Abstract
Three new hydroxylated rhamnolipids, dokdolipids A−C (1−3) were obtained from the marine actinomycete Actinoalloteichus hymeniacidonis, which was isolated from a sediment sample collected off the coasts of Dokdo island, Republic of Korea. The structures of the isolated compounds were elucidated on the basis of 1D and 2D NMR and mass spectrometric data analyses. Their absolute configurations were assigned using the modified Mosher’s method and specific rotation values, as well as acid hydrolysis, chemical derivatizations and subsequent HPLC analysis to determine the configuration of the sugar moieties. All new compounds were evaluated for their cytotoxicity against six cancer cell lines, HCT-15, NUGC-3, NCI-H23, ACHN, PC-3 and MDA-MB-231. Compounds 1−3 displayed moderate cytotoxicity against all the cell lines tested with IC50 values ranging from 13.7−41.5 µM.
1. Introduction
Rhamnolipids belong to a class of biosurfactants composed of rhamnose linked to β-hydroxylated fatty acid chains [1]. Rhamnolipids are classified as mono-rhamnolipids, which contain a single rhamnose molecule and di-rhamnolipids, which contain two rhamnose sugar rings [2]. These biosurfactants are mainly produced by Pseudomonas species [3] such as P. aeruginosa [4], P. chlororaphis, P. plantarii, P. putida, and P. fluorescens [5]. Over the past three decades, rhamnolipids have been broadly investigated and extensively reviewed due to their biodegradability and reduced toxicity compared to synthetic surfactants as well as their various applications [6,7,8]. It has been widely recognized that rhamnolipids have surface active properties such as emulsification, dispersion, foaming, detergency, wetting and stabilization [9]. Moreover, various researchers have demonstrated that rhamnolipids display low toxicity, antimicrobial activities and the ability to suppress the growth of breast cancer cells [10,11]. These unique and diverse properties make them suitable to be used in a wide range of industrial demands such as the bioremediation of pollutants, cosmetics, food, pharmaceuticals and therapeutics [12].
The marine environment constitutes a significant reservoir of natural products which has offered the potential for new drug development over the last few decades [13]. Specifically, marine microorganisms are considered efficient producers of lead compounds with biomedical potential [14]. In addition, structurally diverse and impressive bioactive natural products have been identified from marine microbes [15]. In our continuous search for secondary metabolites from marine-derived bacteria, the Actinoalloteichus hymeniacidonis strain 179DD−027 was isolated from a deep-sea sediment sample collected off the coasts of Dokdo Island, East Sea, Republic of Korea. Dokdo Island is a large volcanic island with 89 small islets and rocks containing rich and well-preserved biodiversity [16]. Subsequent fermentation of the producing strain, solvent extraction and chemical investigation procedures led to the isolation of three new rhamnolipids, named dokdolipids A−C (1−3) (Figure 1). Dokdolipids represent the first rhamnolipids containing a hydroxyl (1 and 3) group and a ketone group (2) in the side chains. In this paper, we describe the isolation, structure elucidation and bioactivities of dokdolipids A−C (1−3).
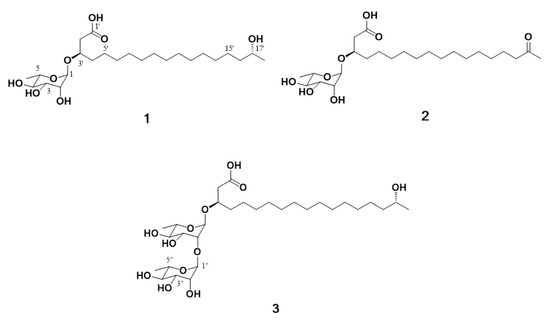
Figure 1.
Structures of compounds 1−3 isolated from Actinoalloteichus hymeniacidonis.
