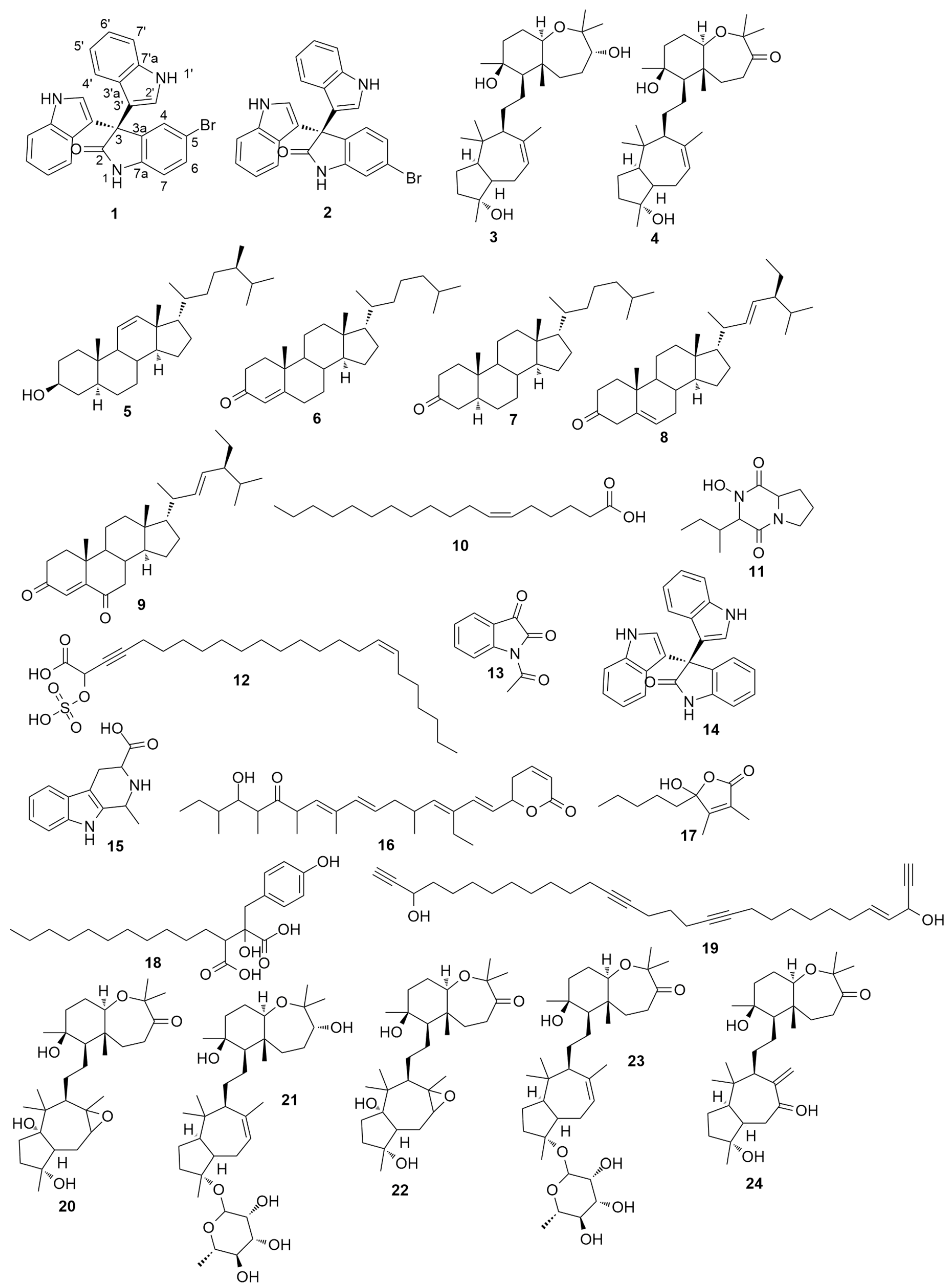
2.1. Metabolomic Profiling
To date, a growing number of secondary metabolites were identified from
Callyspongia siphonella with diverse pharmacological effects [
13]. Sipholane triterpenoids are considered the main characteristic metabolites of this sponge. This class of metabolites was found to show a potent reversal of multidrug resistance in tumor cells that overexpressed P-glycoprotein (P-gp) [
14]. In addition, there are many polyactylenes, polyketides, and alkaloids that were also reported from
C. siphonella. In view of that, metabolomic profiling of the marine sponge
C. siphonella using LC-HRESIMS for dereplication purpose resulted in the characterization of a variety of metabolites (
Figure 1 and
Figure S1) of which sipholane triterpenes were found to be prevalent (
Table 1). From the AntiMarine and Dictionary of Natural Products (DNP) databases, a total of 24 compounds were tentatively identified. Seven compounds out of these were members of the P-glycoprotein inhibitors, sipholane triterpenoids (
3,
4,
20–24). Additionally, the antiproliferative metabolites, callystatin A (
16), hydroxydihydrobovolide (
17), callyspongidic acid (
18), and callyspongendiol (
19), were also dereplicated in the
n-hexane fraction [
15]. All sipholane triterpenes are presumably derived from the cyclization of triepoxysqualenes. Detection of several mass ion peaks proposed to be the biosynthetic intermediates of sipholane triterpenes supported the previously suggested biosynthetic pathway (
