Abstract
Gram-negative bacteria utilize N-acylhomoserine lactones (AHLs) as quorum sensing (QS) signaling molecules for intercellular communication. Cell-to-cell communication depends on cell population density, and AHL-dependent QS is related to the production of multiple genes including virulence factors. Quorum quenching (QQ), signal inactivation by enzymatic degradation, is a potential strategy for attenuating QS regulated bacterial infections. Both Gram-positive and -negative bacteria have QQ enzymes that can degrade AHLs. In our previous study, strain Ruegeria mobilis YJ3, isolated from healthy shrimp, showed strong AHLs degradative activity. In the current study, an AHL lactonase (designated RmmL) was cloned and characterized from Ruegeria mobilis YJ3. Amino acid sequence analysis showed that RmmL has a conserved “HXHXDH” motif and clusters together with lactonase AidC that belongs to the metallo-β-lactamase superfamily. Recombinant RmmL could degrade either short- or long-chain AHLs in vitro. High-performance liquid chromatography analysis indicated that RmmL works as an AHL lactonase catalyzing AHL ring-opening by hydrolyzing lactones. Furthermore, RmmL can reduce the production of pyocyanin by Pseudomonas aeruginosa PAO1, while for the violacein and the extracellular protease activities by Chromobacterium violaceum CV026 and Vibrio anguillarum VIB72, no significant reduction was observed. This study suggests that RmmL might be used as a therapeutic agent in aquaculture.
1. Introduction
Quorum sensing (QS), also known as “auto-induction”, was originally found in Vibrio fisheri []. QS is a bacterial cell-to-cell communication system, in which bacteria regulate gene expression in the environment in accordance with cell density by using diffusible signal molecules. Therefore, by bacterial communication, QS can regulate gene expression and harmonize their behaviors including biofilm formation, bioluminescence, antibiotic production, and virulence factor secretion. Many different types of QS molecules were identified including N-acylhomoserine lactones (AHLs), autoinducer-2 (AI-2), 2-heptyl-3-hydroxy-4-quinoline (Pseudomonas quinolone signal, PQS), and 2-heptyl-4(1H)-hydroxyquinoline, HHQ) []. Among them, AHLs and AI-2 are the two most studied types.
By contrast, quorum quenching (QQ) can disrupt the QS communication system by enzymatic degradation of signal molecules. Currently, these enzymes might be sorted into three major categories based on the type of reaction catalyzed: AHL lactonases (lactone hydrolysis), AHL acylases (cleavage of amide bond), and AHL oxidoreductases (oxidoreduction) (Figure 1) []. Among the three types of QQ enzymes, AHL lactonase and AHL acylase have been extensively studied. The first AHL-degrading enzyme, AiiA, was identified in Bacillus sp. strain 240B1, is a member of the metallo-β-lactamase superfamily, and can attenuate the virulence of Erwinia carotovora []. AiiA shows a wide substrate specificity and there is a “HXHXDH~60aa~H” motif in its amino acid sequence. Heterologous expression of aiiA reduced the production of virulence factors. Since then, several AHL lactonases such as AhlD, AiiM, AidC, and MomL, were found in both Gram-positive (Arthrobacter and Microbacterium) and -negative (Chryseobacterium and Muricauda) bacteria, respectively [,,,]. AiiD was the first identified AHL acylase from Ralstonia sp. XJ12B [] in 2003. Subsequently, several AHL acylases were identified from different bacteria including Anabaena (AiiC) [], Streptomyces (ahlM) [], Pseudomonas (pvdQ) [], and Shewanella (Aac) []. In addition, expression of Aac from the intestinal Shewanella in fish pathogenic Vibrio anguilarum, contributed to the reduction of AHL production and biofilm formation []. Therefore, QQ could serve as a promising strategy to control bacterial virulence.
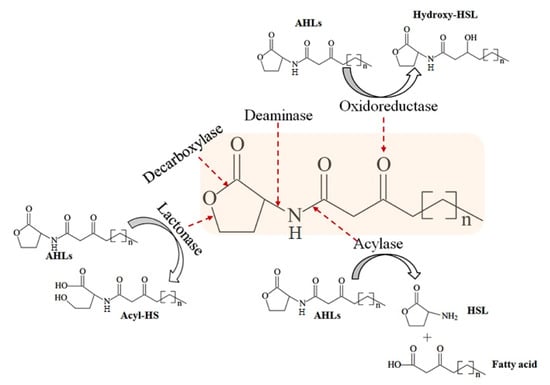
Figure 1.
Enzymatic hydrolysis of N-acylhomoserine lactones (AHLs) [].
Ruegeria mobilis is a Gram-negative marine bacterium belonging to the clade Roseobacter. It was reported that R. mobilis was a globally distributed marine bacterium [], could produce antibacterial compound tropodithietic acid (TDA), and thus could be considered as a potential probiotic bacterium [,]. It remains to be determined whether QS and QQ are present in R. mobilis.
