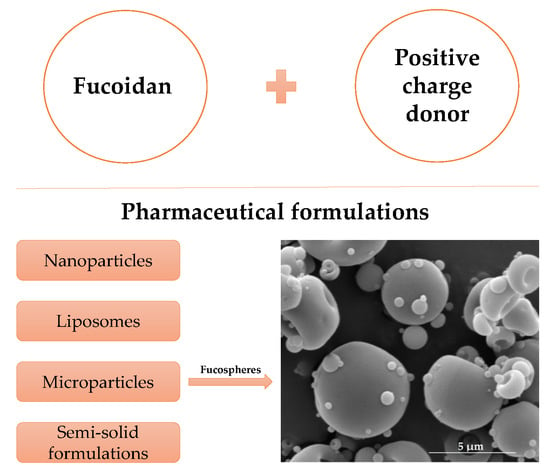
Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms
Abstract
1. Introduction
2. Pharmaceutical Features of Fucoidan
3. Toxicity of Fucoidan
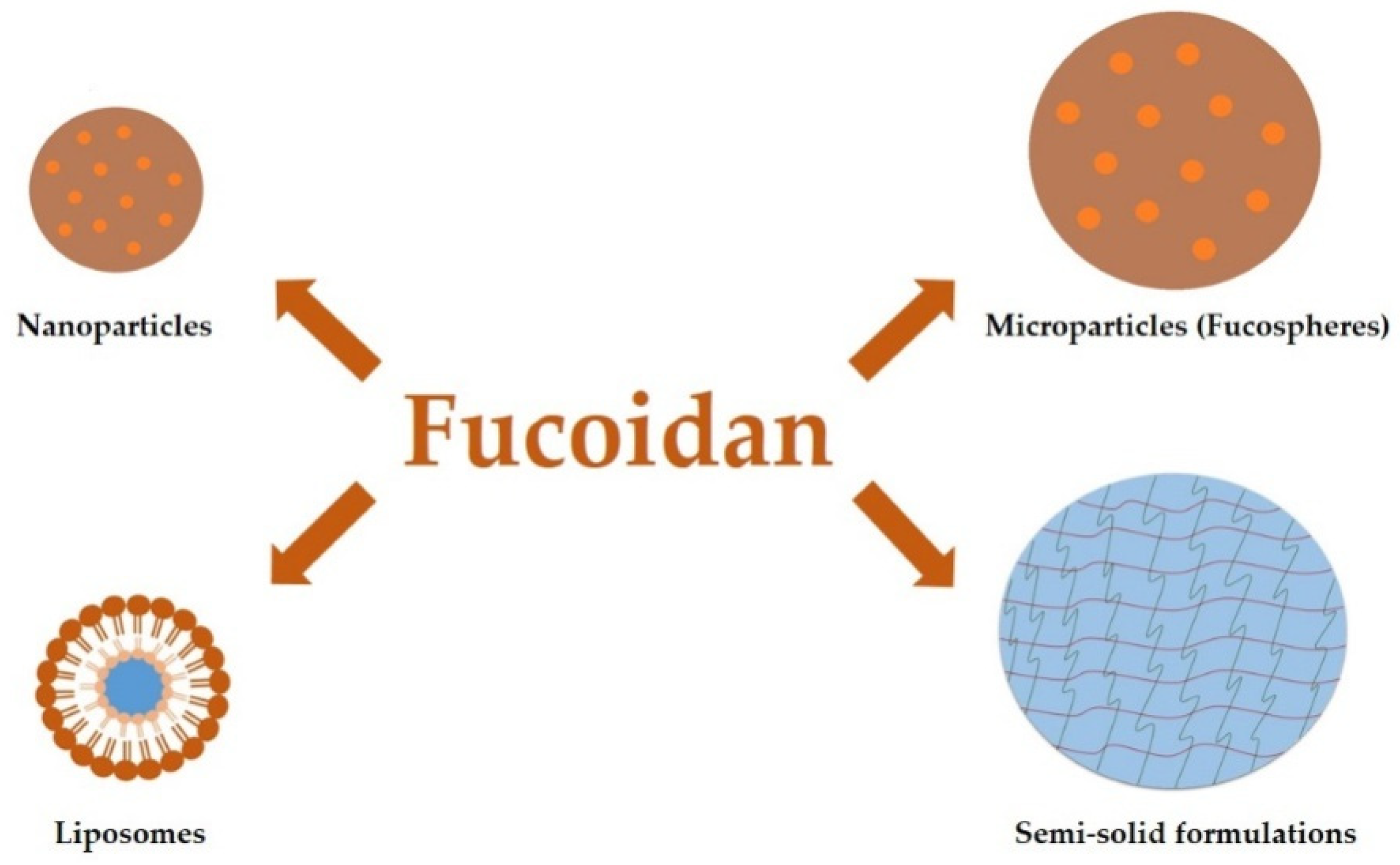
4. Fucoidan Application in the Pharmaceutical Technology
4.1. Nanoparticles
4.2. Liposomes
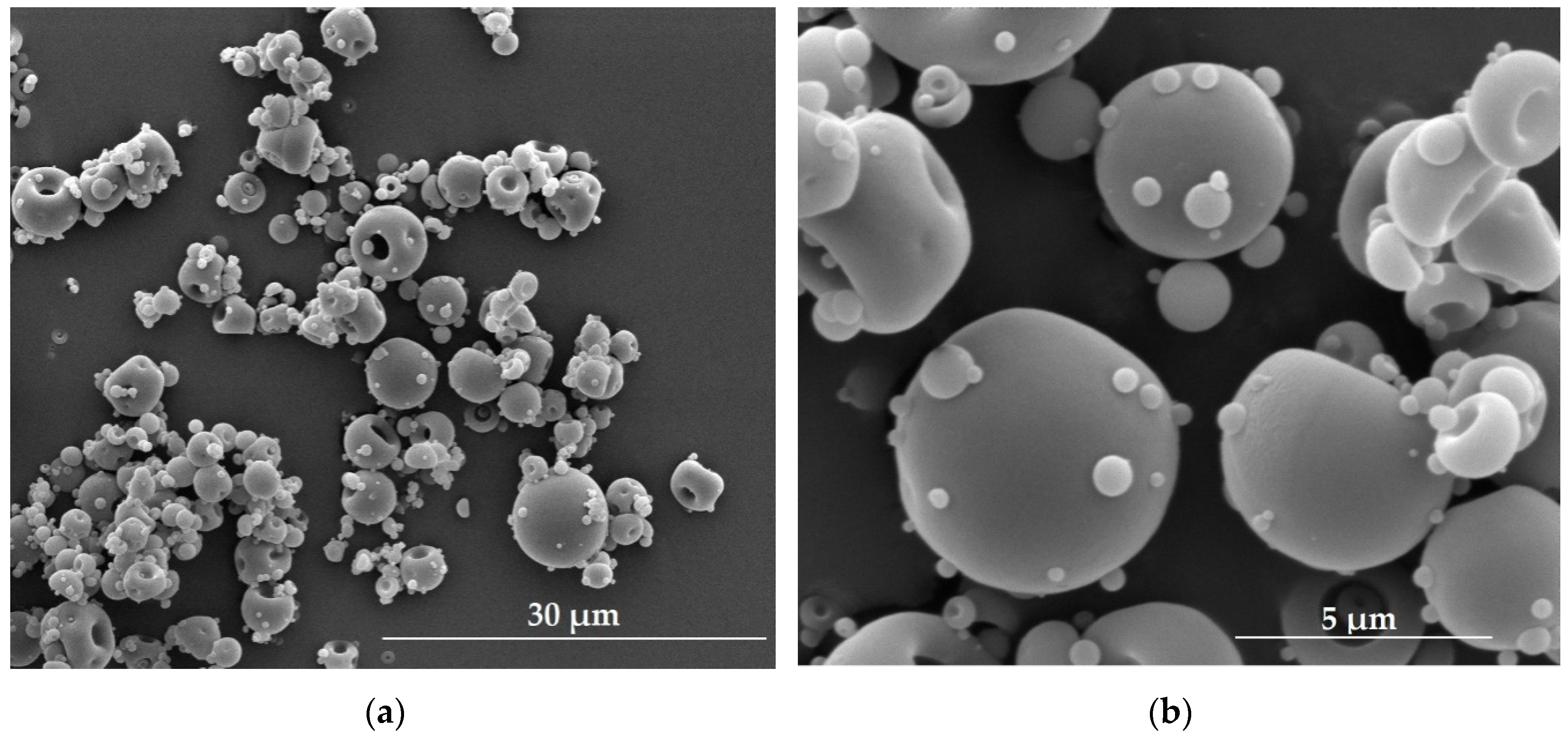
4.3. Microparticles
4.4. Semi-Solid Formulations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kylin, H. Zur biochemie der meeresalgen. Z. Physiol. Chem. 1913, 83, 171–197. [Google Scholar] [CrossRef]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.N.; Letourneur, D.; Chaubet, F. Fucoidans in nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine origin polysaccharides in drug delivery systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xu, J.; Xu, X. Bioactivity of fucoidan extracted from Laminaria Japonica using a novel procedure with high yield. Food Chem. 2018, 245, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 199–223. [Google Scholar] [CrossRef]
- Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M.; Gomaa, M. Upgrading the antioxidant properties of fucoidan and alginate from Cystoseira trinodis by fungal fermentation or enzymatic pretreatment of the seaweed biomass. Food Chem. 2018, 269, 387–395. [Google Scholar] [CrossRef]
