Heterologous Expression of the Marine-Derived Quorum Quenching Enzyme MomL Can Expand the Antibacterial Spectrum of Bacillus brevis
Abstract
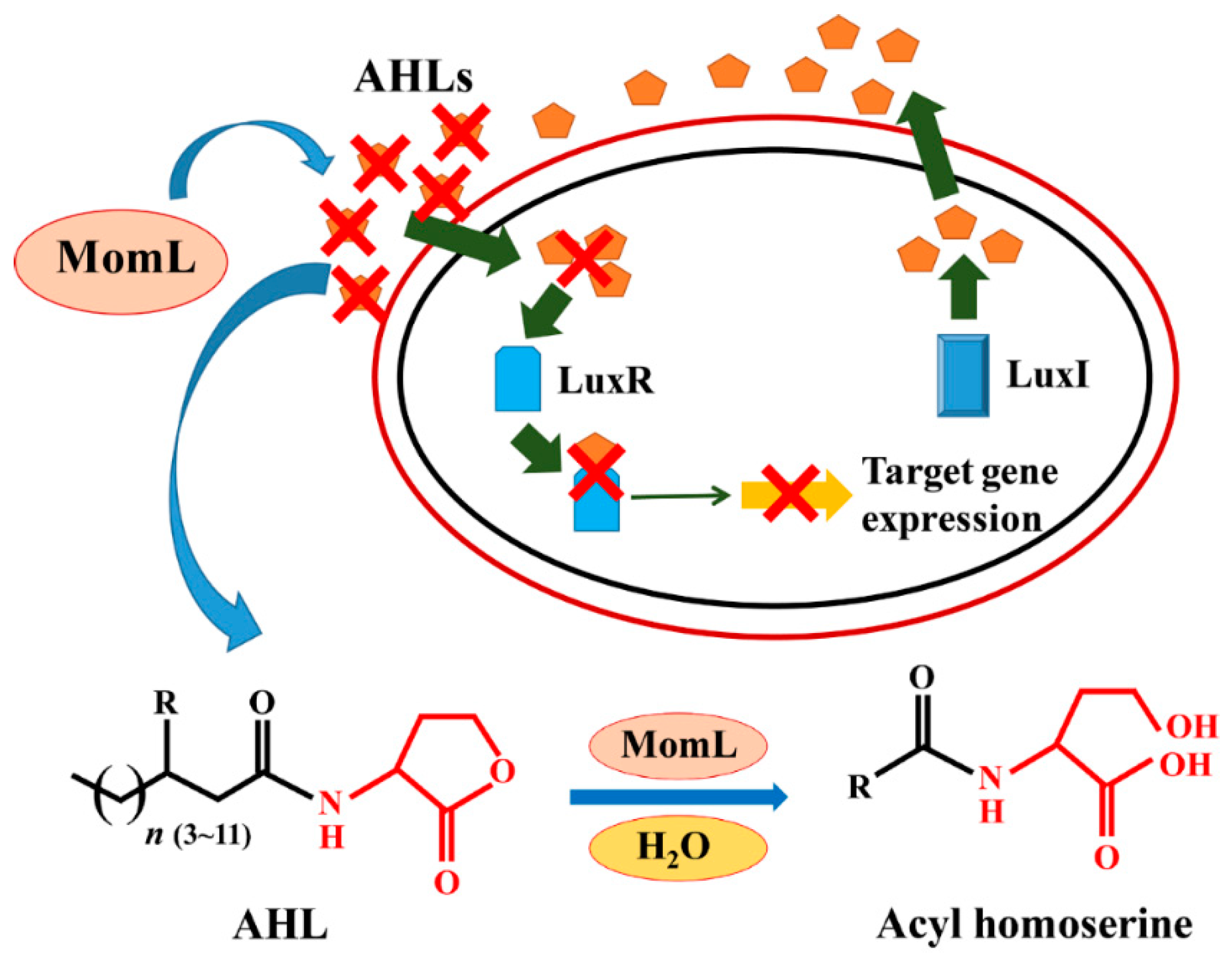
1. Introduction
2. Results
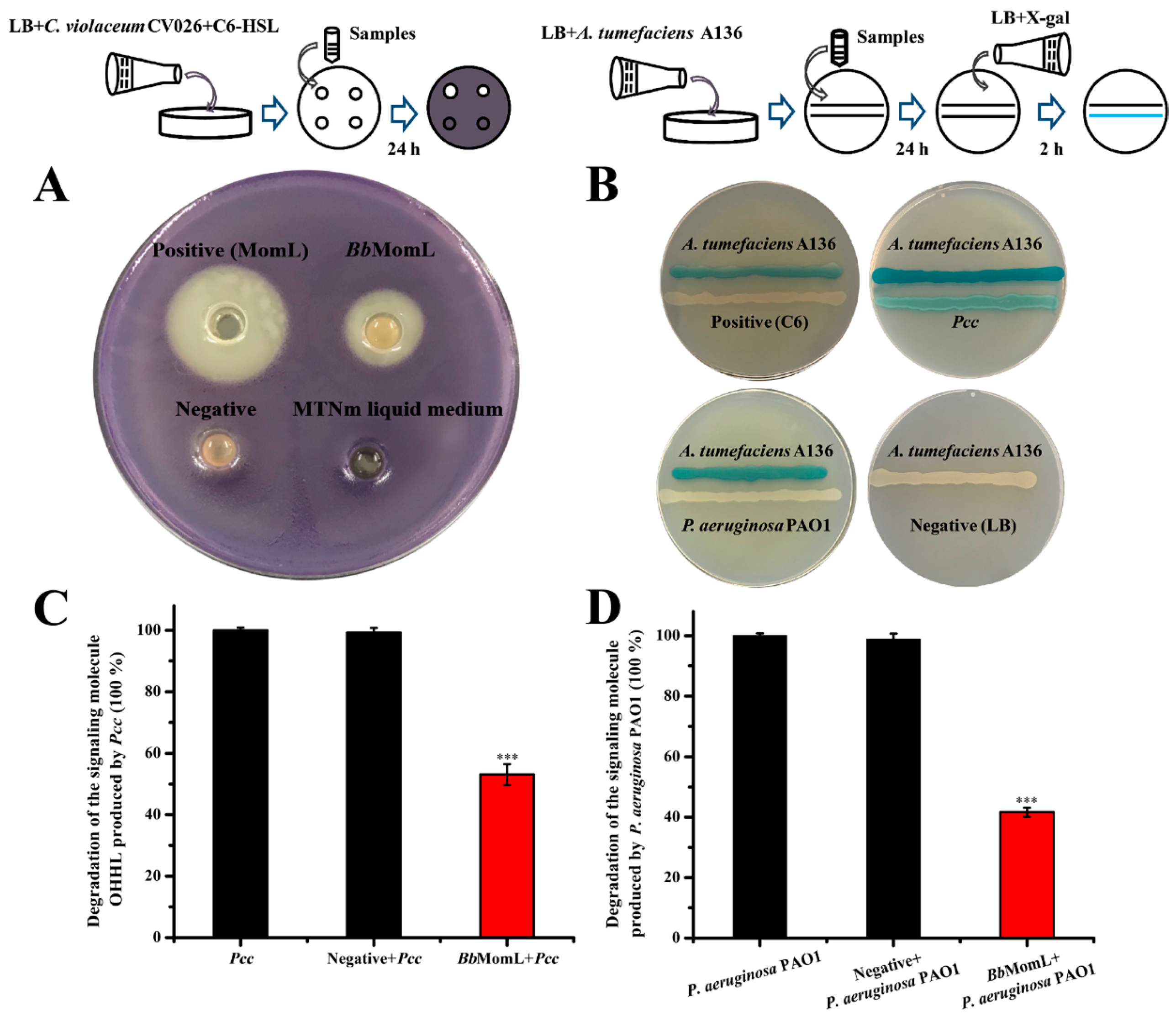
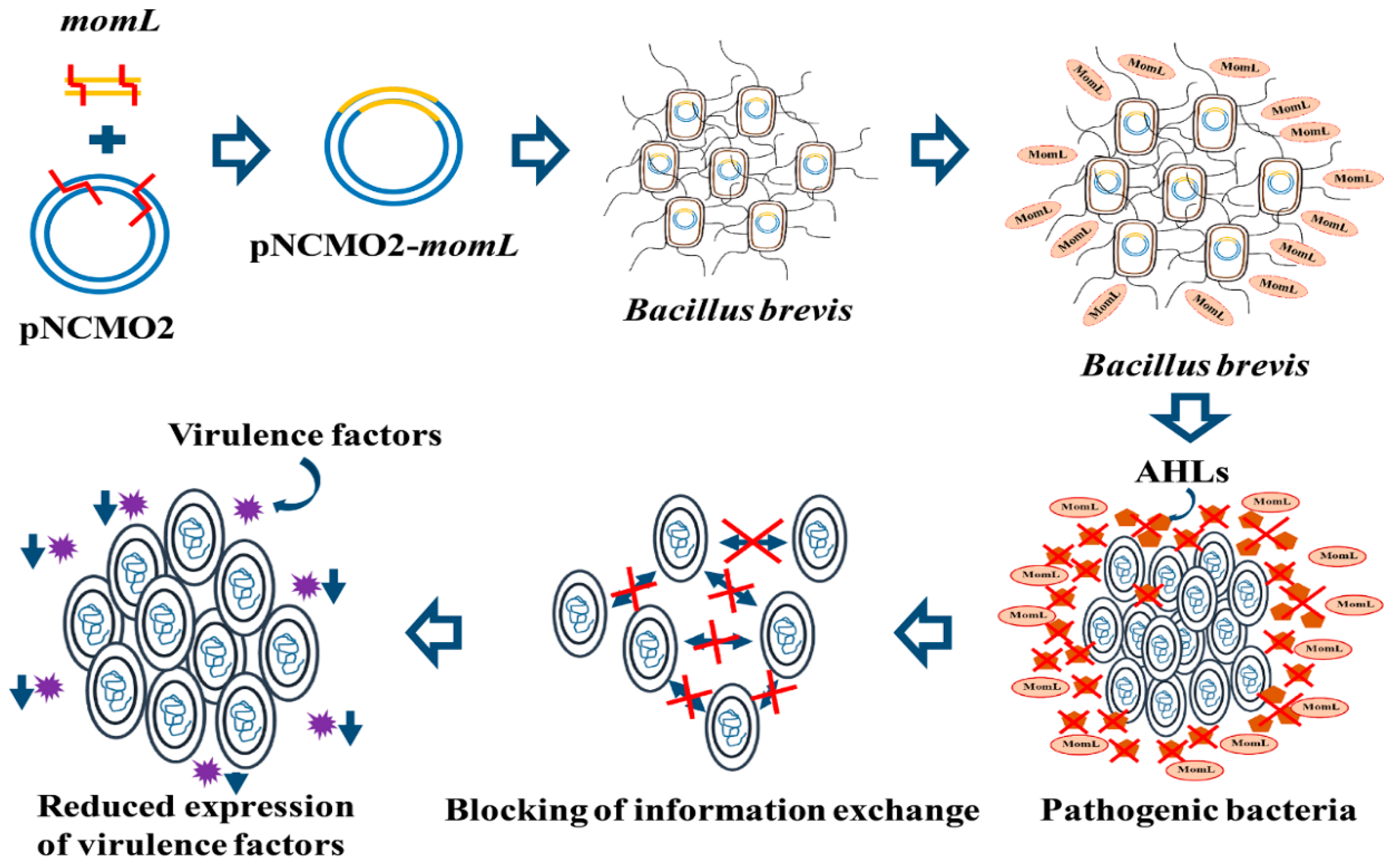
2.1. Construction of the Recombinant Expression Strain BbMomL and Detection of AHL Degradation Activity
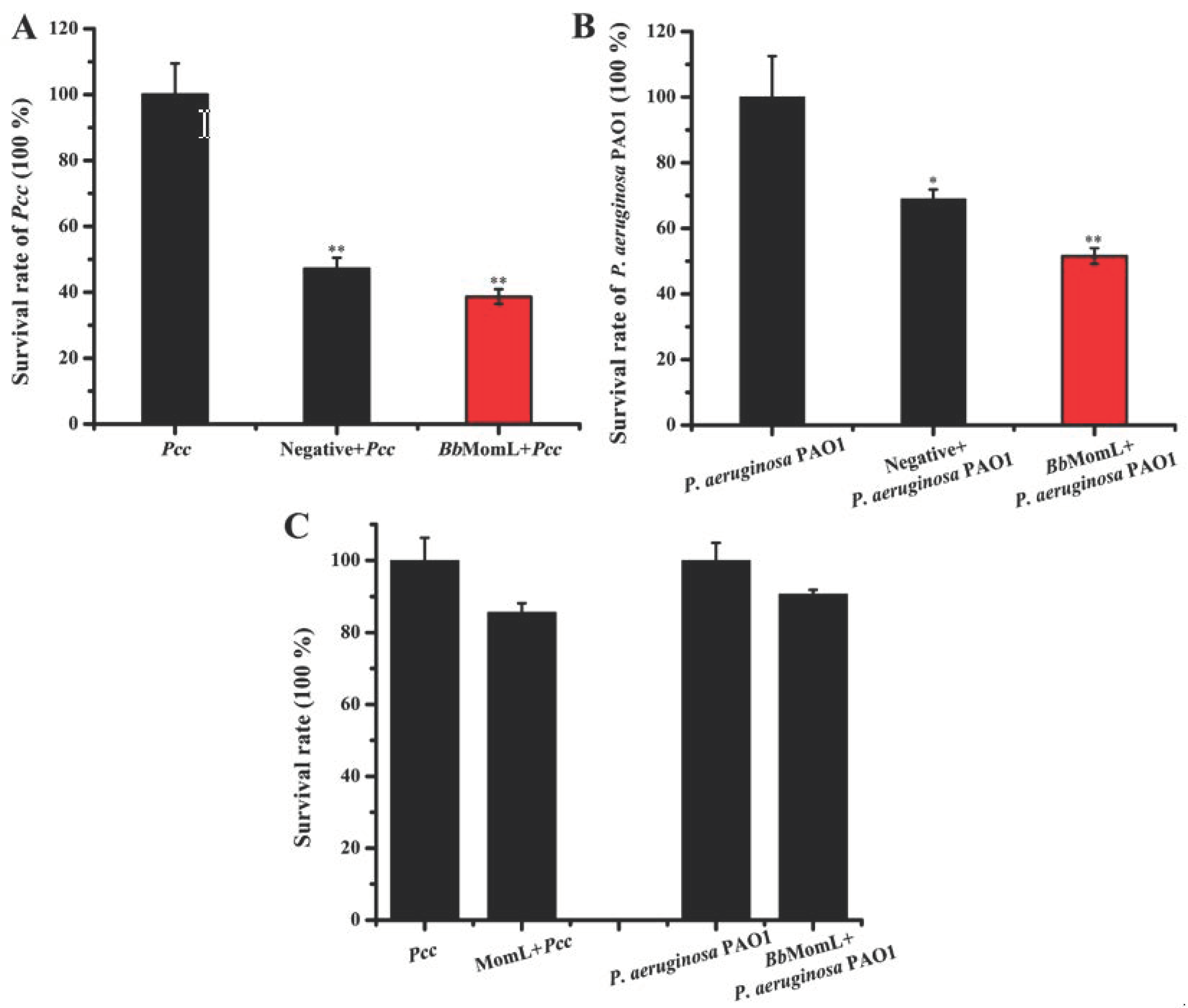
2.2. Effects of BbMomL on the Growth of Pcc and P. aeruginosa PAO1
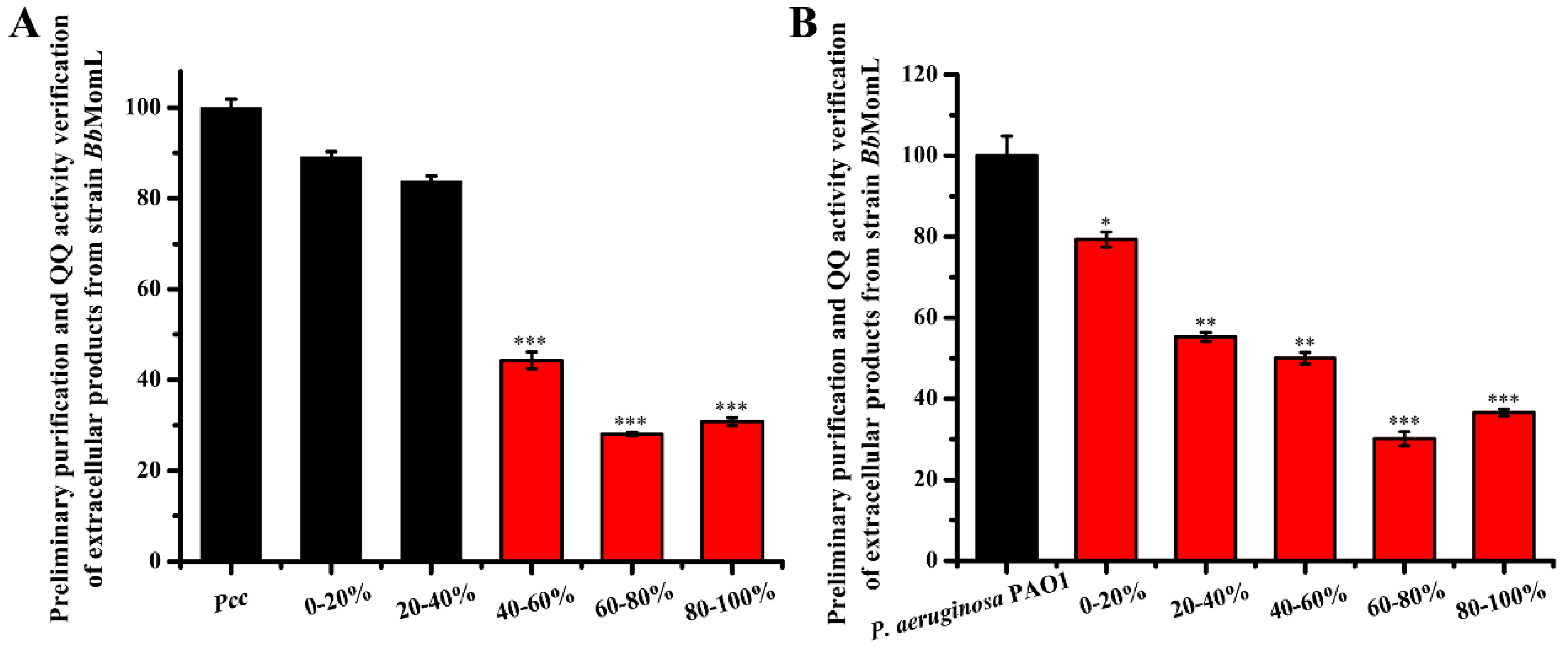
2.3. Isolation of the BbMomL Extracellular Protein and Analysis of AHL Degradation Activity
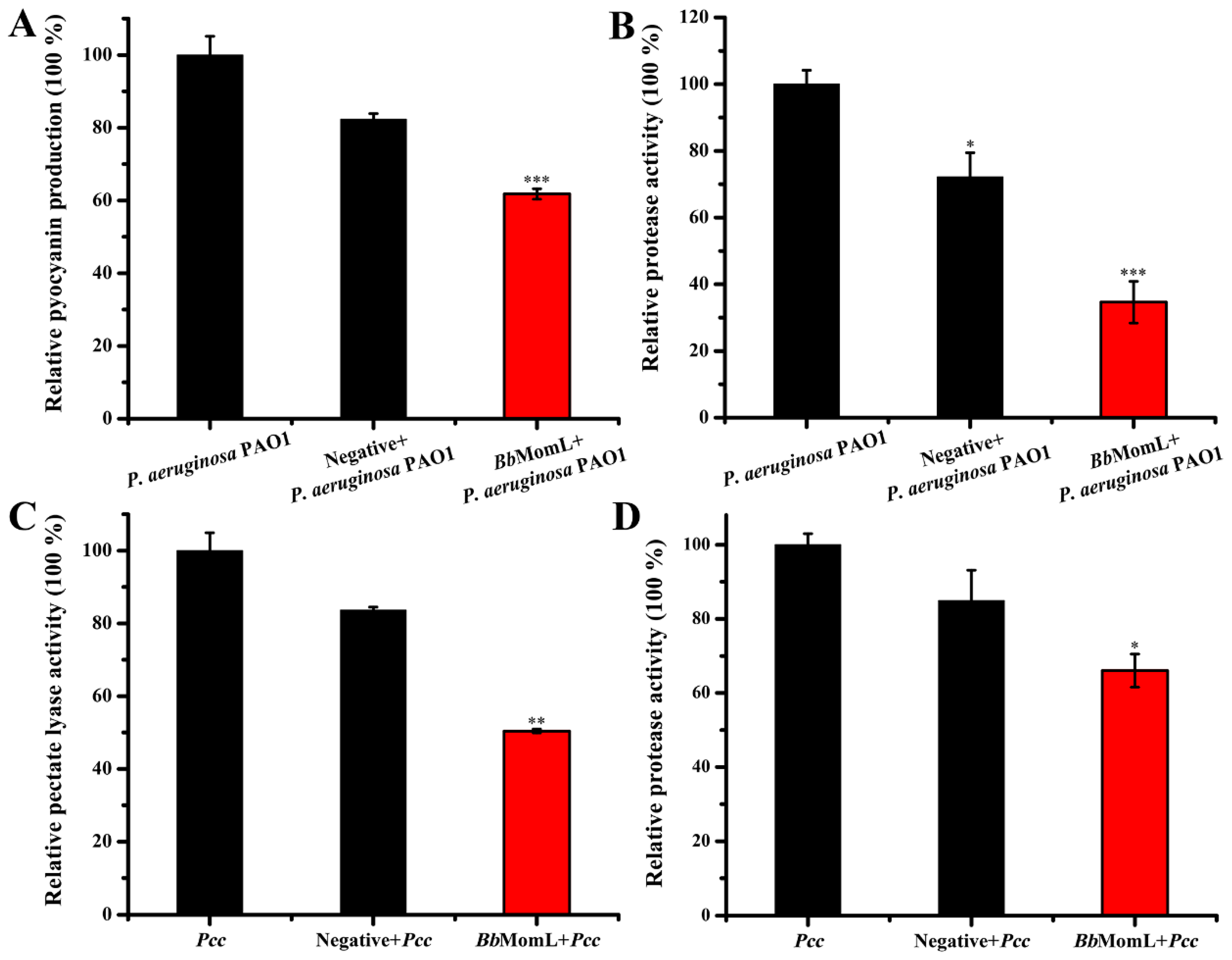
2.4. In Vitro Experiments to Evaluate the Ability of BbMomL to Inhibit the Virulence Factors of Pathogenic Bacteria
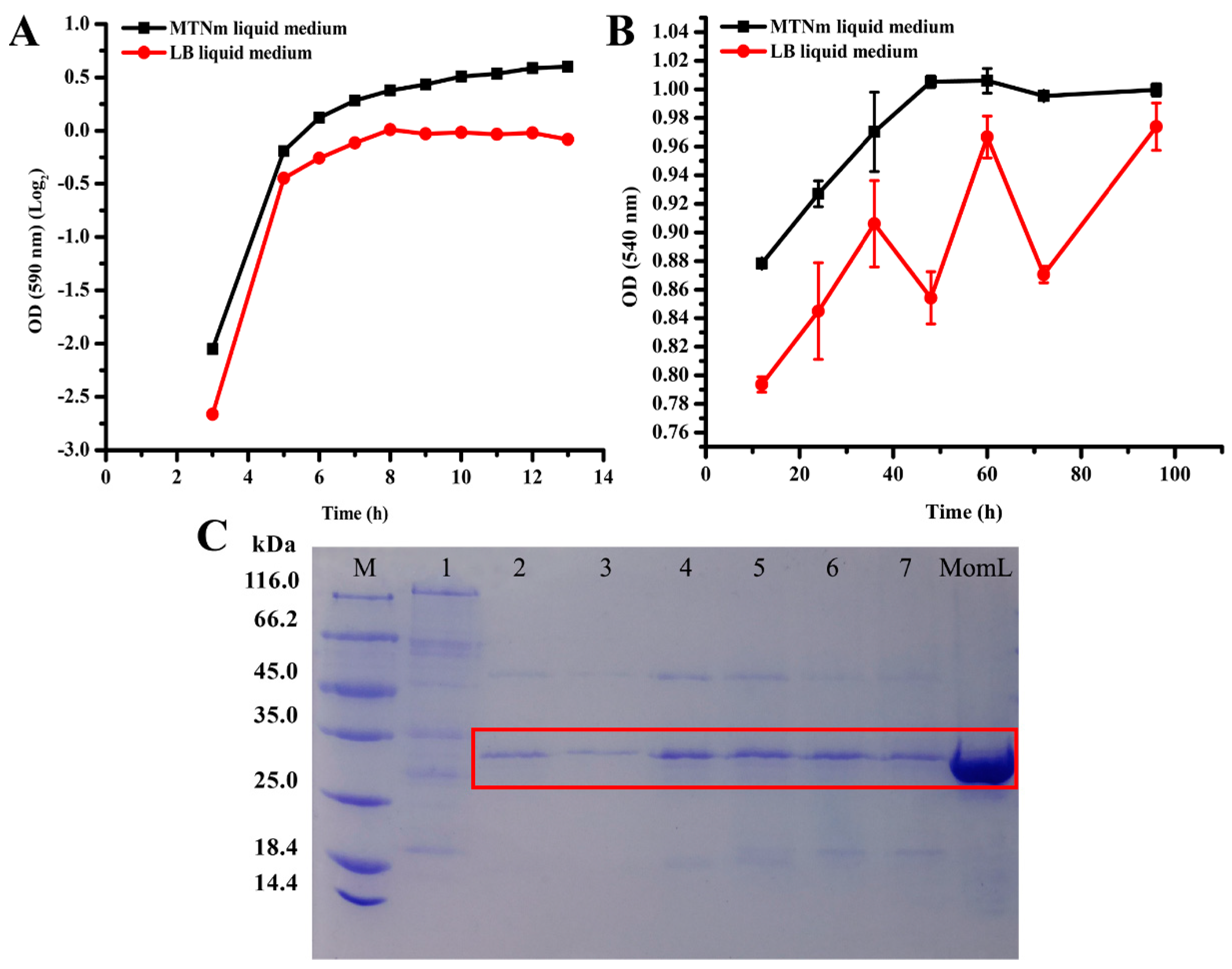
2.5. Analysis of Growth and Protein Content of BbMomL in Different Media and Secretion of the Target Protein at Different Times
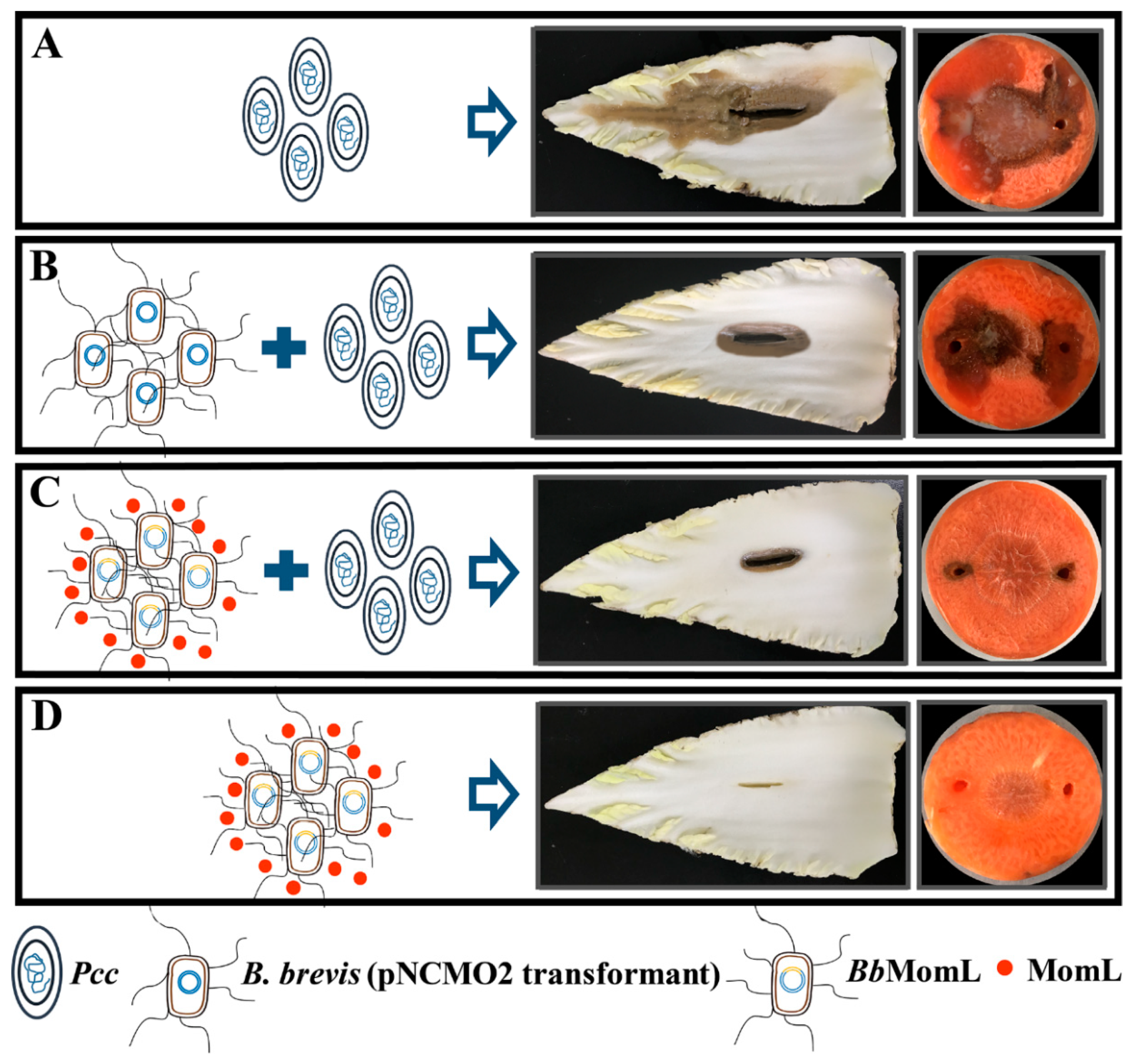
2.6. Inhibitory Effect of BbMomL on Bacterial Soft Rot of Plants
3. Discussion
4. Materials and Methods
4.1. Strains, Plasmids and Culture Conditions
4.2. Plasmids Construction
4.3. Bioassay for AHL Degradation Activity
4.4. Effects of BbMomL on the Survival of Pcc and P. aeruginosa PAO1
4.5. Isolation and Purification of Recombinant Enzymes and AHL Bioassay of Pcc and P. aeruginosa PAO1
4.6. Effects of BbMomL on Virulence Factor Production in Pcc and P. aeruginosa PAO1
4.7. Comparison of the Growth and Protein Content of BbMomL in Different Media and by SDS-PAGE Analysis
4.8. Inhibition of Plant Bacterial Soft Rot
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Corbett, M.; Virtue, S.; Bell, K.; Birch, P.; Burr, T.; Hyman, L.; Lilley, K.; Poock, S.; Toth, I.; Salmond, G. Identification of a new quorum-sensing-controlled virulence factor in Erwinia carotovora subsp. atroseptica secreted via the type II targeting pathway. Mol. Plant Microbe Interact. 2005, 18, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Burr, T.; Barnard, A.M.; Corbett, M.J.; Pemberton, C.L.; Simpson, N.J.; Salmond, G.P. Identification of the central quorum sensing regulator of virulence in the enteric phytopathogen, Erwinia carotovora: The VirR repressor. Mol. Microbiol. 