Two New Cytotoxic Compounds from a Deep-Sea Penicillum citreonigrum XT20-134
Abstract
1. Introduction
2. Results
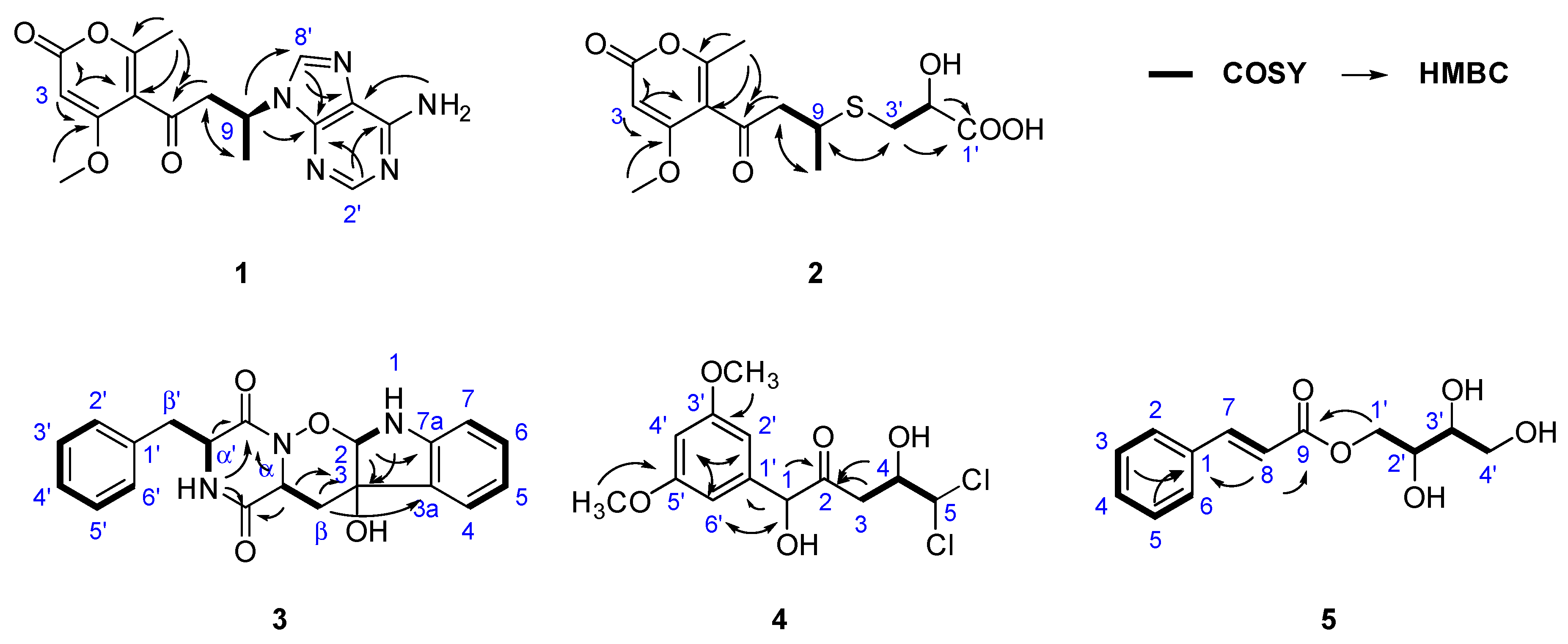
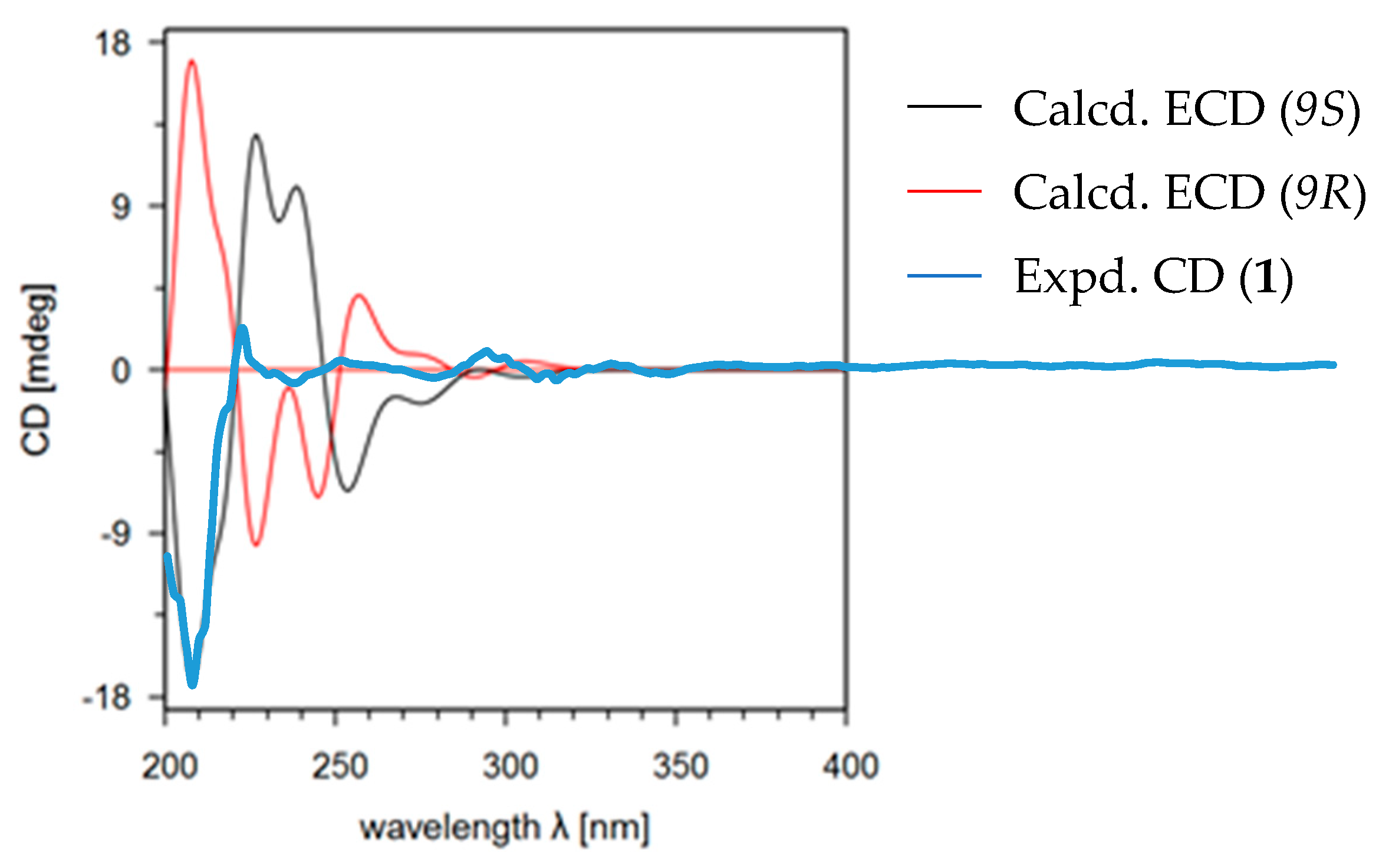
2.1. Structural Identification of New Compounds
2.2. Cytotoxicity Evaluation
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Eletronic Circular Dichroism (ECD) Calculations
4.3. Fungal Strain and Fermentation
4.4. Extraction and Isolation
4.5. Structrural Elucidation of the New Compounds 1–5
4.6. Cytotoxicity Assay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chunxiao, S.; Shah, M.; Zhenzhen, Z.; Yanyan, F.; Yimin, C.; Qian, C.; Qianqun, G.; Tianjiao, Z.; Guojian, Z.; Dehai, L. Secondary Metabolites from Deep-sea Derived Microorganisms. Curr. Med. Chem. 2019, 26, 1. [Google Scholar]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine Fungi: A Source of Potential Anticancer Compounds. Front. Microbiol. 2018, 8, 2536. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Xue, Y.R.; Liu, C.H. A Brief Review of Bioactive Metabolites Derived from Deep-Sea Fungi. Mar. Drugs 2015, 13, 4594–4616. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Sun, Z.H.; Liu, Z.; Chen, Y.C.; Liu, H.X.; Li, H.H.; Zhang, W.M. Dichotocejpins A-C: New Diketopiperazines from a Deep-Sea-Derived Fungus Dichotomomyces cejpii FS110. Mar. Drugs 2016, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, Z.M.; Chen, Y.C.; Tan, H.B.; Li, S.N.; Li, H.H.; Gao, X.X.; Liu, H.X.; Zhang, W.M. Chromone-Derived Polyketides from the Deep-Sea Fungus Diaporthe phaseolorum FS431. Mar. Drugs 2019, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Min, X.T.; Huang, J.J.; Zhong, Y.; Wu, Y.H.; Li, X.X.; Deng, Y.Y.; Jiang, Z.D.; Shao, Z.Z.; Zhang, L.H.; et al. Cytoglobosins H and I, New Antiproliferative Cytochalasans from Deep-Sea-Derived Fungus Chaetomium globosum. Mar. Drugs 2016, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tang, X.X.; Qin, D.; Yi, Z.W.; Fang, M.J.; Wu, Z.; Qiu, Y.K. Biosynthetic Functional Gene Analysis of Bis-Indole Metabolites from 25D7, a Clone Derived from a Deep-Sea Sediment Metagenomic Library. Mar. Drugs 2016, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tang, X.X.; Chen, L.; Yi, Z.W.; Fang, M.J.; Wu, Z.; Qiu, Y.K. Two