R. mobilis YJ3 was isolated from healthy shrimp larvae and confirmed to show excellent antibacterial activities against many fish pathogens, such as Vibrio spp., Edwardsiella tarda, and Aeromonas hydrophila. Furthermore, the ICPs and ECPs of YJ3 showed quorum quenching activity, and it disappeared when treated by heat or protease K (unpublished data). Here, based on the genome sequence of YJ3, a novel AHL-lactonase gene RmmL, from R. mobilis YJ3 was identified and cloned. Further characterization of enzymatic activity and the effects of RmmL on in vitro virulence factor production were performed.
2. Results
2.1. Identification of the Extracelluar QQ Enzyme in YJ3
Local BLASTP (Basic Local Alignment Search Tool Program) of the annotated genome of R. mobilis YJ3, against a known QQ enzymes database, revealed that a protein, (L131) with 322 amino acid residues, is closely related to the known AHL lactonases. There was only 23–35% of amino-acid sequence identity shared with the known QQ enzymes (AiiA, AiiB, AttM, AhlS, AidC, AhlD) [,,,,,]. L131 could represent a novel marine-derived AHL lactonase, which was termed, RmmL (Ruegeria Mobilis Marine Lactonase). Phylogenetic analysis of RmmL and the other AHL lactonases from various bacteria showed that RmmL closely correlates to AidC under the AidC cluster previously reported (Figure 2) []. RmmL was predicted to be extracelluar with an N-terminal signal peptide of 30 amino acid residues through SignalP analysis []. Further sequence comparison analysis showed that RmmL has a “HXHXDH” zinc-binding motif conserved across different types of AHL lactonases (Figure 3).
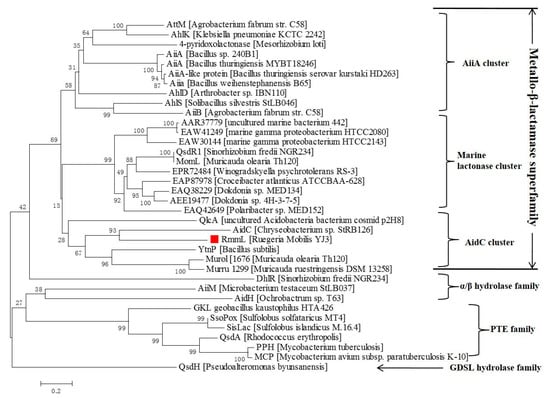
Figure 2.
Neighbor-joining tree of AHL lactonases based on amino acid sequences. The sequences were from the AHL lactonases experimentally identified and unidentified (labeled with GenBank accession numbers). The dendrogram was constructed by the neighbor joining method with the MUSCLE program in the MEGA 7.0 software package (1000 bootstrap replicates). Bootstrap coefficients below 50% were not shown. Scale bar, 0.2 substitutions per amino acid position.
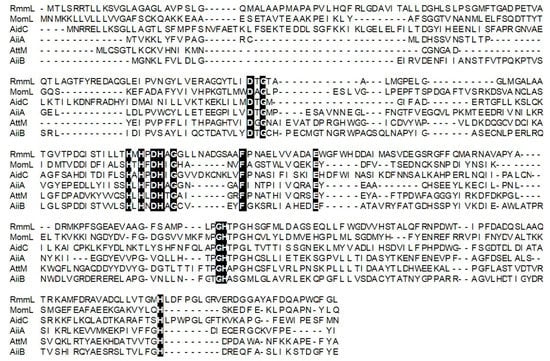
Figure 3.
Multiple-sequence alignment of amino acid sequences of Ruegeria Mobilis Marine Lactonase (RmmL) and other representative AHL lactonases (MomL, AidC, AiiA, AttM, and AiiB). Sequence alignment was performed by the MUSCLE program in the MEGA 7.0 software. RmmL harbors an N-terminal signal peptide and the predicted cleavage site was marked by arrow. RmmL shares the “HXHXDH” motif with other AHL lactonases. The conserved sites are marked by a black background.
2.2. Expression, Purification and Activity Test of RmmL in YJ3
The recombinant RmmL was then cloned into PET-32a and expressed in E. coli BL21 (DE3). The majority of the recombinant RmmL was expressed in a soluble form. The purified Trx-tag fusion protein Trx-RmmL and RmmL without Trx-tag produced a single band of 50 kDa and 33 kDa in size, respectively, which was matched with the predicted molecular weight of RmmL (Figure 4). The purified RmmL can degrade both short- and long-chain AHLs, and C6-, C10-, C12-, 3-oxo-C14-HSL are more efficiently degraded than C8-, 3-oxo-C6-, 3-oxo-C8-, 3-oxo-C10-, 3-oxo-C12-HSL (Figure 5), which proved that recombinant RmmL has AHL degradation activity.