2006, 59, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Koutsoudis, M.D.; Tsaltas, D.; Minogue, T.D.; von Bodman, S.B. Quorum-sensing regulation governs bacterial adhesion, biofilm development, and host colonization in Pantoea stewartii subspecies stewartii. Proc. Natl. Acad. Sci. USA 2006, 103, 5983–5988. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Ni, H.; Meng, S.; He, Y.; Yu, Z.N.; Li, L. Suppressing Erwinia carotovora pathogenicity by projecting N-acyl homoserine lactonase onto the surface of Pseudomonas putida cells. J. Microbiol. Biotechnol. 2011, 21, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Su, Y.; Brackman, G.; Cui, F.; Zhang, Y.; Shi, X.; Coenye, T.; Zhang, X.H. MomL, a novel marine-derived N-acyl homoserine lactonase from Muricauda olearia. Appl. Environ. Microbiol. 2015, 81, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.L. Quorum quenching: Enzymatic disruption of N-acylhomoserine lactone-mediated bacterial communication in Burkholderia thailandensis. Appl. Environ. Microbiol. 2004, 70, 6173–6180. [Google Scholar] [CrossRef]
- Dong, Y.H.; Wang, L.H.; Xu, J.L.; Zhang, H.B.; Zhang, X.F.; Zhang, L.H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar] [CrossRef]
- Wenzel, S.C.; Muller, R. Recent developments towards the heterologous expression of complex bacterial natural product biosynthetic pathways. Curr. Opin. Biotechnol. 2005, 16, 594–606. [Google Scholar] [CrossRef]
- Baneyx, F. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol. 1999, 10, 411–421. [Google Scholar] [CrossRef]
- Bolhuis, A.; Tjalsma, H.; Smith, H.E.; de Jong, A.; Meima, R.; Venema, G.; Bron, S.; van Dijl, J.M. Evaluation of bottlenecks in the late stages of protein secretion in Bacillus subtilis. Appl. Environ. Microbiol. 1999, 65, 2934–2941. [Google Scholar] [PubMed]