New Cytotoxic Indole Alkaloids from a Deep-Sea Sediment Derived Metagenomic Clone. Mar. Drugs 2014, 12, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Tang, X.X.; Yi, Z.W.; Qiu, Y.K.; Wu, Z. Two new compounds from marine-derived fungus Penicillium sp F11. J. Asian Nat. Prod. Res. 2012, 14, 197–203. [Google Scholar] [CrossRef]
- Liu, D.; Lin, H.; Proksch, P.; Tang, X.X.; Shao, Z.Z.; Lin, W.H. Microbacterins A and B, New Peptaibols from the Deep Sea Actinomycete Microbacterium sediminis sp. nov. YLB-01(T). Org. Lett. 2015, 17, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, X.X.; Shao, Z.Z.; Ren, J.W.; Liu, D.; Proksch, P.; Lin, W.H. Indole-based alkaloids from deep-sea bacterium Shewanella piezotolerans with antitumor activities. J. Antibiot. 2014, 67, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Shizuri, Y.; Nagahama, M.; Yamamura, S.; Kawai, K.; Kawai, N.; Furukawa, H. Isolation and Structures of Citreovirenone and Citreovirone. Chem. Lett. 1986, 15, 1129–1132. [Google Scholar] [CrossRef]
- Yang, M.H.; Li, T.X.; Wang, Y.; Liu, R.H.; Luo, J.; Kong, L.Y. Antimicrobial metabolites from the plant endophytic fungus Penicillium sp. Fitoterapia 2017, 116, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tani, K.; Kojima, A.; Sotoma, G.; Okada, K.; Shimada, A. Cyclo-(l-tryptophyl-l-phenylalanyl), a plant growth regulator produced by the fungus Penicillium. Phytochemistry 1996, 41, 665–669. [Google Scholar] [CrossRef]
- Shintani, R.; Hayashi, T. Rhodium-Catalyzed Asymmetric 1,4-Addition of Sodium Tetraarylborates to β, β-Disubstituted α, β-Unsaturated Esters. Org. Lett. 2011, 13, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Sudo, S.; Kosemura, S.; Yamamura, S. Two new metabolites of hybrid strains KO 0201 and 0211 derived from Penicillium citreo-viride B. IFO 6200 and 4692. Tetrahedron Lett. 1999, 40, 6831–6834. [Google Scholar] [CrossRef]
- Sato, H.; Konoma, K.; Sakamura, S. Phytotoxins Produced by Onion Pink Root Fungus, Pyrenochaeta terrestris. J. Agric. Chem. Soc. Jpn. 1979, 43, 2409–2411. [Google Scholar]
