Antifouling Napyradiomycins from Marine-Derived Actinomycetes Streptomyces aculeolatus †
Abstract
1. Introduction
2. Results and Discussion
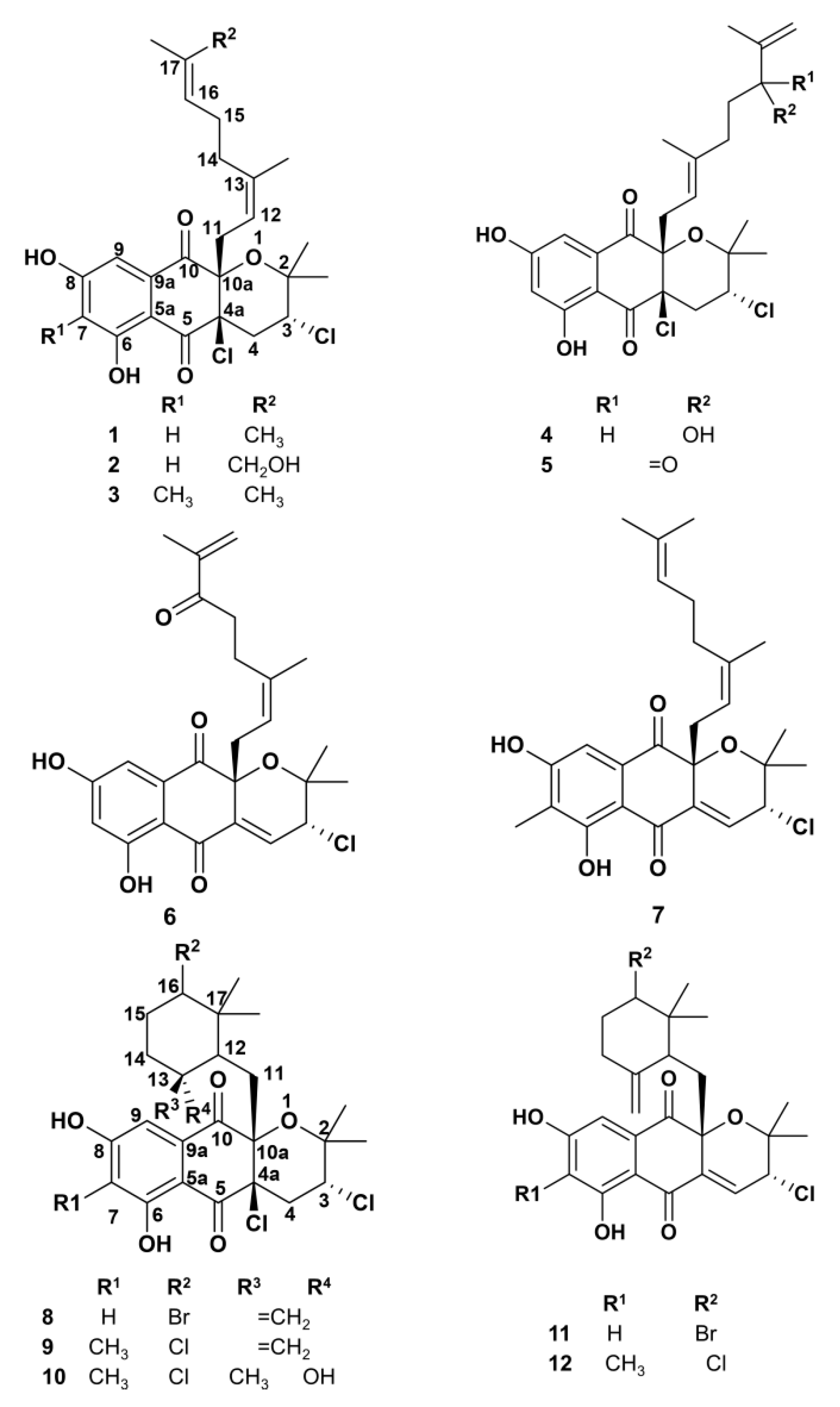
2.1. Napyradiomycin Derivatives Description
2.2. Assessment of Napyradiomycin Derivatives Micro and Macrofouling Inhibitory Activity
2.2.1. Antibacterial Activity
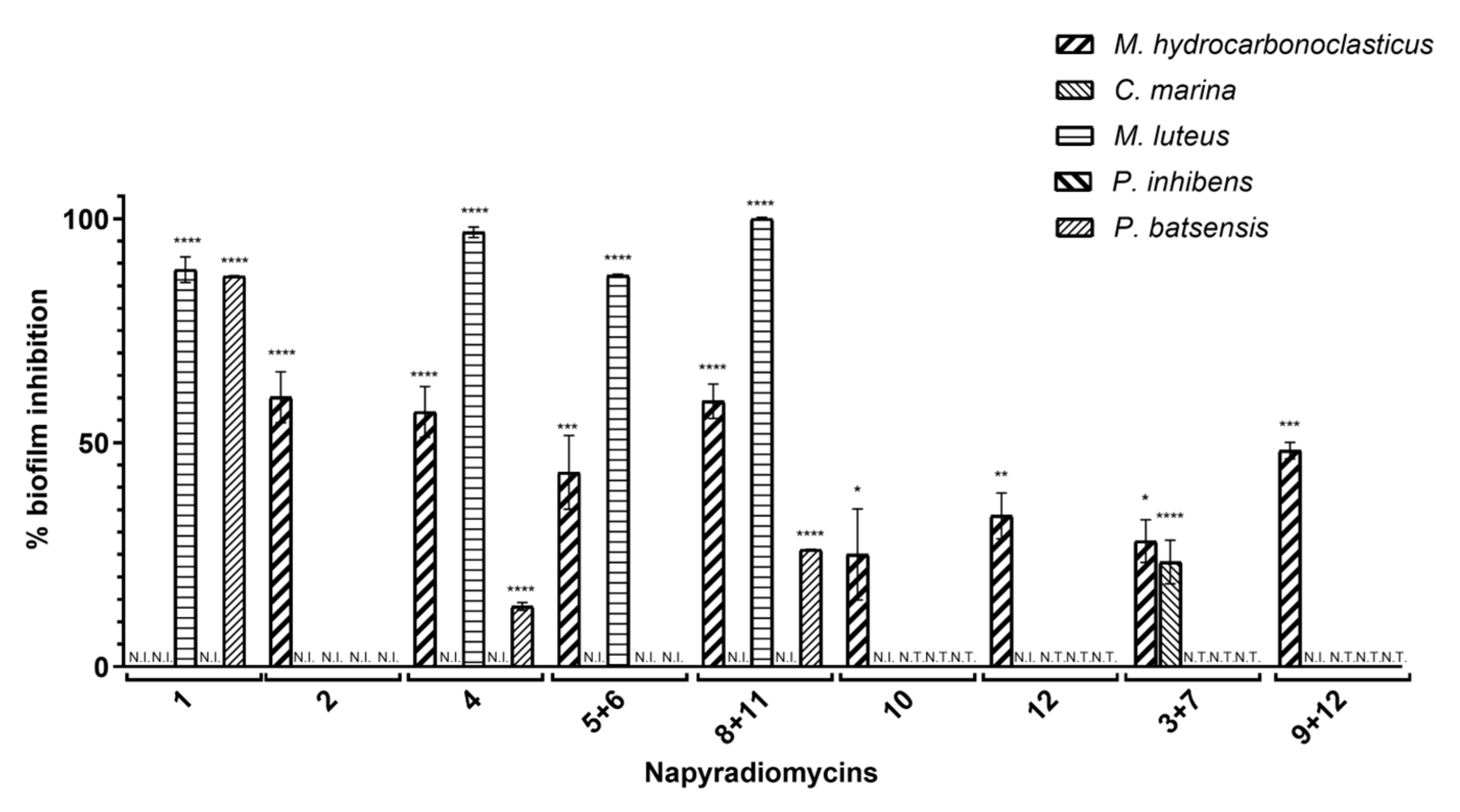
2.2.2. Antibiofilm Activity
2.3. Antifouling Evaluation against Mytilus Galloprovincialis Larval Settlement
2.4. Napyradiomycins in Silico Ecotoxicity Evaluation
2.5. SAR Analysis
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Collection and Isolation of Marine-Derived Actinomycetes
3.3. Phylogenetic Analysis of Strains PTM-029 and PTM-420
3.4. Growth Conditions and Crude Extract Production
3.5. Isolation of Napyradiomycins
3.6. Antimicrofouling Evaluation
3.6.1. Bacterial Growth Conditions
3.6.2. Antibacterial Activity Evaluation Assays
3.6.3. Antibiofilm Activity Evaluation Assays
3.7. Antimacrofouling Evaluation: Mussel Larvae Mytilus Galloprovincialis Acute Toxicity Assay
3.8. In Silico Environmental Toxicity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magin, C.M.; Cooper, S.P.; Brennan, A.B. Non-toxic antifouling strategies. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Abarzua, S.; Jakubowski, S. Biotechnological Investigation for the Prevention of Biofouling. 1. Biological and Biochemical Principles for the Prevention of Biofouling. Mar. Ecol. Prog. Ser. 1995, 123, 301–312. [Google Scholar] [CrossRef]
- Conrad, J.C.; Poling-Skutvik, R. Confined Flow: Consequences and Implications for Bacteria and Biofilms. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.P. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling 2007, 23, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.P.; Walker, J.M.; Steppe, C.N.; Flack, K.A. Impact of diatomaceous biofilms on the frictional drag of fouling-release coatings. Biofouling 2015, 31, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B. Biomimetics: Lessons from nature—An overview. Philos. Trans. A Math Phys. Eng. Sci. 2009, 367, 1445–1486. [Google Scholar] [CrossRef] [PubMed]
- Ware, C.; Berge, J.; Sundet, J.H.; Kirkpatrick, J.B.; Coutts, A.D.M.; Jelmert, A.; Olsen, S.M.; Floerl, O.; Wisz, M.S.; Alsos, I.G. Climate change, non-indigenous species and shipping: Assessing the risk of species introduction to a high-Arctic archipelago. Divers. Distrib. 2014, 20, 10–19. [Google Scholar] [CrossRef]
- Ashton, G.V.; Davidson, I.C.; Geller, J.; Ruiz, G.M. Disentangling the biogeography of ship biofouling: Barnacles in the Northeast Pacific. Glob. Ecol. Biogeogr. 2016, 25, 739–750. [Google Scholar] [CrossRef]
