Redesigning Arenicin-1, an Antimicrobial Peptide from the Marine Polychaeta Arenicola marina, by Strand Rearrangement or Branching, Substitution of Specific Residues, and Backbone Linearization or Cyclization
Abstract
1. Introduction
2. Results
2.1. AR Variants Design
2.2. Assessing the Structure Using Molecular Dynamics Simulations
2.3. Antimicrobial Activity
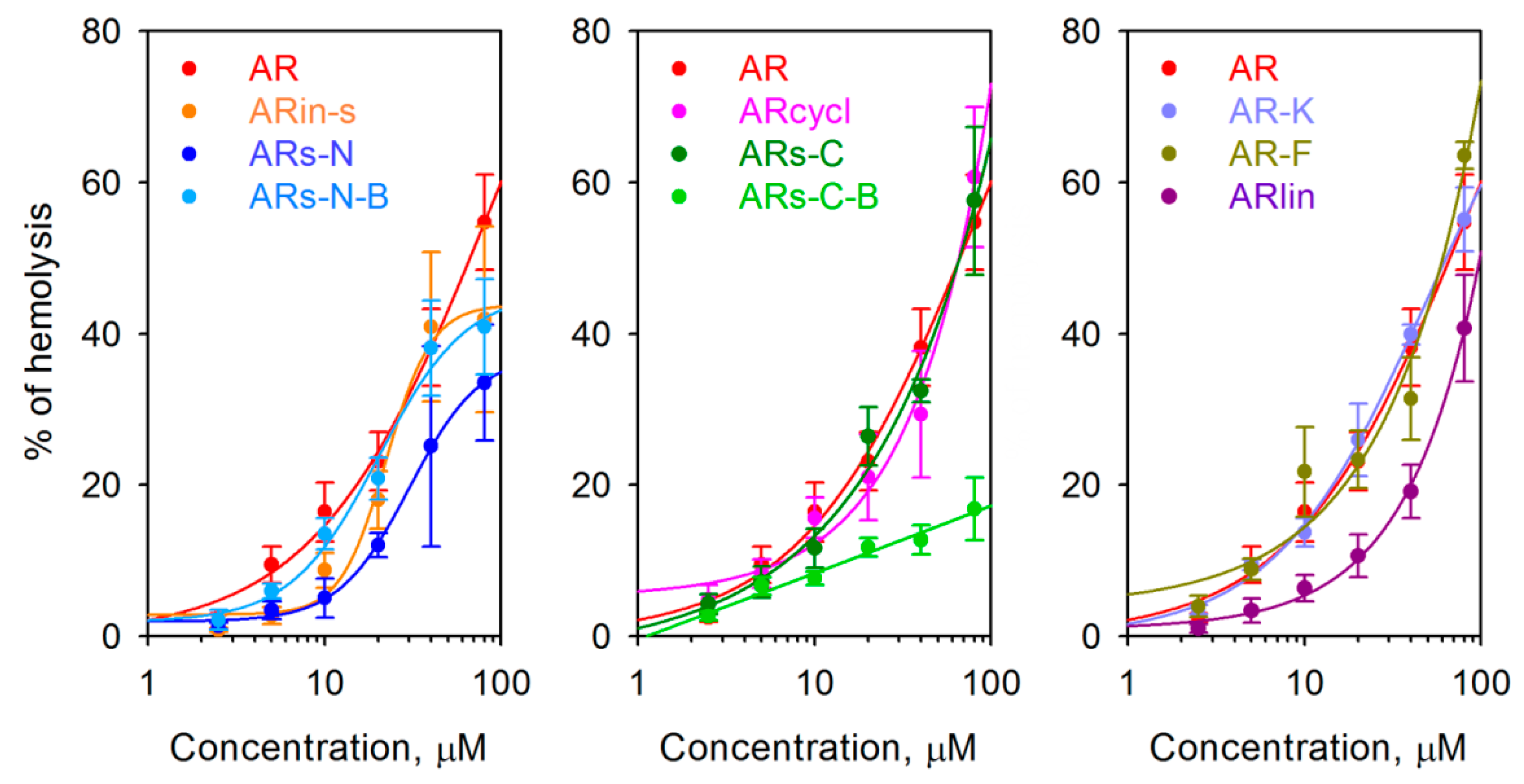
2.4. Effect of Arenicin Variants on Bacterial Membrane Integrity
2.5. Cytotoxicity of Arenicin-1 Variants towards Mammalian Cells
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.1.1. Antiparallel β-Hairpins
4.1.2. Branched, Parallel Hairpins, and Simulation of the Turn Region
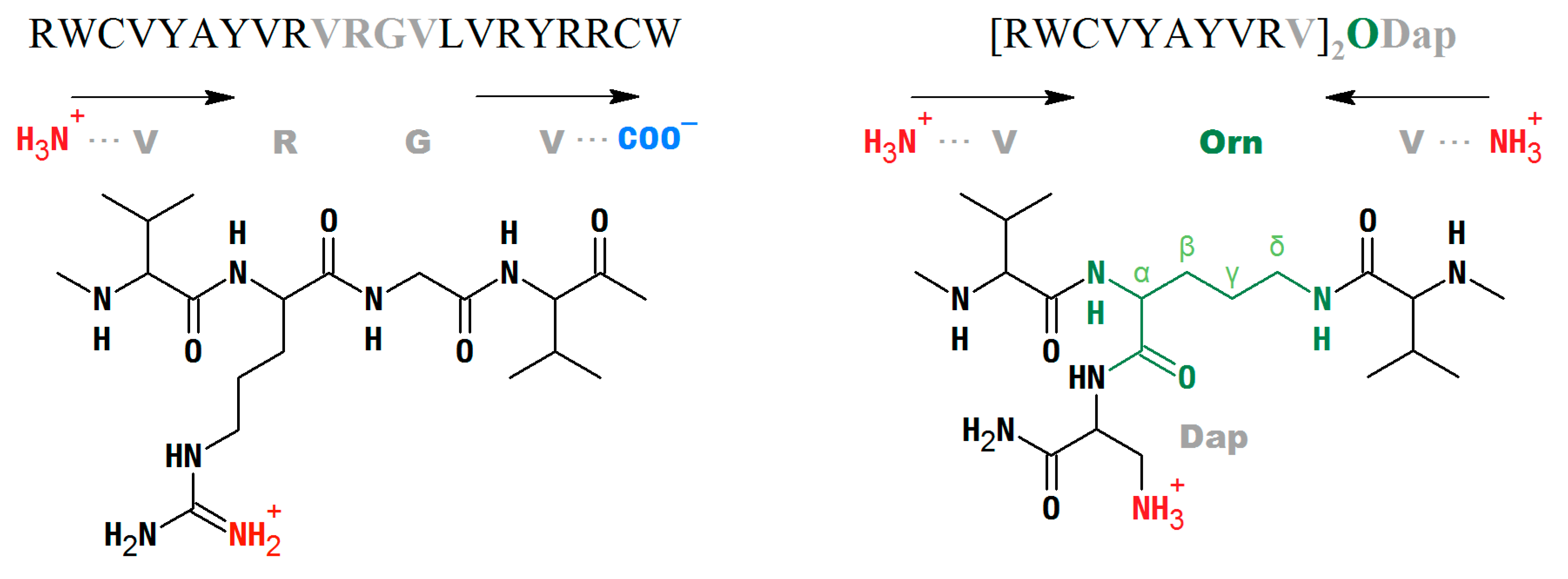
4.1.3. Backbone-Cyclized Arenicin
4.1.4. Disulphide Bond Formation
4.2. Molecular Modeling
4.2.1. Molecular Dynamics Simulations
4.2.2. Analysis of the Simulation Results
4.3. Antibacterial Assays
4.3.1. Bacterial Strains
4.3.2. Broth Microdilution Assay
4.3.3. Membrane Permeabilization Assay
4.4. Cytotoxicity Assays
4.4.1. Hemolytic Activity
4.4.2. MTT Test
5. Conclusions
- Stand inversion or palyndromic symmetrization of the arenicin scaffold does not greatly affect its twisted and kinked antiparallel β-sheet conformation, whereas symmetrization by artificially branching strands results in a flattened and more regular parallel β-hairpin;
- Inverting the strand residue arrangement of the native peptide causes a decrease in activity. This may be due to decreased capacity to oligomerize via the inverted N-terminal strand;
- A more symmetric, palindromic strand arrangement did not improve the activity and decreased it if accompanied by a reduced net charge;
- Increasing symmetry by artificially “branching” strands in a parallel hairpin arrangement allowed to recover the antimicrobial activity while reducing the cytotoxic activity;
- All variants with a modified symmetry demonstrated a reduced capacity to permeabilize the inner membrane of E. coli ML35, possibly pointing to a reduced capacity for oligomerization and/or pore formation.
- The backbone cyclization of the arenicin-1 molecule resulted in improved activity towards drug-resistant clinical isolates but did not markedly affect cytotoxicity.
