Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products
Abstract
1. Introduction
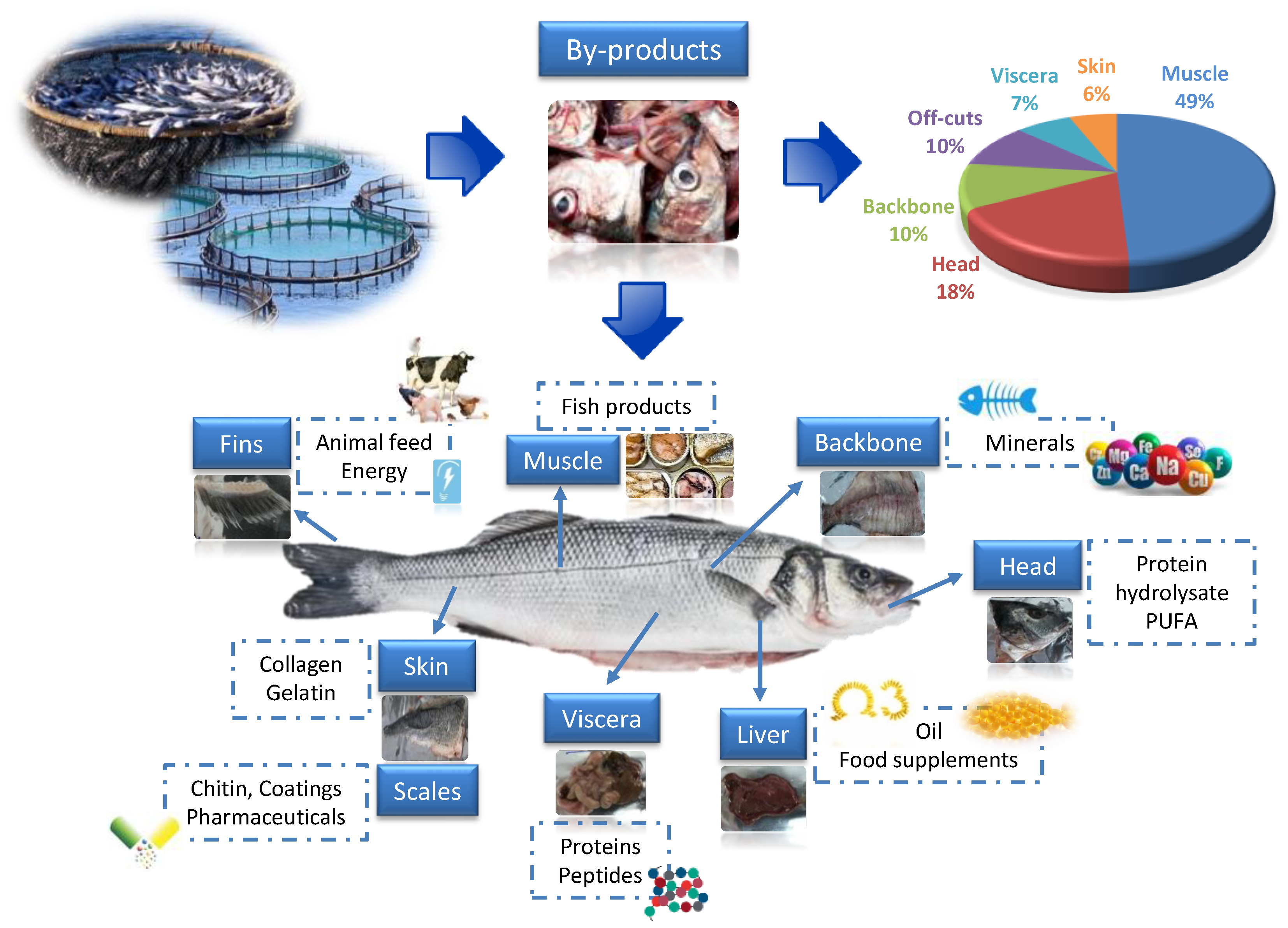
2. Valorization of Fish By-Products
3. Emerging Technologies for the Extraction of Bioactive Compounds from Fishery By-Products
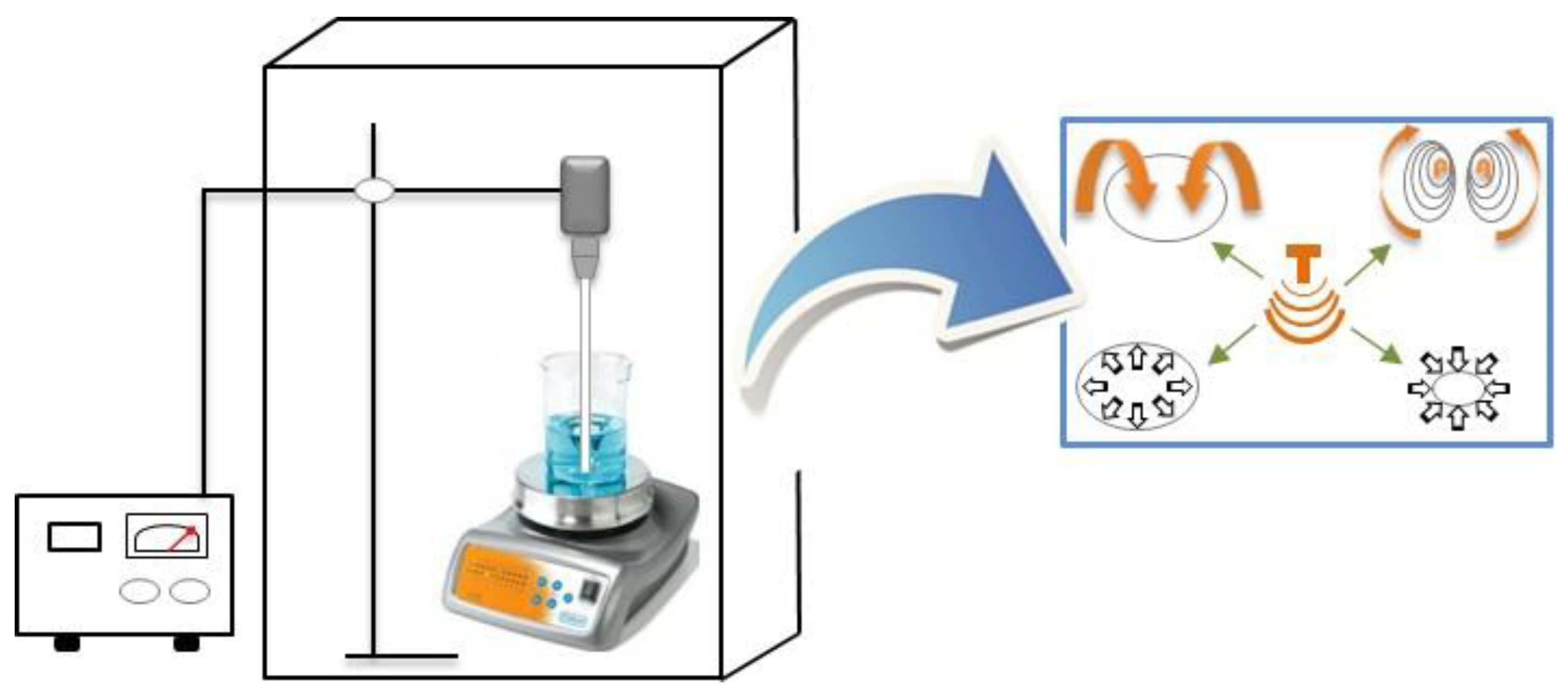
3.1. Ultrasound-assisted Extraction (UAE)
3.1.1. Fundamentals
3.1.2. Use of UAE in Fish Industry
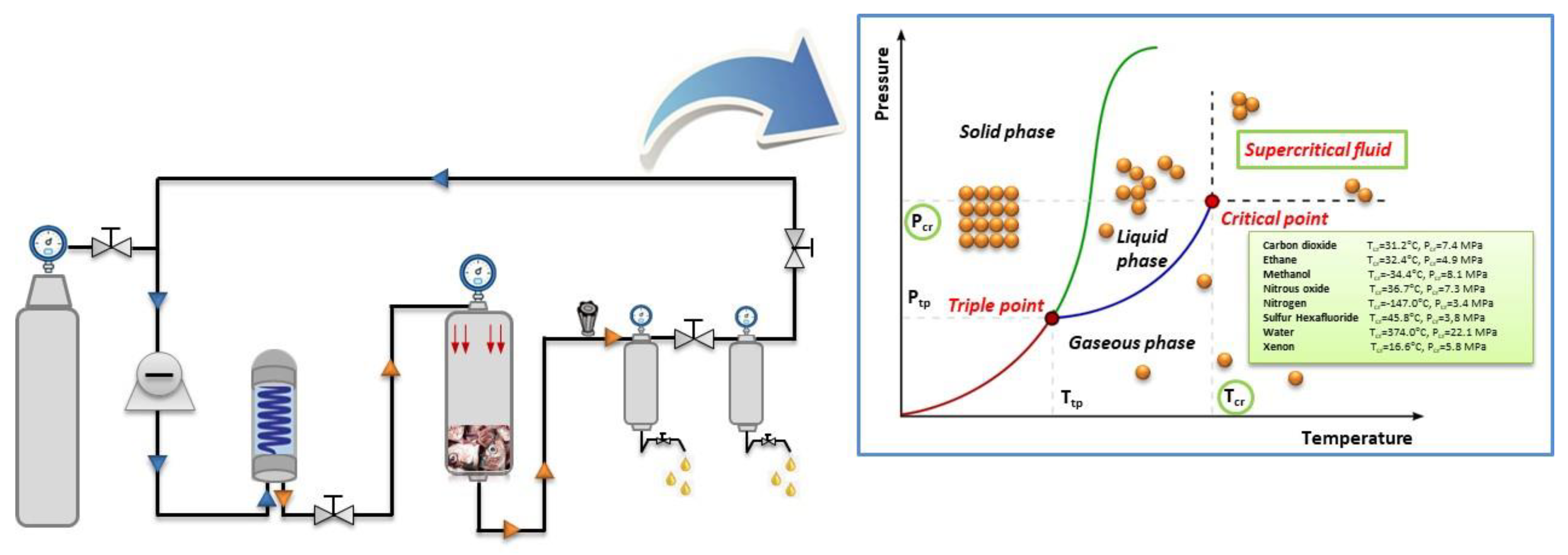
3.2. Supercritical Fluid Extraction (SFE)
3.2.1. Fundamentals
3.2.2. Application of SFE in By-Products from Fish Industry
3.2.3. Application of SFE in by-Products from Processing Shellfish
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Organización de las Naciones Unidas para la Agricultura y la Alimentación Focus: Desperdicio en la Pesca Artisanal. Available online: http://www.fao.org/focus/s/fisheries/proc.htm (accessed on 29 July 2019).
