Antiviral Activity of a Turbot (Scophthalmus maximus) NK-Lysin Peptide by Inhibition of Low-pH Virus-Induced Membrane Fusion
Abstract
1. Introduction
2. Results
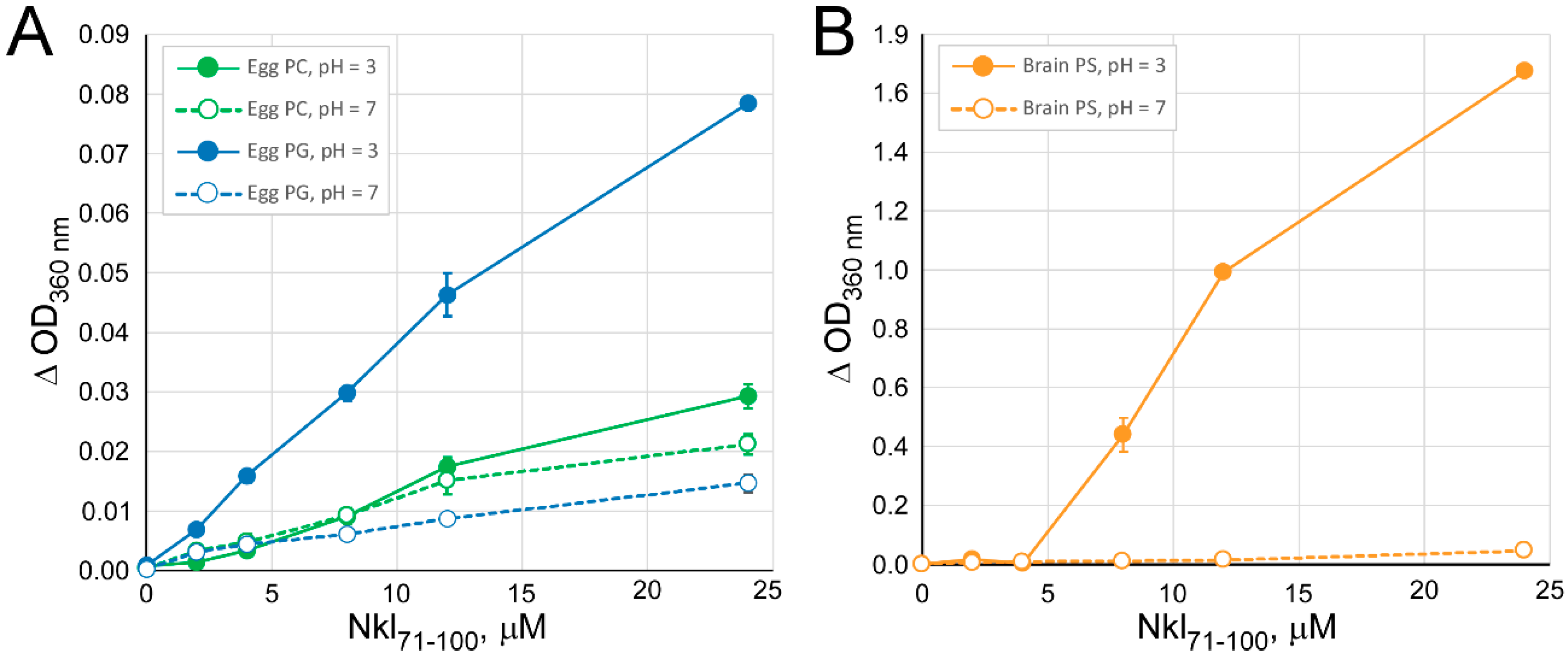
2.1. Interaction of Nkl71–100 with Membranes
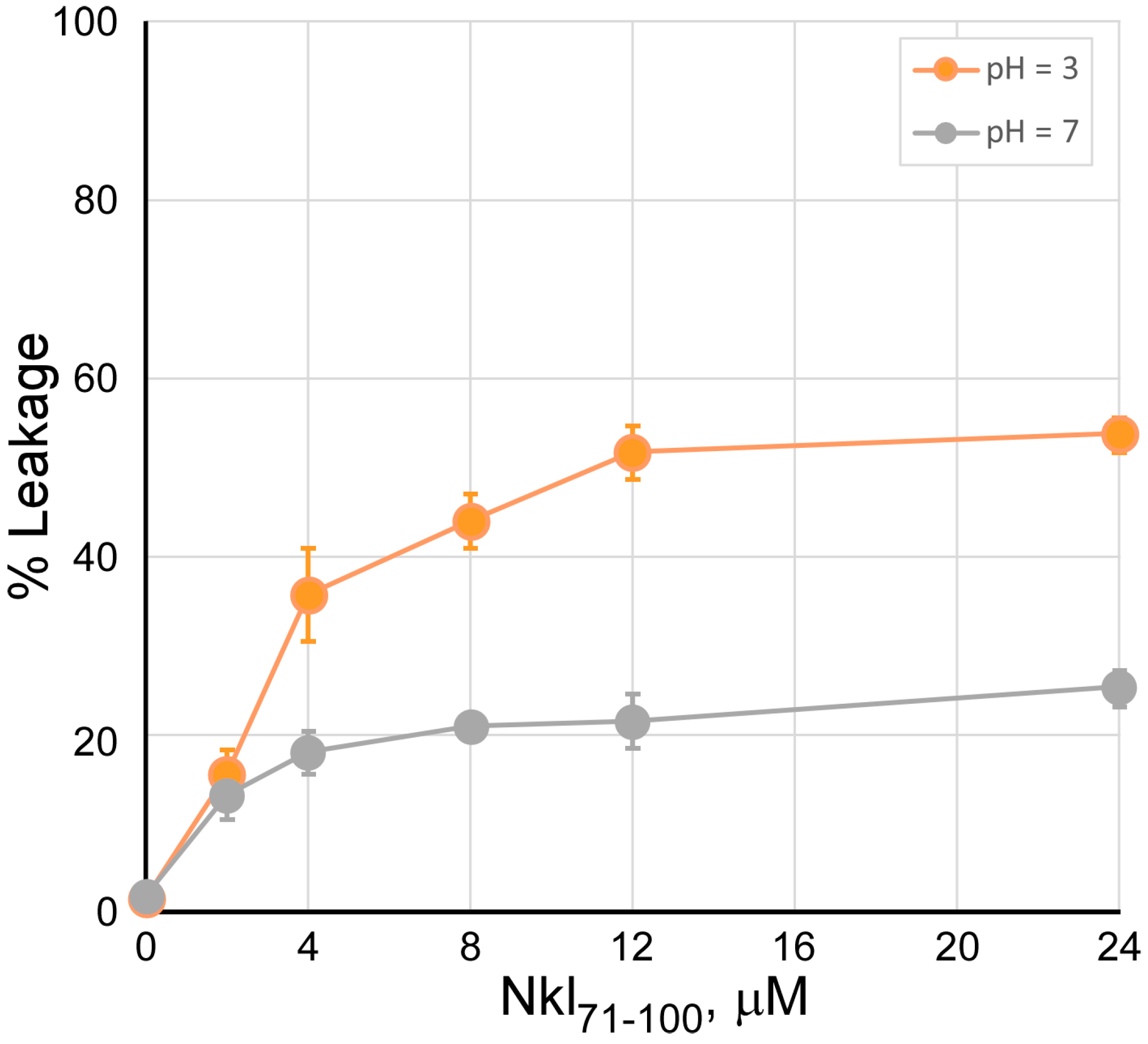
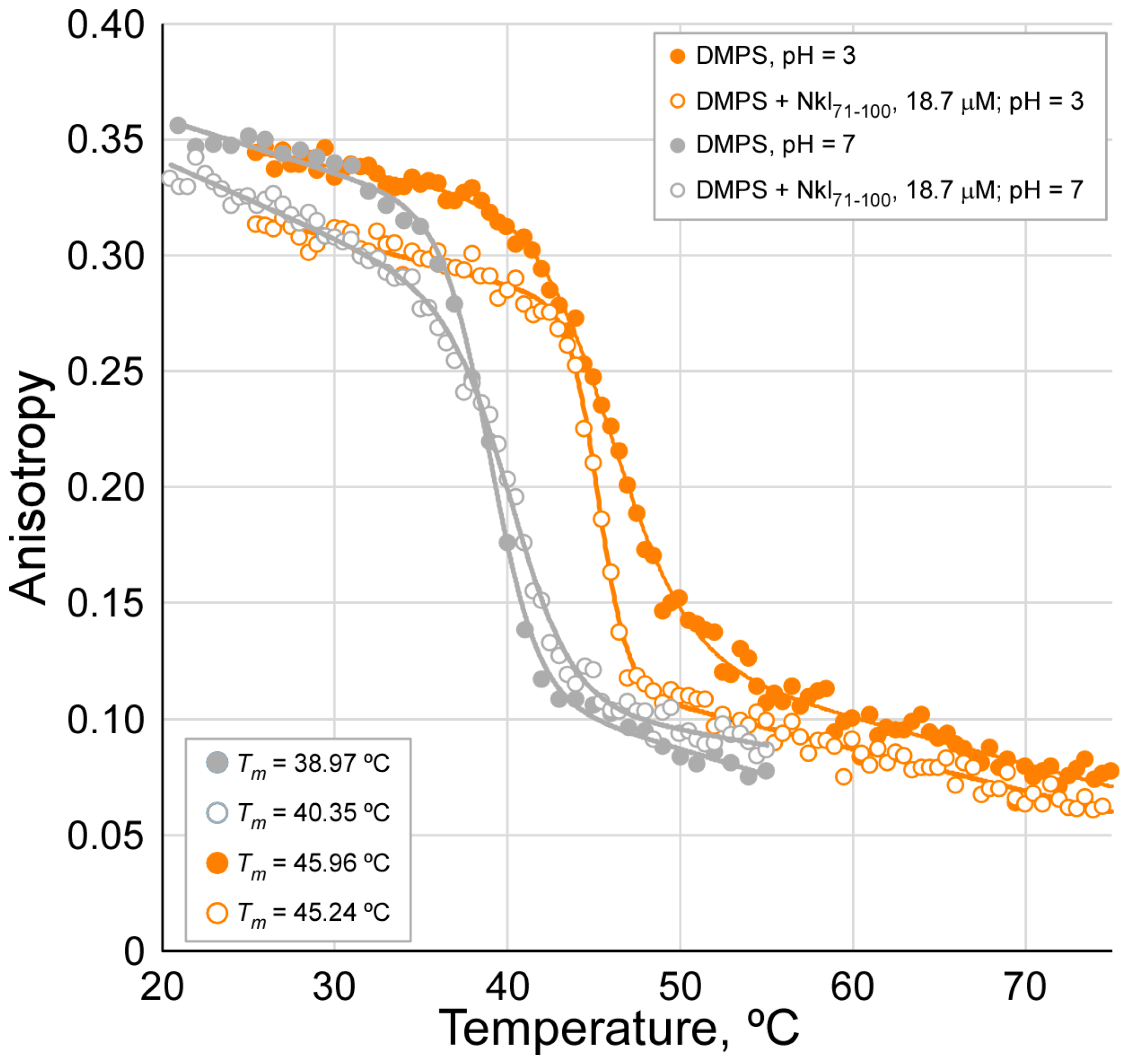
2.2. Studies of Peptide Insertion into Phospholipid Bilayers
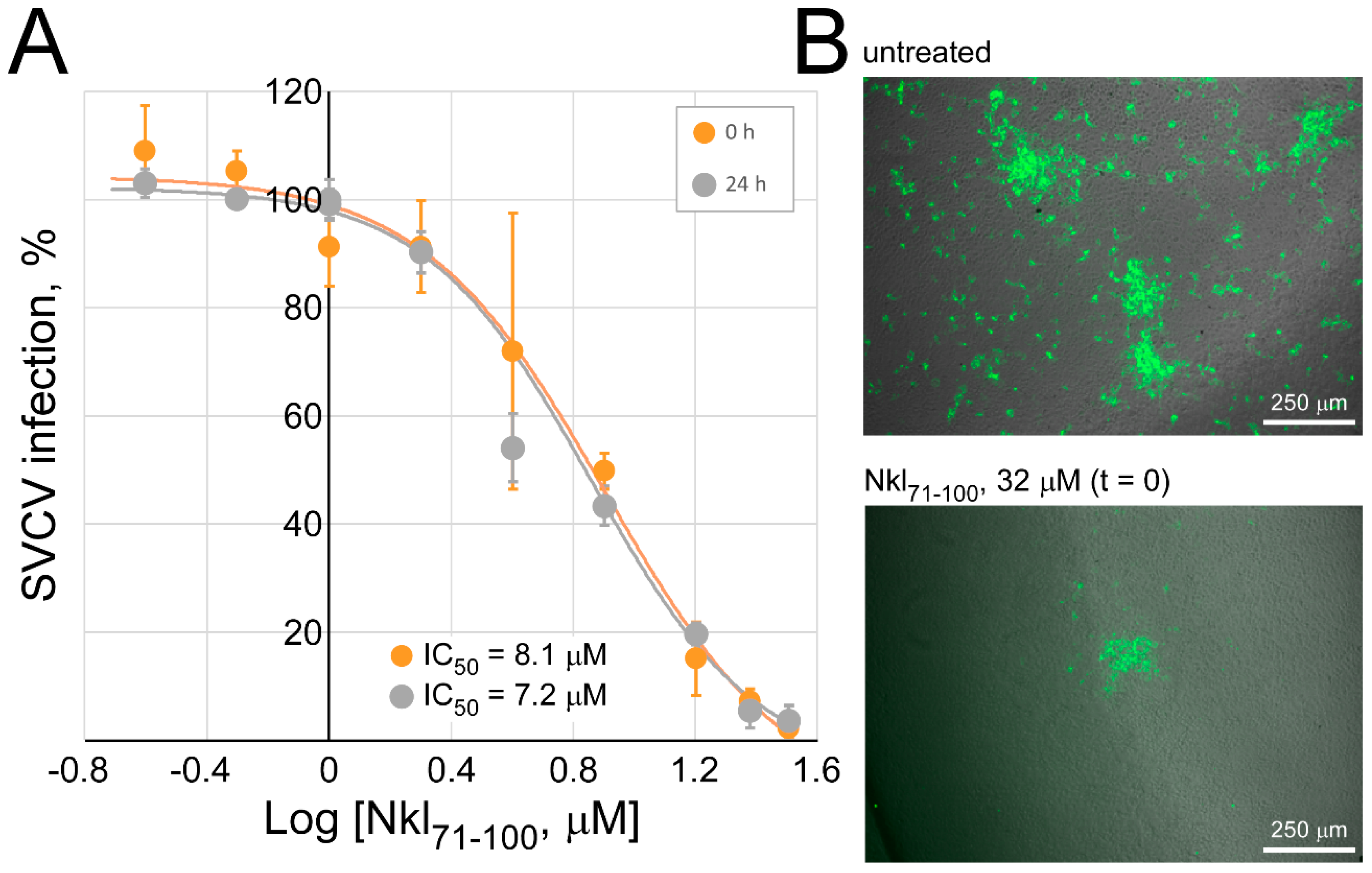
2.3. Assessment of the Nkl71–100 Antiviral Activity
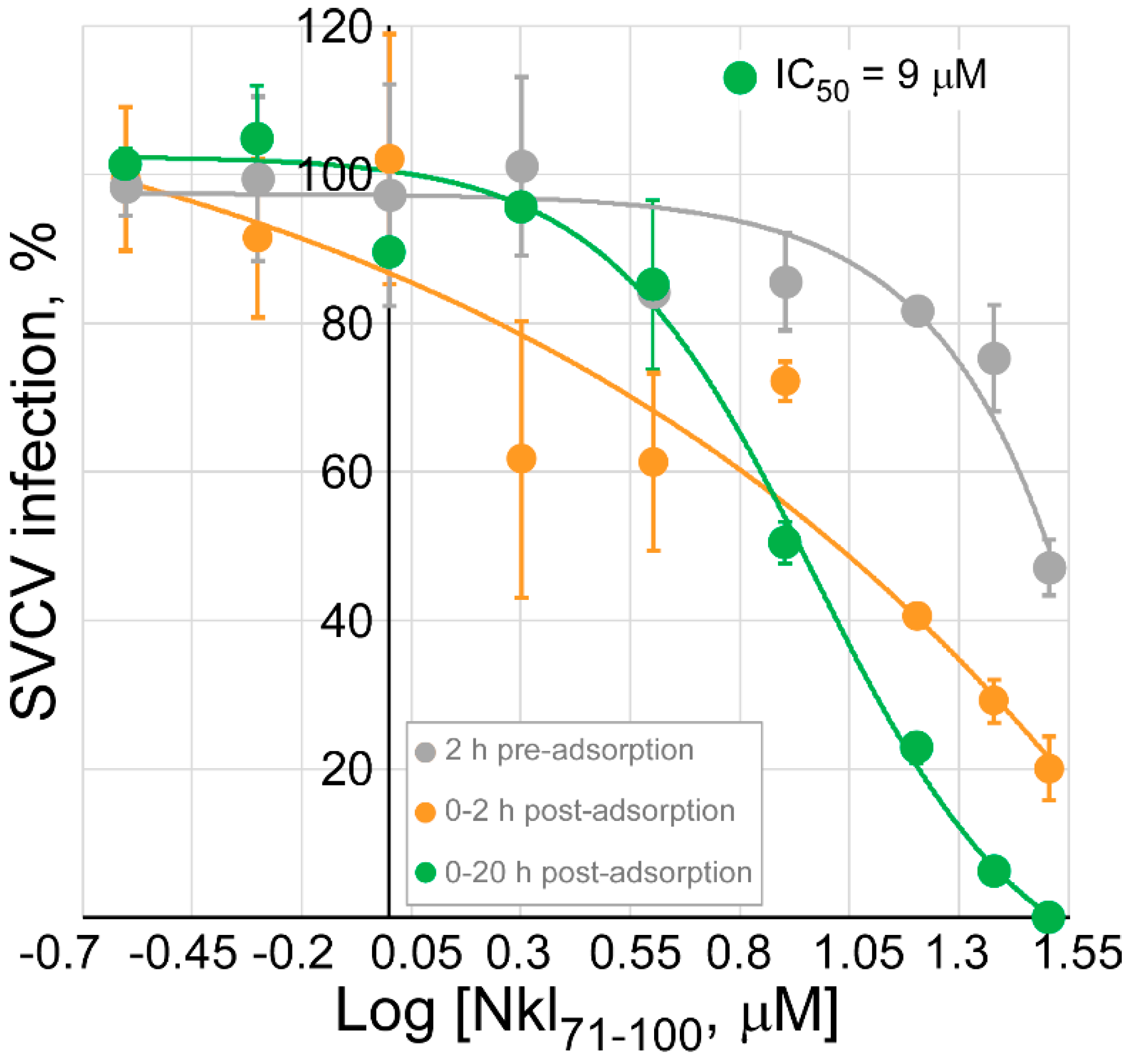
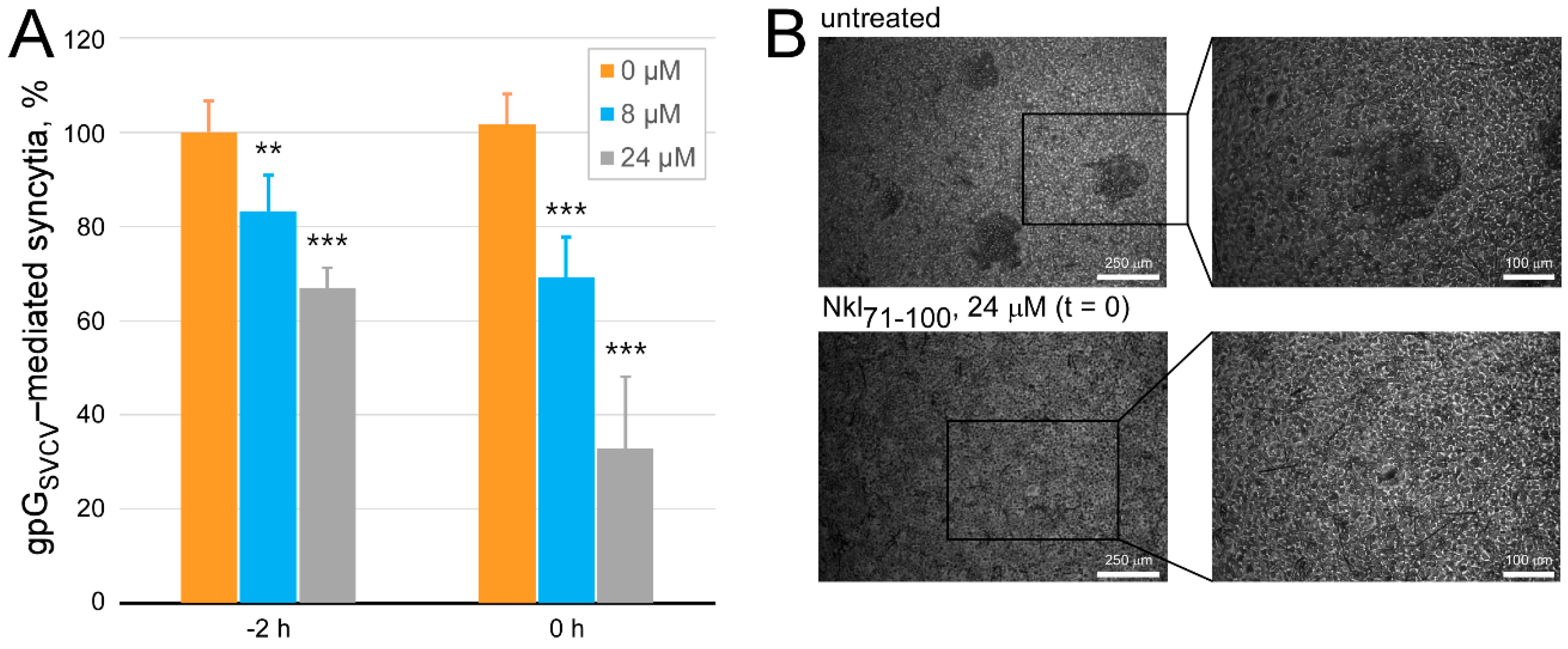
2.4. Determination of the Antiviral Effect of Nkl71–100 on the Viral Entry Stage
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Large Unilamellar Vesicles (LUVs)
4.3. Vesicle Aggregation Assay
4.4. Leakage Assay
4.5. Fluorescence Anisotropy
4.6. Cell lines and Virus
4.7. Cytotoxicity Assays
4.8. Microscopy
4.9. In vitro Viral Infections
4.10. Viral Binding Assays
4.11. RNA Isolation, cDNA Synthesis and qPCR
4.12. Fusion Assays
4.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reperant, L.A.; Osterhaus, A. Aids, avian flu, sars, mers, ebola, zika… What next? Vaccine 2017, 35, 4470–4474. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef]
- Ensoli, B.; Cafaro, A.; Monini, P.; Marcotullio, S.; Ensoli, F. Challenges in HIV vaccine research for treatment and prevention. Front Immunol. 2014, 5, 417. [Google Scholar] [CrossRef] [PubMed]
- Zingaretti, C.; De Francesco, R.; Abrignani, S. Why is it so difficult to develop a hepatitis C virus preventive vaccine? Clin. Microbiol. Infect. 2014, 20 (Suppl. 5), 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R. Vaccines: The real issues in vaccine safety. Nature 2011, 473, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Findlay, E.G.; Currie, S.M.; Davidson, D.J. Cationic host defence peptides: Potential as antiviral therapeutics. BioDrugs 2013, 27, 479–493. [Google Scholar] [CrossRef]
- Vigant, F.; Santos, N.C.; Lee, B. Broad-spectrum antivirals against viral fusion. Nat. Rev. Microbiol. 2015, 13, 426. [Google Scholar] [CrossRef]
- Bello-Pérez, M.; Falcó, A.; Galiano, V.; Coll, J.; Perez, L.; Encinar, J.A. Discovery of nonnucleoside inhibitors of polymerase from infectious pancreatic necrosis virus (IPNV). Drug Des. Devel. Ther. 2018, 12, 2337. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Klotman, M.E.; Chang, T.L. Defensins in innate antiviral immunity. Nat. Rev. Immunol. 2006, 6, 447–456. [Google Scholar] [CrossRef]