- Linearization of the peptide somewhat increased selectivity, while not greatly altering antimicrobial activity.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ovchinnikova, T.V.; Aleshina, G.M.; Balandin, S.V.; Krasnosdembskaya, A.D.; Markelov, M.L.; Frolova, E.I.; Leonova, Y.F.; Tagaev, A.A.; Krasnodembsky, E.G.; Kokryakov, V.N. Purification and primary structure of two isoforms of arenicin, a novel antimicrobial peptide from marine polychaeta Arenicola marina. FEBS Lett. 2004, 577, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, T.V.; Shenkarev, Z.O.; Nadezhdin, K.D.; Balandin, S.V.; Zhmak, M.N.; Kudelina, I.A.; Finkina, E.I.; Kokryakov, V.N.; Arseniev, A.S. Recombinant expression, synthesis, purification, and solution structure of arenicin. Biochem. Biophys. Res. Commun. 2007, 360, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Kang, D.I.; Zhu, W.L.; Shin, S.Y.; Hahm, K.S.; Kim, Y. Solution structures and biological functions of the antimicrobial peptide, arenicin-1, and its linear derivative. Biopolymers 2007, 88, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Andrä, J.; Jakovkin, I.; Grötzinger, J.; Hecht, O.; Krasnosdembskaya, A.D.; Goldmann, T.; Gutsmann, T.; Leippe, M. Structure and mode of action of the antimicrobial peptide arenicin. Biochem. J. 2008, 410, 113–122. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Shenkarev, Z.O.; Balandin, S.V.; Nadezhdin, K.D.; Paramonov, A.S.; Kokryakov, V.N.; Arseniev, A.S. Molecular insight into mechanism of antimicrobial action of the β-hairpin peptide arenicin: Specific oligomerization in detergent micelles. Biopolymers 2008, 89, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Stavrakoudis, A.; Tsoulos, I.G.; Shenkarev, Z.O.; Ovchinnikova, T.V. Molecular dynamics simulation of antimicrobial peptide arenicin-2: Beta-hairpin stabilization by noncovalent interactions. Biopolymers 2009, 92, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Andrä, J.; Hammer, M.U.; Grötzinger, J.; Jakovkin, I.; Lindner, B.; Vollmer, E.; Fedders, H.; Leippe, M.; Gutsmann, T. Significance of the cyclic structure and of arginine residues for the antibacterial activity of arenicin-1 and its interaction with phospholipid and lipopolysaccharide model membranes. Biol. Chem. 2009, 390, 337–349. [Google Scholar] [CrossRef]
- Salnikov, E.S.; Aisenbrey, C.; Balandin, S.V.; Zhmak, M.N.; Ovchinnikova, T.V.; Bechinger, B. Structure and alignment of the membrane-associated antimicrobial peptide arenicin by oriented solid-state NMR spectroscopy. Biochemistry 2011, 50, 3784–3795. [Google Scholar] [CrossRef]
- Cho, J.; Lee, D.G. The characteristic region of arenicin-1 involved with a bacterial membrane targeting mechanism. Biochem. Biophys. Res. Commun. 2011, 405, 422–427. [Google Scholar] [CrossRef]
