Penigrisacids A–D, Four New Sesquiterpenes from the Deep-Sea-Derived Penicillium griseofulvum
Abstract
:1. Introduction
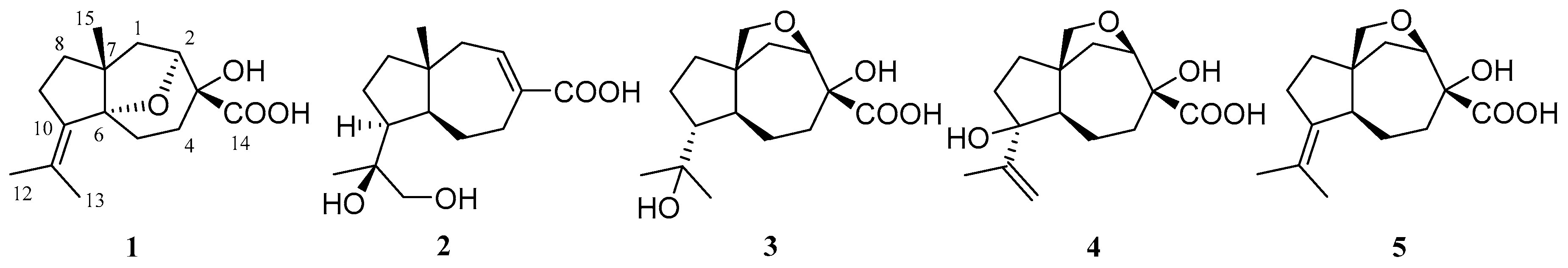
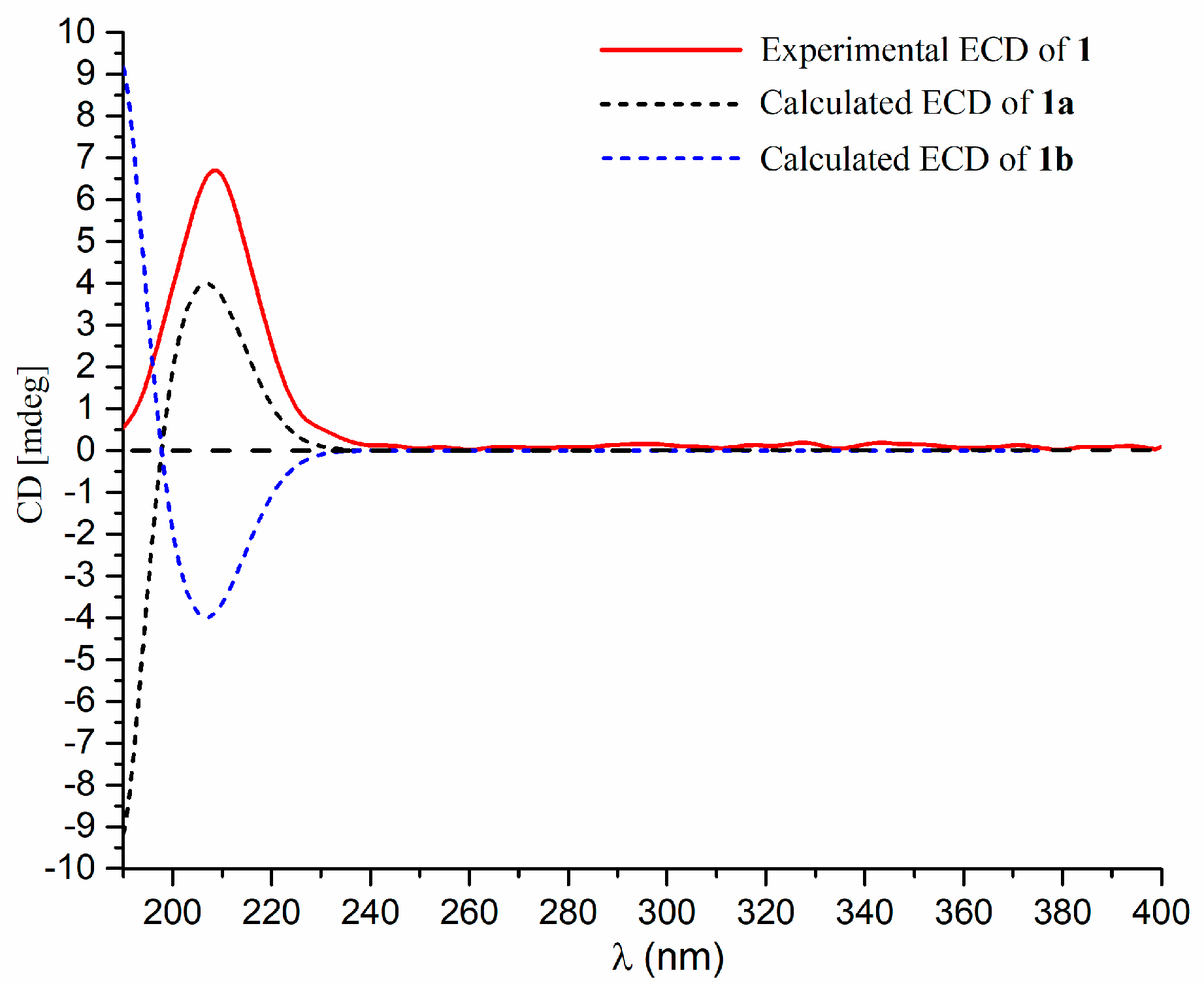
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fermentation and Extraction
3.3. Isolation and Purification
3.4. Theoretical Calculations
3.5. Anti-Allergic Experiment
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sýkora, V.; Novotný, L.; Holub, M.; Herout, V.; Šorm, F. On terpenes. CXVII. The proof of structure of carotol and daucol. Collect. Czech. Chem. Commun. 1961, 26, 788–802. [Google Scholar]
- Bates, R.B.; Green, C.D.; Sneath, T.C. The molecular structure of daucyl d,l-alaninate hydrobromide. Tetrahedron Lett. 1969, 10, 3461–3463. [Google Scholar] [CrossRef]
- Sriraman, M.C.; Nagasampagi, B.A.; Pandey, R.C.; Dev, S. Studies in sesquiterpenes—XLIX: Sesquiterpenes from Ferula jaeschkeana vatke (part 1): Jaeschkeanadiol—structure, stereochemistry. Tetrahedron 1973, 29, 985–991. [Google Scholar] [CrossRef]
- González, A.G.; Fraga, B.M.; Hernández, M.G.; Luis, J.G.; Estevez, R.; Báeza, J.L.; Riverob, M. Linkiol, a new sesquiterpene from Ferula linkii. Phytochemistry 1977, 16, 265–267. [Google Scholar]
- Shimada, A.; Kusano, M.; Takeuchi, S.; Fujioka, S.; Inokuchi, T.; Kimura, Y. Aspterric acid and 6-hydroxymellein, inhibitors of pollen development in Arabidopsis thaliana, produced by Aspergillus terreus Z. Naturforsch. C 2002, 57, 459–464. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Q.K.; Zang, X.; Yuan, S.G.; Bat-Erdene, U.; Nguyen, C.; Gan, J.H.; Zhou, J.H.; Jacobsen, S.E.; Tang, Y. Resistance-gene-directed discovery of a natural-product herbicide with a new mode of action. Nature 2018, 559, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.Q.; Xie, C.L.; Xia, J.M.; Xing, C.P.; Luo, Z.H.; Shao, Z.; Yan, X.J.; He, S.; Yang, X.W. Sarocladione, a unique 5,10:8,9-diseco-steroid from the deep-sea-derived fungus Sarocladium kiliense. Org. Biomol. Chem. 2019, 17, 5925–5928. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.L.; Zhang, D.; Xia, J.M.; Hu, C.C.; Lin, T.; Lin, Y.K.; Wang, G.H.; Tian, W.J.; Li, Z.P.; Zhang, X.K.; et al. Steroids from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475 induced apoptosis via retinoid X receptor (RXR)-α pathway. Mar. Drugs 2019, 17, 178. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Liu, Q.; Xia, J.M.; Xie, C.L.; Luo, Z.H.; Shao, Z.; Liu, G.M.; Yang, X.W. Polyketides from the deep-sea-derived fungus Graphostroma sp. MCCC 3A00421 showed potent antifood allergic activities. J. Agric. Food Chem. 2018, 66, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Fan, Z.W.; Xie, C.L.; Liu, Q.; Luo, Z.H.; Liu, G.; Yang, X.W. Spirograterpene A, a tetracyclic spiro-diterpene with a fused 5/5/5/5 ring system from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475. J. Nat. Prod. 2017, 80, 2174–2177. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Kaneda, M.; Tada, A.; Nitta, K.; Yamamoto, Y.; Iitaka, Y. Aspterric acid, a new sesquiterpenoid of the carotane group, a metabolite from Aspergillus terreus IFO-6123. X-Ray crystal and molecular structure of its p-bromobenzoate. J. Chem. Soc. Chem. Commun. 1978, 160–161. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Miura, S.; Yamashita, Y.; Ohta, T. Aspermytin A: A new neurotrophic polyketide isolated from a marine-derived fungus of the genus Aspergillus Bioorg. Med. Chem. Lett. 2004, 14, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Huang, M.; Liu, L.; Wang, J.; Lin, Y. Six new polyketide decalin compounds from mangrove endophytic fungus Penicillium aurantiogriseum 328. Mar. Drugs 2015, 13, 6306–6318. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Feng, T.; Li, Z.H.; Liu, J.K. Five new polyketides from the basidiomycete Craterellus odoratus. Nat. Prod. Bioprospect. 2012, 2, 170–173. [Google Scholar] [CrossRef]
- Ayer, W.A.; Browne, L.M.; Lin, G. Metabolites of Leptographium wageneri, the causative agent of black stain toot disease of conifers. J. Nat. Prod. 1989, 52, 119–129. [Google Scholar] [CrossRef]
- RDKit: Open-Source Cheminformatics Software. Available online: http://www.rdkit.org/ (accessed on 18 April 2019).