- Holzl, M.A.; Hofer, J.; Steinberger, P.; Pfistershammer, K.; Zlabinger, G.J. Host antimicrobial proteins as endogenous immunomodulators. Immunol. Lett. 2008, 119, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, R.; Kolusheva, S. Membrane interactions of host-defense peptides studied in model systems. Curr. Protein Pept. Sci. 2005, 6, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000, 8, 402–410. [Google Scholar] [CrossRef]
- Douglas, S.E.; Patrzykat, A.; Pytyck, J.; Gallant, J.W. Identification, structure and differential expression of novel pleurocidins clustered on the genome of the winter flounder, Pseudopleuronectes americanus (Walbaum). Eur. J. Biochem. 2003, 270, 3720–3730. [Google Scholar] [CrossRef] [PubMed]
- Falco, A.; Ortega-Villaizan, M.; Chico, V.; Brocal, I.; Perez, L.; Coll, J.M.; Estepa, A. Antimicrobial peptides as model molecules for the development of novel antiviral agents in aquaculture. Mini Rev. Med. Chem. 2009, 9, 1159–1164. [Google Scholar] [CrossRef]
- Masso-Silva, J.A.; Diamond, G. Antimicrobial peptides from fish. Pharmaceuticals 2014, 7, 265–310. [Google Scholar] [CrossRef]
- Cai, S.; Wang, J.; Wang, K.; Chen, D.; Dong, X.; Liu, T.; Zeng, Y.; Wang, X.; Wu, D. Expression, purification and antibacterial activity of NK-lysin mature peptides from the channel catfish (Ictalurus punctatus). Appl. Sci. 2016, 6, 240. [Google Scholar] [CrossRef]
- Wang, G.L.; Wang, M.C.; Liu, Y.L.; Zhang, Q.; Li, C.F.; Liu, P.T.; Li, E.Z.; Nie, P.; Xie, H.X. Identification, expression analysis, and antibacterial activity of NK-lysin from common carp Cyprinus carpio. Fish Shellfish Immunol. 2018, 73, 11–21. [Google Scholar] [CrossRef]
- Hirono, I.; Kondo, H.; Koyama, T.; Arma, N.R.; Hwang, J.Y.; Nozaki, R.; Midorikawa, N.; Aoki, T. Characterization of japanese flounder (Paralichthys olivaceus) NK-lysin, an antimicrobial peptide. Fish Shellfish Immunol. 2007, 22, 567–575. [Google Scholar] [CrossRef]
- Zhou, Q.-J.; Wang, J.; Liu, M.; Qiao, Y.; Hong, W.-S.; Su, Y.-Q.; Han, K.-H.; Ke, Q.-Z.; Zheng, W.-Q. Identification, expression and antibacterial activities of an antimicrobial peptide NK-lysin from a marine fish Larimichthys crocea. Fish Shellfish Immunol. 2016, 55, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Stenger, S.; Hanson, D.A.; Teitelbaum, R.; Dewan, P.; Niazi, K.R.; Froelich, C.J.; Ganz, T.; Thoma-Uszynski, S.; Melian, A.; Bogdan, C.; et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998, 282, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Gansert, J.L.; Kiessler, V.; Engele, M.; Wittke, F.; Rollinghoff, M.; Krensky, A.M.; Porcelli, S.A.; Modlin, R.L.; Stenger, S. Human NKT cells express granulysin and exhibit antimycobacterial activity. J. Immunol. 2003, 170, 3154–3161. [Google Scholar] [CrossRef] [PubMed]