- Park, C.; Cho, J.; Lee, J.; Lee, D.G. Membranolytic antifungal activity of arenicin-1 requires the N-terminal tryptophan and the beta-turn arginine. Biotechnol. Lett. 2011, 33, 185–189. [Google Scholar] [CrossRef]
- Panteleev, P.V.; Bolosov, I.A.; Ovchinnikova, T.V. Bioengineering and functional characterization of arenicin shortened analogs with enhanced antibacterial activity and cell selectivity. J. Pept. Sci. 2016, 22, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Panteleev, P.V.; Myshkin, M.Y.; Shenkarev, Z.O.; Ovchinnikova, T.V. Dimerization of the antimicrobial peptide arenicin plays a key role in the cytotoxicity but not in the antibacterial activity. Biochem. Biophys. Res. Commun. 2017, 482, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Shenkarev, Z.O.; Balandin, S.V.; Trunov, K.I.; Paramonov, A.S.; Sukhanov, S.V.; Barsukov, L.I.; Arseniev, A.S.; Ovchinnikova, T.V. Molecular mechanism of action of β-hairpin antimicrobial peptide arenicin: Oligomeric structure in dodecylphosphocholine micelles and pore formation in planar lipid bilayers. Biochemistry 2011, 50, 6255–6265. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Park, K.H.; Lee, J.Y.; Kim, J.; Shin, S.Y.; Park, Y.; Hahm, K.S.; Kim, Y. Cell Selectivity of Arenicin-1 and Its Derivative with Two Disulfide Bonds. Bull. Korean Chem. Soc. 2008, 29, 1190–1194. [Google Scholar] [CrossRef][Green Version]
- Lehrer, R.I.; Barton, A.; Ganz, T. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J. Immunol. Methods 1988, 108, 153–158. [Google Scholar] [CrossRef]
- Edwards, I.A.; Elliott, A.G.; Kavanagh, A.M.; Zuegg, J.; Blaskovich, M.A.; Cooper, M.A. Contribution of Amphipathicity and Hydrophobicity to the Antimicrobial Activity and Cytotoxicity of β-Hairpin Peptides. ACS Infect. Dis. 2016, 2, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Jindal, H.M.; Le, C.F.; Yusof, M.Y.M.; Velayuthan, R.D.; Lee, V.S.; Zain, S.M.; Isa, D.M.; Sekaran, S.D. Antimicrobial Activity of Novel Synthetic Peptides Derived from Indolicidin and Ranalexin against Streptococcus pneumoniae. PLoS ONE 2015, 10, e0128532. [Google Scholar] [CrossRef] [PubMed]
- Tassanakajon, A.; Amparyup, P.; Somboonwiwat, K.; Supungul, P. Cationic antimicrobial peptides in penaeid shrimp. Mar. Biotechnol. 2010, 12, 487–505. [Google Scholar] [CrossRef]
- Charlet, M.; Chernysh, S.; Philippe, H.; Hetru, C.; Hoffmann, J.A.; Bulet, P. Innate immunity. Isolation of several cystein-rich antimicrobial peptides from the blood of a mollusc, Mytilus edulis. J. Biol. Chem. 1996, 271, 21808–21813. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Balandin, S.V.; Aleshina, G.M.; Tagaev, A.A.; Leonova, Y.F.; Krasnodembsky, E.D.; Men’shenin, A.V.; Kokryakov, V.N. Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins. Biochem. Biophys. Res. Commun. 2006, 348, 514–523. [Google Scholar] [CrossRef]