2. Results and Discussion
Compound 1 was obtained as a dark brown oil and gave a [M + Na]+ ion peak at m/z 485.3094 (calcd 485.3090) in the HRESIMS, consistent with a molecular formula C24H46O8. The 1H NMR spectrum of 1 showed the signals of seven oxygenated methines (δH 4.80, 4.08, 3.75, 3.70, 3.65, 3.60 and 3.35), 14 methylene protons (δH 2.49, 1.55 and 12 overlapped protons at 1.29–1.45) and two methyls (δH 1.23 and 1.13). The 1H and 13C NMR data, in conjunction with HSQC of 1, supported the presence of 24 carbons, which can be classified as one carbonyl (δC 173.9), seven sp3 methines (δC 98.9, 75.2, 72.5, 71.2, 70.9, 68.7 and 67.1), 14 sp3 methylene (δC 40.0–24.5) and two sp3 methyl (δC 22.0 and 16.2) carbon (Table 1). The planar structure of compound 1 was elucidated by analyzing the 2D NMR data, including the 1H–1H COSY and 1H–13C HMBC spectra (Figure 2). The COSY correlation from H2-2′ to highly overlapped proton signals, a terminal methyl group and methylene carbons at δC 24.5-29.4 suggested the presence of an aliphatic chain. The HMBC correlations from H2-2′ (δH 2.48, 2.53) to C-1′ (δC 173.9), C-3′ (δC 74.2) and C-4′ (δC 33.1) and from H-3′ (δH 4.08) to C-1′ (δC 173.9), C-2′ (δC 40.0) and C-5′ (δC 24.5) established the position of the carbonyl carbon C-1′ at δC 173.9 and the secondary alcohol H-3′ at δH 4.08. In addition, the chemical shift value of H-17′ (δH 3.70) and the HMBC correlation from the methyl doublet H3-18′ (δH 1.13, d, J = 6.2 Hz) to C-17′ (δC 67.1) and C-16′ (δC 38.8) indicated that a hydroxyl group was attached to C-17′. Detailed analysis of the 2D NMR spectra revealed the presence of a linear hydroxylated and saturated fatty acid as a 3, 17-dihydroxyoctadecanoic acid. Another spin system was identified from the H-1/H-2/H-3/H-4/H-5/H3-6 COSY correlations. A hexose moiety was confirmed by H-1/C-5 and H-5/C-1 HMBC correlations. The hexose ring was connected to C-3′ through an ether linkage which was confirmed by the H-3′/C-1 and H-1/C-3′ HMBC correlations. Thus, the planar structure of dokdolipid A (1) was elucidated as a new rhamnolipid.

Table 1.
1H and 13C NMR data for 1−3 in CD3OD.
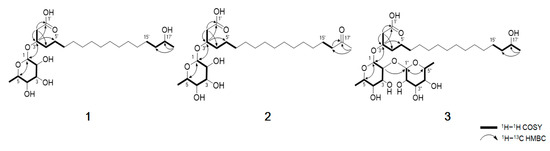
Figure 2.
Key COSY and HMBC correlations of 1−3.
The relative configuration of the sugar moiety was established by analyzing the vicinal coupling constant 3JHH values and ROESY correlations, as seen in Figure 3A. The axial positions of H-3, H-4 and H-5 were assigned by the large coupling constants (3J3,4 = 9.6 Hz and 3J4,5 = 9.6 Hz). The broad singlet of the anomeric proton (H-1) and the ROESY correlations from H-2 to H-3 and from H-4 to H3-6 also suggested that the sugar moiety was rhamnose. To determine the absolute configuration, dokdolipid A (1) was subjected to a chemical degradation. The acid hydrolysis in MeOH of 1 afforded the methylated aglycone of 1 and rhamnopyranose. The absolute configuration of the methylated aglycone was confirmed using the modified Mosher’s method [17]. The observed chemical shift differences ΔδS−R suggested the 3′R,17′R configurations in 1 (Figure 3B). In addition, the rhamnose obtained from hydrolysis and the authentic l- and d-rhamnose were separately derivatized with l-cysteine methyl ester hydrochloride and σ-Tolyl isothiocyanate to establish the absolute configuration of rhamnose [18]. On the basis of the HPLC analysis of the derivatives, the l-rhamnose moiety in 1 was confirmed by chemical derivatization and comparison with standards. Thus, the structure of 1 was identified to be (3′R,17′R)-3′-O-(α-l-rhamnopyranosyl)-17′- dihydroxyoctadecanoic acid and named as dokdolipid A (1).