- Pettengill, J.B.; Wendt, D.E.; Schug, M.D.; Hadfield, M.G. Biofouling likely serves as a major mode of dispersal for the polychaete tubeworm Hydroides elegans as inferred from microsatellite loci. Biofouling 2007, 23, 161–169. [Google Scholar] [CrossRef]
- Piola, R.F.; Johnston, E.L. The potential for translocation of marine species via small-scale disruptions to antifouling surfaces. Biofouling 2008, 24, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Prabowo, R.E.; Ohshiro, Y.; Shimono, T.; Jones, D.; Kawai, H.; Otani, M.; Oshino, A.; Inagawa, S.; Akaya, T.; et al. The introduction to Japan of the Titan barnacle, Megabalanus coccopoma (Darwin, 1854) (Cirripedia: Balanomorpha) and the role of shipping in its translocation. Biofouling 2009, 25, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Sonak, S.; Pangam, P.; Giriyan, A.; Hawaldar, K. Implications of the ban on organotins for protection of global coastal and marine ecology. J. Environ. Manag. 2009, 90, S96–S108. [Google Scholar] [CrossRef] [PubMed]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, C.M.; Brennan, A.B. Bio-Inspired Antifouling Strategies. Annu. Rev. Mater. Res. 2012, 42, 211–229. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Othmani, A.; Bunet, R.; Bonnefont, J.L.; Briand, J.F.; Culioli, G. Settlement inhibition of marine biofilm bacteria and barnacle larvae by compounds isolated from the Mediterranean brown alga Taonia atomaria. J. Appl. Phycol. 2016, 28, 1975–1986. [Google Scholar] [CrossRef]
- Satheesh, S.; Ba-akdah, M.A.; Al-Sofyani, A.A. Natural antifouling compound production by microbes associated with marine macroorganisms—A review. Electron. J. Biotechnol. 2016, 21, 26–35. [Google Scholar] [CrossRef]
- Almeida, J.R.; Vasconcelos, V. Natural antifouling compounds: Effectiveness in preventing invertebrate settlement and adhesion. Biotechnol. Adv. 2015, 33, 343–357. [Google Scholar] [CrossRef]
- Qian, P.Y.; Li, Z.R.; Xu, Y.; Li, Y.X.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef]
- Qian, P.Y.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2010, 26, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Dahms, H.U.; Qian, P.Y. Inhibition of biofouling by marine microorganisms and their metabolites. Biofouling 2006, 22, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-L.; Wu, Z.-H.; Wang, Y.; Wang, C.-Y.; Xu, Y. Mini-Review: Antifouling Natural Products from Marine Microorganisms and Their Synthetic Analogs. Mar. Drugs 2017, 15, 266. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H.; Ma, X. Antifouling Compounds from Marine Invertebrates. Mar. Drugs 2017, 15, 263. [Google Scholar] [CrossRef]
- Dahms, H.U.; Dobretsov, S. Antifouling Compounds from Marine Macroalgae. Mar. Drugs 2017, 15, 265. [Google Scholar] [CrossRef]