- Nakamura, T.; Furunaka, H.; Miyata, T.; Tokunaga, F.; Muta, T.; Iwanaga, S.; Niwa, M.; Takao, T.; Shimonishi, Y. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J. Biol. Chem. 1988, 263, 16709–16713. [Google Scholar] [PubMed]
- Stensvåg, K.; Haug, T.; Sperstad, S.V.; Rekdal, O.; Indrevoll, B.; Styrvold, O.B. Arasin 1, a prolinearginine-rich antimicrobial peptide isolated from the spider crab, Hyas araneus. Dev. Comp. Immunol. 2008, 32, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Shin, S.Y.; Hahm, K.S.; Kim, J.I. Design of perfectly symmetric Trp-rich peptides with potent and broad-spectrum antimicrobial activities. Int. J. Antimicrob. Agents 2006, 27, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chou, S.; Wang, J.; Shao, C.; Li, W.; Zhu, X.; Shan, A. Antimicrobial activity and membrane-active mechanism of tryptophan zipper-like β-hairpin antimicrobial peptides. Amino Acids 2015, 47, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Ma, Q.; Shan, A.; Lv, Y.; Hu, W.; Gu, Y.; Li, Y. Strand length-dependent antimicrobial activity and membrane-active mechanism of arginine- and valine-rich β-hairpin-like antimicrobial peptides. Antimicrob. Agents Chemother. 2012, 56, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Shukla, S.K.; Prakash, O.; Zhang, G. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 2010, 92, 1236–1241. [Google Scholar] [CrossRef]
- Teixeira, V.; Feio, M.J.; Bastos, M. Role of lipids in the interaction of antimicrobial peptides with membranes. Prog. Lipid Res. 2012, 51, 149–177. [Google Scholar] [CrossRef]
- Feder, R.; Dagan, A.; Mor, A. Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J. Biol. Chem. 2000, 275, 4230–4238. [Google Scholar] [CrossRef]
- Jiang, Z.; Vasil, A.I.; Vasil, M.L.; Hodges, R.S. “Specificity Determinants” Improve Therapeutic Indices of Two Antimicrobial Peptides Piscidin 1 and Dermaseptin S4 against the Gram-Negative Pathogens Acinetobacter baumannii and Pseudomonas aeruginosa. Pharmaceuticals 2014, 7, 366–391. [Google Scholar] [CrossRef]