- Amagata, T.; Minoura, K.; Numata, A. Cytotoxic metabolites produced by a fungal strain from a Sargassum alga. J. Antibiot. 1998, 51, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Zhang, H.P. Isolation of Secondary Metabolites of 9F Series Marine Fungi and Their Bioactivities against Pyricularia oryzae. Nat. Prod. Res. Dev. 2005, 17, 677–680. [Google Scholar]
- Hashida, J.; Niitsuma, M.; Iwatsuki, M.; Mori, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tsukashima, A.; Nonaka, K.; Ui, H.; Masuma, R.; et al. Pyrenocine I, a new pyrenocine analog produced by Paecilomyces sp. FKI-3573. J. Antibiot. 2010, 63, 559–561. [Google Scholar] [CrossRef]
- Guo, F.; Yang, S.X.; Li, L.; Wang, Y. Chemical Constituents and Their Toxic Activity from the Endophytic Fungus Phomopsissp. KY-12,Isolated from Pleioblastus amarus. Nat. Prod. Res. Dev. 2014, 26, 1389–1392. [Google Scholar]
- Da Rocha, M.W.; Resck, I.S.; Caldas, E.D. Purification and full characterisation of citreoviridin produced by Penicillium citreonigrum in yeast extract sucrose (YES) medium. Food Addit. Contam. A 2015, 32, 584–595. [Google Scholar] [CrossRef]
- Sarubbi, E.; Seneci, P.F.; Angelastro, M.R.; Peet, N.P.; Denaro, M.; Islam, K. Peptide Aldehydes as Inhibitors of Hiv Protease. FEBS Lett. 1993, 319, 253–256. [Google Scholar] [CrossRef]
- Teng, X.; Zhuang, Y.; Wang, Y.; Liu, P.; Xu, Z.; Zhu, W. Secondary metabolites from Penicillium sp.gxwz406 symbiotic with the gorgonian Echinogorgia flora. Chin. J. Mar. Drugs 2010, 29, 11–15. [Google Scholar]
- Shizuri, Y.; Shigemori, H.; Sato, R.; Yamamura, S.; Kawai, K.; Furukawa, H. 4 New Metabolites Produced by Penicillium-Citreo-Viride B on Addition of Nabr. Chem. Lett. 1988, 17, 1419–1422. [Google Scholar] [CrossRef]
- Matsunaga, K.; Shizuri, Y.; Yamamura, S.; Kawai, K.; Furukawa, H. Isolation and Structure of Citreoindole, a New Metabolite of Hybrid Strain Ko-0052 Derived from Penicillium-Citreo-Viride B—Ifo-6200 and B—Ifo-4692. Tetrahedron Lett. 1991, 32, 6883–6884. [Google Scholar] [CrossRef]
- Jimenez, C. Marine sulfur-containing natural products. Stud. Nat. Prod. Chem. 2001, 25, 811–917. [Google Scholar]
- Wang, X.; Wang, P.; Zhu, T.; Zhu, W. Advances in studies of bioactive halo-natural products from microorganisms. Zhongguo Haiyang Yaowu 2011, 30, 40–51. [Google Scholar]