- Dworjanyn, S.A.; de Nys, R.; Steinberg, P.D. Chemically mediated antifouling in the red alga Delisea pulchra. Mar. Ecol. Prog. Ser. 2006, 318, 153–163. [Google Scholar] [CrossRef][Green Version]
- Richards, J.J.; Ballard, T.E.; Huigens, R.W.; Melander, C. Synthesis and screening of an oroidin library against Pseudomonas aeruginosa biofilms. Chembiochem 2008, 9, 1267–1279. [Google Scholar] [CrossRef]
- Melander, C.; Moeller, P.D.R.; Ballard, T.E.; Richards, J.J.; Huigens, R.W.; Cavanagh, J. Evaluation of dihydrooroidin as an antifouling additive in marine paint. Inter. Biodeterior. Biodegrad. 2009, 63, 529–532. [Google Scholar] [CrossRef]
- Yee, L.H.; Holmstrom, C.; Fuary, E.T.; Lewin, N.C.; Kjelleberg, S.; Steinberg, P.D. Inhibition of fouling by marine bacteria immobilised in kappa-carrageenan beads. Biofouling 2007, 23, 287–294. [Google Scholar] [CrossRef]
- Perry, T.D.; Zinn, M.; Mitchell, R. Settlement inhibition of fouling invertebrate larvae by metabolites of the marine bacterium Halomonas marina within a polyurethane coating. Biofouling 2001, 17, 147–153. [Google Scholar] [CrossRef]
- Gatenholm, P.; Holmstrom, C.; Maki, J.S.; Kjelleberg, S. Toward Biological Antifouling Surface-Coatings—Marine -Bacteria Immobilized in Hydrogel Inhibit Barnacle Larvae. Biofouling 1995, 8, 293–301. [Google Scholar] [CrossRef]
- Bavya, M.; Mohanapriya, P.; Pazhanimurugan, R.; Balagurunathan, R. Potential bioactive compound from marine actinomycetes against biofouling bacteria. Indian J. Geo-Mar. Sci. 2011, 40, 578–582. [Google Scholar]
- Cho, J.Y.; Kim, M.S. Induction of Antifouling Diterpene Production by Streptomyces cinnabarinus PK209 in Co-Culture with Marine-Derived Alteromonas sp KNS-16. Biosci. Biotechnol. Biochem. 2012, 76, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kang, J.Y.; Hong, Y.K.; Baek, H.H.; Shin, H.W.; Kim, M.S. Isolation and Structural Determination of the Antifouling Diketopiperazines from Marine-Derived Streptomyces praecox 291-11. Biosci. Biotechnol. Biochem. 2012, 76, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dobretsov, S.; Xu, Y.; Xiao, X.; Hung, O.S.; Qian, P.-Y. Antifouling diketopiperazines produced by a deep-sea bacterium, Streptomyces fungicidicus. Biofouling 2006, 22, 201–208. [Google Scholar] [CrossRef]
- Xu, Y.; He, H.P.; Schulz, S.; Liu, X.; Fusetani, N.; Xiong, H.R.; Xiao, X.; Qian, P.Y. Potent antifouling compounds produced by marine Streptomyces. Bioresour. Technol. 2010, 101, 1331–1336. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.L.; Li, X.C.; Xiao, X.; Qian, P.Y. Inhibitory Effects of a Branched-Chain Fatty Acid on Larval Settlement of the Polychaete Hydroides elegans. Mar. Biotechnol. 2009, 11, 495–504. [Google Scholar] [CrossRef]