- Arias, M.; Piga, K.B.; Hyndman, M.E.; Vogel, H.J. Improving the Activity of Trp-Rich Antimicrobial Peptides by Arg/Lys Substitutions and Changing the Length of Cationic Residues. Biomolecules 2018, 8, 19. [Google Scholar] [CrossRef]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta 2006, 1758, 1184–1202. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.W.; Wong, G.C. Antimicrobial peptides and induced membrane curvature: Geometry, coordination chemistry, and molecular engineering. Curr. Opin. Solid State Mater. Sci. 2013, 17, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Wu, C.; Yang, J.-L. Membranolytic selectivity of cystine-stabilized cyclic protegrins. Eur. J. Biochem. 2000, 267, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Lu, Y.-A.; Yang, J.-L. Marked Increase in Membranolytic Selectivity of Novel Cyclic Tachyplesins Constrained with an Antiparallel Two-β Strand Cystine Knot Framework. Biochem. Biophys. Res. Commun. 2000, 267, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.Y.; Zhang, V.M.; Huang, Y.H.; Waters, N.C.; Bansal, P.S.; Craik, D.J.; Daly, N.L. Cyclization of the antimicrobial peptide gomesin with native chemical ligation: Influences on stability and bioactivity. ChemBioChem 2013, 14, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Hemu, X.; Tam, J.P. Macrocyclic Antimicrobial Peptides Engineered from ω-Conotoxin. Curr. Pharm. Des. 2017, 23, 2131–2138. [Google Scholar] [CrossRef]
- Wu, M.; Hancock, R.E. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J. Biol. Chem. 1999, 274, 29–35. [Google Scholar] [CrossRef]
- Lai, J.R.; Huck, B.R.; Weisblum, B.; Gellman, S.H. Design of non-cysteine-containing antimicrobial beta-hairpins: Structure-activity relationship studies with linear protegrin-1 analogues. Biochemistry 2002, 41, 12835–12842. [Google Scholar] [CrossRef]
- Hai Nan, Y.; Jacob, B.; Kim, Y.; Yub Shin, S. Linear bactenecin analogs with cell selectivity and anti-endotoxic activity. J. Pept. Sci. 2012, 18, 740–747. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yang, S.T.; Lee, S.K.; Jung, H.H.; Shin, S.Y.; Hahm, K.S.; Kim, J.I. Salt-resistant homodimeric bactenecin, a cathelicidin-derived antimicrobial peptide. FEBS J. 2008, 275, 3911–3920. [Google Scholar] [CrossRef]
- Mosco, A.; Zlatev, V.; Guarnaccia, C.; Pongor, S.; Campanella, A.; Zahariev, S.; Giulianini, P.G. Novel protocol for the chemical synthesis of crustacean hyperglycemic hormone analogues—An efficient experimental tool for studying their functions. PLoS ONE 2012, 7, e30052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benincasa, M.; Zahariev, S.; Pelillo, C.; Milan, A.; Gennaro, R.; Scocchi, M. PEGylation of the peptide Bac7(1-35) reduces renal clearance while retaining antibacterial activity and bacterial cell penetration capacity. Eur. J. Med. Chem. 2015, 95, 210–219. [Google Scholar] [CrossRef] [PubMed]