- Stephens, P.J.; Harada, N. ECD cotton effect approximated by the Gaussian curve and other methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.L.; Liu, Q.; Xia, J.M.; Gao, Y.; Yang, Q.; Shao, Z.Z.; Liu, G.; Yang, X.W. Anti-allergic compounds from the deep-sea-derived actinomycete Nesterenkonia flava MCCC 1K00610. Mar. Drugs 2017, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Zhou, T.T.; Xie, C.L.; Zhang, G.Y.; Yang, X.W. Microindolinone A, a novel 4,5,6,7-tetrahydroindole, from the deep-sea-derived actinomycete Microbacterium sp. MCCC 1A11207. Mar. Drugs 2017, 15, 230. [Google Scholar] [CrossRef] [PubMed]
 ), HMBC (
), HMBC (  ), and NOESY (
), and NOESY (  ) correlations of 1.
) correlations of 1.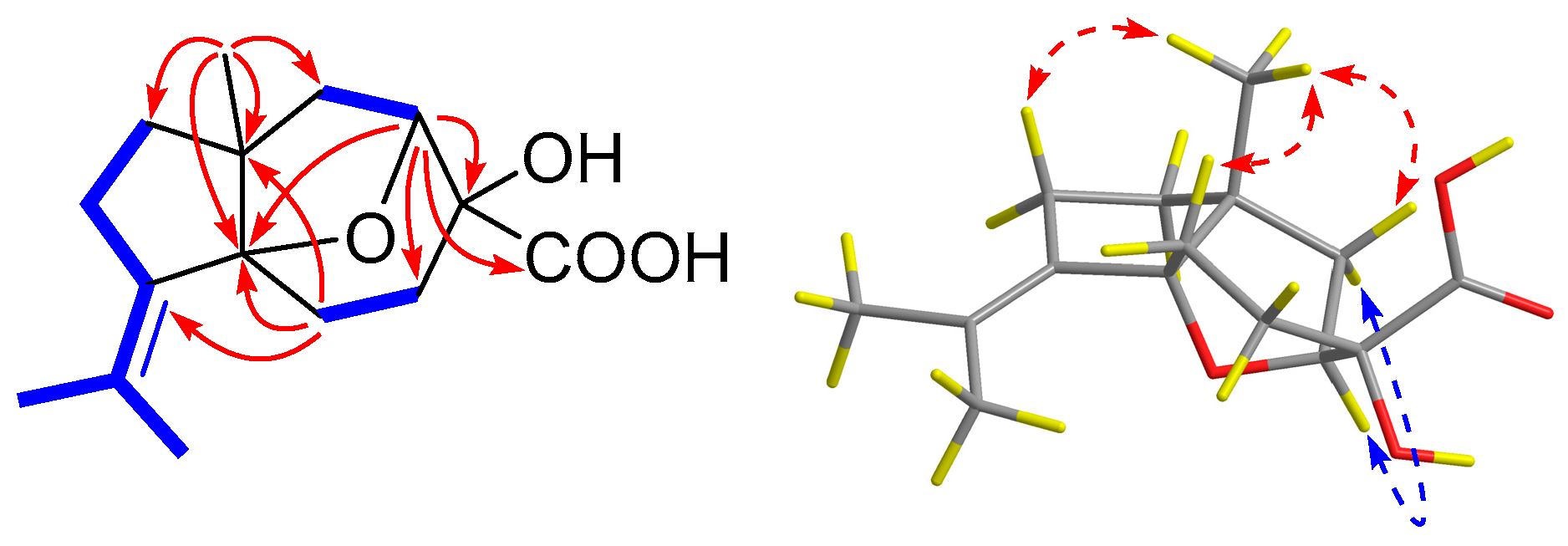
 ), HMBC (
), HMBC (  ), and NOESY (
), and NOESY (  ) correlations of 2.