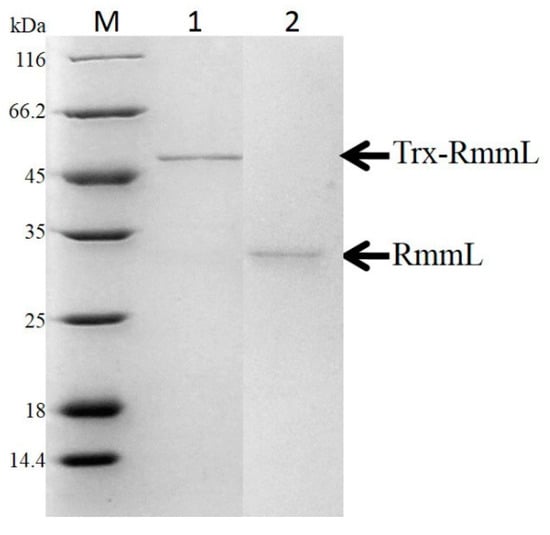
Figure 4.
SDS-PAGE analysis of the affinity column-purified Trx-RmmL (50 kDa, lane 1) and RmmL protein (33 kDa, lane 2) after Trx-tag removal by enterokinase.
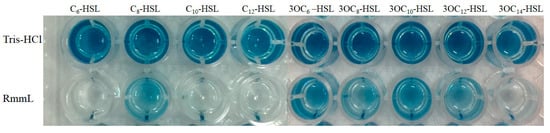
Figure 5.
Characterization of the AHL degradative activity of RmmL by the A136 liquid X-Gal assay. Tris-HCl was used as the negative control. Signal molecules used are C6-HSL, C8-HSL, C10-HSL, C12-HSL, 3OC6-HSL, 3OC8-HSL, 3OC10-HSL, 3OC12-HSL, 3OC14-HSL.
2.3. RmmL is an AHL Lactonase
To further elucidate the mechanism of RmmL catalysis, the resulting degradation products of C12-HSL, treated with purified RmmL, were analyzed by UPLC-MS. One major product with a retention time of 8.743 min was produced by the enzymatic digestion of C12-HSL. The catalytic product of RmmL reaction with C12-HSL gave a peak m/z of 302.22 with a mass increase of 18.09 in comparison with the m/z of intact C12-HSL (284.13) with a retention time of 9.234 min (Figure 6). The increased mass from C12-HSL to C12-HS resulted from an addition of a water molecule to the ester bond of C12-HSL due to the hydrolytic cleavage of lactone ring. This result proved that RmmL functions as an AHL lactonase which can hydrolyze the lactone ring of C12-HSL (Figure 1).
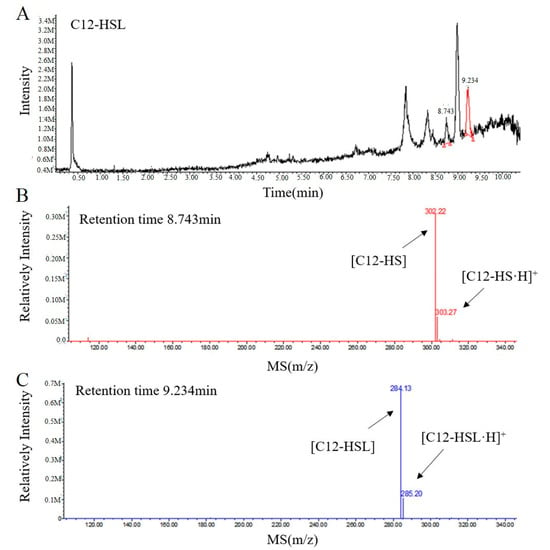
Figure 6.
UPLC-MS analysis of the RmmL-hydrolyzed C12-HSL product. (A) UPLC profile of the RmmL-hydrolyzed C12-HSL product. ESI-MS analysis of UPLC fractions containing the 8.743-min product (B) and 9.234-min undigested C12-HSL (C).
2.4. Biochemical Characterization of RmmL
RmmL exhibited high enzymatic activity at a relatively broad range of temperatures from 10 to 60 °C, with the optimal activity at 50 °C (Figure 7A). RmmL still retained 100% activity after heat treatment at 40 °C for 30 min but dropped at higher temperatures of 60 °C, 80 °C, and 100 °C (Figure 7B). In addition, the CV026 plate assay showed that RmmL still retained activity after incubation at 100 °C for 1 min, whereas it lost activity when treated at 100 °C for 5 min, 10 min, or 30 min (Figure 7C). Lower pH treatment at 4 °C for 3 h reduced the RmmL activity while higher pH treatment at 4 °C for 3 h retained or even increased enzymatic activity (Figure 7D). Either EDTA alone or together with various metal ions Ni2+, Mg2+, Ca2+, Cu2+, and Fe2+ partially inhibited RmmL activity, however, Zn2+, Mn2+, and Co2+ together with EDTA enhanced RmmL activity (Figure 7E).