- von Eggelkraut-Gottanka, R.; Klose, A.; Beck-Sickinger, A.G.; Beyermann, M. Peptide αthioester formation using standard Fmoc-chemistry. Tetrahedron Lett. 2003, 44, 3551–3554. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- van Gunsteren, W.F.; Billeter, S.R.; Eising, A.A.; Hünenberger, P.H.; Krüger, P.K.H.C.; Mark, A.E.; Scott, W.R.P.; Tironi, I.G. Biomolecular Simulation: The GROMOS96 Manual and User Guide; Vdf Hochschulverlag AG an der ETH Zürich: Zürich, Switzerland, 1996; ISBN 9783728124227. [Google Scholar]
- Kabsch, W.; Sander, C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 1983, 22, 2577–2637. [Google Scholar] [CrossRef] [PubMed]
- Frishman, D.; Argos, P. Knowledge-based protein secondary structure assignment. Proteins 1995, 23, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Needleman, S.B.; Wunsch, C.D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 1970, 48, 443–453. [Google Scholar] [CrossRef]
- Schrödinger, LLC. The PyMOL Molecular Graphics System; Version 2.2.0; Schrödinger, LLC: New York, NY, USA, 2018. [Google Scholar]
- Xu, D.; Zhang, Y. Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins 2012, 80, 1715–1735. [Google Scholar] [CrossRef]
- Giangaspero, A.; Sandri, L.; Tossi, A. Amphipathic alpha helical antimicrobial peptides. Eur. J. Biochem. 2001, 268, 5589–5600. [Google Scholar] [CrossRef]
- Tossi, A.; Scocchi, M.; Zanetti, M.; Gennaro, R.; Storici, P.; Romeo, D. An Approach Combining Rapid cDNA Amplification and Chemical Synthesis for the Identification of Novel, Cathelicidin-Derived, Antimicrobial Peptides. In Antibacterial Peptide Protocols. Methods in Molecular Biology™; Shafer, W.M., Ed.; Humana Press: Totowa, NJ, USA, 1997; Volume 78, pp. 133–151. ISBN 978-0-89603-408-2. [Google Scholar]
- Lyu, Y.; Yang, Y.; Lyu, X.; Dong, N.; Shan, A. Antimicrobial activity, improved cell selectivity and mode of action of short PMAP-36-derived peptides against bacteria and Candida. Sci. Rep. 2016, 6, 27258. [Google Scholar] [CrossRef]
- Davies, B.I. The importance of the geometric mean MIC. J. Antimicrob. Chemother. 1990, 25, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, S. Measures of central tendency: The mean. J. Pharmacol. Pharmacother. 2011, 2, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Eliseev, I.E.; Terterov, I.N.; Yudenko, A.N.; Shamova, O.V. Linking sequence patterns and functionality of alpha-helical antimicrobial peptides. Bioinformatics 2018. [Google Scholar] [CrossRef] [PubMed]