- Tørris, C.; Småstuen, M.C.; Molin, M. Nutrients in fish and possible associations with cardiovascular disease risk factors in metabolic syndrome. Nutrients 2018, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Kundam, D.N.; Acham, I.O.; Girgih, A.T. Bioactive compounds in fish and their health benefits. Asian Food Sci. J. 2018, 4, 1–14. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; ISBN 978-92-5-130562-1. [Google Scholar]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Falch, E.; Rustad, T.; Jonsdottir, R.; Shaw, N.B.; Dumay, J.; Berge, J.P.; Arason, S.; Kerry, J.P.; Sandbakk, M.; Aursand, M. Geographical and seasonal differences in lipid composition and relative weight of by-products from gadiform species. J. Food Compos. Anal. 2006, 19, 727–736. [Google Scholar] [CrossRef]
- Vázquez, J.; Meduíña, A.; Durán, A.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.; Rodríguez-Amado, I. Production of valuable compounds and bioactive metabolites from by-products of fish discards using chemical processing, enzymatic hydrolysis, and bacterial fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Schmidsdorff, W. Fish meal and fish oil-not only by-products. In Fish and Fishery Products, Composition, Nutritive Properties and Stability; Ruiter, A., Ed.; CAB International: Wallingford, UK, 1995; pp. 347–376. [Google Scholar]
- Breivik, H. Long-Chain Omega-3 Specialty Oils; The Oily Press: Cambridge, UK, 2007. [Google Scholar]
- Singh, A.; Ahmad, S.; Ahmad, A. Green extraction methods and environmental applications of carotenoids-a review. RSC Adv. 2015, 5, 62358–62393. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Barba, F.J.; Roselló-Soto, E.; Brnčić, M.; Lorenzo, J.M. Green Extraction and Valorization of By-Products from Food Processing from Food Processing, CRC Press: Boca Raton, FL, USA, 2020; ISBN 978100054.