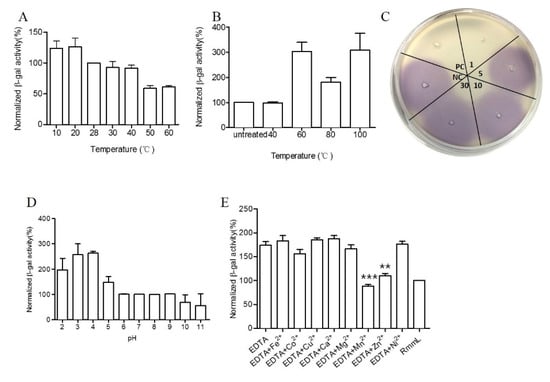
Figure 7.
Biochemical characterization of RmmL activity. The optimal temperature (A), temperature stability at 40 °C, 60 °C, 80 °C, and 100 °C treatment for 30 min (B), and at 100 °C at 1 min, 5 min, 10 min 30 min (C), pH stability (D) of RmmL activity and the effect of metal ions and EDTA on RmmL activity (E) were measured. Bars indicate standard deviation of the mean. NC, negative control (Tris-HCl); PC, positive control (purified RmmL); Two-tailed unpaired t-test was used for statistical significance analysis; (**, p < 0.01; ***, p < 0.001; n ≥ 3).
2.5. The Effect of RmmL on In Vitro Production of Virulence Factors
The effect of RmmL on violacien and pyocyanin production of C. violaceum VIR24 and P. aeruginosa PAO1, respectively, and the effect of RmmL on the extracellular proteolytic activity of P. aeruginosa PAO1 and V. anguillarum VIB72 were evaluated in vitro. RmmL reduced the violacien and pyocyanin production by C. violaceum VIR24 and P. aeruginosa PAO1, respectively, in a concentration–dependent response (Figure 8A,B). Similarly, RmmL reduced the extracellular protease activity of V. anguillarum VIB72 in a concentration–dependent manner (Figure 8C). Instead, the reduction of the extracellular protease activity of P. aeruginosa PAO1 was not detected, and the data was not shown.
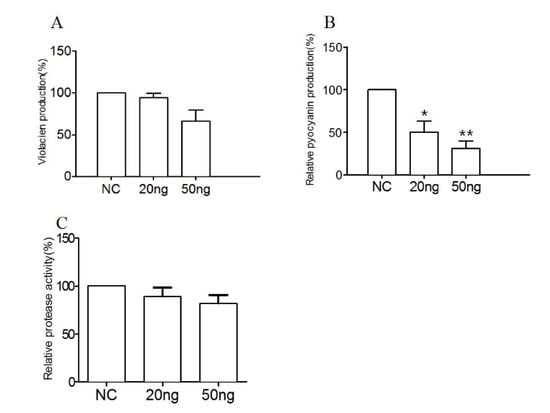
Figure 8.
The effect of RmmL on virulence factor production. The violacein (A) and pyocyanin (B) production of C. violaceum and P. aeruginosa, respectively, and the extracellular protease activity (C) of V. anguillarum were determined. NC, negative control; Two-tailed unpaired t-test was used for statistical significance analysis; (*, p < 0.05; **, p < 0.01; n ≥ 3).
3. Discussion
There are more than 30 types of AHL lactonases identified and reported. Phylogenetic analysis of amino acid sequences of all known lactonases has classified them into four different families including the metallo-β-lactamase superfamily, the phosphotriesterase (PTE) family, GDSL hydrolase family, and α/β hydrolase family []. Within the metallo-β-lactamase superfamily, three clusters of AiiA, marine lactonase, and AidC were further defined. In this study, a novel QQ enzyme of AHL lactonase, RmmL, from a marine R. mobilis YJ3 strain was identified and characterized. To our knowledge, this is the first study that has identified and characterized the QQ enzyme in R. mobilis. Phylogenetic tree analysis revealed that RmmL is distantly related to the marine lactonase cluster, and closely related to AidC derived from a terrestrial bacterium in the AidC cluster (Figure 2). It remains unclear if RmmL is an exception, or if there are two or more marine lactonase clusters. Several reports demonstrated that hydrolysis of the lactone ring by AHL lactonases results from the addition of a water molecule into AHLs to produce acylhomoserine [,,,], and in this research the UPLC-MS was used to confirm the AHL degradation activity of RmmL via a lactonase mechanism. The mass spectrum of the degraded product of C12-HSL treated with RmmL produced a correct peak corresponding to acylhomoserine at 302.22 m/z. This result proved that RmmL is an AHL lactonase.