) correlations of 2.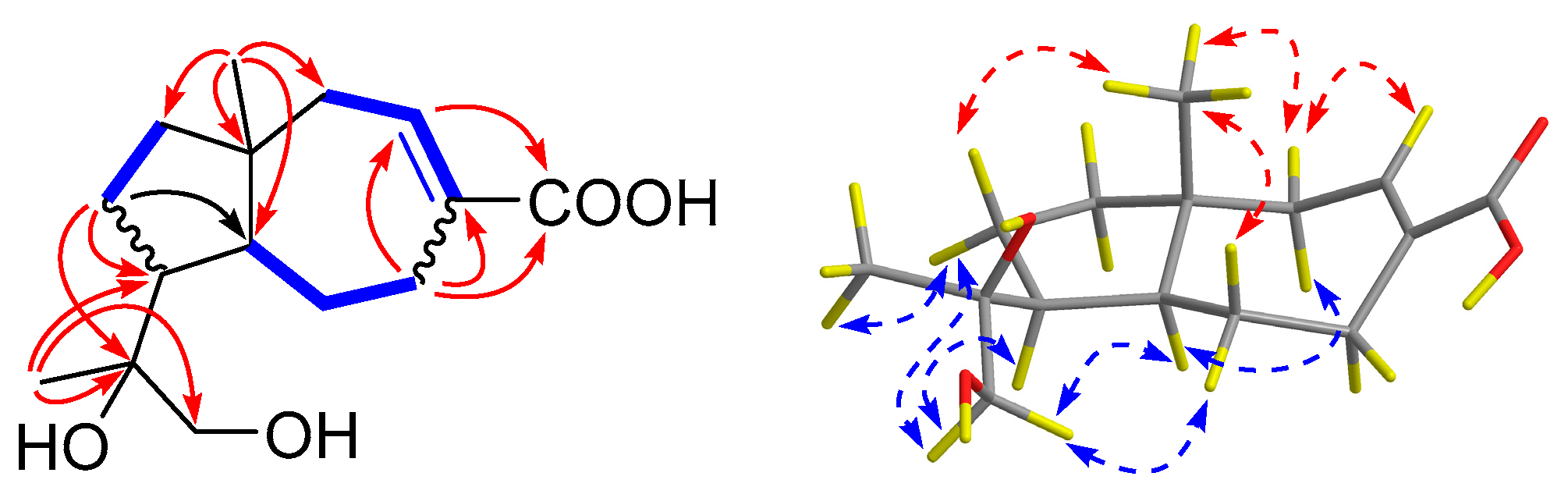

| No. | 1 a | 2 a | 3 b | 4 a | 5 a |
|---|---|---|---|---|---|
| 1 | 2.01, dd (13.2, 7.6) | 2.41, dd (14.9, 9.1) | 2.00, dd (12.7, 8.5) | 2.20, dd (13.0, 8.5) | 2.40, overlap |
| 1.90, d (13.2) | 2.14, br d (14.2) | 1.85, d (12.7) | 2.00, d (13.0) | 1.48, overlap | |
| 2 | 4.48, d (7.5) | 7.07, dt (8.6, 3.1) | 4.13, d (8.4) | 4.28, d (8.4) | 4.31, d (8.4) |
| 4 | 2.18, dd (13.5, 6.4) | 2.99, dd (15.8, 5.8) | 2.18, dd (13.8, 7.6) | 2.42, dd (13.7, 7.5) | 2.28, m |
| 1.69, m | 1.93, overlap | 1.20, m | 1.33, m | 2.03, overlap | |
| 5 | 2.74, dt (12.6, 6.2) | 1.93, overlap | 1.93, dd (11.0, 8.0) | 1.77, m | 2.16, dd (13.1, 8.4) |
| 1.55, overlap | 1.25, d (13.2) | 1.43, overlap | 1.45, dd (13.6, 7.6) | 2.03, overlap | |
| 6 | - | 1.59, t (10.5) | 1.42, overlap | 1.72, m | 2.32, m |
| 8 | 1.63, m | 1.41, m | 1.56, m | 1.84, m | 2.40, overlap |
| 1.55, overlap | 1.26, dd (11.1, 7.6) | 1.65, m | 1.67, m | ||
| 9 | 2.38, m | 1.78, m | 1.41, overlap | 2.10, m | 1.69, m |
| 1.68, m | 1.61, m | 1.48, overlap | |||
| 10 | - | 1.93, overlap | 1.58, m | - | - |
| 12 | 1.82, br s | 1.19, s | 1.03, s | 1.76, s | 1.73, br s |
| 13 | 1.63, br s | 3.45, br s | 1.05, s | 5.01, s | 1.60, br s |
| 4.84, s | |||||
| 15 | 1.18, s | 0.79, s | 3.64, d (8.0) | 4.62, d (8.0) | 3.70, d (8.0) |
| 3.18, d (8.1) | 3.34, d (7.9) | 3.35, d (8.7) |
| No. | 1 a | 2 a | 3 b | 4 a | 5 a |
|---|---|---|---|---|---|
| 1 | 40.4 CH2 | 42.3 CH2 | 35.8 CH2 | 38.9 CH2 | 35.2 CH2 |
| 2 | 82.6 CH | 142.8 CH | 82.8 CH | 84.0 CH | 84.9 CH |
| 3 | 74.4 C | 136.5 C | 77.6 C | 79.6 C | 79.5 C |
| 4 | 28.5 CH2 | 28.5 CH2 | 34.3 CH2 | 35.3 CH2 | 33.0 CH2 |
| 5 | 27.7 CH2 | 27.0 CH2 | 24.8 CH2 | 19.8 CH2 | 36.8 CH2 |