- European Commission DIRECTIVE 2008/98/EC on waste and repealing certain Directives. Off. J. Eur. Union 2008, L 312, 3–30.
- European Commission Discarding and the Landing Obligation Fisheries. Available online: https://ec.europa.eu/fisheries/cfp/fishing_rules/discards_en (accessed on 29 July 2019).
- Kim, S.-K.; Mendis, E.; Shahidi, F. Marine fisheries by-products as potential nutraceuticals: An overview. In Marine Nutraceuticals and Functional Foods; Barrow, C.J., Shahidi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–23. ISBN 9781420015812. [Google Scholar]
- Ferraro, V.; Cruz, I.B.; Jorge, R.F.; Malcata, F.X.; Pintado, M.E.; Castro, P.M.L. Valorisation of natural extracts from marine source focused on marine by-products: A review. Food Res. Int. 2010, 43, 2221–2233. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Novel functional food ingredients from marine sources. Curr. Opin. Food Sci. 2015, 2, 123–129. [Google Scholar] [CrossRef]
- Teves, J.F.C.; Ragaza, J.A. The quest for indigenous aquafeed ingredients: A review. Rev. Aquac. 2016, 8, 154–171. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef]
- Aspevik, T.; Oterhals, Å.; Rønning, S.B.; Altintzoglou, T.; Wubshet, S.G.; Gildberg, A.; Afseth, N.K.; Whitaker, R.D.; Diana Lindberg, D. Valorization of proteins from co- and by-products from the fish and meat industry. In Chemistry and Chemical Technologies in Waste Valorization; Springer: Cham, Switzerland, 2017; pp. 123–150. [Google Scholar]
- Kudre, T.G.; Bhaskar, N.; Sakhare, P.Z. Optimization and characterization of biodiesel production from rohu (Labeo rohita) processing waste. Renew. Energy 2017, 113, 1408–1418. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.M. Bio-diesel and bio-gas production from seafood processing by-products. In Maximising the Value of Marine By-Products; Woodhead Publishing: Cambridge, England, 2007; pp. 460–485. ISBN 9781845690137. [Google Scholar]
- Blanco, M.; Sotelo, C.G.; Chapela, M.J.; Pérez-Martín, R.I. Towards sustainable and efficient use of fishery resources: Present and future trends. Trends Food Sci. Technol. 2007, 18, 29–36. [Google Scholar] [CrossRef]
- Ordóñez-Del Pazo, T.; Antelo, L.T.; Franco-Uría, A.; Pérez-Martín, R.I.; Sotelo, C.G.; Alonso, A.A. Fish discards management in selected Spanish and Portuguese métiers: Identification and potential valorisation. Trends Food Sci. Technol. 2014, 36, 29–43. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Durán, A.I.; Menduíña, A.; Nogueira, M.; Fraguas, J.; Mirón, J.; Valcárcel, J. Tailor-made process to recover high added value compounds from fishery by-products. In Green Extraction and Valorization of By-Products from Food Processing; Barba, F.J., Roselló-Soto, E., Brncic, M., Lorenzo, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 91–140. ISBN 9781138544048. [Google Scholar]
- Silva, T.; Moreira-Silva, J.; Marques, A.; Domingues, A.; Bayon, Y.; Reis, R.; Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; et al. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Alves, M.H.M.E.; Nascimento, G.A.; Cabrera, M.P.; Silvério, S.I.; Da, C.; Nobre, C.; Teixeira, J.A.; de Carvalho, L.B. Trypsin purification using magnetic particles of azocasein-iron composite. Food Chem. 2017, 226, 75–78. [Google Scholar] [CrossRef]
- Lassoued, I.; Mora, L.; Nasri, R.; Jridi, M.; Toldrá, F.; Aristoy, M.-C.; Barkia, A.; Nasri, M. Characterization and comparative assessment of antioxidant and ACE inhibitory activities of thornback ray gelatin hydrolysates. J. Funct. Foods 2015, 13, 225–238. [Google Scholar] [CrossRef]