- Ernst, W.A.; Thoma-Uszynski, S.; Teitelbaum, R.; Ko, C.; Hanson, D.A.; Clayberger, C.; Krensky, A.M.; Leippe, M.; Bloom, B.R.; Ganz, T.; et al. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J. Immunol. 2000, 165, 7102–7108. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Zerboni, L.; Sommer, M.; Kaspar, A.A.; Clayberger, C.; Krensky, A.M.; Arvin, A.M. Granulysin blocks replication of varicella-zoster virus and triggers apoptosis of infected cells. Viral Immunol. 2001, 14, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.; Bruhn, H.; Gaworski, I.; Fleischer, B.; Leippe, M. NK-lysin and its shortened analog NK-2 exhibit potent activities against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2003, 47, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Lama, R.; Pereiro, P.; Costa, M.M.; Encinar, J.A.; Medina-Gali, R.M.; Perez, L.; Lamas, J.; Leiro, J.; Figueras, A.; Novoa, B. Turbot (Scophthalmus maximus) NK-lysin induces protection against the pathogenic parasite philasterides dicentrarchi via membrane disruption. Fish Shellfish Immunol. 2018, 82, 190–199. [Google Scholar] [CrossRef]
- Hong, Y.H.; Lillehoj, H.S.; Siragusa, G.R.; Bannerman, D.D.; Lillehoj, E.P. Antimicrobial activity of chicken NK-lysin against Eimeria sporozoites. Avian Dis. 2008, 52, 302–305. [Google Scholar] [CrossRef]
- Fan, K.; Li, H.; Wang, Z.; Du, W.; Yin, W.; Sun, Y.; Jiang, J. Expression and purification of the recombinant porcine NK-lysin in Pichia pastoris and observation of anticancer activity in vitro. Prep. Biochem. Biotechnol. 2016, 46, 65–70. [Google Scholar] [CrossRef]
- Andersson, M.; Gunne, H.; Agerberth, B.; Boman, A.; Bergman, T.; Sillard, R.; Jörnvall, H.; Mutt, V.; Olsson, B.; Wigzell, H. NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. EMBO J. 1995, 14, 1615–1625. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, B.; Wang, Y.; Peatman, E.; Liu, Z. Characterization of a NK-lysin antimicrobial peptide gene from channel catfish. Fish Shellfish Immunol. 2006, 20, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Lillehoj, H.S.; Dalloul, R.A.; Min, W.; Miska, K.B.; Tuo, W.; Lee, S.H.; Han, J.Y.; Lillehoj, E.P. Molecular cloning and characterization of chicken NK-lysin. Vet. Immunol. Immunopathol. 2006, 110, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Pena, S.V.; Krensky, A.M. Granulysin, a new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity. Semin. Immunol. 1997, 9, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Munford, R.S.; Sheppard, P.O.; O’Hara, P.J. Saposin-like proteins (SAPLIP) carry out diverse functions on a common backbone structure. J. Lipid Res. 1995, 36, 1653–1663. [Google Scholar] [PubMed]