- Seok, J.H.; Kim, H.S.; Hatada, Y.; Nam, S.W.; Kim, Y.H. Construction of an expression system for the secretory production of recombinant alpha-agarase in yeast. Biotechnol. Lett. 2012, 34, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Kashima, Y.; Udaka, S. High-level production of hyperthermophilic cellulase in the Bacillus brevis expression and secretion system. Biosci. Biotechnol. Biochem. 2004, 68, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liu, Q.; Guo, H.; Ju, R.; Zhao, Y.; Li, J.; Liu, X. Tostadin, a novel antibacterial peptide from an antagonistic microorganism Brevibacillus brevis XDH. Bioresour. Technol. 2012, 111, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Mogi, T.; Kita, K. Gramicidin S and polymyxins: The revival of cationic cyclic peptide antibiotics. Cell. Mol. Life Sci. 2009, 66, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-H.; Zhang, X.-F.; Xu, J.-L.; Zhang, L.-H. Insecticidal Bacillus thuringiensis silences Erwinia carotovora virulence by a new form of microbial antagonism, signal interference. Appl. Environ. Microbiol. 2004, 70, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-H.; Xu, J.-L.; Li, X.-Z.; Zhang, L.-H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Grandclément, C.; Tannières, M.; Moréra, S.; Dessaux, Y.; Faure, D. Quorum quenching: Role in nature and applied developments. FEMS Microbiol. Rev. 2015, 40, 86–116. [Google Scholar] [CrossRef]
- He, P.; Zhang, Z.; Cai, D.; Chen, Y.; Wang, H.; Wei, X.; Li, S.; Chen, S. High-level production of alpha-amylase by manipulating the expression of alanine racamase in Bacillus licheniformis. Biotechnol. Lett. 2017, 39, 1389–1394. [Google Scholar] [CrossRef]
- Mizukami, M.; Hanagata, H.; Miyauchi, A. Brevibacillus expression system: Host-vector system for efficient production of secretory proteins. Curr. Pharm. Biotechnol. 2010, 11, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Miyauchi, A.; Takagi, H.; Kadowaki, K.; Yamane, K.; Kobayashi, S. Expression of the Cyclodextrin Glucanotransferase Gene of Bacillus macerans in Bacillus brevis. Biosci. Biotechnol. Biochem. 1992, 56, 808–809. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Kikuchi, A.; Kobayashi, M.; Yamaguchi, M.; Ifuku, S.; Yamashoji, S.; Ando, A.; Saito, A. Characterization of antifungal activity of the GH-46 subclass III chitosanase from Bacillus circulans MH-K1. Antonie Van Leeuwenhoek 2013, 104, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Su, L.; Duan, X.; Wu, D.; Wu, J. Efficient Expression of Maltohexaose-Forming α-Amylase from Bacillus stearothermophilus in Brevibacillus choshinensis SP3 and Its Use in Maltose Production. Biomed. Res. Int. 2017, 2017, 5479762. [Google Scholar] [CrossRef] [PubMed]
- Wasels, F.; Ferreira, N.L.; Collas, F.; Contreras, A.L. Genetic Tool for the Transformation of Clostridium Bacteria. Google Patents WO2017064439, 12 April 2017. [Google Scholar]
- Tojo, H.; Asano, T.; Kato, K.; Udaka, S.; Horiuchi, R.; Kakinuma, A. Production of human protein disulfide isomerase by Bacillus brevis. J. Biotechnol. 1994, 33, 55–62. [Google Scholar] [CrossRef]
- Yamagata, H.; Nakahama, K.; Suzuki, Y.; Kakinuma, A.; Tsukagoshi, N.; Udaka, S. Use of Bacillus brevis for efficient synthesis and secretion of human epidermal growth factor. Proc. Natl. Acad. Sci. USA 1989, 86, 3589–3593. [Google Scholar] [CrossRef] [PubMed]
- Takimura, Y.; Kato, M.; Ohta, T.; Yamagata, H.; Udaka, S. Secretion of human interleukin-2 in biologically active form by Bacillus brevis directly into cultute medium. Biosci. Biotechnol. Biochem. 1997, 61, 1858–1861. [Google Scholar] [CrossRef]
- Yashiro, K.; Lowenthal, J.W.; O’Neil, T.E.; Ebisu, S.; Takagi, H.; Moore, R.J. High-level production of recombinant chicken interferon-γ by Brevibacillus choshinensis. Protein Expr. Purif. 2001, 23, 113–120. [Google Scholar] [CrossRef]
- Mukai, H.; Takahashi, M.; Watanabe, Y. Potential usefulness of Brevibacillus for bacterial cancer therapy: Intratumoral provision of tumor necrosis factor-α and anticancer effects. Cancer Gene Ther. 2018, 25, 47–57. [Google Scholar] [CrossRef]
- Ando, A.; Saito, A.; Arai, S.; Usuda, S.; Furuno, M.; Kaneko, N.; Shida, O.; Nagata, Y. Molecular characterization of a novel family-46 chitosanase from Pseudomonas sp. A-01. Biosci. Biotechnol. Biochem. 2008, 72, 2074–2081. [Google Scholar] [CrossRef]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Beaber, J.W.; More, M.I.; Fuqua, C.; Eberhard, A.; Winans, S.C. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J. Bacteriol. 1998, 180, 5398–5405. [Google Scholar] [PubMed]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Foster, K.R.; Comstock, L.E. The evolution of cooperation within the gut microbiota. Nature 2016, 533, 255. [Google Scholar] [CrossRef] [PubMed]
- Pader, V.; Hakim, S.; Painter, K.L.; Wigneshweraraj, S.; Clarke, T.B.; Edwards, A.M. Staphylococcus aureus inactivates daptomycin by releasing membrane phospholipids. Nat. Microbiol. 2016, 2, 16194. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhaya, A.; Tsurumi, A.; Maura, D.; Jeffrey, K.L.; Rahme, L.G. A quorum-sensing signal promotes host tolerance training through HDAC1-mediated epigenetic reprogramming. Nat. Microbiol. 2016, 1, 16174. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J. Indole as an intercellular signal in microbial communities. Fems Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.-S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Jensen, P.O.; Burmolle, M.; Hentzer, M.; Haagensen, J.A.; Hougen, H.P.; Calum, H.; Madsen, K.G.; Moser, C.; Molin, S.; et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 2005, 151, 373–383. [Google Scholar] [CrossRef]
- Dou, Y.; Song, F.; Guo, F.; Zhou, Z.; Zhu, C.; Xiang, J.; Huan, J. Acinetobacter baumannii quorum-sensing signalling molecule induces the expression of drug-resistance genes. Mol. Med. Rep. 2017, 15, 4061–4068. [Google Scholar] [CrossRef]
- Koch, G.; Nadal-Jimenez, P.; Reis, C.R.; Muntendam, R.; Bokhove, M.; Melillo, E.; Dijkstra, B.W.; Cool, R.H.; Quax, W.J. Reducing virulence of the human pathogen Burkholderia by altering the substrate specificity of the quorum-quenching acylase PvdQ. Proc. Natl. Acad. Sci. USA 2014, 111, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Sokol, P.A.; Sajjan, U.; Visser, M.B.; Gingues, S.; Forstner, J.; Kooi, C. The CepIR quorum-sensing system contributes to the virulence of Burkholderia cenocepacia respiratory infections. Microbiology 2003, 149, 3649–3658. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.J.; Swift, S.; Kirke, D.F.; Keevil, C.W.; Dodd, C.E.R.; Williams, P. The regulation of biofilm development by quorum sensing in Aeromonas hydrophila. Environ. Microbiol. 2002, 4, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.; Karlyshev, A.V.; Fish, L.; Durant, E.L.; Winson, M.K.; Chhabra, S.R.; Williams, P.; Macintyre, S.; Stewart, G.S. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: Identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J. Bacteriol. 1997, 179, 5271–5281. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, aaf4268. [Google Scholar] [CrossRef] [PubMed]
- Alekshun, M.N.; Levy, S.B. Molecular Mechanisms of Antibacterial Multidrug Resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Romero, M.; Muras, A.; Otero, A. Aii20J, a wide-spectrum thermostable N-acylhomoserine lactonase from the marine bacterium Tenacibaculum sp. 20J, can quench AHL-mediated acid resistance in Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 99, 9523–9539. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Momb, J.; Thomas, P.W.; Moulin, A.; Petsko, G.A.; Fast, W.; Ringe, D. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 1. Product-bound structures. Biochemistry 2008, 47, 7706–7714. [Google Scholar] [CrossRef]
- Momb, J.; Wang, C.; Liu, D.; Thomas, P.W.; Petsko, G.A.; Guo, H.; Ringe, D.; Fast, W. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 2. Substrate modeling and active site mutations. Biochemistry 2008, 47, 7715–7725. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Lu, F.-P.; Li, Y.; Yin, X.-B.; Wang, Y.; Gao, C. Characterisation of mutagenised acid-resistant alpha-amylase expressed in Bacillus subtilis WB600. Appl. Microbiol. Biotechnol. 2008, 78, 85–94. [Google Scholar] [CrossRef]
- Sarvas, M. Gene Expression in Recombinant Bacillus; Marcel Dekker Inc.: New York, NY, USA, 1995. [Google Scholar]
- Liu, G.; Xing, M.; Yu, S. High-effective expression of thermostable alpha-amylase from a bacterial phage based recombinant Bacillus subtilis. Chin. J. Appl. Environ. Biol. 2005, 11, 368. [Google Scholar]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Udaka, S.; Tsukagoshi, N.; Yamagata, H. Bacillus brevis, a host bacterium for efficient extracellular production of useful proteins. Biotechnol. Genet. Eng. Rev. 1989, 7, 113–146. [Google Scholar] [CrossRef]