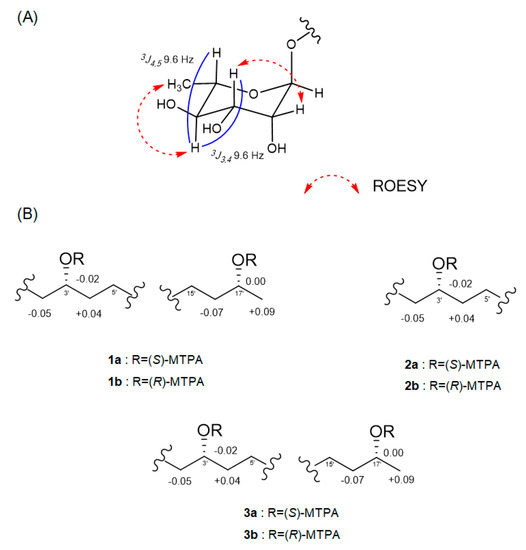
Figure 3.
(A) ROESY correlations in the sugar moiety of 1. (B) ΔδS−R values in ppm of the MTPA esters of the methylated aglycones of 1–3.
Compound 2 was isolated as a dark brown oil, and its molecular formula was determined as C24H44O8 by the [M + Na]+ ion peak at m/z 483.2936 (calcd 483.2934) in the HRESIMS. The 1H and 13C NMR spectra of 2 were similar to those of 1, suggesting that 2 shared the same carbon skeleton as 1. The obvious differences were the disappearance of a doublet methyl and appearance of a singlet methyl. In addition, a 13C NMR signal of a carbonyl carbon at δC 210.8 was observed. The HMBC correlations from H3-18′ (δH 2.12) to C-16′ (δC 42.9) and C-17′ (δC 210.8) and from H2-16′ (δH 2.47) to C-17′ (δC 210.8) suggested that the oxygenated methine C-17′ in 1 was replaced by the carbonyl carbon in 2. The absolute configuration of C-3′ in 2 was confirmed by the same method as that of 1, as seen in Figure 3B. The results indicated the R-configuration of C-3′ in 2. The comparison of the specific rotation values of 1 and 2 ([α]D25 −33.3 (c 0.3, MeOH) and [α]D25 −10.0 (c 0.3, MeOH), respectively) and identical chemical shifts also supported that 2 had the same absolute configuration as 1. Thus, the structure of 2 was determined as a new derivative of 1 and named dokdolipid B (2).
Compound 3 was purified as a dark brown oil and gave a [M + Na]+ ion peak at m/z 631.3669 (calcd 631.3669) in the HRESIMS, consistent with a molecular formula C30H56O12. Comparison of the NMR spectroscopic data of 3 with 1 revealed that 3 has a very similar structure to that of 1. However, the singlet at δH 1.23 corresponded to two methyl groups while the proton signal at δH 4.79 belonged to two anomeric protons. In addition, the presence of eight methine protons at δH 3.35–3.72 suggested that 3 possessed two hexose units. Further analysis of its 2D NMR data and coupling constants 3JHH confirmed that the structure of 3 was analogous to that of 1 with two rhamnoses. The absolute configurations of C-3′ and C-17′ in 3 were also determined using the same method as 1 and the comparison of the specific rotation values of 1 and 3, as well as similar chemical shifts, as seen in Figure 3B. By considering all the experimental data and the biosynthetic pathway of 1 and 3, the absolute configuration of 3 was determined to be the same as 1. Thus, the structure of 3 was elucidated and named dokdolipid C (3).
Compounds 1–3 were tested for their cytotoxicity against cancer cell lines including HCT-15, NUGC-3, NCI-H23, ACHN, PC-3 and MDA-MB-231 using sulforhodamine B (SRB) assay, with adriamycin as a positive control. As shown in Table 2, 1−3 showed moderate activity against these cells, with GI50 values ranging from 13.7 to 41.5 μM. Among the tested compounds, 2 displayed the strongest cytotoxicity in all the cell lines except for MDA-MB-231 (Breast cancer), whereas 3 showed better activity against the MDA-MB-231 cell line than other compounds.

Table 2.
Growth inhibition (GI50, μM) values of 1–3 against human tumor cell lines.