- Olmeda, B.; Garcia-Alvarez, B.; Perez-Gil, J. Structure-function correlations of pulmonary surfactant protein SP-B and the saposin-like family of proteins. Eur. Biophys. J. 2013, 42, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Saier, M.H., Jr. The amoebapore superfamily. Biochim. Biophys. Acta 2000, 1469, 87–99. [Google Scholar] [CrossRef]
- Gonzalez, C.; Langdon, G.M.; Bruix, M.; Galvez, A.; Valdivia, E.; Maqueda, M.; Rico, M. Bacteriocin AS-48, a microbial cyclic polypeptide structurally and functionally related to mammalian NK-lysin. Proc. Natl. Acad. Sci. USA 2000, 97, 11221–11226. [Google Scholar] [CrossRef]
- Kervinen, J.; Tobin, G.J.; Costa, J.; Waugh, D.S.; Wlodawer, A.; Zdanov, A. Crystal structure of plant aspartic proteinase prophytepsin: Inactivation and vacuolar targeting. EMBO J. 1999, 18, 3947–3955. [Google Scholar] [CrossRef]
- Ponting, C.P. Acid sphingomyelinase possesses a domain homologous to its activator proteins: Saposins B and D. Protein Sci. 1994, 3, 359–361. [Google Scholar] [CrossRef]
- Hagen, F.S.; Grant, F.J.; Kuijper, J.L.; Slaughter, C.A.; Moomaw, C.R.; Orth, K.; O’Hara, P.J.; Munford, R.S. Expression and characterization of recombinant human acyloxyacyl hydrolase, a leukocyte enzyme that deacylates bacterial lipopolysaccharides. Biochemistry 1991, 30, 8415–8423. [Google Scholar] [CrossRef]
- Anderson, D.H.; Sawaya, M.R.; Cascio, D.; Ernst, W.; Modlin, R.; Krensky, A.; Eisenberg, D. Granulysin crystal structure and a structure-derived lytic mechanism. J. Mol. Biol. 2003, 325, 355–365. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.F.; Sun, L. NKLP27: A teleost NK-lysin peptide that modulates immune response, induces degradation of bacterial DNA, and inhibits bacterial and viral infection. PLoS ONE 2014, 9, e106543. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Choice, E.; Kaspar, A.; Hanson, D.; Okada, S.; Lyu, S.C.; Krensky, A.M.; Clayberger, C. Bactericidal and tumoricidal activities of synthetic peptides derived from granulysin. J. Immunol. 2000, 165, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Carreno, C.; Linde, C.; Boman, H.G.; Andersson, M. Identification of an anti-mycobacterial domain in NK-lysin and granulysin. Biochem. J. 1999, 344 Pt 3, 845–849. [Google Scholar] [CrossRef]