RmmL can degrade both short- and long-chain AHLs, but is somewhat more effective at degrading C6-, C10-, C12-, 3-oxo-C14-HSL (Figure 5). The activity of degrading C4-HSL was not detected in this research. Further biochemical characterization demonstrated that RmmL has the same optimal temperature (50 °C) to AiiM derived from the leaf-associated bacterium Microbacterium testaceum, while other QQ enzymes, AidC, AdeH, and MomL, have lower optimum temperatures (30 °C, 35 °C, and 40 °C, respectively) [,,,]. Different from M. testaceum, which has a wide range of growth temperatures (10–60 °C), R. mobilis has a narrow range of growth temperatures, 5–35 °C. In addition, RmmL retained 100% activity after 30 min treatment at 40 °C and 1 min at 100 °C. These results suggest RmmL could tolerate a relatively high temperature and maintain better enzymatic activity. Similar to MomL, a low range of pH affects the stability of RmmL. This is likely due to the conventional chelating agent, citric acid, used in the buffer, as previously speculated [].
RmmL might have a different AHL-degrading mechanism with other lactonase, even though the same “HXHXDH” motif, which agrees with the metallohydrolase criterion and conserved in the sequences of the metallo-β-lactamase superfamily, were found in RmmL as other AHL lactonases. EDTA (metal-chelating reagent) alone and most of the tested metal ions with EDTA inhibited the enzyme activity of RmmL, whereas Zn2+, Mn2+ and Co2+ with EDTA enhanced RmmL activity (Figure 6E), which is similar as AidC []. Despite the presence of “HXHXDH” zinc-binding motif in RmmL, its different metal-binding capability might result from the interaction involved with multiple sites, which remains to be determined by using site-directed mutagenesis in our future work.
The bacterial pathogen C. violaceum uses a LuxIR-type quorum-sensing system and the QS molecule C10-HSL can regulate the production of the violacein []; While 3O-C12-HSL and C4-HSL, C6-HSL and 3O-C6-HSL, respectively, are the important AHL signal molecules of P. aeruginosa [] and V. anguillarum []. RmmL significantly reduced the pyocyanin production of P. aeruginosa, while there was no significant reduction of the violacien production of C. violaceum, and extracellualar protease activity of V. anguillarum was observed. These results indicate that RmmL shows potential as a new therapeutic agent for attenuation of QS phenotypes in pathogenic bacteria. Future studies are warranted to test the therapeutic effect of RmmL on pathogens in vivo.
There are increasing numbers of studies identifying QS molecules and QQ enzymes from both Gram-positive and -negative bacteria of different families. In the case of the Rhodobacteriaceae family, only a few (Phaeobacter inhibens, Ruegeria sp. and Dinoroseobacter shibae) out of 17 different genera were reported to have produced AHL [], but there is no report about identification of QQ enzymes from any genus of the Rhodobacteriaceae family except R. mobilis from this study. RmmL identified and characterized represents a novel AHL lactonase and might have the potential to be used as a therapeutic agent against fish pathogens in aquaculture.
4. Materials and Methods
4.1. Bacterial Strains, Media, Growth Conditions, and Chemicals
R. mobilis YJ3 was cultured in marine broth 2216 (MB; Becton Dickinson, Franklin Lakes, NJ, USA) at 28 °C. Escherichia coli strain BL21 (DE3) was cultured on Luria–Bertani (LB) agar at 37 °C and used as a host for expressing proteins whose encoding genes were cloned into pET-32a (Novagen, Madison, WI, USA). The AHL biosensor Agrobacterium tumefaciens A136 (pCF218) (pCF372) [] was maintained on LB agar and grown in AT minimal medium (KH2PO4, 10.7 g/liter; MgSO4·7H2O, 160 mg/liter; CaCl2·2H2O, 10 mg/liter; FeSO4·7H2O, 5 mg/liter; MnSO4·7H2O, 2 mg/liter; (NH4)2SO4, 2 g/liter; glucose, 2 g/liter) [] containing 0.5% (w/v) glucose for detecting AHLs in liquid X-Gal assay. The AHL biosensor Chromobacterium violaceum CV026 [] and VIR24 [], which were used to detect short-chain (C4 to C8) and long-chain (C8 to C14) AHLs, were maintained on LB agar at 28 °C. Pseudomonas aeruginosa PAO1 was cultured on tryptic soy agar (TSA, Oxoid, Lenexa, KS, USA) at 37 °C. Appropriate antibiotics were added at the following concentrations: Ampicillin, 100 µg mL−1; Spectinomycin, 50 µg mL−1; Tetracycline, 4.5 µg mL−1. C4-HSL, C6-HSL, 3-oxo-C6-HSL, and C8-HSL were purchased from Cayman Chemical Company (Ann Arbor, MI, USA); 3-oxo-C8-HSL, C10-HSL, 3-oxo-C10-HSL, C12-HSL, 3-oxo-C12-HSL, C14-HSL, and 3-oxo-C14-HSL were purchased from Sigma-Aldrich (St. Louis, MO, USA). All of the AHL stock solutions (10 to 500 mM) were prepared in dimethyl sulfoxide (DMSO) and stored under −20 °C.