3. Materials and Methods
3.1. General Experimental Procedures
The 1D (1H and 13C) and 2D (COSY, ROESY, HSQC, and HMBC) NMR spectra were acquired on a Bruker 600 MHz spectrometer. UV spectra were obtained on a Shimadzu UV-1650PC spectrophotometer. IR spectra were recorded on a JASCO FT/IR-4100 spectrophotometer. Optical rotations were measured on a Rudolph Research Analytical (Autopol III) polarimeter. HRESIMS spectra were recorded on a hybrid ion-trap time-of-flight mass spectrometer (Shimadzu LC/MS-IT-TOF). HPLC was performed on a PrimeLine Binary pump with RI-101 (Shodex). Analytical HPLC was conducted on an ODS column (YMC-Pack-ODS-A, 250 × 4.6 mm i.d, 5 µm).
3.2. Isolation and Cultivation of the Strain 179DD-027 (Actinoalloteichus hymeniacidonis)
The strain 179DD-027 was isolated from a sediment sample, collected off the coasts of Dokdo island, Republic of Korea. The strain was identified as Actinoalloteichus hymeniacidonis on the basis of the 16s rRNA gene sequence analysis (GenBank accession number MH681580). The strain 179DD-027 was grown on a Bennett’s (BN) agar plate for 7 days at 28 °C and then incubated in BN medium (composed of 10 g of glucose, 1 g of yeast extract, 2 g of tryptone, 1 g of beef extract, 5 g of glycerol and 32 g of NaCl in 1 L of H2O) in a 50 mL flask. After a four-day cultivation at 28 °C with shaking at 130 rpm, 10 mL of the seed culture in a 50 mL flask was used to inoculate 1 L of the culture medium in a 2 L flask for four days. For mass culture, 1 L of the culture in a 2 L flask was used to inoculate 40 L cultivation in BN medium in a 100 L fermenter. A total of 40 L of bacterial culture was incubated at 28 °C for 7 days.
3.3. Isolation of Compounds
The culture broth (40 L) was separated into cells and supernatant by centrifugation. The supernatant was extracted with EtOAc (40 L × 2) at room temperature and then concentrated under reduced pressure to yield the crude extract (3 g). The crude extract was fractionated by flash column chromatography on ODS using a stepwise elution (each fraction 300 mL × 3) with combinations of MeOH/H2O (1:4, 2:3, 3:2, 4:1 and 100% MeOH). The second fraction eluted with MeOH/H2O (4:1) was purified by an analytical, reversed-phase HPLC (YMC-Pack-ODS-A, 250 × 4.6 mm i.d, 5 µm, flow rate 2.0 mL/min, RI detector) using isocratic elution with 40% MeCN in H2O to yield 1 (35.2 mg, tR = 14 min), 2 (4.5 mg, tR = 20 min), and 3 (5.8 mg, tR = 9 min).
3.4. Spectral Data
Dokdolipid A(1): dark brown oil; [α]D25 −33.3 (c 0.3, MeOH); IR νmax 3345, 2918, 2851, 1710, 1646, 1127, 1049 cm−1; UV(MeOH) λmax (log ε) 318 (3.09), 218 (3.42) nm; HRESIMS m/z 485.3094 [M + Na]+ (calcd for 485.3090, C24H46O8Na); 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 125 MHz) see Table 1.
Dokdolipid B (2): dark brown oil; [α]D25 −10.0 (c 0.3, MeOH); IR νmax 3377, 2910, 2851, 1710, 1371, 1068, 1017 cm−1; UV(MeOH) λmax (log ε) 406 (3.29), 312 (3.50), 238 (3.60) nm; HRESIMS m/z 483.2936 [M + Na]+ (calcd for 483.2934, C24H44O8Na); 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 125 MHz) see Table 1.
Dokdolipid C (3): dark brown oil; [α]D25 −40.0 (c 0.3, MeOH); IR νmax 3693, 3328, 2971, 2858, 1632, 1349, 1058, 1010 cm−1; UV(MeOH) λmax (log ε) 310 (3.15), 216 (3.51) nm; HRESIMS m/z 631.3669 [M + Na]+ (calcd for 631.3669, C30H56O12Na); 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 125 MHz) see Table 1.