- Mansour, M.B.; Balti, R.; Yacoubi, L.; Ollivier, V.; Chaubet, F.; Maaroufi, R.M. Primary structure and anticoagulant activity of fucoidan from the sea cucumber Holothuria polii. Int. J. Biol. Macromol. 2019, 121, 1145–1153. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, M.R.; Choi, S.M.; Na, S.S.; Cha, J.D. Synergistic effect of fucoidan with antibiotics against oral pathogenic bacteria. Arch. Oral Biol. 2013, 58, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Ali, A.; Ahmed, M.; Zia, M.; Haq, I.; Kim, S.J. In vitro antileishmanial, antibacterial, antifungal and anticancer activity of fucoidan from Undaria pinnatifida. Int. J. Biosci. 2017, 11, 219–227. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, J.Y. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Lee, J.S.; Kim, W.S.; Jeon, Y.J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Hwang, P.A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Tocaciu, S.; Oliver, L.J.; Lowenthal, R.M.; Peterson, G.M.; Patel, R.; Shastri, M.; McGuinness, G.; Olesen, I.; Fitton, J.H. The effect of Undaria pinnatifida fucoidan on the pharmacokinetics of letrozole and tamoxifen in patients with breast cancer. Integr. Cancer. Ther. 2018, 17, 99–105. [Google Scholar] [CrossRef]
- Clinical Trials of Oligo Fucoidan. Available online: https://clinicaltrials.gov/ct2/show/NCT03130829 (accessed on 24 July 2019).
- Tsai, H.L.; Tai, C.J.; Huang, C.W.; Chang, F.R.; Wang, J.Y. Efficacy of low-molecular-weight fucoidan as a supplemental therapy in metastatic colorectal cancer patients: A double-blind randomized controlled trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef]