- Maccari, F.; Galeotti, F.; Volpi, N. Isolation and structural characterization of chondroitin sulfate from bony fishes. Carbohydr. Polym. 2015, 129, 143–147. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Duan, W.W.; Duan, Z.H.; Hai, Y.; Lei, X.G.; Chang, H. Extraction of chondroitin sulfate from tilapia byproducts with ultrasonic-microwave synergistic. Adv. Mater. Res. 2013, 726–731, 4381–4385. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Enhancing and improving the extraction of omega-3 from fish oil. Sustain. Chem. Pharm. 2017, 5, 54–59. [Google Scholar] [CrossRef]
- Ferraro, V.; Carvalho, A.P.; Piccirillo, C.; Santos, M.M.; Castro, P.M.; Pintado, M.E. Extraction of high added value biological compounds from sardine, sardine-type fish and mackerel canning residues—A review. Mater. Sci. Eng. C 2013, 33, 3111–3120. [Google Scholar] [CrossRef]
- Toppe, J.; Albrektsen, S.; Hope, B.; Aksnes, A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from fish by-product protein hydrolysates and its functional properties: An overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Ishak, N.H.; Sarbon, N.M. A review of protein hydrolysates and bioactive peptides deriving from wastes generated by fish processing. Food Bioprocess Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Létisse, M.; Comeau, L. Enrichment of eicosapentaenoic acid and docosahexaenoic acid from sardine by-products by supercritical fluid fractionation. J. Sep. Sci. 2008, 31, 1374–1380. [Google Scholar] [CrossRef]
- Atef, M.; Mahdi Ojagh, S. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- He, S.; Franco, C.; Zhang, W. Functions, applications and production of protein hydrolysates from fish processing co-products (FPCP). Food Res. Int. 2013, 50, 289–297. [Google Scholar] [CrossRef]
- Hansen, J.S.; Rosnes, J.T.; Skuland, A.V.; Solstad, R.G.; Kousoulaki, K. Technical and sensory properties of salmon burgers added soluble peptide-rich fish proteins. In Proceedings of the WEFTA, Tórshavn, Faroe Islands; 15–17 October 2019. [Google Scholar]
- Galanakis, C.M. Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques; Academic Press: Cambridge, MA, USA, 2017. ISBN 978012809 6505.
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: A short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; de Alba, M.; Sun, D.-W.; Tiwari, B. Principles and recent applications of novel non-thermal processing technologies for the fish industry—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of ultrasound in food science and technology: A perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V.; Savva, A.G. Use of ultrasounds in the food industry–Methods and effects on quality, safety, and organoleptic characteristics of foods: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Shangguan, X.; Wang, H.; Zhang, L.; Sha, X. Rheological and structural properties of fish scales gelatin: Effects of conventional and ultrasound-assisted extraction. Int. J. Food Prop. 2017, 20, 1210–1220. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, Y.H.; Park, H.J.; Lee, N.H. Application of ultrasonic treatment to extraction of collagen from the skins of sea bass Lateolabrax japonicus. Fish. Sci. 2013, 79, 849–856. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913–922. [Google Scholar]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Tu, Z.-C.; Huang, T.; Wang, H.; Sha, X.-M.; Shi, Y.; Huang, X.-Q.; Man, Z.-Z.; Li, D.-J. Physico-chemical properties of gelatin from bighead carp (Hypophthalmichthys nobilis) scales by ultrasound-assisted extraction. J. Food Sci. Technol. 2015, 52, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.F.; Kudre, T.G.; Bhaskar, N. Impact of pretreatment-assisted enzymatic extraction on recovery, physicochemical and rheological properties of oil from Labeo rohita head. J. Food Process Eng. 2019, 42. [Google Scholar] [CrossRef]