- Gopikrishnan, V.; Radhakrishnan, M.; Shanmugasundaram, T.; Pazhanimurugan, R.; Balagurunathan, R. Antibiofouling potential of quercetin compound from marine-derived actinobacterium, Streptomyces fradiae PE7 and its characterization. Environ. Sci. Pollut. Res. 2016, 23, 13832–13842. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Jensen, P.R.; Fenical, W. Cytotoxic and Antimicrobial Napyradiomycins from Two Marine-Derived Streptomyces Strains. Eur. J. Organ. Chem. 2013, 3751–3757. [Google Scholar] [CrossRef]
- Farnaes, L.; Coufal, N.G.; Kauffman, C.A.; Rheingold, A.L.; DiPasquale, A.G.; Jensen, P.R.; Fenical, W. Napyradiomycin Derivatives, Produced by a Marine-Derived Actinomycete, Illustrate Cytotoxicity by Induction of Apoptosis. J. Nat. Prod. 2014, 77, 15–21. [Google Scholar] [CrossRef]
- Prieto-Davo, A.; Dias, T.; Gomes, S.E.; Rodrigues, S.; Parera-Valadezl, Y.; Borralho, P.M.; Pereira, F.; Rodrigues, C.M.P.; Santos-Sanches, I.; Gaudencio, S.P. The Madeira Archipelago As a Significant Source of Marine-Derived Actinomycete Diversity with Anticancer and Antimicrobial Potential. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, A.; Pereira, F.; Grilo, I.R.; Godinho, C.C.; Paulino, M.; Almeida, V.; Gobbo-Neto, L.; Prieto-Davo, A.; Sobral, R.G.; Lopes, N.P.; et al. Intra-clade metabolomic profiling of MAR4 Streptomyces from the Macaronesia Atlantic region reveals a source of anti-biofilm metabolites. Environ. Microbial. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gaudêncio, S.P.; Sobral, R.G.; Pereira, F.; Santos-Sanches, I.; Gonçalves, S.P.; Cunha, I.; Almeida, J.R.; Vasconcelos, V. Utilização de napiradiomicinas com atividade anti-incrustante e suas composições. Portuguese Patent PT115055, 4 October 2018. [Google Scholar]
- Shiomi, K.; Nakamura, H.; Iinuma, H.; Naganawa, H.; Isshiki, K.; Takeuchi, T.; Umezawa, H. Structures of New Antibiotics Napyradiomycins. J. Antibiot. 1986, 39, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, K.; Sue, M.; Furihata, K.; Ito, S.; Seto, H. Terpenoids produced by actinomycetes: Napyradiomycins from Streptomyces antimycoticus NT17. J. Nat. Prod. 2008, 71, 595–601. [Google Scholar] [CrossRef]
- Wu, Z.C.; Li, S.M.; Li, J.; Chen, Y.C.; Saurav, K.; Zhang, Q.B.; Zhang, H.B.; Zhang, W.J.; Zhang, W.M.; Zhang, S.; et al. Antibacterial and Cytotoxic New Napyradiomycins from the Marine-Derived Streptomyces sp SCSIO 10428. Mar. Drugs 2013, 11, 2113–2125. [Google Scholar] [CrossRef]
- Fukuda, D.S.; Mynderse, J.S.; Baker, P.J.; Berry, D.M.; Boeck, L.D.; Yao, R.C.; Mertz, F.P.; Nakatsukasa, W.M.; Mabe, J.; Ott, J.; et al. A80915, A New Antibiotic Complex Produced By Streptomyces aculeolatus—Discovery, Taxonomy, Fermentation, Isolation, Caharacterization, and Antibacterial Evaluation. J. Antibiot. 1990, 43, 623–633. [Google Scholar] [CrossRef]