Scheme S1) [
6]. Similarly, compounds
11,
12,
13,
14, and
15 were detected in the ethyl acetate fraction. Moreover, intermediate compounds suggesting that they were involved in the biosynthesis of trisindoline (
14) and its brominated derivatives (compounds
1 and
2) from tryptophan [
16,
17] were also found (
Scheme S2). Tisindoline (
14) is an antibiotic indole trimer previously isolated from marine
Vibrio and
Shewanella species [
18,
19]. In addition to the identified hits (
Figure 1), other unidentified molecular formulae were detected, particularly in the ethyl acetate fraction, suggesting the presence of further unknown metabolites. These findings, together with the primary antibacterial screening, prioritized the ethyl acetate fraction for a further chemical investigation to isolate the active metabolites.
2.2. Structure Characterization of the Isolated Compounds
The ethanolic extract of C. siphonella was partitioned with different organic solvents with increasing polarity. The n-hexane and ethyl acetate fractions displayed weak to moderate antibacterial activities. Further bioactivity-guided fractionation of the ethyl acetate fraction, which showed several new hits upon LC-HRESIMS dereplication, led to the isolation of compounds 1 and 2.
Compound
1 was isolated as buff powder; the molecular formula C
24H
16ON
3Br was suggested on the basis of a positive HRESIMS ion at