- Kjartansson, G.T.; Zivanovic, S.; Kristbergsson, K.; Weiss, J. Sonication-assisted extraction of chitin from shells of fresh water prawns (Macrobrachium rosenbergii). J. Agric. Food Chem. 2006, 54, 3317–3323. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; Lélu, P.; Lynch, S.A.; Tiwari, B.K. Optimised protein recovery from mackerel whole fish by using sequential acid/alkaline isoelectric solubilization precipitation (ISP) extraction assisted by ultrasound. LWT Food Sci. Technol. 2018, 88, 210–216. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Hayes, M.; Tiwari, B.K. Bioactive carbohydrates and peptides in foods: An overview of sources, downstream processing steps and associated bioactivities. Int. J. Mol. Sci. 2015, 16, 22485–22508. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Barber, A.R.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioresour. Bioprocess. 2017, 4, 27. [Google Scholar] [CrossRef]
- Bruno, S.F.; Ekorong, F.J.A.A.; Karkal, S.S.; Cathrine, M.S.B.; Kudre, T.G. Green and innovative techniques for recovery of valuable compounds from seafood by-products and discards: A review. Trends Food Sci. Technol. 2019, 85, 10–22. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Saari, N.; Jahurul, H.A.; Abbas, K.A.; Norulaini, N.A. PUFAs in fish: Extraction, fractionation, importance in health. Compr. Rev. Food Sci. Food Saf. 2009, 8, 59–74. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Rodríguez-Meizoso, I. Advanced extraction processes to obtain bioactives from marine foods. In Bioactive Compounds from Marine Foods; John Wiley & Sons Ltd: Chichester, UK, 2013; pp. 343–371. [Google Scholar]
- Nautiyal Omprakash, H. Food processing by supercritical carbon dioxide-Review Omprakash. EC Chem. 2016, 2, 111–135. [Google Scholar]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Bucio, S.L.; Sanz, M.T.; Beltrán, S.; Melgosa, R.; Solaesa, Á.G.; Ruiz, M.O. Study of the influence of process parameters on liquid and supercritical CO2 extraction of oil from rendered materials: Fish meal and oil characterization. J. Supercrit. Fluids 2016, 107, 270–277. [Google Scholar] [CrossRef]
- Taati, M.M.; Shabanpour, B.; Ojagh, M. Extraction of oil from tuna by-product by supercritical fluid extraction (SFE) and comparison with wet reduction method. AACL Bioflux 2017, 10, 1546–1553. [Google Scholar]
- Kuvendziev, S.; Lisichkov, K.; Zeković, Z.; Marinkovski, M.; Musliu, Z.H. Supercritical fluid extraction of fish oil from common carp (Cyprinus carpio L.) tissues. J. Supercrit. Fluids 2018, 133, 528–534. [Google Scholar] [CrossRef]
- Ferdosh, S.; Sarker, M.Z.I.; Rahman, N.N.N.A.; Akanda, M.J.H.; Ghafoor, K.; Kadir, M.O.A. Simultaneous extraction and fractionation of fish oil from tuna by-product using supercritical carbon dioxide (SC-CO2). J. Aquat. Food Prod. Technol. 2016, 25, 230–239. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Norulaini, N.N.A.; Jinap, S.; Jahurul, M.H.A.; Omar, M.A.K. Storage stability and quality of polyunsaturated fatty acid rich oil fraction from Longtail tuna (Thunnus tonggol) head using supercritical extraction. CYTA J. Food 2014, 12, 183–188. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, S.; Hu, W.; Zhang, J.; Ding, Y.; Liu, J. Extraction of oil from high-moisture tuna livers by subcritical dimethyl ether: A comparison with different extraction methods. Eur. J. Lipid Sci. Technol. 2019, 121, 1800087. [Google Scholar] [CrossRef]
- Haque, A.S.M.; Asaduzzaman, A.K.M.; Chun, B.S. Fatty acid composition and stability of extracted mackerel muscle oil and oil-polyethylene glycol particles formed by gas saturated solution process. Fish. Aquat. Sci. 2014, 17, 67–73. [Google Scholar] [CrossRef]