| 6 | 93.9 C | 55.8 CH | 52.3 CH | 58.4 CH | 56.7 CH |
| 7 | 54.0 C | 43.5 C | 52.7 C | 52.7 C | 54.2 C |
| 8 | 41.6 CH2 | 42.4 CH2 | 33.6 CH2 | 33.3 CH2 | 24.9 CH2 |
| 9 | 33.3 CH2 | 24.6 CH2 | 26.8 CH2 | 40.0 CH2 | 35.1 CH2 |
| 10 | 135.6 C | 50.1 CH | 51.8 CH | 86.0 C | 136.7 C |
| 11 | 131.6 C | 76.3 C | 71.5 C | 150.7 C | 125.2 C |
| 12 | 21.3 CH3 | 22.9 CH3 | 26.6 CH3 | 19.7 CH3 | 21.0 CH3 |
| 13 | 23.5 CH3 | 69.5 CH2 | 29.2 CH3 | 110.3 CH2 | 23.4 CH3 |
| 14 | 178.0 C | 172.0 C | 175.8 C | 177.9 C | 177.9 C |
| 15 | 20.1 CH3 | 19.2 CH3 | 74.5 CH2 | 76.2 CH2 | 76.5 CH2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, C.-P.; Xie, C.-L.; Xia, J.-M.; Liu, Q.-M.; Lin, W.-X.; Ye, D.-Z.; Liu, G.-M.; Yang, X.-W. Penigrisacids A–D, Four New Sesquiterpenes from the Deep-Sea-Derived Penicillium griseofulvum. Mar. Drugs 2019, 17, 507. https://doi.org/10.3390/md17090507
Xing C-P, Xie C-L, Xia J-M, Liu Q-M, Lin W-X, Ye D-Z, Liu G-M, Yang X-W. Penigrisacids A–D, Four New Sesquiterpenes from the Deep-Sea-Derived Penicillium griseofulvum. Marine Drugs. 2019; 17(9):507. https://doi.org/10.3390/md17090507
Chicago/Turabian StyleXing, Cui-Ping, Chun-Lan Xie, Jin-Mei Xia, Qing-Mei Liu, Wei-Xiang Lin, De-Zan Ye, Guang-Ming Liu, and Xian-Wen Yang. 2019. "Penigrisacids A–D, Four New Sesquiterpenes from the Deep-Sea-Derived Penicillium griseofulvum" Marine Drugs 17, no. 9: 507. https://doi.org/10.3390/md17090507
APA StyleXing, C.-P., Xie, C.-L., Xia, J.-M., Liu, Q.-M., Lin, W.-X., Ye, D.-Z., Liu, G.-M., & Yang, X.-W. (2019). Penigrisacids A–D, Four New Sesquiterpenes from the Deep-Sea-Derived Penicillium griseofulvum. Marine Drugs, 17(9), 507. https://doi.org/10.3390/md17090507