m/z 442.0554 [M + H]
+, indicating 18 degrees of unsaturation.
1H-NMR spectral data of
1 (
Table 2) in deuterated dimethyl sulfoxide (DMSO-
d6) suggested the presence of two exchangeable protons (
δH 10.77, 11.03) due to NH. In addition, the splitting pattern of resonances at
δH 6.97 (H-7), 7.42 (H-6), and 7.30 (H-4), together with the
1H–
1H COSY correlation between (H-6) and (H-7), suggested the presence of a 1,3,4-trisubstituted benzene ring. The DEPTQ spectrum (
Table 2) displayed 16 signals, with 15
sp2 aromatic carbons including eight CH groups and seven quaternary carbons, and one
sp3 aliphatic quaternary carbon (C-3). The spectra also revealed an aminocarbonyl at
δC178.9 (C-2). The assignment of protonated carbons was achieved by the
1H–
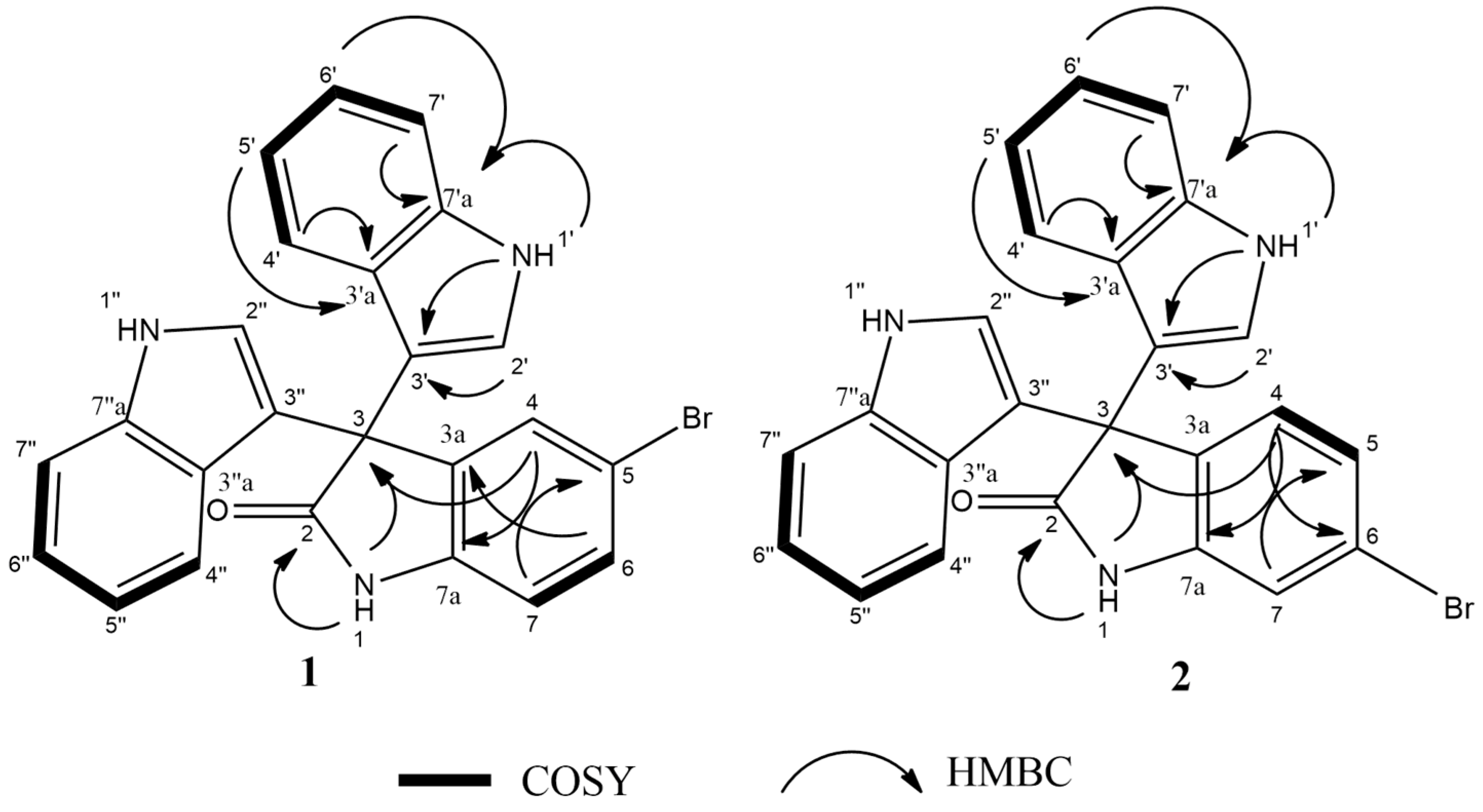
13C HSQC data. The key HMBC correlations (
Figure 2) from H-4 (
δH 7.30, s, 1H) to C-3 (
δC 53.2), and from the exchangeable proton H-1(
δH 10.77) to the carbonyl carbons C-2 (
δC 178.9) and C-3a (
δC 137.44) together with the previous reported data [
18], suggested the presence of a brominated indolin-2-one skeleton at C-5 (
δC 114). The presence of two identical indole moieties linked to brominated oxindole moity at C-3 (
δC 53.2) was figured out from the
1H-NMR spectral data (
Table 2) and the
1H–
13C HMBC correlations (
Figure 2). According to these data, HRESIMS analysis, and previously published data [
20], compound
1 was identified as 5-bromo trisindoline.