4.2. Cloning, Expression, and Purification of QQ Enzyme of YJ3
The putative lactonase gene rmmL of R. mobilis YJ3 was predicted from the whole genome sequence of YJ3 (X. Cai and X-H Zhang, unpublished data) by means of local BLASTP against several known QQ enzymes sequences. Genomic DNA of R. mobilis YJ3 was extracted through a phenol-chloroform method and used as the template of PCR (polymerase chain reaction) amplification for the rmmL gene using a pair of primers: Lactonase131F: 5′-CGCGGATCCATGACCCTTTCCCGT-3′ and Lactonase131R: 5′-CCCAAGCTTTTAGAGGCCGAATTGC-3′. The restriction sites for BamHI and HindIII are underlined both in the forward and reverse primers, respectively. PCR was performed with the following conditions: 1 cycle of 98 °C for 30 s; 30 cycles of 98 °C for 10 s, 55 °C for 10 s, and 72 °C for 1 min; and a final extension step at 72 °C for 2 min. The amplified DNA fragment, digested by BamHI and HindIII, was cloned into pET-32a vector (Novagen) to get the plasmid pET-32a-rmmL, which was then transformed into E. coli BL21 (DE3). Protein expression and purification were performed as previously described []. Briefly, E. coli BL21 (DE3) was grown in LB at 37 °C until the OD600 of the culture was between 0.4 to 0.6. Following this, 0.1 mM IPTG (isopropyl-d-thiogalactopyranoside) was added into the culture to induce protein expression at 16 °C with moderate shaking overnight. Following sonication and centrifugation, the Trx-tag fusion protein was purified from the supernatant by NTA-Ni column (Qiagen, Hilden, Germany). For purification of the RmmL without Trx-tag sequence, the fusion protein was incubated with enterokinase at room temperature overnight followed by purification using NTA-Ni column. The purified proteins were analyzed by SDS-PAGE and stored at 20 °C with 25% glycerol.
4.3. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis of AHL Degradation Products by RmmL
To analyze the chemical structure of products formed by RmmL catalysis, 100 µM C12-HSL was subjected to degradation by 10 µg purified RmmL in 500 µL reaction volume containing 50 µL 1,4-piperazinediethanesulfonic acid (PIPES, 1 M, pH 6.7) buffer. The hydrolysis reaction was carried out at 28 °C for 30 h and the mixture was extracted with 0.1% ethyl acetate three times and analyzed by ultra-performance liquid chromatography (UPLC) using a SunFire C18 reversed-phase column (3.5 µm; 4.6 by 50 mm) with a mobile phase of acetonitrile-water (0.01% trifluoroacetic acid; a linear gradient [v/v] of acetonitrile from 5 to 100% over 1.0 min at a flow rate of 0.5 mL min−1). The electrospray ionization (ESI)-mass spectrometry (MS) was used to analyze the resulting fractions.
4.4. Detection of AHL-Degrading Activity of RmmL against Different Signaling Molecules
The A. tumefaciens A136 liquid X-gal assay was carried out to determine the AHL-degrading activity of RmmL according to Tang et al. []. The normalized β-galactosidase activity was used to characterize the remaining AHLs, which is exactly the opposite of the activity of the RmmL. Briefly, a mixture of 8.9 µL recombinant RmmL, 1 µL PIPES, and 0.1 µL of 10 mM signaling molecule (C6-, C8-, C10-, C12-, 3-oxo-C6-, 3-oxo-C8-, 3-oxo-C10-, 3-oxo-C12-, 3-oxo-C14-HSL) were incubated for 30 h at 28 °C and then mixed with 190 µL A136 X-gal assay solution (A. tumefaciens A136 was cultured in broth overnight and then inoculated in AT minimal-glucose medium containing a final X-gal concentration of 250 µg mL−1) in 96-well plate and incubated at 28 °C for 24 h, The normalized β-galactosidase activities were calculated as described by Tang et al. [].