- Soria-Mercado, I.E.; Prieto-Davo, A.; Jensen, P.R.; Fenical, W. Antibiotic terpenoid chloro-dihydroquinones from a new marine actinomycete. J. Nat. Prod. 2005, 68, 904–910. [Google Scholar] [CrossRef]
- Gomi, S.; Ohuchi, S.; Sasaki, T.; Itoh, J.; Sezaki, M. Studies on New Antibiotics-SF2415. 2. The Structure Elucidation. J. Nat. Prod. 1987, 40, 740–749. [Google Scholar] [CrossRef]
- Pinori, E.; Berglin, M.; Brive, L.M.; Hulander, M.; Dahlstrom, M.; Elwing, H. Multi-seasonal barnacle (Balanus improvisus) protection achieved by trace amounts of a macrocyclic lactone (ivermectin) included in rosin-based coatings. Biofouling 2011, 27, 941–953. [Google Scholar] [CrossRef]
- Dang, H.; Li, T.; Chen, M.; Huang, G. Cross-ocean distribution of Rhodobacterales bacteria as primary surface colonizers in temperate coastal marine waters. Appl. Environ. Microbiol. 2008, 74, 52–60. [Google Scholar] [CrossRef]
- Ekblad, T.; Bergstroem, G.; Ederth, T.; Conlan, S.L.; Mutton, R.; Clare, A.S.; Wang, S.; Liu, Y.L.; Zhao, Q.; D’Souza, F.; et al. Poly(ethylene glycol)-Containing Hydrogel Surfaces for Antifouling Applications in Marine and Freshwater Environments. Biomacromolecules 2008, 9, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Akesso, L.; Pettitt, M.; Callow, J.; Callow, M.; Stallard, J.; Teer, D.; Liu, C.; Wang, S.; Zhao, Q.; D’Souza, F.; et al. The potential of nano-structured silicon oxide type coatings deposited by PACVD for control of aquatic biofouling. Biofouling 2009, 25, 55–67. [Google Scholar] [CrossRef]
- D’Souza, F.; Bruin, A.; Biersteker, R.; Donnelly, G.; Klijnstra, J.; Rentrop, C.; Willemsen, P. Bacterial assay for the rapid assessment of antifouling and fouling release properties of coatings and materials. J. Ind. Microbiol. Biotechnol. 2010, 37, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Michael, V.; Frank, O.; Bartling, P.; Scheuner, C.; Göker, M.; Brinkmann, H.; Petersen, J. Biofilm plasmids with a rhamnose operon are widely distributed determinants of the ’swim-or-stick’ lifestyle in roseobacters. ISME J. 2016, 10, 2498–2513. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, M.E.; McElroy, K.; Maczka, M.; Thomas, T.; Egan, S. Causes and Consequences of a Variant Strain of. Front. Microbiol. 2018, 9, 2601. [Google Scholar] [CrossRef] [PubMed]
- Inbakandan, D.; Murthy, P.S.; Venkatesan, R.; Khan, S.A. 16S rDNA sequence analysis of culturable marine biofilm forming bacteria from a ship’s hull. Biofouling 2010, 26, 893–899. [Google Scholar] [CrossRef]
- El-Masry, M.H.; Hassouna, M.S.; El-Rakshy, N.; Mousa, I.E. Bacterial populations in the biofilm and non-biofilm components of a sand filter used in water treatment. FEMS Microbiol. Lett. 1995, 131, 263–269. [Google Scholar] [CrossRef]