- Veltri, D.; Kamath, U.; Shehu, A. Deep learning improves antimicrobial peptide recognition. Bioinformatics 2018, 34, 2740–2747. [Google Scholar] [CrossRef]

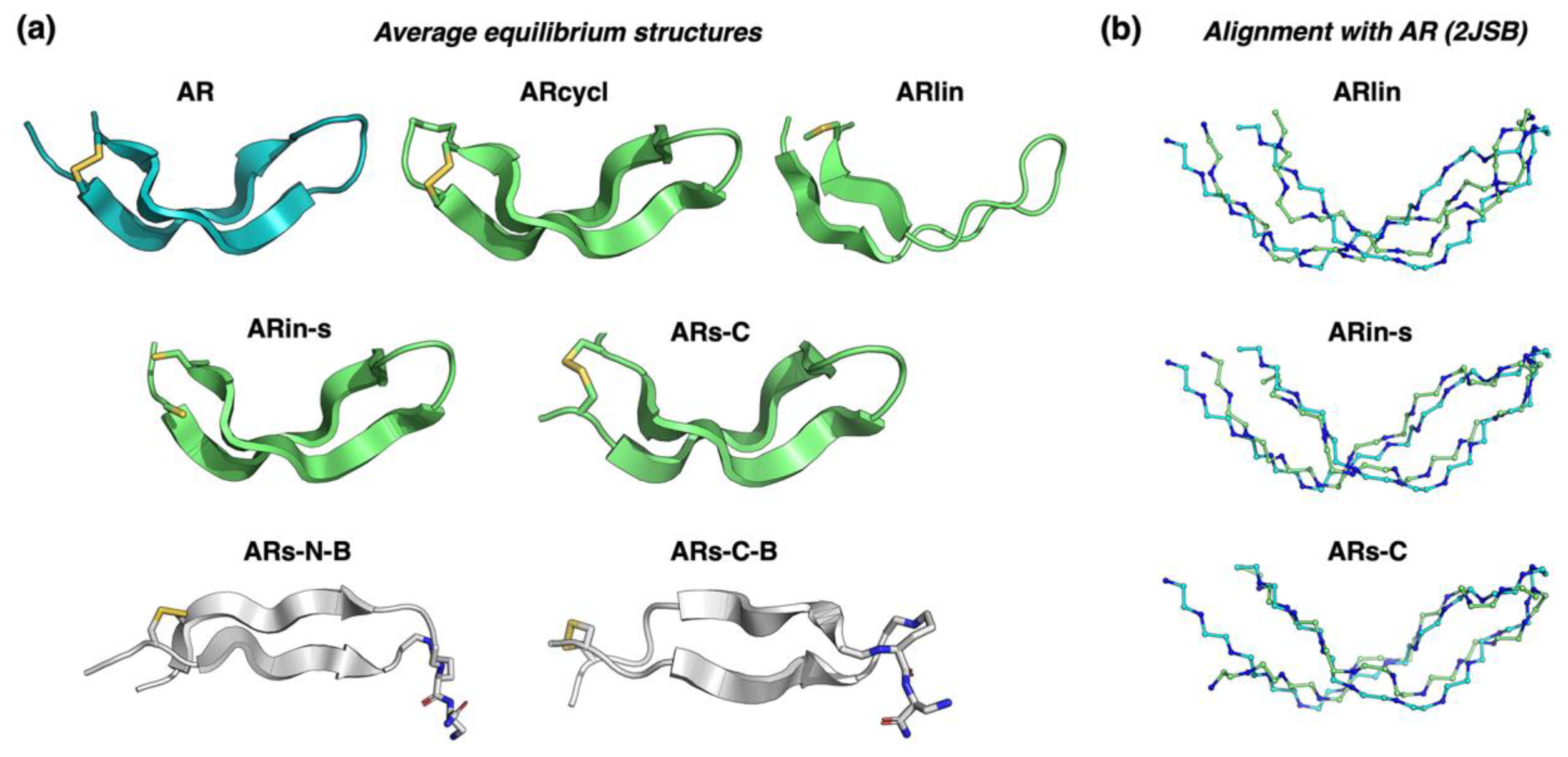
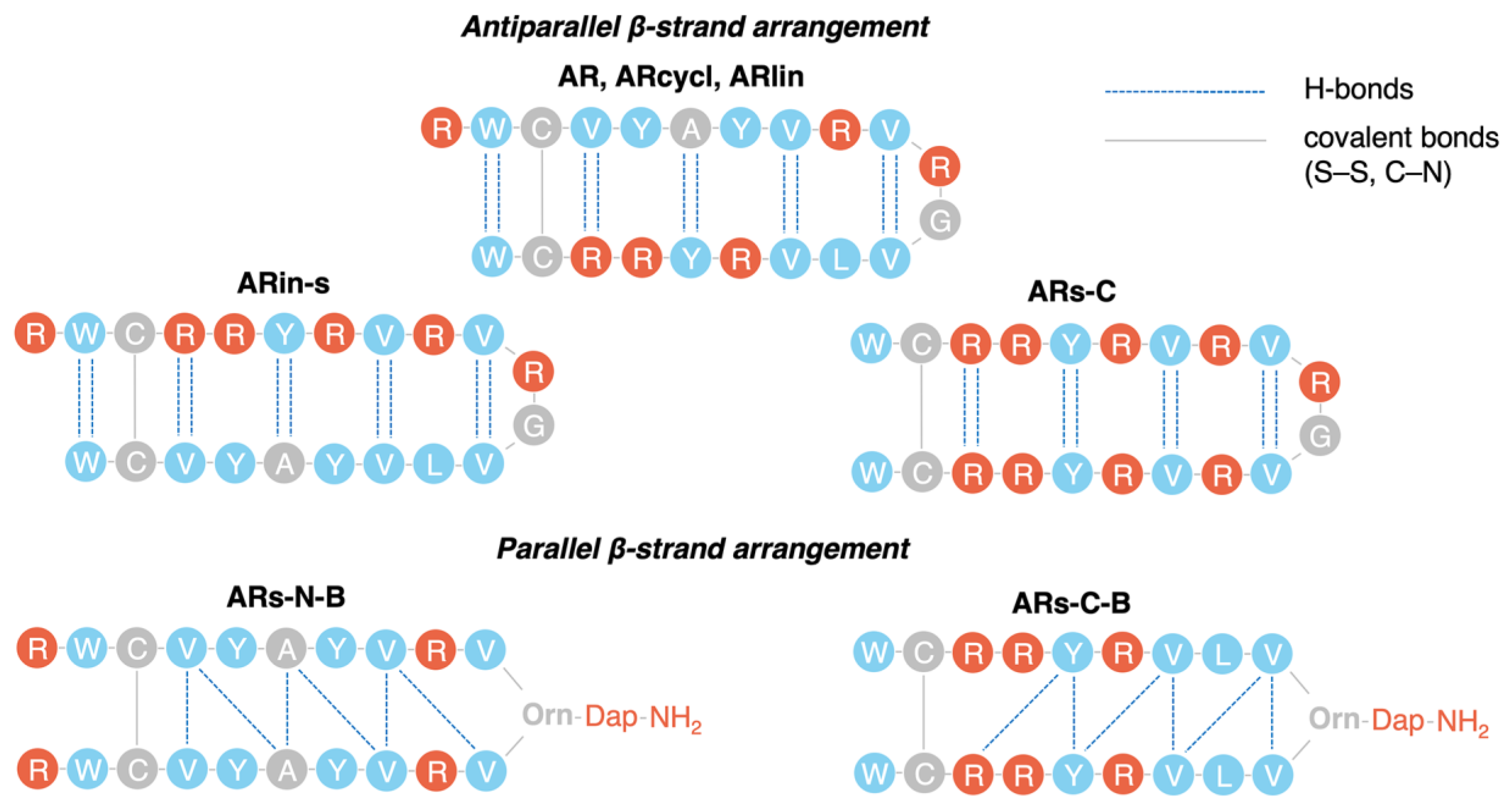
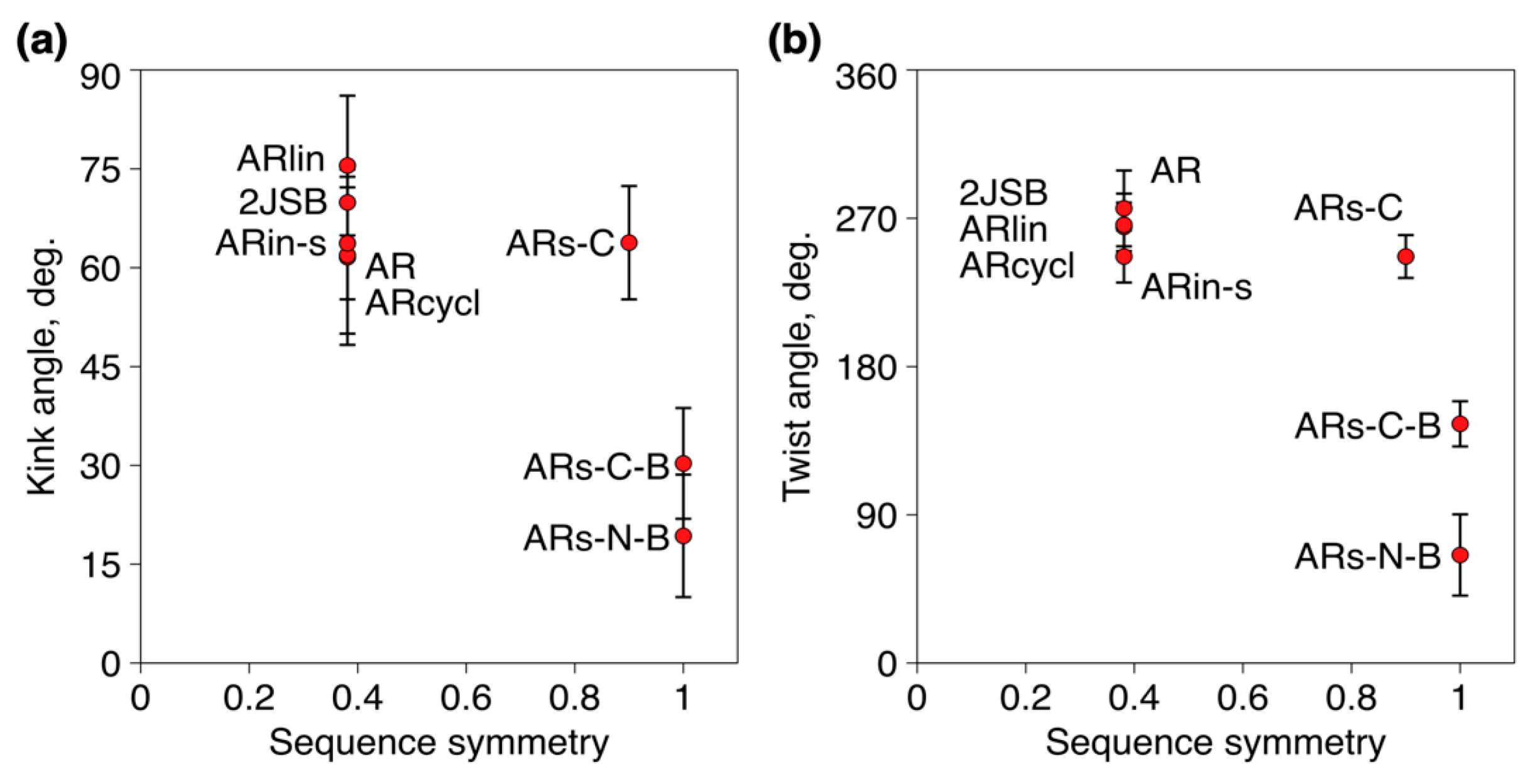

| Peptide | (a) Cα RMSD with 2JSB (Å) | (b) Average N° of H-Bonds (±SD) | (c) Average 2y Structure Content |
|---|---|---|---|
| AR | 1.37 | 8.3 ± 1.2 | 14/21 |
| ARin-s | 1.67 | 8.3 ± 1.2 | 16/21 |
| ARs-C | 1.90 | 6.4 ± 1.1 | 14/20 |
| ARs-N-B | 4.29 | 7.1 ± 1.3 | 13/22 |
| ARs-C-B | 3.30 | 6.2 ± 1.1 | 10/20 |
| ARlin | 3.13 | 5.4 ± 1.2 | 9/21 |
| ARcycl | 1.57 | 7.1 ± 1.2 | 16/21 |
| Minimal Inhibitory Concentrations (MIC) a, µM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AR | ARin-s | ARs-N | ARs-C | ARs-N-B | ARs-C-B | AR K | AR F | ARlin | ARcycl | |
| Gram-negative laboratory strains | ||||||||||
| Escherichia coli ML35p | 1–2 | 4–8 | 16 | 1 | 4 | 1–2 | 1 | 1 | 1–2 | 1–2 |
| E. coli ATCC 25922 | 2 | 8 | >16 | 2 | 8 | 4 | 2 | 1 | 2 | 2 |
| E. coli M15 | 2 | 8 | >16 | 2 | 4 | 8 | 1 | 1 | 2 | 2 |
| Pseudomonas aeruginosa ATCC 27853 | 2 | 4–8 | >16 | 1 | 2 | 1 | 1–2 | 1 | 2 | 1 |
| Gram-positive laboratory strains | ||||||||||
| Listeria monocytogenes EGD | 1–2 | 2 | 8 | 1 | 2–4 | 2 | 2 | 2 | 2 | 2 |
| Staphylococcus aureus 710A | 2 | 16 | >16 | 2–4 | 8 | 4 | 2 | 2 | 2 | 2 |
| S. aureus ATCC 25923 | 2 | 16 | >16 | 4 | 8 | 4 | 2 | 2 | 2 | 2 |
| MRSA ATCC 33591 | 4 | >16 | >16 | 4–8 | 8 | 8 | 2 | 2 | 4 | 4 |
| Clinical isolates | ||||||||||
| P. aeruginosa c.i. | 4 | >16 | >16 | 16 | 16 | 16 | 2–4 | 2 | 4–8 | 2 |
| Acinetobacter baumanii c.i. | 4 | 16 | >16 | 2 | 4 | 8–16 | 1 | 2 | 4 | 4 |
| Staphylococcus intermidius | 8 | >16 | >16 | 8 | 8 | 4 | 4 | 4 | 8 | 4 |
| S. aureus c.i. | 4 | >16 | >16 | 16 | 8 | 4–8 | 4 | 4 | 8–16 | 4 |
| Overall statistics | ||||||||||
| G-MIC b | 2.7 | ≥12.8 | ≥26.9 | 3.2 | 5.9 | 4.4 | 1.9 | 1.8 | 3.1 | 2.3 |
| G-MIC improvement ratio in comparison with AR c | 1.0 | ≤0.2 | ≤0.1 | 0.8 | 0.5 | 0.6 | 1.4 * | 1.5 * | 0.9 | 1.2 * |
| Effects of the Peptides | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AR | ARin-s | ARs-N | ARs-C | ARs-N-B | ARs-C-B | AR-K | AR-F | ARlin | ARcyclic | |
| Cytotoxicity towards K-562 cell line (human erythroleukemia cells) | ||||||||||
| IC50a, µM (MTT-assay) | 17.9 | 37.7 | 18.0 | 11.6 | 35.1 | 39.0 | 7.7 | 9.3 | >40 | 16.2 |
| SI1 assessment IC50/G-MIC | 6.6 | ≤2.9 | ≤0.7 | 3.6 | 6.0 | 8.8 | 4.0 | 5.2 | >12.8 | 7.0 |
| SI1 improvement ratio in comparison with ARb | 1.0 | ≤0.4 | ≤0.1 | 0.5 | 0.9 | 1.3 * | 0.6 | 0.8 | >1.9 * | 1.1 |