- Marakalala, M.B.; Mmutlane, E.M.; Kinfe, H.H. β-hydroxy sulfides and their syntheses. Beilstein J. Org. Chem. 2018, 14, 1668–1692. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Hu, X.; Ma, Y.; Mu, J.; Li, Z.; Bai, J.; Hua, H.; Li, D.; Liu, W.; Xu, F. Marine-Derived Natural Lead Compound Disulfide-Linked Dimer Psammaplin A: Biological Activity and Structural Modification. Mar. Drugs 2019, 17, 384. [Google Scholar] [CrossRef]
- Tang, X.X.; Yan, X.; Fu, W.H.; Yi, L.Q.; Tang, B.W.; Yu, L.B.; Fang, M.J.; Wu, Z.; Qiu, Y.K. New beta-Lactone with Tea Pathogenic Fungus Inhibitory Effect from Marine-Derived Fungus MCCC3A00957. J. Agric. Food Chem. 2019, 67, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Ou-Yang, H.; Yan, X.; Tang, B.W.; Fang, M.J.; Wu, Z.; Chen, J.W.; Qiu, Y.K. Open-Ring Butenolides from a Marine-Derived Anti-Neuroinflammatory Fungus Aspergillus terreus Y10. Mar. Drugs 2018, 16, 428. [Google Scholar] [CrossRef] [PubMed]

| Positions | 1 | 2 | 2’ | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 2 | 162.0 | 162.2 | 162.2 | |||
| 3 | 87.8 | 5.62, s | 87.9 | 5.69, s | 87.9 | 5.69, s |
| 4 | 168.3 | 168.5 | 168.5 | |||
| 5 | 115.1 | 115.4 | 115.4 | |||
| 6 | 162.8 | 163.2 | 163.2 | |||
| 7 | 198.8 | 199.5 | 199.5 | |||
| 8 | 49.3 | 3.78, dd (17.6, 7.9) | 51.7 | 2.99, dd (17.1, 6.6) | 51.6 | 2.99, dd (17.1, 6.6) |
| 3.35, dd (17.6, 6.0) | 2.90, dd (17.1, 3.7) | 2.89, dd (17.1, 3.7) | ||||
| 9 | 47.7 | 5.06, m | 36.3 | 3.30, m | 36.2 | 3.30, m |
| 10 | 21.1 | 1.54, br. d (6.6) | 21.8 | 1.23, d (6.8) | 21.8 | 1.23, d (6.8) |
| 6-CH3 | 18.0 | 1.85, s | 18.5 | 2.18, d (2.6) | 18.5 | 2.18, d (2.6) |
| 4-OCH3 | 57.4 | 3.74, s | 57.5 | 3.87, s | 57.5 | 3.87, s |
| 2′ | 152.6 | 8.11, br. s | ||||
| 4′ | 149.5 | |||||
| 5′ | 119.5 | |||||
| 6′ | 156.4 | |||||
| 8′ | 140.3 | 8.21, br. s | ||||
| 6′-NH2 | 7.16, br. s | |||||
| 1′ | 174.5 | 174.5 | ||||
| 2′ | 71.1 | 4.09, m | 71.0 | 4.09, m | ||
| 3′ | 34.7 | 2.83, dd (13.5, 5.1) | 34.6 | 2.82, dd (13.5, 5.1) | ||
| 2.72, dd (13.6, 9.5) | 2.71, dd (13.6, 9.5) | |||||
| Compd. | Bel7402 | HT1080 | Cne2 | A549 |
|---|---|---|---|---|
| 2 | 7.63 ± 1.46 | 10.22 ± 1.32 | 73.14 ± 5.32 | 87.08 ± 7.32 |
| 4 | 13.14 ± 1.41 | 16.53 ± 1.67 | 83.56 ± 6.49 | 92.47 ± 6/33 |
| paclitaxel | <1 | <1 | <1 | <1 |
