films. Int. J. Biol. Macromol. 2019, 127, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Kokabi, M.; Hassan, Z. Poly (vinyl alcohol)/chitosan/honey/clay responsive nanocomposite hydrogel wound dressing. J. Appl. Polym. Sci. 2018, 135, 46311. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P. In Vivo evaluation of chitosan–PVP–titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, J.; He, Q.; Wu, J.; Liang, D.; Yang, H.; Chen, R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr. Polym. 2017, 156, 460–469. [Google Scholar] [CrossRef]
- Zhai, M.; Xu, Y.; Zhou, B.; Jing, W. Keratin-chitosan/n-ZnO nanocomposite hydrogel for antimicrobial treatment of burn wound healing: Characterization and biomedical application. J. Photochem. Photobiol. B Biol. 2018, 180, 253–258. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Kumar, K.D.; Akbari-Fakhrabadi, A.; Mangalaraja, R.; Amalraj, J. Chitosan capped copper oxide/copper nanoparticles encapsulated microbial resistant nanocomposite films. Int. J. Biol. Macromol. 2019, 128, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel asymmetric wettable AgNPs/chitosan wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M.; El-Sherbiny, I.M. Honey/chitosan nanofiber wound dressing enriched with Allium sativum and Cleome droserifolia: Enhanced antimicrobial and wound healing activity. ACS Appl. Mater. Interfaces 2016, 8, 6379–6390. [Google Scholar] [CrossRef] [PubMed]
- Pulat, M.; Kahraman, A.S.; Tan, N.; Gümüşderelioğlu, M. Sequential antibiotic and growth factor releasing chitosan-PAAm semi-IPN hydrogel as a novel wound dressing. J. Biomater. Sci. Polym. Ed. 2013, 24, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Kakehata, S.; Hirose, Y.; Kitani, R.; Futai, K.; Maruya, S.-I.; Ishii, K.; Shinkawa, H. Autologous serum eardrops therapy with a chitin membrane for closing tympanic membrane perforations. Otol. Neurotol. 2008, 29, 791–795. [Google Scholar] [CrossRef]
- Carles, G.; Dabiri, C.; Mchirgui, A. Different uses of chitosan for treating serious obstetric hemorrhages. J. Gynecol. Obstet. Hum. Reprod. 2016, 46, 693–695. [Google Scholar] [CrossRef]
- Chung, Y.-J.; An, S.-Y.; Yeon, J.-Y.; Shim, W.S.; Mo, J.-H. Effect of a chitosan gel on hemostasis and prevention of adhesion after endoscopic sinus surgery. Clin. Exp. Otorhinolaryngol. 2016, 9, 143. [Google Scholar] [CrossRef]
- Rahmani, F.; Moghadamnia, A.A.; Kazemi, S.; Shirzad, A.; Motallebnejad, M. Effect of 0.5% Chitosan mouthwash on recurrent aphthous stomatitis: A randomized double-blind crossover clinical trial. Electron. Physician 2018, 10, 6912. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, H. Clinical evaluation of an oral mucoadhesive film containing chitosan for the treatment of recurrent aphthous stomatitis: A randomized, double-blind study. J. Dermatol. Treat. 2019, 1–5. [Google Scholar] [CrossRef]
- Kumar, K.A.; Kumar, J.; Sarvagna, J.; Gadde, P.; Chikkaboriah, S. Hemostasis and post-operative care of oral surgical wounds by hemcon dental dressing in patients on oral anticoagulant therapy: A split mouth randomized controlled clinical trial. J. Clin. Diagn. Res. 2016, 10, ZC37. [Google Scholar] [CrossRef]
- Madrazo-Jiménez, M.; Rodríguez-Caballero, Á.; Serrera-Figallo, M.-Á.; Garrido-Serrano, R.; Gutiérrez-Corrales, A.; Gutiérrez-Pérez, J.-L.; Torres-Lagares, D. The effects of a topical gel containing chitosan, 0, 2% chlorhexidine, allantoin and despanthenol on the wound healing process subsequent to impacted lower third molar extraction. Med. Oral Patol. Oral Y Cir. Bucal 2016, 21, e696. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shafaeifard, S.; Sarkarat, F.; Pahlevan, R.; Ezati, A.; Keyhanlou, F. Investigating the effect of Chitohem powder on coagulation time and the complications following tooth extraction. J. Res. Dent. Sci. 2017, 14, 138–143. [Google Scholar]
- Latańska, I.; Kozera-Żywczyk, A.; Paluchowska, E.B.; Owczarek, W.; Kaszuba, A.; Noweta, M.; Tazbir, J.; Kolesińska, B.; Draczyński, Z.; Sujka, W. Characteristic Features of Wound Dressings Based on Butyric-Acetic Chitin Copolyesters—Results of Clinical Trials. Materials 2019, 12, 4170. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69, 216–222. [Google Scholar] [CrossRef]
- Angspatt, A.; Taweerattanasil, B.; Janvikul, W.; Chokrungvaranont, P.; Sirimaharaj, W. Carboxymethylchitosan, alginate and tulle gauze wound dressings: A comparative study in the treatment of partial-thickness wounds. Asian Biomed. 2011, 5, 413–416. [Google Scholar]
- Bachtell, N.; Goodell, T.; Grunkemeier, G.; Jin, R.; Gregory, K. Treatment of dialysis access puncture wound bleeding with chitosan dressings. Dial. Transplant. 2006, 35, 672–681. [Google Scholar] [CrossRef]