| Hemolysis of human red blood cells | ||||||||||
| HC50c, µM | 66.3 | >80 | >80 | 66.0 | >80 | >80 | 63.0 | 60.5 | >80 | 65.5 |
| SI2 assessment HC50/G-MIC | 24.6 | - | - | 20.6 | >13.7 | >18.1 | 33.1 | 34.0 | >25.6 | 28.2 |
| SI2 improvement ratio in comparison with ARb | 1.0 | - | - | 0.8 | >0.6 | >0.7 | 1.3 | 1.4 | >1.0 | 1.1 |
| HC15c, µM | 10.3 | 17.2 | 23.5 | 11.8 | 12.4 | 56.4 | 10.1 | 10.6 | 29.9 | 14.0 |
| SI3 assessment HC15/G-MIC | 3.8 | ≤1.3 | ≤0.9 | 3.7 | 2.1 | 12.7 | 5.3 | 6.0 | 9.5 | 6.0 |
| SI3 improvement ratio in comparison with ARb | 1.0 | ≤0.4 | ≤0.2 | 1.0 | 0.6 | 3.3 * | 1.4 | 1.6 | 2.5 * | 1.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlov, D.S.; Shamova, O.V.; Eliseev, I.E.; Zharkova, M.S.; Chakchir, O.B.; Antcheva, N.; Zachariev, S.; Panteleev, P.V.; Kokryakov, V.N.; Ovchinnikova, T.V.; et al. Redesigning Arenicin-1, an Antimicrobial Peptide from the Marine Polychaeta Arenicola marina, by Strand Rearrangement or Branching, Substitution of Specific Residues, and Backbone Linearization or Cyclization. Mar. Drugs 2019, 17, 376. https://doi.org/10.3390/md17060376
Orlov DS, Shamova OV, Eliseev IE, Zharkova MS, Chakchir OB, Antcheva N, Zachariev S, Panteleev PV, Kokryakov VN, Ovchinnikova TV, et al. Redesigning Arenicin-1, an Antimicrobial Peptide from the Marine Polychaeta Arenicola marina, by Strand Rearrangement or Branching, Substitution of Specific Residues, and Backbone Linearization or Cyclization. Marine Drugs. 2019; 17(6):376. https://doi.org/10.3390/md17060376
Chicago/Turabian StyleOrlov, Dmitriy S., Olga V. Shamova, Igor E. Eliseev, Maria S. Zharkova, Oleg B. Chakchir, Nikolinka Antcheva, Sotir Zachariev, Pavel V. Panteleev, Vladimir N. Kokryakov, Tatiana V. Ovchinnikova, and et al. 2019. "Redesigning Arenicin-1, an Antimicrobial Peptide from the Marine Polychaeta Arenicola marina, by Strand Rearrangement or Branching, Substitution of Specific Residues, and Backbone Linearization or Cyclization" Marine Drugs 17, no. 6: 376. https://doi.org/10.3390/md17060376
APA StyleOrlov, D. S., Shamova, O. V., Eliseev, I. E., Zharkova, M. S., Chakchir, O. B., Antcheva, N., Zachariev, S., Panteleev, P. V., Kokryakov, V. N., Ovchinnikova, T. V., & Tossi, A. (2019). Redesigning Arenicin-1, an Antimicrobial Peptide from the Marine Polychaeta Arenicola marina, by Strand Rearrangement or Branching, Substitution of Specific Residues, and Backbone Linearization or Cyclization. Marine Drugs, 17(6), 376. https://doi.org/10.3390/md17060376