Compound
2 was isolated as a white amorphous powder. The molecular formula C
24H
16ON
3Br was suggested on the basis of the positive HRESIMS ion at
m/z 442.0554 [M + H]
+, indicating 18 degrees of unsaturation. HRESIMS and
1H and
13C-NMR spectra of
2 (
Table 2) were almost identical to that of compound
1. In addition, the similar two-dimensional (2D)-NMR correlations indicate that compounds
1 and
2 are positional isomers, where the bromine is attached to C-6 instead of C-5. This different attachment was confirmed from the
1H–
13C HMBC correlation between H-4 (
δH 7.16, d, 8Hz, 1H) and C-2 (
δC 179); hence, compound
2 was identified as 6-bromo trisindoline.
Both 5-bromotrisindoline (
1) and 6-bromotrisindoline (
2) are brominated indole trimers, previously reported as synthetic intermediates [
20,
21] and isolated herein for the first time from a natural source. Their parent non-halogenated trisindoline (
14) was previously isolated from a marine bacterium and exhibited significant antibacterial activity [
18]. Only 5-bromotrisindoline (
1) was reported as an α-glycosidase enzyme inhibitor [
20].
1H-NMR and
13C-NMR assignments of 5-bromotrisindoline (
1) and 6-bromotrisindoline (
2) are reported here for the first time based on extensive HSQC, COSY, and HMBC analysis (
Figures S3–S21,
Supplementary Materials).
Further known metabolites, sipholenol A (
3) and sipholenone A (
4) [
22], along with five steroidal compounds, callysterol (
5) [
9], cholestenone (
6), 5α-cholestanone (
7) [
23], stigmasterone (
8) [
24], and stigmasta-4,22-dien-3,6-dione (
9) [
25], and one major fatty acid, petroselenic acid (
10) [
26,
27], were also isolated from the
n-hexane fraction of
C. siphonella (
Figure 1). All isolated known metabolites were identified based on their accurate mass analyses and comparison of their NMR spectroscopic data with those reported in the literature (
Figures S22–S47,
Supplementary Materials). The triterpenes sipholenol A (
3) and sipholenone A (
4) are major metabolites in
C. siphonella. Sipholenol A (
3) was previously reported as a potent P-glycoprotein inhibitor in multidrug-resistant cancer cells [
14]. Callysterol (
5) is a characteristic sterol in
C. siphonella which exhibited in vitro anti-inflammatory activity [
9]. The steroidal compounds (
6–9), along with the fatty acid petroselenic acid (
10), were isolated here from
C. siphonella for the first time.
2.3. Antibacterial Activity
For primary antibacterial screening, the crude ethanolic extracts, all fractions, and the isolated compounds were evaluated against
Staphylococcus aureus (ATCC 25923),
Bacillus subtilis (ATCC 5230),
Escherichia coli (ATCC 25922), and
Pseudomonas aeruginosa (ATCC 9027). The antibacterial activity was recorded as an inhibition zone diameter and measured as “mm” (
Table 3). Ampicillin and gentamicin were used as a positive control. The ethyl acetate fraction showed the highest inhibition against
Staphylococcus aureus (6.6 mm),
Bacillus subtilis (5.4 mm), and
Escherichia coli (1.5 mm), while the
n-hexane fraction exhibited mild antibacterial activity with inhibition zones ranging from 1 to 2 mm. These findings are in great accordance with the previously reported antibacterial activity of
C. siphonella extracts [
28]. Compounds
1 and
2 derived from the ethyl acetate fraction displayed potent antibacterial activity against Gram-positive bacteria
S. aureus and
B. subtilis with inhibition zones of 17.5 mm and 18 mm, and 15 mm and 16.4 mm, respectively (
Table 4). With respect to Gram-negative bacteria
E. coli and
P. aeruginosa,
1 and
2 showed weak antibacterial activity with inhibition zones of 0.5 mm and 1.3 mm, and 0.2 mm and 1.1 mm, respectively. The other isolated compounds (
3–10) did not exhibit any inhibition against the tested bacterial strains. The minimum inhibitory concentration (MIC) values of
1 and
2 that showed antibiotic activity in disc diffusion tests were determined (
Table 5). It was observed that the MIC value of
2 against
S. aureus (16 µg/mL) was higher than that of
1 (8 µg/mL). However, both compounds exhibited potent inhibitory effect toward
B. subtilis with an MIC value of 4 µg/mL. In a previous study [
18], their parent compound, trisindoline (
14), showed antibacterial activity against both Gram-positive and Gram-negative bacterial strains; hence, incorporation of a bromine atom into the oxindole moiety of trisindoline (
14) reduces the antibacterial spectrum of these molecules. From the previous observations, we can conclude that the bromotrisindolines (