- Udaka, S.; Yamagata, H. Protein secretion in Bacillus brevis. Antonie Van Leeuwenhoek 1993, 64, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Udaka, S.; Yamagata, H. High-level secretion of heterologous proteins by Bacillus brevis. Methods Enzym. 1993, 217, 23–33. [Google Scholar]
- Inoue, Y.; Ohta, T.; Tada, H.; Iwasa, S.; Udaka, S.; Yamagata, H. Efficient production of a functional mouse/human chimeric Fab′ against human urokinase-type plasminogen activator by Bacillus brevis. Appl. Microbiol. Biotechnol. 1997, 48, 487–492. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, W.; Zhu, H. The construction of shuttle vectors of Brevibacillus brevis-Escherichia coli. Sheng Wu Gong Cheng Xue Bao (Chin. J. Biotechnol.) 2002, 18, 438–441. [Google Scholar]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef]
- Pedro, A.Q.; Bonifacio, M.J.; Queiroz, J.A.; Maia, C.J.; Passarinha, L.A. A novel prokaryotic expression system for biosynthesis of recombinant human membrane-bound catechol-O-methyltransferase. J. Biotechnol. 2011, 156, 141–146. [Google Scholar] [CrossRef]
- Kang, J.E.; Han, J.W.; Jeon, B.J.; Kim, B.S. Efficacies of quorum sensing inhibitors, piericidin A and glucopiericidin A, produced by Streptomyces xanthocidicus KPP01532 for the control of potato soft rot caused by Erwinia carotovora subsp. atroseptica. Microbiol. Res. 2016, 184, 32–41. [Google Scholar] [CrossRef]
- Essar, D.W.; Eberly, L.; Hadero, A.; Crawford, I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990, 172, 884–900. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Hoiby, N.; et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Ayora, S.; Gotz, F. Genetic and biochemical properties of an extracellular neutral metalloprotease from Staphylococcus hyicus subsp. hyicus. Mol. Gen. Genet. 1994, 242, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Jafra, S.; Przysowa, J.; Czajkowski, R.; Michta, A.; Garbeva, P.; van der Wolf, J.M. Detection and characterization of bacteria from the potato rhizosphere degrading N-acyl-homoserine lactone. Can. J. Microbiol. 2006, 52, 1006–1015. [Google Scholar] [CrossRef]