- Hellio, C.; Marechal, J.P.; Veron, B.; Bremer, G.; Clare, A.S.; Le Gal, Y. Seasonal variation of antifouling activities of marine algae from the Brittany coast (France). Mar. Biotechnol. 2004, 6, 67–82. [Google Scholar] [CrossRef]
- Briand, J.F. Marine antifouling laboratory bioassays: An overview of their diversity. Biofouling 2009, 25, 297–311. [Google Scholar] [CrossRef]
- Che, Q.; Zhu, T.; Qi, X.; Mandi, A.; Kurtan, T.; Mo, X.; Li, J.; Gu, Q.; Li, D. Hybrid Isoprenoids from a Reeds Rhizosphere Soil Derived Actinomycete Streptomyces sp CHQ-64. Org. Lett. 2012, 14, 3438–3441. [Google Scholar] [CrossRef]
- Davies, I.M.; McHenery, J.G.; Rae, G.H. Environmental risk from dissolved ivermectin to marine organisms. Aquaculture 1997, 158, 263–275. [Google Scholar] [CrossRef]
- Young, D.M. The Toxicity Estimation Software Tool (T.E.S.T.); New England Green Chemistry Networking Forum: Boston, MA, USA, 2010. [Google Scholar]
- Tisler, T.; Zagorc-Koncan, J. Aquatic toxicity of selected chemicals as a basic criterion for environmental classification. Arh. Hig. Rada. Toksikol. 2003, 54, 207–213. [Google Scholar] [PubMed]
- Schipper, C.A.; Rietjens, I.M.C.M.; Burgess, R.M.; Murk, A.J. Application of bioassays in toxicological hazard, risk and impact assessments of dredged sediments. Mar. Pollut. Bull. 2010, 60, 2026–2042. [Google Scholar] [CrossRef] [PubMed]
- Diaza, R.G.; Manganelli, S.; Esposito, A.; Roncaglioni, A.; Manganaro, A.; Benfenati, E. Comparison of in silico tools for evaluating rat oral acute toxicity. SAR QSAR Environ. Res. 2015, 26, 1–27. [Google Scholar] [CrossRef]
- Gini, G.; Franchi, A.M.; Manganaro, A.; Golbamaki, A.; Benfenati, E. ToxRead: A tool to assist in read across and its use to assess mutagenicity of chemicals. SAR QSAR Environ. Res. 2014, 25, 999–1011. [Google Scholar] [CrossRef]
- Winter, J.M.; Moffitt, M.C.; Zazopoulos, E.; McAlpine, J.B.; Dorrestein, P.C.; Moore, B.S. Molecular basis for chloronium-mediated meroterpene cyclization—Cloning, sequencing, and heterologous expression of the napyradiomycin biosynthetic gene cluster. J. Biol. Chem. 2007, 282, 16362–16368. [Google Scholar] [CrossRef]
- Gontang, E.A.; Fenical, W.; Jensen, P.R. Phylogenetic diversity of gram-positive bacteria cultured from marine sediments. Appl. Environ. Microbiol. 2007, 73, 3272–3282. [Google Scholar] [CrossRef]
- Almeida, J.R.; Correia-da-Silva, M.; Sousa, E.; Antunes, J.; Pinto, M.; Vasconcelos, V.; Cunha, I. Antifouling potential of Nature-inspired sulfated compounds. Sci. Rep. 2017, 7, 42424. [Google Scholar] [CrossRef]
- Antunes, J.; Pereira, S.; Ribeiro, T.; Plowman, J.E.; Thomas, A.; Clerens, S.; Campos, A.; Vasconcelos, V.; Almeida, J.R. A Multi-Bioassay Integrated Approach to Assess the Antifouling Potential of the Cyanobacterial Metabolites Portoamides. Mar. drugs 2019, 17, 111. [Google Scholar] [CrossRef]