3.5. Acid Hydrolysis and Determination of Absolute Configuration of Rhamnose
Each of the dokdolipids A−C (1−3) was dissolved in 3 N HCl (0.5 mL) in methanol and heated to 100 °C for 30 min. The solution was cooled and extracted with EtOAc twice. The EtOAc layer and the aqueous layer gave a methylated aglycone and a sugar residue after removal of the solvent respectively. The sugar residue was dissolved in pyridine (0.5 mL) containing l-cysteine methyl ester hydrochloride (0.5 mg) and heated to 60 °C for 1 h. σ-Tolylisothiocyanate (10 μL) was added to the mixture and heating was continued for an additional 1 h. The reaction mixture was directly analyzed using HPLC (10 to 100% MeCN gradient with 0.1% formic acid over 40 min). The sugar residue was detected at 17.9 min. The retention times of the authentic rhamnose samples were 15.5 (d-rhamnose) and 17.9 (l-rhamnose) min under the same HPLC conditions. Therefore, the absolute configuration of the rhamnose unit was established as l-configuration. All dokdolipids were also assigned using the chemical derivatization and HPLC analysis as described above.
3.6. Preparation of MTPA and Esters of 1−3 using the Modified Mosher’s Method
(R)-MTPA-Cl (10 μL) or (S)-MTPA-Cl (10 μL) and anhydrous pyridine (200 μL) were added to a methylated aglycone (0.6 mg for each). The mixture was stirred overnight at room temperature. The reaction mixture was evaporated to dryness and extracted with EtOAc twice. The EtOAc extracts were purified using an analytical reversed-phase HPLC (YMC-Pack-ODS-A, 250 × 4.6 mm i.d, 5 µm, flow rate 2.0 mL/min, RI detector) using gradient elution from 70% to 100% MeOH in 40 min to yield 1a (0.4 mg, tR = 33 min) and 1b (0.5 mg, tR = 33 min). Using the same procedure, 2a (0.3 mg, tR = 27 min), 2b (0.3 mg, tR = 28 min), 3a (0.3 mg, tR = 32 min) and 3b (0.4 mg, tR = 33 min) were prepared from dokdolipids B and C (2 and 3, 1.0 mg for each), respectively.
Compound 1a: 1H NMR (CD3OD, 600 MHz) δH 7.52−7.42 (10H, m, aromatic), 5.49 (1H, m, H-3′), 5.13 (1H, m, H-17′), 3.57 (3H, s, OMe), 3.55 (3H, s, OMe), 3.50 (3H, s, OMe), 2.64 (2H, dd, H-2′), 1.67 (2H, m, H-4′), 1.54 (2H, m, H-16′), 1.35−1.17 (22H, m), 1.32 (3H, d, H-18′); ESIMS m/z 785.2 [M + Na]+ (Supporting information).
Compound 1b: 1H-NMR (CD3OD, 600 MHz) δH 7.52−7.42 (10H, m, aromatic), 5.46 (1H, m, H-3′), 5.13 (1H, m, H-17′), 3.66 (3H, s, OMe), 3.53 (3H, s, OMe), 3.52 (3H, s, OMe), 2.69 (2H, dd, H-2′), 1.63 (2H, m, H-4′), 1.61 (2H, m, H-16′), 1.35−1.17 (22H, m), 1.23 (3H, d, H-18′); ESIMS m/z 785.4 [M + Na]+ (Supporting information).
Compound 2a: 1H NMR (CD3OD, 600 MHz) δH 7.52−7.42 (10H, m, aromatic), 5.50 (1H, m, H-3′), 3.57 (3H, s, OMe), 3.50 (3H, s, OMe), 2.64 (2H, dd, H-2′), 1.70 (2H, m, H-4′), 2.47 (2H, t, H-16′), 1.35−1.17 (22H, m), 2.12 (3H, s, H-18′); ESIMS m/z 567.4 [M + Na]+ (Supporting information).
Compound 2b: 1H NMR (CD3OD, 600 MHz) δH 7.50−7.42 (10H, m, aromatic), 5.47 (1H, m, H-3′), 3.66 (3H, s, OMe), 3.53 (3H, s, OMe), 2.70 (2H, dd, H-2′), 1.62 (2H, m, H-4′), 2.47 (2H, t, H-16′), 1.35−1.17 (22H, m), 2.12 (3H, s, H-18′); ESIMS m/z 567.3 [M + Na]+ (Supporting information).