- Dassanayake, R.P.; Falkenberg, S.M.; Briggs, R.E.; Tatum, F.M.; Sacco, R.E. Antimicrobial activity of bovine NK-lysin-derived peptides on bovine respiratory pathogen Histophilus somni. PLoS ONE 2017, 12, e0183610. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huddleston, J.; Buckley, R.M.; Malig, M.; Lawhon, S.D.; Skow, L.C.; Lee, M.O.; Eichler, E.E.; Andersson, L.; Womack, J.E. Bovine NK-lysin: Copy number variation and functional diversification. Proc. Natl. Acad. Sci. USA 2015, 112, E7223–E7229. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, C.; Tizioto, P.C.; Huang, H.; Lee, M.O.; Payne, H.R.; Lawhon, S.D.; Schroeder, F.; Taylor, J.F.; Womack, J.E. Expression of the bovine NK-lysin gene family and activity against respiratory pathogens. PLoS ONE 2016, 11, e0158882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Long, H.; Sun, L. A NK-lysin from Cynoglossus semilaevis enhances antimicrobial defense against bacterial and viral pathogens. Dev. Comp. Immunol. 2013, 40, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, P.; Varela, M.; Diaz-Rosales, P.; Romero, A.; Dios, S.; Figueras, A.; Novoa, B. Zebrafish NK-lysins: First insights about their cellular and functional diversification. Dev. Comp. Immunol. 2015, 51, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, Q.; Niu, J.; Tang, J.; Wang, B.; Abarike, E.D.; Lu, Y.; Cai, J.; Jian, J. NK-lysin from Oreochromis niloticus improves antimicrobial defence against bacterial pathogens. Fish Shellfish Immunol. 2018, 72, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, P.; Romero, A.; Diaz-Rosales, P.; Estepa, A.; Figueras, A.; Novoa, B. Nucleated teleost erythrocytes play an NK-lysin- and autophagy-dependent role in antiviral immunity. Front. Immunol. 2017, 8, 1458. [Google Scholar] [CrossRef] [PubMed]
- Ellens, H.; Bentz, J.; Szoka, F.C. Proton-and calcium-induced fusion and destabilization of liposomes. Biochemistry 1985, 24, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Lentz, B.R.; Barenholz, Y.; Thompson, T.E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 2 two-component phosphatidylcholine liposomes. Biochemistry 1976, 15, 4529–4537. [Google Scholar] [CrossRef] [PubMed]
- Tsui, F.C.; Ojcius, D.M.; Hubbell, W.L. The intrinsic pka values for phosphatidylserine and phosphatidylethanolamine in phosphatidylcholine host bilayers. Biophys. J. 1986, 49, 459–468. [Google Scholar] [CrossRef]
- Pöhlmann, S.; Simmons, G. Viral Entry into Host Cells; Springer: New York, NY, USA, 2013. [Google Scholar]