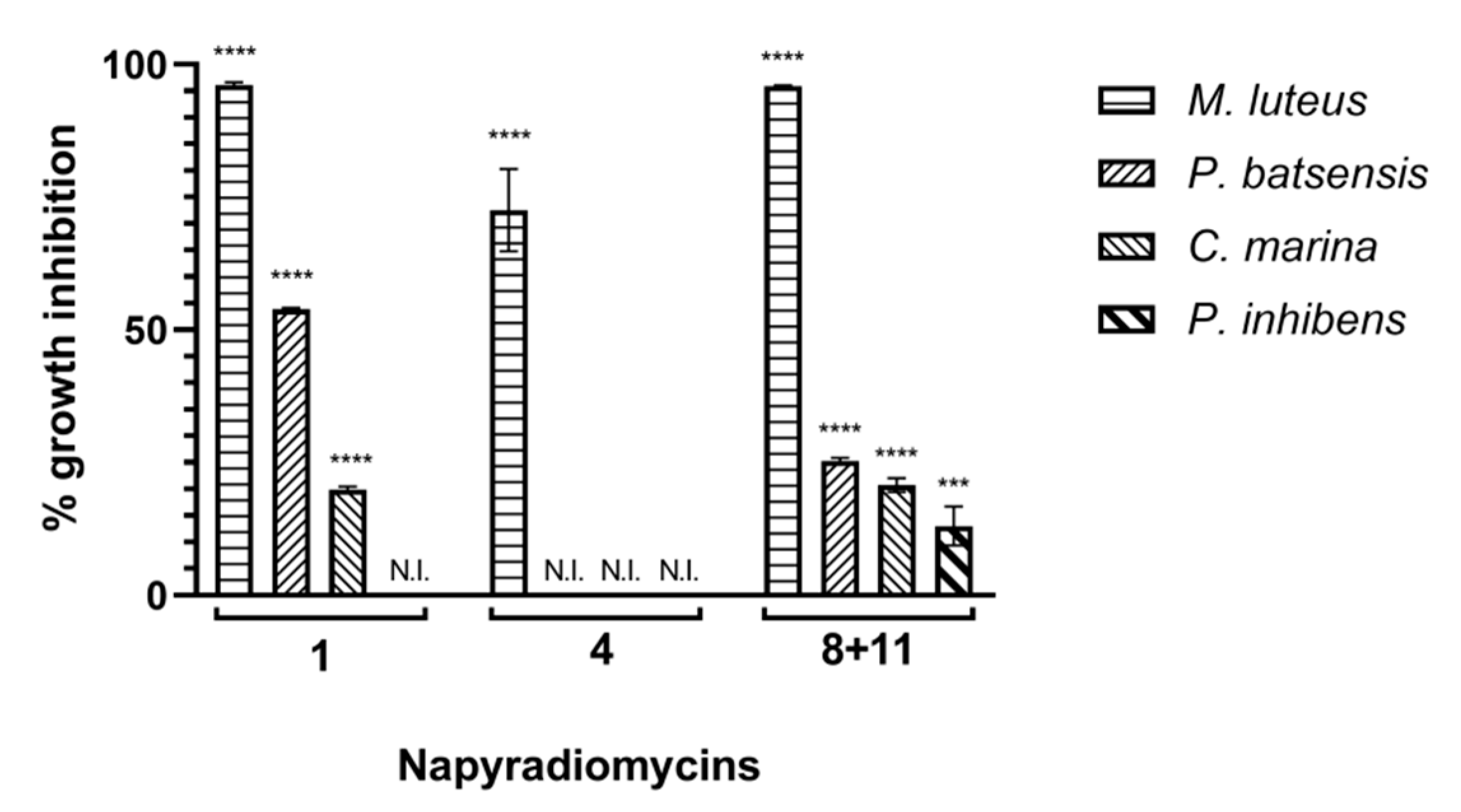
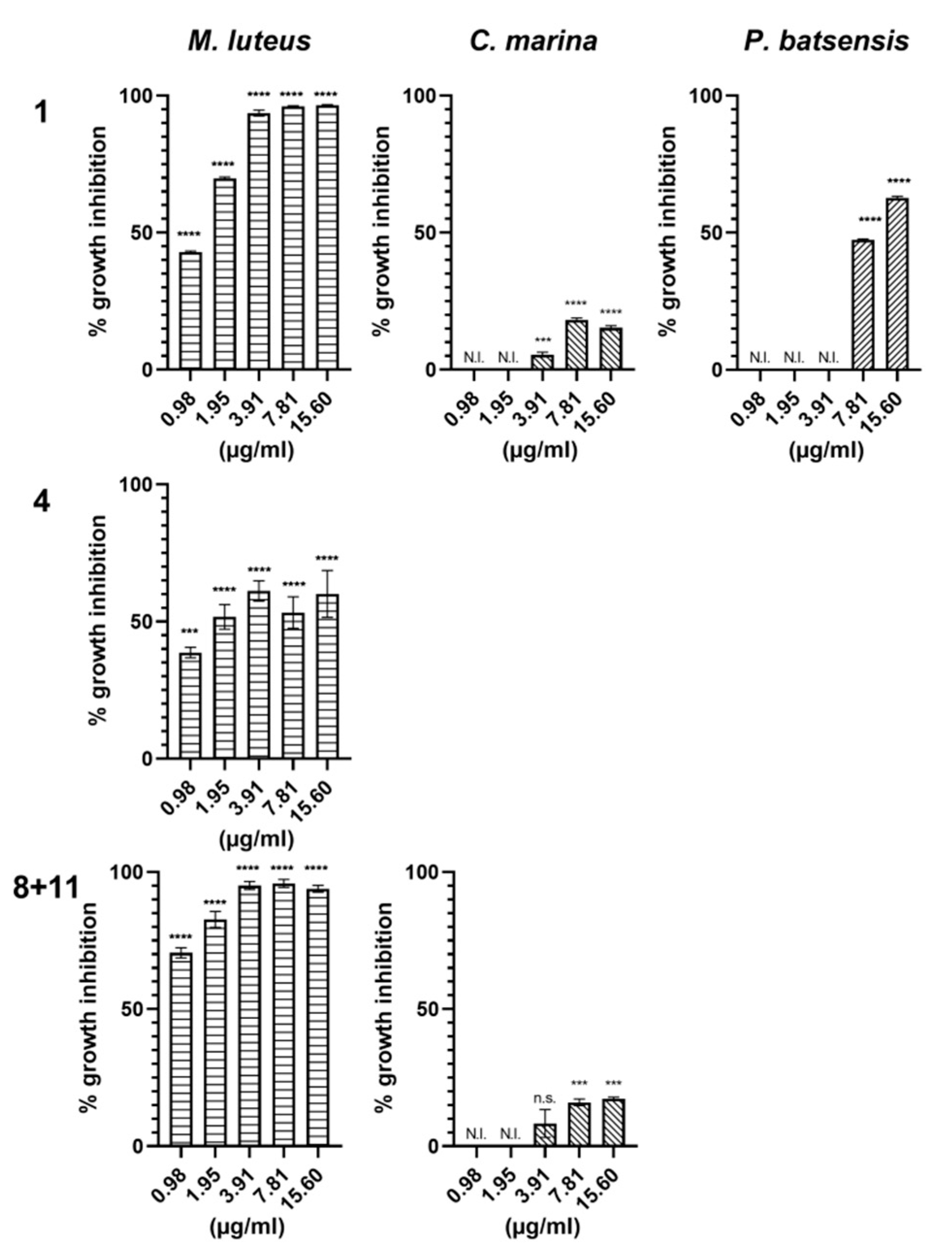
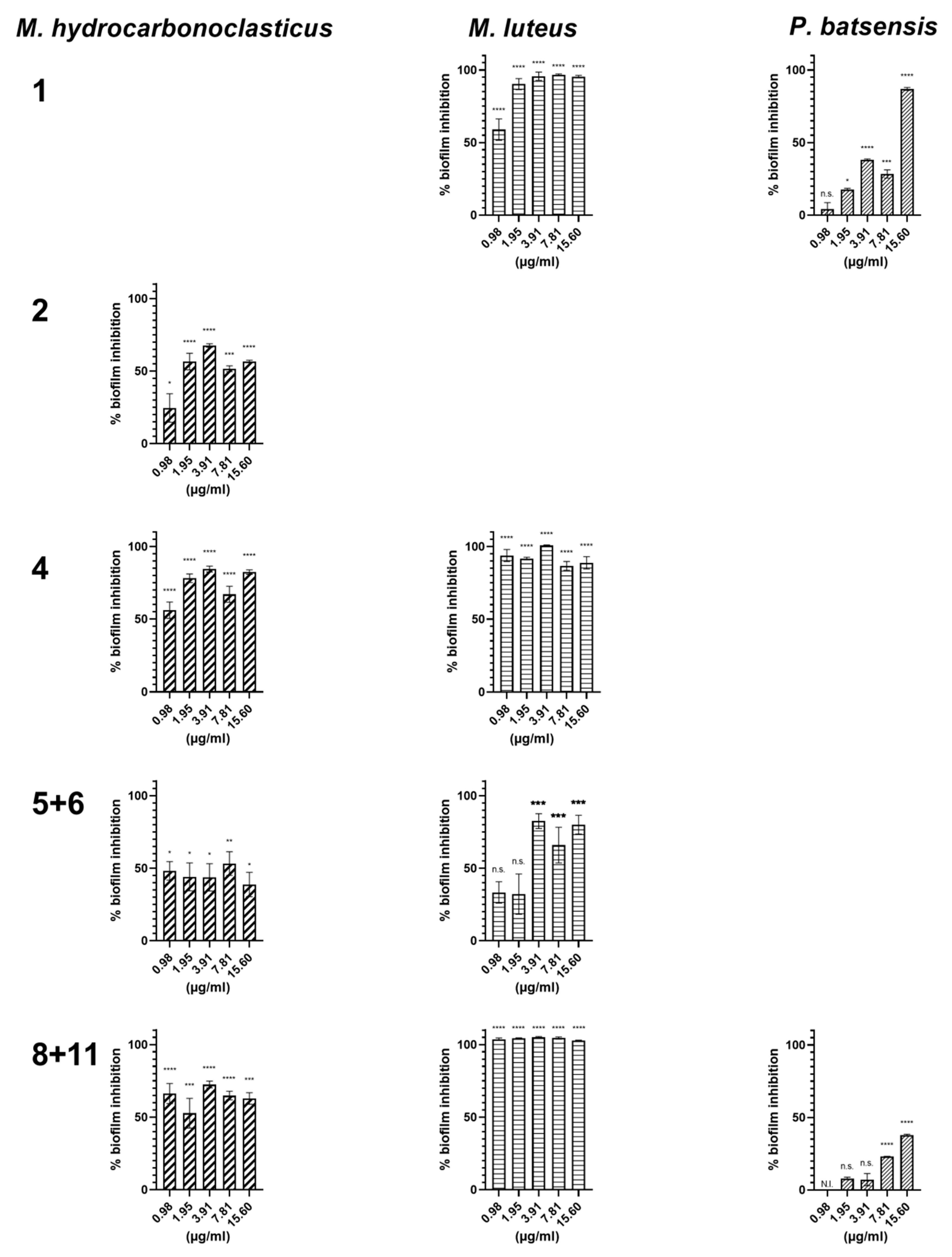
| Napyradiomycin | (1) | (4) | (8 and 11) | |||
|---|---|---|---|---|---|---|
| Concentration (µg/mL) | M. luteus | C. marina | P. batsensis | M. luteus | M. luteus | C. marina |
| 15.60 | 96.5 ± 0.2 | 15.3 ± 0.7 | 62.7 ± 0.6 | 60.1 ± 8.5 | 93.9 ± 1.3 | 17.4 ± 0.5 |
| 7.81 | 96.0 ± 0.3 | 18.2 ± 0.7 | 47.4 ± 0.3 | 53.3 ± 5.7 | 96.8 ± 1.4 | 16.0 ± 1.2 |
| 3.91 | 93.6 ± 1.1 | 5.5 ± 0.8 | N.I. | 61.2 ± 3.7 | 95.1 ± 1.5 | 8.3 ± 5.1 |
| 1.95 | 69.8 ± 0.5 | N.I. | N.I. | 51.7 ± 4.5 | 82.7 ± 2.9 | N.I. |
| 0.98 | 42.9 ± 0.4 | N.I. | N.I. | 38.7 ± 1.9 | 70.5 ± 1.9 | N.I. |
| % Biofilm Inhibition ± SEM | |||||
|---|---|---|---|---|---|
| Napyradiomycin | M. hydrocarbonoclasticus | C. marina | M. luteus | P. batsensis | P. inhibens |
| (1) | N.I. | N.I. | 88.6 ± 2.9 | 87.2 ± 0.1 | N.I. |
| (2) | 60.2 ± 5.7 | N.I. | N.I. | N.I. | N.I. |
| (4) | 56.9 ± 5.7 | N.I. | 97.0 ± 1.2 | 13.4 ± 0.8 | N.I. |
| (5 and 6) | 43.4 ± 8.2 | N.I. | 87.3 ± 0.3 | N.I. | N.I. |
| (8 and 11) | 59.3 ± 3.8 | N.I. | 100 ± 0.3 | 26.2 ± 0.0 | N.I. |
| (10) | 25.1 ± 10.2 | N.I. | N.T. | N.T. | N.T. |
| (12) | 33.7 ± 5.1 | N.I. | N.T. | N.T. | N.T. |
| (3 and 7) | 28.0 ± 4.8 | 23.4 ± 4.9 | N.T. | N.T. | N.T. |
| (9 and 12) | 48.2 ± 1.9 | N.I. | N.T. | N.T. | N.T. |
| Napyradiomycin | (1) | (2) | (4) | (5 and 6) | (8 and 11) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (µg/mL) | M. luteus | P. batsensis | M. hydro. | M. hydro. | M. luteus | M. hydro. | M. luteus | M. hydro. | M. luteus | P. batsensis |
| 15.6 | 95.4 ± 0.9 | 86.9 ± 1.0 | 56.6 ± 0.9 | 82.3 ± 1.5 | 88.8 ± 4.2 | 38.8 ± 8.4 | 80.1 ± 6.5 | 62.8 ± 4.0 | 100 ± 0.3 | 37.8 ± 0.7 |
| 7.81 | 96.7 ± 0.5 | 28.4 ± 2.8 | 51. 7± 2.0 | 67.0 ± 5.5 | 86.7 ± 3.0 | 53.2 ± 8.2 | 65.9 ± 12.3 | 64.9 ± 3.0 | 100 ± 0.8 | 23.1 ± 0.2 |
| 3.91 | 95.8 ± 2.9 | 38.1 ± 0.6 | 67. 7± 1.2 | 84.5 ± 2.0 | 100 ± 0.4 | 43.8 ± 9.4 | 82.5 ± 5.1 | 72.5 ± 2.3 | 100 ± 0.6 | 7.1 ± 4.2 |
| 1.95 | 90.3 ± 3.8 | 17.7 ± 0.8 | 56.5 ± 5.7 | 78.1 ± 3.0 | 91.7 ± 0.9 | 44.1 ± 9.6 | 32.2 ± 13.9 | 52.7 ± 10.2 | 100 ± 0.3 | 7.9 ± 1.0 |
| 0.98 | 59.0 ±7.4 | 4.3 ± 4.3 | 24.6 ± 9.8 | 56.1 ± 5.6 | 93.8 ± 4.1 | 48.2 ± 6.4 | 33.3 ± 7.4 | 66.3 ± 6.9 | 100 ± 0.9 | N.I. |
| Napyradiomycin | EC50 [Conf. limits] (µg/mL) | Chi-Square Test | LC50 (µg/mL) | LC50/EC50 |
|---|---|---|---|---|
| (1) | 0.655 [0.300; 0.906] | χ2 = 217.986; df = 18; p < 0.001 | >12 | 18.32 |
| (2) | 1.999 [1.581; 2.547] | χ2 = 414.500; df = 18; p < 0.001 | >12 | 6.00 |
| (3 and 7) | 1.092 [0.225; 2.933] | χ2 = 555.409; df = 18; p < 0.001 | >12 | 10.99 |