- Clinical Trials of Fucoidan. Available online: https://clinicaltrials.gov/ct2/show/record/NCT02875392?view=record (accessed on 24 July 2019).
- Jae-Geun, K.; Kil-Suk, J.; Jin-Hee, P.; Koo, J.G.; Jo, K.S.; Park, J.H. Rheological properties of fucoidans from Laminaria religiosa, Sporophylls of Undaria pinnatifida, Hizikia fusiforme and Sagassum fulvellum in Korea. Korean J. Fish. Aquat. Sci. 1997, 30, 329–333. [Google Scholar]
- Tako, M. Rheological characteristics of fucoidan isolated from commercially cultured Cladosiphon okamuranus. Bot. Mar. 2003, 46, 461–465. [Google Scholar] [CrossRef]
- MyoungLae, C.; Won-Seok, C.; Sangguan, Y.; Cho, M.L.; Choi, W.S.; You, S.G. Steady and dynamic shear rheology of fucoidan-buckwheat starch mixtures. Starch J. 2009, 61, 282–290. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E. Fucoidan: A versatile biopolymer for biomedical applications. In Active Implants and Scaffolds for Tissue Regeneration; Zilberman, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 8, pp. 377–406. [Google Scholar]
- Rioux, L.; Turgeon, S.L.; Beaulieu, M. Rheological characterisation of polysaccharides extracted from brown seaweeds. J. Sci. Food Agric. 2007, 87, 1630–1638. [Google Scholar] [CrossRef]
- Venugopal, V. Polysaccharide from seaweed and microalgae. In Marine Polysaccharides: Food Applications; Zollo, S., Ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2011; pp. 111–122. [Google Scholar]
- Re: GRAS Notice, No. GRN 000661. Available online: https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm549588.pdf (accessed on 4 July 2019).