| DMSO | None | None | None | None |
| Positions | 3 | 19 | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 2 | 99.3 | 5.10, d (2.8) | 121.4 | 6.88, br. s |
| 3 | 74.6 | 109.2 | ||
| 4 | 123.1 | 6.90, br. d (7.2) | 118.9 | 7.43, br. d (7.2) |
| 5 | 118.3 | 6.56, br. t (7.0) | 119.2 | 6.93, br. t (7.0) |
| 6 | 129.1 | 7.00, br. t (7.5) | 124.9 | 7.01, br. t (7.4) |
| 7 | 109.6 | 6.56, br. d (7.7) | 111.8 | 7.26, br. d (7.7) |
| 3a | 131.9 | 128.0 | ||
| 7a | 148.1 | 136.5 | ||
| 1′ | 136.0 | 137.0 | ||
| 2′, 6′ | 130.4 | 6.98, br. d (7.2) | 130.2 | 7.10, overlapped |
| 3′, 5′ | 128.2 | 6.86, br. t (7.4) | 128.5 | 6.64, br. t (7.4) |
| 4′ | 126.5 | 6.75, m | 126.9 | 7.12, overlapped |
| C=O | 166.1 | 166.7 | ||
| C=O′ | 161.5 | 167.3 | ||
| α | 54.6 | 4.17, dd (9.1, 4.8) | 55.8 | 3.92, m |
| α′ | 54.8 | 4.19, dd (5.0, 2.8) | 56.1 | 3.79, m |
| β′ | 37.7 | 2.87, dd (13.9, 2.8) | 39.5 | 2.40, overlapped |
| 2.74, dd (13.8, 5.0) | 1.78, dd (13.5, 7.0) | |||
| β | 36.0 | 1.91, dd (13.6, 4.8) | 30.2 | 2.74, dd (14.0, 4.4) |
| 1.03, dd (13.5, 9.1) | 2.44, overlapped | |||
| 1-NH | 6.57, br. s | 10.83, br. s | ||
| α-NH | 7.85, br. s | |||
| α′-NH | 7.96, br. s | 7.64, br. s | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.-X.; Liu, S.-Z.; Yan, X.; Tang, B.-W.; Fang, M.-J.; Wang, X.-M.; Wu, Z.; Qiu, Y.-K. Two New Cytotoxic Compounds from a Deep-Sea Penicillum citreonigrum XT20-134. Mar. Drugs 2019, 17, 509. https://doi.org/10.3390/md17090509
Tang X-X, Liu S-Z, Yan X, Tang B-W, Fang M-J, Wang X-M, Wu Z, Qiu Y-K. Two New Cytotoxic Compounds from a Deep-Sea Penicillum citreonigrum XT20-134. Marine Drugs. 2019; 17(9):509. https://doi.org/10.3390/md17090509
Chicago/Turabian StyleTang, Xi-Xiang, Shun-Zhi Liu, Xia Yan, Bo-Wen Tang, Mei-Juan Fang, Xiu-Min Wang, Zhen Wu, and Ying-Kun Qiu. 2019. "Two New Cytotoxic Compounds from a Deep-Sea Penicillum citreonigrum XT20-134" Marine Drugs 17, no. 9: 509. https://doi.org/10.3390/md17090509
APA StyleTang, X.-X., Liu, S.-Z., Yan, X., Tang, B.-W., Fang, M.-J., Wang, X.-M., Wu, Z., & Qiu, Y.-K. (2019). Two New Cytotoxic Compounds from a Deep-Sea Penicillum citreonigrum XT20-134. Marine Drugs, 17(9), 509. https://doi.org/10.3390/md17090509