- Haq, M.; Ahmed, R.; Cho, Y.J.; Chun, B.S. Quality properties and bio-potentiality of edible oils from atlantic salmon by-products extracted by supercritial carbon dioxide and conventional methods. Waste Biomass Valoriz. 2017, 8, 1953–1967. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; de Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods. J. Food Eng. 2012, 109, 238–248. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Jahurul, M.H.A.; Khatib, A.; Norulaini, N.A.N. Extraction of fish oil from the skin of Indian mackerel using supercritical fluids. J. Food Eng. 2010, 99, 63–69. [Google Scholar] [CrossRef]
- Uddin, M.S.; Ahn, H.-M.; Kishimura, H.; Chun, B.-S. Comparative study of digestive enzymes of squid (Todarodes pacificus) viscera after supercritical carbon dioxide and organic solvent extraction. Biotechnol. Bioprocess Eng. 2009, 14, 338–344. [Google Scholar] [CrossRef]
- Uddin, M.S.; Ahn, H.-M.; Kishimura, H.; Chun, B.-S. Production of valued materials from squid viscera by subcritical water hydrolysis. J. Environ. Biol. 2010, 31, 675–679. [Google Scholar]
- Uddin, M.S.; Kishimura, H.; Chun, B.-S. Isolation and characterization of lecithin from squid (Todarodes pacificus) viscera deoiled by supercritical carbon dioxide extraction. J. Food Sci. 2011, 76, C350–C354. [Google Scholar] [CrossRef]
- Lisichkov, K.; Kuvendziev, S.; Zeković, Z.; Marinkovski, M. Influence of operating parameters on the supercritical carbon dioxide extraction of bioactive components from common carp (Cyprinus carpio L.) viscera. Sep. Purif. Technol. 2014, 138, 191–197. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; de Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of the omega-3 rich oil contained in hake (Merluccius capensis–Merluccius paradoxus) by-products: Study of the influence of process parameters on the extraction yield and oil quality. J. Supercrit. Fluids 2008, 47, 215–226. [Google Scholar] [CrossRef]
- Khaw, K.; Parat, M.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhu, X.; Zhu, C.; Qian, J.; Zhu, N.; Zhao, L.; Chen, J. Hydrolysis technology of biomass waste to produce amino acids in sub-critical water. Bioresour. Technol. 2008, 99, 3337–3341. [Google Scholar] [CrossRef] [PubMed]
- Amiguet, V.T.; Kramp, K.L.; Mao, J.; McRae, C.; Goulah, A.; Kimpe, L.E.; Blais, J.M.; Arnason, J.T. Supercritical carbon dioxide extraction of polyunsaturated fatty acids from Northern shrimp (Pandalus borealis Kreyer) processing by-products. Food Chem. 2012, 130, 853–858. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Almeida Meireles, M.Â.; Lopes, B.L.F.; Cabral, F.A. Proximate composition and extraction of carotenoids and lipids from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Food Eng. 2011, 102, 87–93. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Martinez-Correa, H.A.; Paviani, L.C.; Cabral, F.A. Supercritical CO2 extraction of lipids and astaxanthin from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Supercrit. Fluids 2011, 56, 164–173. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Zhang, W.; Barber, A.R.; Su, P.; He, S. Significant enrichment of polyunsaturated fatty acids (PUFAs) in the lipids extracted by supercritical CO2 from the livers of australian rock lobsters (Jasus edwardsii). J. Agric. Food Chem. 2015, 63, 4621–4628. [Google Scholar] [CrossRef]
- Charest, D.J.; Balaban, M.O.; Marshall, M.R.; Cornell, J.A. Astaxanthin extraction from crawfish shells by supercritical CO2 with ethanol as cosolvent. J. Aquat. Food Prod. Technol. 2001, 10, 81–96. [Google Scholar] [CrossRef]
- Shahidi, F.; Brown, J.A. Carotenoid pigments in seafoods and aquaculture. Crit. Rev. Food Sci. Nutr. 1998, 38, 1–67. [Google Scholar] [CrossRef]
- López, M.; Arce, L.; Garrido, J.; Ríos, A.; Valcárcel, M. Selective extraction of astaxanthin from crustaceans by use of supercritical carbon dioxide. Talanta 2004, 64, 726–731. [Google Scholar] [CrossRef]