- Final Assessment Report on Fucus vesiculosus, L., thallus. Available online: https://www.ema.europa.eu/documents/herbal-report/final-assessment-report-fucus-vesiculosus-l-thallus_en.pdf (accessed on 22 July 2019).
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol. 2005, 43, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Yan, M.D.; Lin, H.T.V.; Li, K.L.; Lin, Y.C. Toxicological evaluation of low molecular weight fucoidan in vitro and in vivo. Mar. Drugs 2016, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Gideon, T.P.; Rengasamy, R. Toxicological evaluation of fucoidan from Cladosiphon okamuranus. J. Med. Food 2008, 11, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Jeun, J.; Houng, S.J.; Jun, H.J.; Kweon, D.K.; Lee, S.J. Toxicological evaluation of fucoidan from Undaria pinnatifida in vitroand in vivo. Phytother. Res. 2010, 24, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Hiramatsu, K.; Ichikawa, O.; Kawamoto, H.; Kasagi, T.; Miki, Y.; Kimura, T.; Ikeda, T. Safety evaluation of excessive ingestion of mozuku fucoidan in human. J. Food Sci. 2013, 78, T648–T651. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.P.; O’Connor, J.; Fitton, J.H.; Brooks, L.; Rolfe, M.; Connellan, P.; Wohlmuth, H.; Cheras, P.A.; Morris, C. A combined phase I and II open label study on the effects of seaweed extract nutrient complex on osteoarthritis. Biologics 2010, 4, 33–44. [Google Scholar] [CrossRef]
- Myers, S.P.; Mulder, A.M.; Baker, D.G.; Robinson, S.R.; Rolfe, M.I.; Brooks, L.; Fitton, J.H. Effects of fucoidan from Fucus vesiculosus in reducing symptoms of osteoarthritis: A randomized placebo-controlled trail. Biologics 2016, 10, 81–88. [Google Scholar] [CrossRef]
- Prasad, S.; Lillicrap, D.; Labelle, A.; Knappe, S.; Keller, T.; Burnett, E.; Powell, S.; Johnson, K.W. Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood 2008, 111, 672–679. [Google Scholar] [CrossRef]
- Morello, S.; Southwood, L.L.; Slack, J.; Crack, A.; Springate, C.M.K. Safety of intraperitoneal fucoidan solution in healthy adult horses undergoing exploratory celiotomy and jejunojejunostomy: Clinical findings. In Proceeding of the American College of Veterinary Surgeons Symposium, Washington, DC, USA, 6–10 October 2009. [Google Scholar]
- Kim, K.J.; Lee, O.H.; Lee, B.Y. Genotoxicity studies on fucoidan from Sporophyll of Undaria pinnatifida. Food Chem. Toxicol. 2010, 48, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Ku, S.K.; Han, J.S. Genotoxicity testing of low molecular weight fucoidan from brown seaweeds. Food Chem. Toxicol. 2012, 50, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Elbi, S.; Nimal, T.R.; Rajan, V.K.; Baranwal, G.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Fucoidan coated ciprofloxacin loaded chitosan nanoparticles for the treatment of intracellular and biofilm infections of Salmonella. Colloids Surf. B Biointerfaces 2017, 160, 40–47. [Google Scholar] [CrossRef]
- Huang, Y.C.; Li, R.Y. Preparation and characterization of antioxidant nanoparticles composed of chitosan and fucoidan for antibiotics delivery. Mar. Drugs 2014, 12, 4379–4398. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Nguyen, V.P.; Manivasagan, P.; Jung, M.J.; Kim, S.W.; Oh, J.; Kang, H.W. Doxorubicin-fucoidan-gold nanoparticles composite for dual-chemo-photothermal treatment on eye tumors. Oncotarget 2017, 8, 113719–113733. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Rosa da Costa, A.M.; Lourenço, J.P.; Buttini, F.; Grenha, A. Spray-dried fucoidan microparticles for pulmonary delivery of antitubelcular drugs. J. Microencapsul. 2018, 35, 392–405. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yang, Y.T. Effect of basic fibroblast growth factor released from chitosan–fucoidan nanoparticles on neurite extension. J. Tissue Eng. Regen. Med. 2016, 10, 418–427. [Google Scholar] [CrossRef]