1,2) may be the main metabolites responsible for the antibacterial activity of
C. siphonella extract against Gram-positive bacteria.
2.4. Antibiofilm Activity
As demonstrated from the antibacterial screening, metabolites
1 and
2 did not exhibit any inhibition against the Gram-negative bacteria,
P. aeruginosa PAO1. However, compounds that have no direct growth inhibitory effect on the pathogenic organisms still have the potential to act as biofilm formation inhibitors. As outlined in a recent review [
29], many categories of natural products, including marine invertebrate-derived metabolites can stop the formation of biofilms and, thus, comprise candidates for adjuvant therapy as boosters of the common antibiotics. Therefore, all isolated compounds were tested against
P. aeruginosa to determine if they could inhibit biofilm formation. Compound
1 reduced biofilm formation slightly more (49.32% inhibition) than
2 (41.76%) at concentrations of 128 µg/mL (0.5 MIC) (
Table 6). The remaining compounds were inactive at tested concentrations. To the best of our knowledge, none of the derivatives of trisindoline antibiotic are so far associated with inhibition of biofilm formation in human pathogens. Therefore, further mechanistic studies on these compounds are needed.
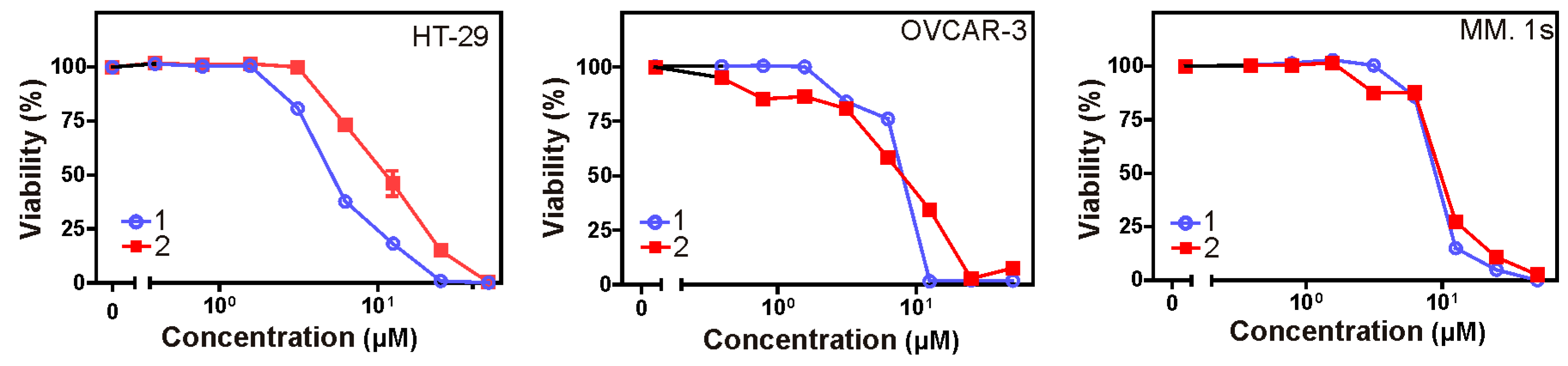
2.6. Cytotoxic Activity
Crystal violet and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were used to assess the cytotoxicity of all isolated metabolites against three different cancer cell lines over a concentration range of 0.01–50 µM. Both
1 and
2 revealed significant cytotoxic effects (
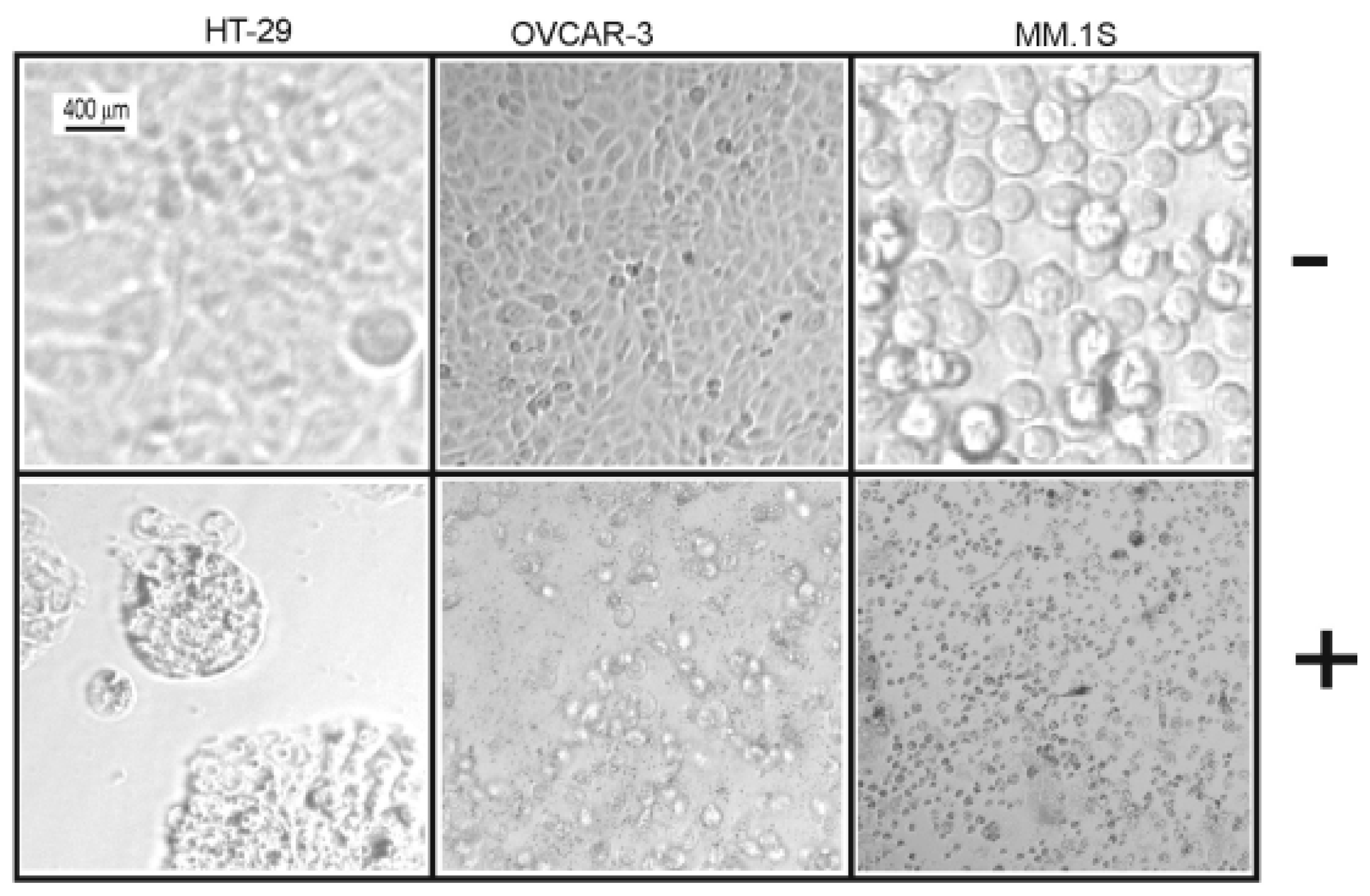
Table 7) against all cell lines under investigation (human colon cancer cell line: HT-29, human ovarian cancer cell line: OVCAR-3, and multiple myeloma cell line: MM.1S). Furthermore, the microscopic pictures showed clear changes in cell morphology after overnight treatment with