- Falco, A.; Mas, V.; Tafalla, C.; Perez, L.; Coll, J.M.; Estepa, A. Dual antiviral activity of human alpha-defensin-1 against viral haemorrhagic septicaemia rhabdovirus (VHSV): Inactivation of virus particles and induction of a type I interferon-related response. Antiviral Res. 2007, 76, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Leippe, M. Ancient weapons: Nk-lysin, is a mammalian homolog to pore-forming peptides of a protozoan parasite. Cell 1995, 83, 17–18. [Google Scholar] [CrossRef]
- Ruysschaert, J.M.; Goormaghtigh, E.; Homble, F.; Andersson, M.; Liepinsh, E.; Otting, G. Lipid membrane binding of Nk-lysin. FEBS Lett. 1998, 425, 341–344. [Google Scholar] [CrossRef]
- Schoggins, J.W.; Randall, G. Lipids in innate antiviral defense. Cell Host Microb. 2013, 14, 379–385. [Google Scholar] [CrossRef]
- Birge, R.B.; Boeltz, S.; Kumar, S.; Carlson, J.; Wanderley, J.; Calianese, D.; Barcinski, M.; Brekken, R.A.; Huang, X.; Hutchins, J.T.; et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016, 23, 962–978. [Google Scholar] [CrossRef]
- Sharma, B.; Kanwar, S.S. Phosphatidylserine: A cancer cell targeting biomarker. Semin. Cancer Biol. 2018, 52, 17–25. [Google Scholar] [CrossRef]
- De, M.; Ghosh, S.; Sen, T.; Shadab, M.; Banerjee, I.; Basu, S.; Ali, N. A novel therapeutic strategy for cancer using phosphatidylserine targeting stearylamine-bearing cationic liposomes. Mol. Ther. Nucleic. Acids 2018, 10, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; de Kroon, A.I. Lipid map of the mammalian cell. J. Cell Sci. 2011, 124, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Gunne, H.; Agerberth, B.; Boman, A.; Bergman, T.; Olsson, B.; Dagerlind, A.; Wigzell, H.; Boman, H.G.; Gudmundsson, G.H. Nk-lysin, structure and function of a novel effector molecule of porcine T and Nk cells. Vet Immunol. Immunopathol. 1996, 54, 123–126. [Google Scholar] [CrossRef]
- Bankovic, J.; Andra, J.; Todorovic, N.; Podolski-Renic, A.; Milosevic, Z.; Miljkovic, D.; Krause, J.; Ruzdijic, S.; Tanic, N.; Pesic, M. The elimination of p-glycoprotein over-expressing cancer cells by antimicrobial cationic peptide Nk-2: The unique way of multi-drug resistance modulation. Exp. Cell Res. 2013, 319, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Schroder-Borm, H.; Bakalova, R.; Andra, J. The Nk-lysin derived peptide Nk-2 preferentially kills cancer cells with increased surface levels of negatively charged phosphatidylserine. FEBS Lett. 2005, 579, 6128–6134. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.X.; Wang, K.R.; Chen, R.; Song, J.J.; Zhang, B.Z.; Dang, W.; Zhang, W.; Wang, R. Membrane active antitumor activity of nk-18, a mammalian nk-lysin-derived cationic antimicrobial peptide. Biochimie 2012, 94, 184–191. [Google Scholar] [CrossRef]