- Sezer, A.D.; Akbuğa, J. Fucosphere – new microsphere carriers for peptide and protein delivery: Preparation and in vitro characterization. J. Microencapsul. 2006, 23, 513–522. [Google Scholar] [CrossRef]
- Park, H.W.; Kim, D.Y.; Shin, W.S. Fucoidan improves the structural integrity and the molecular stability of β-lactoglobulin. Food Sci. Biotechnol. 2018, 27, 1247–1255. [Google Scholar] [CrossRef]
- Sezer, A.D.; Akbuğa, J. Comparison on in vitro characterization of fucospheres and chitosan microspheres encapsulated plasmid DNA (pGM-CSF): Formulation design and release characteristics. AAPS Pharm. Sci. Tech. 2009, 10, 1193–1199. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E.; Hatipoğlu, F.; Oğurtan, Z.; Baş, A.L.; Akbuğa, J. The use of fucosphere in the treatment of dermal burns in rabbits. Eur. J. Pharm. Biopharm. 2008, 69, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Varga, M. Self-assembly of nanobiomaterials. In Fabrication and Self-Assembly of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew: Norwich, NY, USA, 2016; Volume 1, pp. 57–90. [Google Scholar]
- Huang, Y.C.; Li, R.Y.; Chen, J.Y.; Chen, J.K. Biphasic release of gentamycin from chitosan/fucoidan nanoparticles for pulmonary delivery. Carbohydr. Polym. 2016, 138, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Jeong, D.; Na, K. Doxorubicin loading fucoidan acetate nanoparticles for immune and chemotherapy in cancer treatment. Carbohydr. Polym. 2013, 94, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.Y.; Li, R.; Hsu, C.H.; Lin, C.W.; Chou, S.C.; Tsai, M.L.; Mi, F.L. Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohydr. Polym. 2017, 165, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.K.; Singh, Y.; Sharma, K.; Shrivastav, A.; Sharma, A.; Singh, A.; Meher, J.G.; Singh, P.; Raval, K.; Kumar, A.; et al. Improved chemotherapy against breast cancer through immunotherapeutic activity of fucoidan decorated electrostatically assembled nanoparticles bearing doxorubicin. Int. J. Biol. Macromol. 2019, 122, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Moorthy, M.S.; Manivasagan, P.; Xu, L.; Song, K.; Lee, K.D.; Kwak, M.; Oh, J.; Jin, J.O. Fucoidan-coated CuS nanoparticles for chemo- and photothermal therapy against cancer. Oncotarget 2018, 9, 12649–12661. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Kankala, R.K.; Chen, B.; Long, R.; Cai, D.; Liu, Y.; Wang, S. Polyy-allylamine hydrochloride and fucoidan-cased self-assembled polyelectrolyte complex nanoparticles for cancer therapeutics. J. Biomed. Mater. Res. A 2019, 107, 339–347. [Google Scholar] [CrossRef]
- da Silva, L.C.; Garcia, T.; Mori, M.; Sandri, G.; Bonferoni, M.C.; Finotelli, P.V.; Cinelli, L.P.; Caramella, C.; Cabral, L.M. Preparation and characterization of polysaccharide-based nanoparticles with anticoagulant activity. Int. J. Nanomed. 2012, 7, 2975–2986. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Costa Lima, S.S.; Reis, S. Development of methotrexate loaded fucoidan/chitosan nanoparticles with anti-inflammatory potential and enhanced skin permeation. Int. J. Biol. Macromol. 2019, 124, 1115–1122. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lam, U.I. Chitosan/fucoidan pH sensitive nanoparticles for oral delivery system. J. Chin. Chem. Soc. 2011, 58, 779–785. [Google Scholar] [CrossRef]
- Huang, Y.C.; Kuo, T.H. O-carboxylmethyl chitosan/fucoidan nanoparticles increase cellular curcumin uptake. Food Hydrocoll. 2016, 53, 261–269. [Google Scholar] [CrossRef]