4.5. Physical and Chemical Parameters That Affect RmmL Activity
The thermostability and pH stability of RmmL were measured by the A136 liquid X-gal assay as previously described. Purified RmmL were pre-treated at different temperatures (40 °C, 60 °C, 80 °C, and 100 °C) for 30 min and at different pHs (Na2HPO4/citric acid for pH 2.0 to 7.0, Tris-HCl for pH 8.0 to 9.0, and Na2CO3/NaHCO3 for pH 10.0 to 11.0) at 4 °C for 3 h, respectively, and the activity was measured by the A136 liquid X-gal assay method by incubating RmmL with 10 mM C6-HSL substrate in PIPES buffer. The enzymatic activity of RmmL at 28 °C or treated at pH 7.0 was set as 100%. In addition, to test the stability of RmmL at a high temperature, RmmL was treated at 100 °C for 1 min, 5 min, 10 min or 30 min, and then the CV026 plate assay method was used to detect the residual activity []. The optimal temperature of RmmL activity was measured by the A136 liquid X-gal assay method by incubating RmmL with 10 mM C6-HSL substrate in PIPES buffer at different temperatures (10 °C, 20 °C, 30 °C, 40 °C, 50 °C, and 60 °C). The activity of RmmL at 28 °C was used as a control for comparison. To evaluate the effect of metal ions and EDTA on RmmL, purified RmmL was first incubated with 1 mM EDTA only at 4 °C for 1 h, and then further treated with 1.1 mM of different metal ions (Ni2+, Mg2+, Mn2+, Ca2+, Cu2+, Zn2+, Co2+, and Fe2+) at 4 °C for 1 h, followed by measurement of the residue activity using the A136 liquid X-gal assay with 10 mM C6-HSL substrate in PIPES buffer.
4.6. The Effect of RmmL on Violacien and Pyocyanin Production
The biosensor C. violaceum VIR24 was grown in 5 mL LB broth containing different concentrations (20 ng and 50 ng) of purified RmmL protein for 24 h at 28 °C without shaking. The cell density of each culture was then measured at a wavelength of 660 nm. Two hundred microliters of each culture were used for extracting violacein as previously described []. The upper n-butyl alcohol phase was collected and used to measure the absorbance at a wavelength of 590 nm. The amount of violacien was calculated using the equation OD590/(OD660 × 200 µL). No RmmL was added in the negative control group. The effect of RmmL on the pyocyanin production of P. aeruginosa PAO1 was performed as described by Tang et al. [].
4.7. The Effect of RmmL on the Extracellular Proteolytic Activity of V. anguillarum VIB72
V. anguillarum VIB72 (5 mL) was cultured together with different concentrations of RmmL (0, 25 ng, 50 ng), and then the extracellular proteolytic activity of the cultures was determined as described by Tang et al. [].
4.8. Nucleotide Sequence Accession Number
The nucleotide sequence of rmmL gene from YJ3 has been deposited in the GenBank database under accession no. MH289473.
Author Contributions
X.-H.Z. designed the experiments, supervised the research, and revised the manuscript; X.C. performed the experiments, analyzed the data, and wrote the manuscript; M.Y. and H.S. contributed to part of the characterization and analyzed the activity of RmmL; X.T. and Y.Z. provided help in purification of RmmL; C.X. helped to analyze the activity of RmmL.
Funding
This work was supported by projects from the National Natural Science Foundation of China (No. 41476112, No. 31502171 and No. 41521064), the National Key Research and Development Program of China (No. 2016YFA0601303) and the Fundamental Research Funds for the Central Universities (201762009).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hastings, J.W. Bioluminescence. Annu. Rev. Biochem. 1968, 37, 597–630. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, X.H. Quorum quenching agents: Resources for antivirulence therapy. Mar. Drugs 2014, 6, 3245–3282. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Xu, J.L.; Li, X.Z.; Zhang, L.H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 7, 3526–3531. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.J.; Oh, T.K.; Oh, J.W.; Koo, B.T.; Yum, D.Y.; Lee, J.K. AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology 2003, 6, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Su, Y.; Brackman, G.; Cui, F.; Zhang, Y.; Shi, X.; Coenye, T.; Zhang, X.H. MomL, a novel marine-derived N-acyl homoserine lactonase from Muricauda olearia. Appl. Environ. Microbiol. 2015, 2, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Z.; Morohoshi, T.; Ikenoya, M.; Someya, N.; Ikeda, T. AiiM, a novel class of N-acylhomoserine lactonase from the leaf-associated bacterium Microbacterium testaceum. Appl. Environ. Microbiol. 2010, 8, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Z.; Morohoshi, T.; Someya, N.; Ikeda, T. AidC, a novel N-acylhomoserine lactonase from the potato root-associated cytophaga-flavobacteria-bacteroides (CFB) group bacterium Chryseobacterium sp. strain StRB126. Appl. Environ. Microbiol. 2012, 22, 7985–7992. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Xu, J.L.; Hu, J.; Wang, L.H.; Ong, S.L.; Leadbetter, J.R.; Zhang, L.H. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol. Microbiol. 2003, 3, 849–860. [Google Scholar] [CrossRef]