Compound 3a: 1H NMR (CD3OD, 600 MHz) δH 7.52−7.42 (10H, m, aromatic), 5.49 (1H, m, H-3′), 5.13 (1H, m, H-17′), 3.57 (3H, s, OMe), 3.55 (3H, s, OMe), 3.50 (3H, s, OMe), 2.64 (2H, dd, H-2′), 1.70 (2H, m, H-4′), 1.55 (2H, m, H-16′), 1.35−1.17 (22H, m), 1.33 (3H, d, H-18′); ESIMS m/z 785.5 [M + Na]+ (Supporting information).
Compound 3b: 1H NMR (CD3OD, 600 MHz) δH 7.52−7.42 (10H, m, aromatic), 5.46 (1H, m, H-3′), 5.13 (1H, m, H-17′), 3.66 (3H, s, OMe), 3.53 (3H, s, OMe), 3.52 (3H, s, OMe), 2.69 (2H, dd, H-2′), 1.66 (2H, m, H-4′), 1.60 (2H, m, H-16′), 1.35−1.17 (22H, m), 1.24 (3H, d, H-18′); ESIMS m/z 785.2 [M + Na]+ (Supporting information).
3.7. Cytotoxicity Test by SRB Assay
Human cancer cell lines HCT-15 (colon), NUGC-3 (stomach), NCI-H23 (lung), ACHN (renal), PC-3 (prostate) and MDA-MB-231 (breast), were purchased from the American Type Culture Collection (Manassas, VA). The cell lines were cultured RPMI 1640 supplemented with 10% fetal bovine serum (FBS). Cell cultures were maintained at 37 °C under a humidified atmosphere of 5% CO2. The growth inhibition assay against human cancer cell lines was carried out according to a sulforhodamine B (SRB) assay [19]. In brief, 8000 cells/well were seeded in a 96-well plate. Next day, the cells were treated with compounds 1–3 including vehicle control (0.1% DMSO) and positive control (adriamycin). After being incubated for 48 hours, cultures were fixed with 50% trichloroactetic acid (50 μg/mL) and stained with 0.4% sulforhodamine B in 1% acetic acid. Unbound dye was removed by washing with 1% acetic acid, and protein-bound dye was extracted with 10 mM Tris base (pH 10.5) for determination of optical density. The absorbance at 540 nm was determined using a VersaMax microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA). GI50 values were calculated using GraphPad Prism 4.0 software (GraphPad Software, Inc., San Diego, CA, USA).
4. Conclusions
In conclusion, the chemical analysis of the marine bacterium Actinoalloteichus hymeniacidonis 179DD-027 led to the isolation of three new hydroxylated rhamnolipids (1−3), and the identification of the sugar moiety as well as the determination of the absolute configuration of the stereogenic carbon in the carboxylic acid moiety. Dokdolipids A (1) and C (3) possess a hydroxyl group at C-17′ with one rhamnose and two rhamnoses respectively. Dokdolipid B (2) contains a ketone group at C-17′ with one rhamnose. To the best of our knowledge, this is the first report describing rhamnolipids with the hydroxyl and ketone groups at the fatty acid chain. All isolated rhamnolipids were tested for cytotoxicity against cancer cell lines, and compounds 1−3 displayed moderate activity. Additionally, compound 2, possessing a ketone group, showed better activity than other compounds. Our research suggested that the discovery of these new rhamnolipids might be useful in expanding the field of rhamnolipid research and developing various industrial applications.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/4/237/s1, Figures S1–S10: HRESI-MS data, 1H NMR, 13C NMR, COSY, HSQC, HMBC, ROESY and experimental spectra of 1; Figures S11–S26: HRESI-MS data, 1H NMR, 13C NMR, COSY, HSQC, HMBC and experimental spectra of 2 and 3.
Author Contributions
H.J.S. was the principal investigator who proposed ideas for the present work, managed and supervised the whole research work, prepared and corrected the manuscript, and contributed to the structure elucidation of the new compounds. B.-K.C. conducted all experiments for compounds 1–3, including fermentation, isolation, and structure elucidation, and prepared the manuscript. H.S.L. contributed to analyzing data. J.S.K. evaluated the cytotoxicity of 1–3.
Funding
This research was supported in part by the Ministry of Oceans and Fisheries, Korea (Grants PG51010 to C.H.P. and PM61100 to J.S.S.).