1 (
Figure 3 and
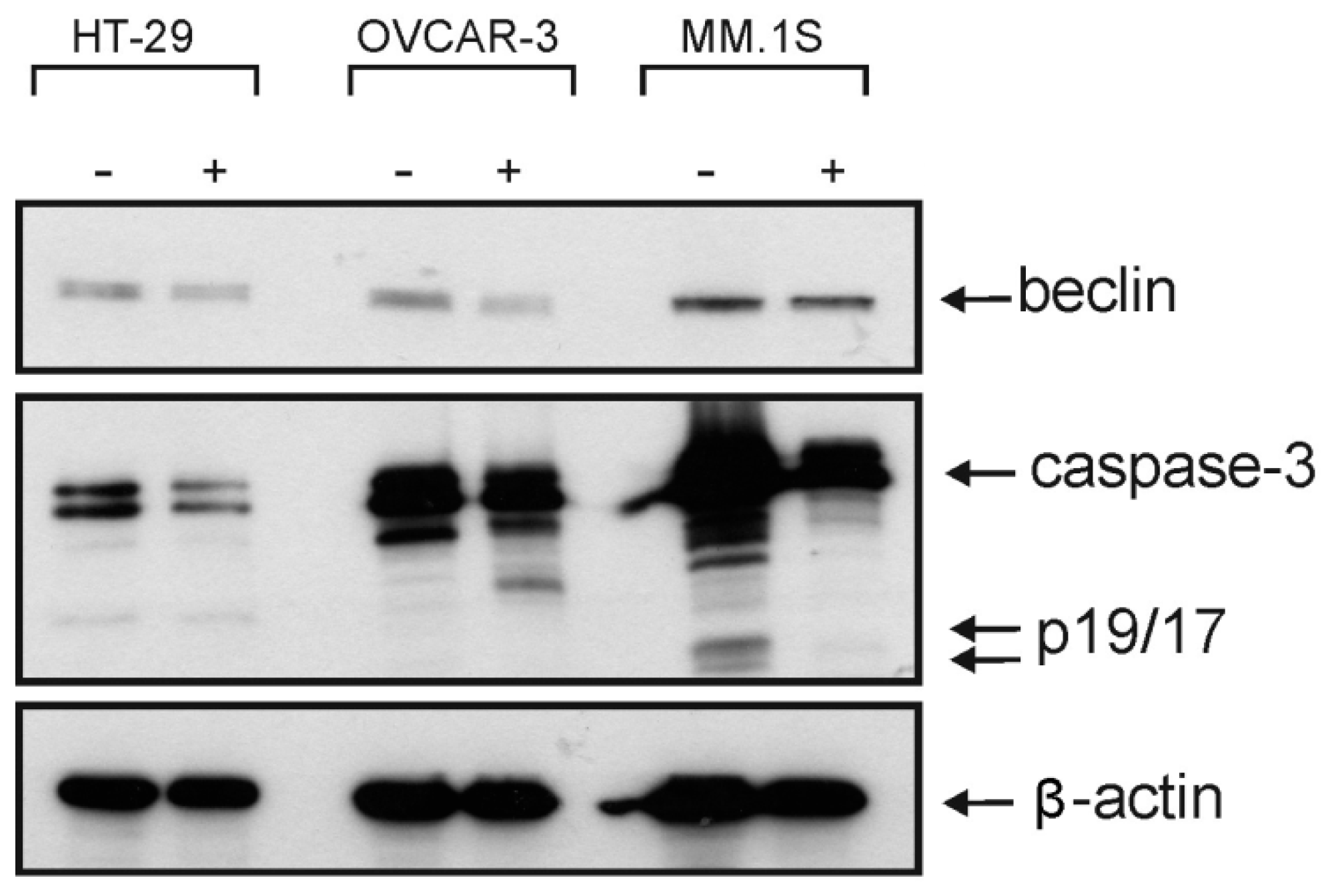
Figure 4). However, the Western blotting analysis showed only a decrease in the level of procaspase-3 but unfortunately, we neither detected any split caspase-3 products, the key protein of apoptosis, nor changes in the level of beclin protein, one of the master proteins of autophagy (
Figure 5). Moreover,
1 was not able to induce the upregulation of proinflammatory cytokines such as interleukin-8 (IL8) in MM.1S and HT-29 (
Figure S2,
Supplementary Materials). Our previous results and many previous publications showed that the programmed cell death is usually accompanied by the induction of proinflammatory signals such as IL8 [
31,
32]. This confirms the Western blotting results that compound
1 kills the cancer cells by non-programmed cell necrosis but not by programmed cell death mechanisms such as necroptosis, apoptosis, or autophagy.