| Protein | Origin | Quantity of Expression (g/L) | References |
|---|---|---|---|
| Enzymes | |||
| Alpha-Amylase | B. licheniformis | 3.7 | [20] |
| Sphingomyelinase | B. cereus | 3.0 | [21] |
| Xylanase | B. halodurans | 0.2 | [21] |
| CGTase | B. macerans | 1.5 | [22] |
| Chitosanase | B. circulans | 1.4 | [23] |
| Hyperthermophilic protease | A. pernix | 0.1 | [24] |
| Hyperthermophilic nuclease | P. horikoshii | 0.7 | [25] |
| PDI | Human | 1.0 | [26] |
| Antigens | |||
| Surface antigen | E. rhusiopathiae | 0.9 | [21] |
| Surface antigen | T. pallidum | 0.8 | [21] |
| Cytokines | |||
| EGF | Human | 1.5 | [27] |
| IL-2 | Human | 0.6 | [28] |
| NGF | Mouse | 0.2 | [21] |
| IFN-γ | Chicken | 0.5 | [29] |
| TNF-α | Cow | 0.4 | [30] |
| GM-CSF | Cow | 0.2 | [21] |
| GH | Flounder | 0.2 | [31] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, J.; Feng, T.; Du, R.; Tian, X.; Wang, Y.; Zhang, X.-H. Heterologous Expression of the Marine-Derived Quorum Quenching Enzyme MomL Can Expand the Antibacterial Spectrum of Bacillus brevis. Mar. Drugs 2019, 17, 128. https://doi.org/10.3390/md17020128
Zhang J, Wang J, Feng T, Du R, Tian X, Wang Y, Zhang X-H. Heterologous Expression of the Marine-Derived Quorum Quenching Enzyme MomL Can Expand the Antibacterial Spectrum of Bacillus brevis. Marine Drugs. 2019; 17(2):128. https://doi.org/10.3390/md17020128
Chicago/Turabian StyleZhang, Jingjing, Jiayi Wang, Tao Feng, Rui Du, Xiaorong Tian, Yan Wang, and Xiao-Hua Zhang. 2019. "Heterologous Expression of the Marine-Derived Quorum Quenching Enzyme MomL Can Expand the Antibacterial Spectrum of Bacillus brevis" Marine Drugs 17, no. 2: 128. https://doi.org/10.3390/md17020128
APA StyleZhang, J., Wang, J., Feng, T., Du, R., Tian, X., Wang, Y., & Zhang, X.-H. (2019). Heterologous Expression of the Marine-Derived Quorum Quenching Enzyme MomL Can Expand the Antibacterial Spectrum of Bacillus brevis. Marine Drugs, 17(2), 128. https://doi.org/10.3390/md17020128