- Chen, C.H.; Lin, Y.S.; Wu, S.J.; Mi, F.L. Multifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym. 2018, 193, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Singh, S.K.; Anil, S.; Kim, S.K.; Shim, M.S. Preparation, characterization and biological applications of biosynthesized silver nanopartciles with chitosan-fucoidan coating. Molecules 2018, 23, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Maricato, É.; Nunes, C.; Coimbra, M.A.; Vicente, A.A. Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr. Polym. 2015, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.C.; Chen, C.H.; Lin, C.W.; Ho, Y.C.; Mi, F.L. Development of multifunctional nanoparticles self-assembled from trimethyl chitosan and fucoidan for enhanced oral delivery of insulin. Int. J. Biol. Macromol. 2019, 126, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Juenet, M.; Aid-Launais, R.; Li, B.; Berger, A.; Aerts, J.; Ollivier, V.; Nicoletti, A.; Letourneur, D.; Chauvierre, C. Thrombolytic therapy based on fucoidan-functionalized polymer nanoparticles targeting P-selectin. Biomaterials 2018, 156, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Lira, M.C.; Santos-Magalhães, N.S.; Nicolas, V.; Marsaud, V.; Silva, M.P.; Ponchel, G.; Vauthier, C. Cytotoxicity and cellular uptake of newly synthesized fucoidan-coated nanoparticles. Eur. J. Pharm. Biopharm. 2011, 79, 162–170. [Google Scholar] [CrossRef]
- Cai, D.; Fan, J.; Wang, S.; Long, R.; Zhou, X.; Liu, Y. Primary biocompatibility tests of poly(lactide-co-glycolide)-(poly-L-orithine/fucoidan) core–shell nanocarriers. R. Soc. Open. Sci. 2018, 5, 180320. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Lin, X.Z.; Kuo, K.L.; Hsu, F.Y. Fabrication and cytotoxicity of fucoidan-cisplatin nanoparticles for macrophage and tumor cells. Materials (Basel) 2017, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Santos, N.; Almeida-Lima, J.; Vidal, A.A.; Gomes, D.L.; Oliveira, R.M.; Santos Pedrosa, S.; Pereira, P.; Gama, F.M.; Oliveira Rocha, H.A. Antiproliferative activity of fucan nanogel. Mar. Drugs 2012, 10, 2002–2022. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Rokkaku, T.; Takeda, S.; Senba, M.; Mori, N. Cytotoxic effects of fucoidan nanoparticles against osteosarcoma. Mar. Drugs 2013, 11, 4267–4278. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Juenet, M.; Aid-Launais, R.; Maire, M.; Ollivier, V.; Letourneur, D.; Chauvierre, C. Development of polymer microcapsules functionalized with fucoidan to target P-selectin overexpressed in cardiovascular diseases. Adv. Health Mater. 2017, 6, 1601200. [Google Scholar] [CrossRef]
- Wang, P.; Kankala, R.K.; Fan, J.; Long, R.; Liu, Y.; Wang, S. Poly-L-ornithine/fucoidan-coated calcium carbonate microparticles by layer-by-layer self-assembly technique for cancer theranostics. J. Mater Sci. Mater Med. 2018, 29, 68. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Akbuğa, J. The design of biodegradable ofloxacin-based core-shell microspheres: Influence of the formulation parameters on in vitro characterization. Pharm. Dev. Technol. 2012, 17, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Rodrigues, S.; Rosa da Costa, A.M.; Faleiro, M.L.; Buttini, F.; Grenha, A. Inhalable fucoidan microparticles combining two antitubercular drugs with potential application in pulmonary tuberculosis therapy. Polymers 2018, 10, 636. [Google Scholar] [CrossRef]
- Dolores, B.M.; Maria, O.R.; Maria, T.J. Hydrogels. In Encyclopedia of Pharmaceutical Technology, 3rd ed.; Swarbrick, J., Ed.; Informa Healthcare USA: New York, NY, USA, 2007; Volume 3, pp. 2021–2039. [Google Scholar]