- Romero, M.; Diggle, S.P.; Heeb, S.; Camara, M.; Otero, A. Quorum quenching activity in Anabaena sp. PCC 7120: Identification of AiiC, a novel AHL-acylase. FEMS Microbiol. Lett. 2008, 1, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kang, H.O.; Jang, H.S.; Lee, J.K.; Koo, B.T.; Yum, D.Y. Identification of extracellular N-acylhomoserine lactone acylase from a Streptomyces sp. and its application to quorum quenching. Appl. Environ. Microbiol. 2005, 5, 2632–2641. [Google Scholar] [CrossRef] [PubMed]
- Nadal Jimenez, P.; Koch, G.; Papaioannou, E.; Wahjudi, M.; Krzeslak, J.; Coenye, T.; Cool, R.H.; Quax, W.J. Role of PvdQ in Pseudomonas aeruginosa virulence under iron-limiting conditions. Microbiology 2010, 1, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, T.; Nakazawa, S.; Ebata, A.; Kato, N.; Ikeda, T. Identification and characterization of N-acylhomoserine lactone-acylase from the fish intestinal Shewanella sp. strain MIB015. Biosci. Biotechnol. Biochem. 2008, 7, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, C. Exploiting quorum sensing interfering strategies in Gram-negative bacteria for the enhancement of environmental applications. Front. Microbiol. 2016, 6, 1535. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, E.C.; Nielsen, K.F.; D’Alvise, P.; Porsby, C.H.; Melchiorsen, J.; Heilmann, J.; Kalatzis, P.G.; Lopez-Perez, M.; Bunk, B.; Sproer, C.; et al. Global occurrence and heterogeneity of the Roseobacter-clade species Ruegeria mobilis. ISME J. 2017, 2, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, J.B.; Gram, L.; Belas, R. Production of antibacterial compounds and biofilm formation by Roseobacter species are influenced by culture conditions. Appl. Environ. Microbiol. 2007, 2, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Porsby, C.H.; Nielsen, K.F.; Gram, L. Phaeobacter and Ruegeria species of the Roseobacter clade colonize separate niches in a Danish Turbot (Scophthalmus maximus)-rearing farm and antagonize Vibrio anguillarum under different growth conditions. Appl. Environ. Microbiol. 2008, 23, 7356–7364. [Google Scholar] [CrossRef] [PubMed]
- Carlier, A.; Uroz, S.; Smadja, B.; Fray, R.; Latour, X.; Dessaux, Y.; Faure, D. The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-Acyl homoserine lactonase activity. Appl. Environ. Microbiol. 2003, 8, 4989–4993. [Google Scholar] [CrossRef]
- Zhang, H.B.; Wang, L.H.; Zhang, L.H. Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 2002, 7, 4638–4643. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, T.; Tominaga, Y.; Someya, N.; Ikeda, T. Complete genome sequence and characterization of the N-acylhomoserine lactone-degrading gene of the potato leaf-associated Solibacillus silvestris. J. Biosci. Bioeng. 2012, 1, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; Heijne, G.; von Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods. 2011, 10, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Garge, S.S.; Nerurkar, A.S. Attenuation of Quorum Sensing Regulated Virulence of Pectobacterium carotovorum subsp. carotovorum through an AHL Lactonase Produced by Lysinibacillus sp. Gs50. PLoS ONE 2016, 12, e0167344. [Google Scholar] [CrossRef] [PubMed]
- Stauff, D.L.; Bassler, B.L. Quorum sensing in Chromobacterium violaceum: DNA recognition and gene regulation by the CviR receptor. J. Bacteriol. 2011, 193, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S.; Iglewski, B.H. Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. J. Clin. Investig. 2003, 112, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Milton, D.L. Quorum sensing in vibrios: Complexity for diversification. Int. J. Med. Microbiol. 2006, 296, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Zan, J.; Liu, Y.; Fuqua, C.; Hill, R.T. Acyl-homoserine lactone quorum sensing in the Roseobacter clade. Int. J. Mol. Sci. 2014, 1, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Beaber, J.W.; Moré, M.I.; Fuqua, C.; Eberhard, A.; Winans, S.C. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J. Bacteriol. 1998, 20, 5398–5405. [Google Scholar]
- Tempé, J.; Petit, A.; Holsters, M.; Montagu, M.; Schell, J. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: Possible relation to transformation in crown gall. Proc. Natl. Acad. Sci. USA 1977, 7, 2848–2849. [Google Scholar] [CrossRef]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycoft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Someya, N.; Morohoshi, T.; Okano, N.; Otsu, E.; Usuki, K.; Sayama, M.; Sekiguchi, H.; Ikeda, T.; Ishida, S. Distribution of N-acylhomoserine lactone-producing fluorescent pseudomonads in the phyllosphere and rhizosphere of potato (Solanum tuberosum L.). Microbes Environ. 2009, 4, 305–314. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, Y.; Yu, M.; Shi, X.; Coenye, T.; Bossier, P.; Zhang, X.H. Evaluation of a new high-throughput method for identifying quorum quenching bacteria. Sci. Rep. 2013, 3, 2935. [Google Scholar] [CrossRef] [PubMed]
- Blosser, R.S.; Gray, K.M. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods 2000, 1, 47–55. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).