- Yan, J.; Liang, X.; Bai, C.; Zhou, L.; Li, J.; Wang, K.; Tang, Y.; Zhao, L. Nk-18, a promising antimicrobial peptide: Anti-multidrug resistant leukemia cells and LPS neutralizing properties. Biochimie 2018, 147, 143–152. [Google Scholar] [CrossRef]
- Amara, A.; Mercer, J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 2015, 13, 461–469. [Google Scholar] [CrossRef]
- Gaudin, Y.; Ruigrok, R.W.; Tuffereau, C.; Knossow, M.; Flamand, A. Rabies virus glycoprotein is a trimer. Virology 1992, 187, 627–632. [Google Scholar] [CrossRef]
- Coll, J.M. Synthetic peptides from the heptad repeats of the glycoproteins of rabies, vesicular stomatitis and fish rhabdoviruses bind phosphatidylserine. Arch. Virol. 1997, 142, 2089–2097. [Google Scholar] [CrossRef]
- Estepa, A.; Coll, J.M. Pepscan mapping and fusion-related properties of the major phosphatidylserine-binding domain of the glycoprotein of viral hemorrhagic septicemia virus, a salmonid rhabdovirus. Virology 1996, 216, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Estepa, A.; Coll, J.M. Phosphatidylserine binding to solid-phase rhabdoviral peptides: A new method to study phospholipid/viral protein interactions. J. Virol. Methods 1996, 61, 37–45. [Google Scholar] [CrossRef]
- Schlegel, R.; Willingham, M.C.; Pastan, I.H. Saturable binding sites for vesicular stomatitis virus on the surface of vero cells. J. Virol. 1982, 43, 871–875. [Google Scholar] [PubMed]
- Coll, J.M. Heptad-repeat sequences in the glycoprotein of rhabdoviruses. Virus Genes 1995, 10, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Estepa, A.; Coll, J. Temperature and pH requirements for viral haemorrhagic septicemia virus induced cell fusion. Dis. Aquat. Org. 1997, 28, 185–189. [Google Scholar] [CrossRef]
- Estepa, A.M.; Rocha, A.I.; Mas, V.; Perez, L.; Encinar, J.A.; Nunez, E.; Fernandez, A.; Gonzalez Ros, J.M.; Gavilanes, F.; Coll, J.M. A protein g fragment from the salmonid viral hemorrhagic septicemia rhabdovirus induces cell-to-cell fusion and membrane phosphatidylserine translocation at low pH. J. Biol. Chem. 2001, 276, 46268–46275. [Google Scholar] [CrossRef] [PubMed]
- Estepa, A.; Fernandez-Alonso, M.; Coll, J.M. Structure, binding and neutralization of VHSV with synthetic peptides. Virus Res. 1999, 63, 27–34. [Google Scholar] [CrossRef]