- Sezer, A.D.; Cevher, E.; Hatipoğlu, F.; Oğurtan, Z.; Baş, A.L.; Akbuğa, J. Preparation of fucoidan-chitosan hydrogel and its application as burn healing accelerator on rabbits. Biol. Pharm. Bull. 2008, 31, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef]
- Murakami, K.; Ishihara, M.; Aoki, H.; Nakamura, S.; Nakamura, S.; Yanagibayashi, S.; Takikawa, M.; Kishimoto, S.; Yokoe, H.; Kiyosawa, T.; et al. Enhanced healing of mitomycin C-treated healing-impaired wounds in rats with hydrosheets composed of chitin/chitosan, fucoidan, and alginate as wound dressings. Wound Repair Regen. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Nakamura, S.; Nambu, M.; Ishizuka, T.; Hattori, H.; Kanatani, Y.; Takase, B.; Kishimoto, S.; Amano, Y.; Aoki, H.; Kiyosawa, T.; et al. Effect of controlled release of fibroblast growth factor-2 from chitosan/fucoidan micro complex-hydrogel on in vitro and in vivo vascularization. J. Biomed. Mater Res. A 2008, 85, 619–627. [Google Scholar] [CrossRef]
- Purnama, A.; Aid-Launais, R.; Haddad, O.; Maire, M.; Mantovani, D.; Letourneur, D.; Hlawaty, H.; Le Visage, C. Fucoidan in a 3D scaffold interacts with vascular endothelial growth factor and promotes neovascularization in mice. Drug Deliv. Transl. Res. 2015, 5, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, J.K.; Cho, T.S. Applications of ophthalmic biomaterials embedded with fucoidan. Ind. Eng. Chem. 2012, 18, 1197–1201. [Google Scholar] [CrossRef]
- Zayed, A.; Ulber, R. Fucoidan production: Approval key challenges and opportunities. Carbohydr. Polym. 2019, 211, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Kim, D.; Nah, J.W.; Jeon, Y.J. Advances in functionalizing fucoidans and alginates (bio)polymers by structural modifications: A review. Chem. Eng. J. 2019, 355, 33–48. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Potential of intensification techniques for the extraction and depolymerization of fucoidan. Algal Res. 2018, 30, 128–148. [Google Scholar] [CrossRef]

| Concentration of Fucoidan [% w/w] | pH Value | Viscosity1 | Color |
|---|---|---|---|
| 1 | 5.23 | - | yellowish |
| 2 | 5.05 | 3.5 | slightly yellow |
| 3 | 4.96 | 4.2 | yellow |
| 5 | 4.72 | 7.2 | dark yellow |
| 10 | 4.61 | 18.8 | brownish |
| 20 | 4.47 | 100.8 | brown |
| 30 | 4.45 | 507.0 | dark brown |
| Fucoidan (Source/Modification/Molecular Weight) | Copolymer/Positive Charge Donor | Drug | Method of Obtaining | Application | Route of Administration | Ref. |
|---|---|---|---|---|---|---|
| Acetylated fucoidan (Fucus vesiculosus) | - | Doxorubicin | Self-assembly and dialysis | Anticancer therapy and immunotherapy | NA1 | 51 |
| Fucoidan (Laminaria japonica, 80 kDa) | Protamine | Doxorubicin | Self-assembly | Anticancer therapy | Intravenous | 52 |
| Fucoidan (Fucus vesiculosus) | Polyethyleneimine | Doxorubicin | Polyelectrolyte complexation method | Anticancer therapy | Intravenous | 53 |
| Fucoidan (Fucus vesiculosus) | Gold nanoparticles | Doxorubicin | Electrostatic physisorption | Anticancer therapy | Ocular | 42 |
| Fucoidan (Fucus vesiculosus) | Polyallylamine hydrochloride | Copper sulfide | Layer-by-layer | Anticancer therapy | Intratumoral | 54 |
| Fucoidan (200–400 kDa) | Polyallyamine hydrochloride | Methotrexate | Self-assembly | Anticancer therapy | NA1 | 55 |
| Fucoidan (Fucus vesiculosus) | Chitosan | - | Coacervation | Thrombolytic therapy | Oral | 56 |
| Fucoidan (Fucus vesiculosus, 50–190 kDa) | Chitosan | Methotrexate | Self-assembly | Skin inflammation | Topical (ear skin) | 57 |
| Fucoidan | Chitosan | Curcumin | Self-assembly | Anticancer therapy | Oral | 58 |