| (4) | 6.339 [5.602; 7.181] | χ2 = 144.409; df = 18; p < 0.001 | >12 | 1.89 |
| (5 and 6) | 4.331 [2.911; 7.091] | χ2 = 617.072; df = 18; p < 0.001 | >12 | 2.77 |
| (8 and 11) | 0.727 [0.065; 1.406] | χ2 = 458.713; df = 18; p < 0.001 | >12 | 16.51 |
| (9 and 12) | 0.451 [0.192; 0.760] | χ2 = 770.695; df = 22; p < 0.001 | >12 | 26.58 |
| (10) | 0.102 [0.072; 0.140] | χ2 = 844.065; df = 42; p < 0.001 | >12 | 117.28 |
| (12) | 0.947 [0.586; 1.473] | χ2 = 729.107; df = 22; p < 0.001 | >12 | 12.67 |
| Toxicity End Points for Consensus Models | |||||||
|---|---|---|---|---|---|---|---|
| # | Fathead Minnow 1 | Daphnia magna 2 | Tetrahymena pyriformis 3 | Oral Rat 4 | Bioconcentration Factor | Developmental Toxicity 5 | Ames Mutagenicity 6 |
| 1 | 0.27 | 0.76 | 3.01 | 495.72 | 22.17 | 0.88; DT | 0.23; MN |
| 2 | 0.22 | 0.45 | 1.22 | 291.93 | 10.25 | 0.72; DT | 0.17; MN |
| 3 | 0.05 | 0.17 | 310 | 505.42 | 42.17 | 0.71; DT | 0.31; MN |
| 4 | 0.13 | 1.64 | 1.22 | 491.94 | 5.31 | 0.51; DT | 0.36; MN |
| 5 | 0.05 | 3.23 | 3.09 | 478.12 | 26.41 | 0.73; DT | 0.15; MN |
| 6 | 0.09 | 2.28 | 2.87 | 1246.67 | 10.36 | 0.92; DT | 0.18; MN |
| 7 | 0.06 | 1.23 | 2.87 | 895.08 | 26.62 | 0.96; DT | 0.17; MN |
| 8 | 0.04 | 0.41 | 1.65 | 1055.66 | 18.59 | 0.95; DT | 0.27; MN |
| 9 | 0.04 | 0.98 | 1.56 | 1516.88 | 22.55 | 0.91; DT | 0.25; MN |
| 10 | 0.63 | 0.91 | 1.62 | 390.57 | 36.37 | 0.74; DT | 0.29; MN |
| 11 | 0.02 | 0.38 | 5.61 | 1414.33 | 31.86 | 0.93; DT | 0.19; MN |
| 12 | 0.05 | 0.45 | 5.29 | 687.01 | 68.24 | 0.90; DT | 0.19; MN |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, F.; Almeida, J.R.; Paulino, M.; Grilo, I.R.; Macedo, H.; Cunha, I.; Sobral, R.G.; Vasconcelos, V.; Gaudêncio, S.P. Antifouling Napyradiomycins from Marine-Derived Actinomycetes Streptomyces aculeolatus. Mar. Drugs 2020, 18, 63. https://doi.org/10.3390/md18010063
Pereira F, Almeida JR, Paulino M, Grilo IR, Macedo H, Cunha I, Sobral RG, Vasconcelos V, Gaudêncio SP. Antifouling Napyradiomycins from Marine-Derived Actinomycetes Streptomyces aculeolatus. Marine Drugs. 2020; 18(1):63. https://doi.org/10.3390/md18010063
Chicago/Turabian StylePereira, Florbela, Joana R. Almeida, Marisa Paulino, Inês R. Grilo, Helena Macedo, Isabel Cunha, Rita G. Sobral, Vitor Vasconcelos, and Susana P. Gaudêncio. 2020. "Antifouling Napyradiomycins from Marine-Derived Actinomycetes Streptomyces aculeolatus" Marine Drugs 18, no. 1: 63. https://doi.org/10.3390/md18010063
APA StylePereira, F., Almeida, J. R., Paulino, M., Grilo, I. R., Macedo, H., Cunha, I., Sobral, R. G., Vasconcelos, V., & Gaudêncio, S. P. (2020). Antifouling Napyradiomycins from Marine-Derived Actinomycetes Streptomyces aculeolatus. Marine Drugs, 18(1), 63. https://doi.org/10.3390/md18010063