Acknowledgments
The authors express gratitude to Y. H. Kim, Korea Basic Science Institute, Ochang, Korea, for providing mass data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chong, H.; Li, Q. Microbial Production of Rhamnolipids: Opportunities, Challenges and Strategies. Microb. Cell Fact. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Rikalovic, M.; Gojgic-Cvijovic, G.M.; Vrvic, M.; Karadzic, I. Production and Characterization of Rhamnolipids from Pseudomonas Aeruginosa San Ai. J. Serb. Chem. Soc. 2012, 77, 27–42. [Google Scholar] [CrossRef]
- Rashedi, H.; Mazaheri Assadi, M.; Bonakdarpour, B.; Jamshidi, E. Environmental Importance of Rhamnolipid Production from Molasses as a Carbon Source. Int. J. Environ. Sci. Technol. 2005, 2, 59–62. [Google Scholar] [CrossRef]
- Chayabutra, C.; Wu, J.; Ju, L.K. Rhamnolipid Production by Pseudomonas Aeruginosa Under Denitrification: Effects of Limiting Nutrients and Carbon Substrates. Biotechnol. Bioeng. 2001, 72, 25–33. [Google Scholar] [CrossRef]
- Gunther, N.W.; Nunez, A.; Fett, W.; Solaiman, D.K. Production of Rhamnolipids by Pseudomonas Chlororaphis, a Nonpathogenic Bacterium. Appl. Environ. Microbiol. 2005, 71, 2288–2293. [Google Scholar] [CrossRef] [PubMed]
- Ławniczak, Ł.; Marecik, R.; Chrzanowski, Ł. Contributions of Biosurfactants to Natural or Induced Bioremediation. Appl. Microbiol. Biotechnol. 2013, 97, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Hoskova, M.; Schreiberova, O.; Jezdik, R.; Chudoba, J.; Masak, J.; Sigler, K.; Rezanka, T. Characterization of Rhamnolipids Produced by Non-Pathogenic Acinetobacter and Enterobacter Bacteria. Bioresour. Technol. 2013, 130, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Sinumvayo, J.P. Agriculture and Food Applications of Rhamnolipids and its Production by Pseudomonas Aeruginosa. J. Chem. Eng. Process Technol. 2015, 6. [Google Scholar] [CrossRef]
- Soares dos Santos, A.; Pereira, N., Jr.; Freire, D.M.G. Strategies for Improved Rhamnolipid Production by Pseudomonas Aeruginosa PA1. PeerJ 2016, 4. [Google Scholar] [CrossRef]
- Lang, S.; Wullbrandt, D. Rhamnose lipids–Biosynthesis, Microbial Production and Application Potential. Appl. Microbiol. Biotechnol. 1999, 51, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.; Mulligan, C.N. Environmental Applications for Biosurfactants. Environ. Pollut. 2005, 133, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Sekhon Randhawa, K.K.; Rahman, P.K.S.M. Rhamnolipid Biosurfactants—Past, Present, and Future Scenario of Global Market. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Trischman, J.A.; Jensen, P.R.; Fenical, W. Halobacillin: A Cytotoxic Cyclic Acylpeptide of the Iturin Class Produced by a Marine Bacillus. Tetrahedron Lett. 1994, 35, 5571–5574. [Google Scholar] [CrossRef]
- Xiong, Z.Q.; Wang, J.F.; Hao, Y.Y.; Wang, Y. Recent Advances in the Discovery and Development of Marine microbial Natural Products. Mar. Drugs 2013, 11, 700–717. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Qadir, M.I.; Janbaz, K.H.; Ali, M. Novel Drugs from Marine Microorganisms. Crit. Rev. Microbiol. 2011, 37, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Park, J.; Ryu, J.; Rho, H.S.; Kim, W.; Kim, J.S. Biodiversity Hotspot for Marine Invertebrates around the Dokdo, East Sea, Korea: Ecological Checklist Revisited. Mar. Pollut. Bull. 2017, 119, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-Field FT NMR Application of Mosher’s Method. The absolute Configurations of Marine Terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Shin, B.; Ahn, S.; Noh, M.; Shin, J.; Oh, D.-C. Suncheonosides A–D, Benzothioate Glycosides from a Marine-Derived Streptomyces Sp. J. Nat. Prod. 2015, 78, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).