| Fucoidan (Fucus vesiculosus) | O-carboxymethyl chitosan | Curcumin | Ionotropic crosslinking | Penetration enhancer | Oral | 59 |
| Thiolated fucoidan (THL-fucoidan) | Arginine-modified chitosan | Dextran/rhodamine/curcumin | Self-assembly | NA1 | Oral | 60 |
| Fucoidan (Fucus vesiculosus) | Chitosan | Gentamicin | Self-assembly | Pulmonary diseases | Pulmonary | 41 |
| Fucoidan (Fucus vesiculosus) | Chitosan | Gentamicin | Ionotropic crosslinking | Pulmonary diseases | Pulmonary | 50 |
| Fucoidan (Fucus vesiculosus) | Chitosan | Silver nitrate | Self-assembly | Antibacterial and anticancer therapy | NA1 | 61 |
| Fucoidan (20–200 kDa) | TPP crosslinked chitosan | Ciprofloxacin | Self-assembly | Infections of Salmonella | NA1 | 40 |
| Fucoidan (Fucus vesiculosus, 57.26 kDa) | Chitosan | Poly-l-lysine | Layer-by-layer | Antibacterial therapy | NA1 | 62 |
| Fucoidan (Fucus vesiculosus, 5–50 kDa) | Trimethyl chitosan | Insulin | Self-assembly | Diabetes | Oral | 63 |
| Fucoidan (Fucus vesiculosus, 80 kDa) | Chitosan | Basic fibroblast growth factor | Ionotropic crosslinking | Neurite extension | Nerve tissue | 44 |
| Fucoidan (104 kDa) | Isobutylcyanoacrylate | Recombinant tissue plasminogen activator | Redox radical emulsion polymerization | Thrombolytic therapy | Retro-orbital (C57BL/6 mice) | 64 |
| Fucoidan (Sargassum cymosum) | Isobutylcyanoacrylate | - | Anionic emulsion polymerization and redox radical emulsion polymerization | Immunotherapy | NA1 | 65 |
| Fucoidan | Poly(lactide-co-glycolide) and poly-l-ornitine (core-shell) | - | Layer-by-layer | Anticancer therapy | NA1 | 66 |
| Fucoidan (Fucus vesiculosus, 20–200 kDa) | - | Cisplatin | Self-assembly | Anticancer therapy and immunotherapy | Colonic drug delivery system | 67 |
| Fucoidan (Spatoglossum schrőederi, 21 kDa) | Hexadecylamine | - | Self-assembly | Anticancer therapy | NA1 | 68 |
| Fucoidan (Source/Molecular Weight) | Copolymer/Positive Charge Donor | Drug | Method of Obtaining | Application | Route of Administration | Ref. |
|---|---|---|---|---|---|---|
| Fucoidan (Fucus vesiculosus, 80 kDa) | Chitosan | Bovine serum albumin | Ionotropic cross-linking | Peptide and protein delivery | NA 1 | 45 |
| Fucoidan (Fucus vesiculosus, 80 kDa) | Chitosan | - | Polyion complexation | Treatment of dermal burns | Topical | 48 |
| Fucoidan | Poly(alkylcyanoacrylate)and dextran | Perfluorooctylbromide | Emulsion-evaporation polymerization | Targeting carrier | Intravenous | 71 |
| Fucoidan (200-400 kDa) | Poly-l-ornithine (shell); calcium carbonate (core) | Doxorubicin | Layer-by-layer self-assembly | Anticancer therapy | NA 1 | 72 |
| Fucoidan (Fucus vesiculosus, 80 kDa) | Chitosan | Ofloxacin | Polyion complexation | Antibiotics carriers | NA 1 | 73 |
| Fucoidan (Laminaria japonica, 598.4 Da–0.598 kDa) | - | Isoniazid or rifabutin | Spray-drying | Tuberculosis therapy | Pulmonary | 43 |
| Fucoidan (Laminaria japonica) | - | Isoniazid and rifabutin | Spray-drying | Tuberculosis therapy | Pulmonary | 74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. https://doi.org/10.3390/md17080458
Citkowska A, Szekalska M, Winnicka K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Marine Drugs. 2019; 17(8):458. https://doi.org/10.3390/md17080458
Chicago/Turabian StyleCitkowska, Aleksandra, Marta Szekalska, and Katarzyna Winnicka. 2019. "Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms" Marine Drugs 17, no. 8: 458. https://doi.org/10.3390/md17080458
APA StyleCitkowska, A., Szekalska, M., & Winnicka, K. (2019). Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Marine Drugs, 17(8), 458. https://doi.org/10.3390/md17080458