- Ketan, P.; Anjali, P.; Rignesh, P.; Bhavika, P.; Priyank, P.; Dev, P. Assessing the Efficacy of Haemostatic Dressing Axiostat® In Trauma Care at a Tertiary Care Hospital in India: A Comparison with Conventional Cotton Gauze. Indian J. Emerg. Med. 2016, 2, 93–99. [Google Scholar] [CrossRef]
- Halim, A.S.; Nor, F.M.; Mat Saad, A.Z.; Mohd Nasir, N.A.; Norsa’adah, B.; Ujang, Z. Efficacy of chitosan derivative films versus hydrocolloid dressing on superficial wounds. J. Taibah Univ. Med. Sci. 2018, 13, 512–520. [Google Scholar] [CrossRef]
- Campani, V.; Pagnozzi, E.; Mataro, I.; Mayol, L.; Perna, A.; D’Urso, F.; Carillo, A.; Cammarota, M.; Maiuri, M.; De Rosa, G. Chitosan Gel to Treat Pressure Ulcers: A Clinical Pilot Study. Pharmaceutics 2018, 10, 15. [Google Scholar] [CrossRef]
- Mo, X.; Cen, J.; Gibson, E.; Wang, R.; Percival, S.L. An open multicenter comparative randomized clinical study on chitosan. Wound Repair Regen. 2015, 23, 518–524. [Google Scholar] [CrossRef]
| Polymorph | Sources | Chitin Content | Other Major Constituents |
|---|---|---|---|
| α | Crustacean shells | (chitinous shell up to 50% of crustacean d.wt.) | |
| Lobster | 16–23% | 20–60% calcium or magnesium carbonate, 20–40% protein | |
| Crab | 25–30% | ||
| Krill | 34–49% | ||
| Insect cuticles | (chitinous cuticle up to 50% of insect d.wt.) | ||
| Cockroach | 18–38% | 20–50% protein, minerals, pigments and fat | |
| Butterfly | 22–64% | ||
| Silkworm | 20–44% | ||
| Fungal cell walls | (chitin–glucan nanofibers up to 26% of fungal biomass d.wt.) | ||
| Mushrooms | 8–43% | 50–60% β-glucan, protein | |
| Mycelium | 5–35% | ||
| Yeast | 1–3% | ||
| Mold | 8–27% | ||
| β | Squid pen | 31–49% | Proteins and minerals |
| Sea tube worms | 25–29% | ||
| Cell Type | Function in Wound Healing | Effects of Chitin or Chitosan |
|---|---|---|
| Red blood cells | Supportive role in fibrin clot formation. | Chitosan forms a coagulum with red blood cells. |
| Polymorphonuclear neutrophils (PMN) | Clean wound site of foreign particles and cell debris. | Chitin and chitosan attract PMNs to wound site. |
| Macrophages | Consume dead cells, attract fibroblasts, support skin and blood vessel replacement and synthesis of the extracellular matrix. | Chitin and chitosan attract macrophages. Chitosan stimulates cytokine production (TGF-β1, PDGF, IL-1). |
| Fibroblasts | Reformation of the dermis and synthesis of extracellular matrix. | Indirect effect through macrophage cytokines and stimulates IL-8 production. |
| Keratinocytes | Reformation of epidermis. | Indirect effect through macrophage cytokines. |
| Application | Wound Type | Treatment Utilized | Treatment Constituents | Ref. |
|---|---|---|---|---|
| Ear | Membrane perforation | Beschitin® W (membrane) | Chitin, unknown | [162] |
| Hemorrhage | Obstetric hemorrhage | Celox® (powder/gauze) | Chitosan, unknown | [163] |
| Nasal | Postoperative | Surgi shield® (gel) | 8% carboxymethyl chitosan, unknown | [164] |
| Oral | Aphthous stomatitis | Mouthwash | 0.5% chitosan powder, polyacrylic acid, methyl-/propylparaben, glycerin | [165] |
| Adhesive film | Chitosan powder, sesame oil | [166] | ||
| Postoperative | HemCon® (dressing) | Chitosan, unknown | [167] | |
| Tooth extraction | Bexident® Post (gel) | Chitosan, chlorhexidine, allantoin, dexpanthenol | [168] | |
| Chitohem® (powder) | Chitosan, unknown | [169] | ||
| Skin | Diabetic | Medisorb® R (membrane/powder) | Butyric-acetic chitin copolyesters, unknown | [170] |
| Postoperative | Membrane | Chitosan only | [171] | |
| Dressing | Carboxymethyl chitosan, unknown | [172] | ||
| Puncture | HemCon® (dressing) | Chitosan, unknown | [173] | |
| Superficial | Axiostat® (dressing) | Chitosan, unknown | [174] | |
| Film | Oligochitosan, glycerol | [175] | ||
| Ulcers | Pressure, vascular, diabetic ulcers | Topical gel | 2% chitosan powder, acetic acid, regenerated cellulose | [176] |
| SEQUA® San Chitosan (dressing) | Chitosan, unknown | [177] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. https://doi.org/10.3390/md18010064
Jones M, Kujundzic M, John S, Bismarck A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Marine Drugs. 2020; 18(1):64. https://doi.org/10.3390/md18010064
Chicago/Turabian StyleJones, Mitchell, Marina Kujundzic, Sabu John, and Alexander Bismarck. 2020. "Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment" Marine Drugs 18, no. 1: 64. https://doi.org/10.3390/md18010064
APA StyleJones, M., Kujundzic, M., John, S., & Bismarck, A. (2020). Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Marine Drugs, 18(1), 64. https://doi.org/10.3390/md18010064