- Nunez, E.; Fernandez, A.M.; Estepa, A.; Gonzalez-Ros, J.M.; Gavilanes, F.; Coll, J.M. Phospholipid interactions of a peptide from the fusion-related domain of the glycoprotein of VHSV, a fish rhabdovirus. Virology 1998, 243, 322–330. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nemesio, H.; Palomares-Jerez, M.F.; Villalain, J. Hydrophobic segment of dengue virus c protein. Interaction with model membranes. Mol. Membr. Biol. 2013, 30, 273–287. [Google Scholar] [CrossRef]
- Fijan, N.; Petrinec, Z.; Sulimanovic, D.; Zwillenberg, L. Isolation of the viral causative agent from the acute form of infectious dropsy of carp. Veterinarski arhiv. 1971, 41, 125–138. [Google Scholar]
- Espin-Palazon, R.; Martinez-Lopez, A.; Roca, F.J.; Lopez-Munoz, A.; Tyrkalska, S.D.; Candel, S.; Garcia-Moreno, D.; Falco, A.; Meseguer, J.; Estepa, A.; et al. TNFα impairs rhabdoviral clearance by inhibiting the host autophagic antiviral response. PLoS Pathog. 2016, 12, e1005699. [Google Scholar] [CrossRef] [PubMed]
- Bello-Perez, M.; Falco, A.; Medina-Gali, R.; Pereiro, P.; Encinar, J.A.; Novoa, B.; Perez, L.; Coll, J. Neutralization of viral infectivity by zebrafish c-reactive protein isoforms. Mol. Immunol. 2017, 91, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Falco, A.; Chico, V.; Marroqui, L.; Perez, L.; Coll, J.M.; Estepa, A. Expression and antiviral activity of a beta-defensin-like peptide identified in the rainbow trout (Oncorhynchus mykiss) EST sequences. Mol. Immunol. 2008, 45, 757–765. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falco, A.; Medina-Gali, R.M.; Poveda, J.A.; Bello-Perez, M.; Novoa, B.; Encinar, J.A. Antiviral Activity of a Turbot (Scophthalmus maximus) NK-Lysin Peptide by Inhibition of Low-pH Virus-Induced Membrane Fusion. Mar. Drugs 2019, 17, 87. https://doi.org/10.3390/md17020087
Falco A, Medina-Gali RM, Poveda JA, Bello-Perez M, Novoa B, Encinar JA. Antiviral Activity of a Turbot (Scophthalmus maximus) NK-Lysin Peptide by Inhibition of Low-pH Virus-Induced Membrane Fusion. Marine Drugs. 2019; 17(2):87. https://doi.org/10.3390/md17020087
Chicago/Turabian StyleFalco, Alberto, Regla María Medina-Gali, José Antonio Poveda, Melissa Bello-Perez, Beatriz Novoa, and José Antonio Encinar. 2019. "Antiviral Activity of a Turbot (Scophthalmus maximus) NK-Lysin Peptide by Inhibition of Low-pH Virus-Induced Membrane Fusion" Marine Drugs 17, no. 2: 87. https://doi.org/10.3390/md17020087
APA StyleFalco, A., Medina-Gali, R. M., Poveda, J. A., Bello-Perez, M., Novoa, B., & Encinar, J. A. (2019). Antiviral Activity of a Turbot (Scophthalmus maximus) NK-Lysin Peptide by Inhibition of Low-pH Virus-Induced Membrane Fusion. Marine Drugs, 17(2), 87. https://doi.org/10.3390/md17020087