- Félix-Valenzuela, L.; Higuera-Ciapara, I.; Goycoolea-Valencia, F.; Argüelles-Monal, W. Supercritical CO2/ethanol extraction of astaxanthin from blue crab (Callinectes sapidus) shell waste. J. Food Process Eng. 2001, 24, 101–112. [Google Scholar] [CrossRef]
- Ali-Nehari, A.; Chun, B.-S. Characterization of purified phospholipids from krill (Euphausia superba) residues deoiled by supercritical carbon dioxide. Korean J. Chem. Eng. 2012, 29, 918–924. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Meireles, M.Â.A.; Ferreira, A.L.K.; Saito, E.; Cabral, F.A. Extraction of ω-3 fatty acids and astaxanthin from Brazilian redspotted shrimp waste using supercritical CO2 + ethanol mixtures. J. Supercrit. Fluids 2012, 61, 71–77. [Google Scholar] [CrossRef]
| Extraction Technique | Advantages | Drawbacks | Extraction Conditions | Solvents |
|---|---|---|---|---|
| UAE | Reduction of energy, time and solvent consumption | Can induce lipid oxidation: increasing temperature by cavitation; formation of free radicals by sonolysis; mechanical forces generated by shockwaves and microstreaming. | 25 kHz 200–2450 W 30-60 min | Ethanol, cyclohexane, other organic solvents |
| Safe; does not produce toxic compounds | High power consumption | |||
| Higher penetration of solvent into cellular material and enhanced release in medium | Difficult to scale up |
| By-Product | Source | Bioactive Compound and Product | Extraction Conditions | Main Effects | Ref. |
|---|---|---|---|---|---|
| Head | Labeo rohita | Oil | UAE: 20 kHz, 40% amplitude, for 5, 10 and 15 min. Enzymatic hydrolysis: Protamex ratio of 1:100 (w/w), 2 h, 150 rpm, 55 °C. | Pretreatments with UAE improved the extraction yield of oil, showing higher oil recoveries (67.48% vs. 58.74 % for SFE and untreated samples, respectively). | [64] |
| Scales | Bighead carp (Hypophthalmichthys nobilis) | Gelatin | Temperature: 60, 70 and 80 °C Extraction time: 1 h | Improved technological properties: highest storage modulus (5000 Pa), gelation point (22.94 °C), and melting point (29.54 °C). | [59] |
| Bighead carp (Hypophthalmichthys nobilis) | Gelatin | Temperature: 60 °C Extraction time: 1, 3 and 5 h | Extraction yield: 46.67% for ultrasound bath versus 36.39% for water bath. | [63] | |
| Shells | Prawns (Macrobrachium rosenbergii) | Chitin | Extraction time: 0, 1, and 4 h 0.25M NaOH at solid to liquid ratio of 1:40 (w/v) Power: 41 W/cm. | Decrease of the crystallinity indices and extraction yield of chitin as the time of sonication increased. | [65] |
| Skin | Japanese sea bass (Lateolabrax japonicus) | Collagen | UAE: 20 kHz, 80% amplitude, 0.1 M acetic acid, 3 h. | UAE did not alter the major components of collagen (α1, α2 and β chains). | [60] |
| Whole fish | Mackerel | Proteins | ISP: Isoelectric solubilization precipitation. UAE: 40 kHz, 60% amplitude, 0.1 M NaOH, 10 min. | Significant increase of protein recovery, recovering more than 95% of total protein from mackerel by-products. | [66] |
| Extraction Technique | Advantages | Drawbacks | Extraction Conditions | Solvents |
|---|---|---|---|---|
| SFE | Green extraction Technique. No need for organic solvent, and therefore the extract is very pure. Lipids can be used immediately | Very expensive and complex equipment operating at elevated pressures | 25–40 MPa 40–80 °C CO2 flow > 2 mL/min 45 min-6 h | Co-solvent: Ethanol |
| Maintain the quality of the final product. Low operating temperatures (40–80 °C) | No polar substances are extracted | |||
| Free of heavy metals and inorganic salts | High power consumption | |||
| Very effective because of its low viscosity and high diffusivity. Fast and high yield |
| By-Product | Source | Bioactive Compound and Product | SC-CO2 Conditions | Outcomes | Ref. | |
|---|---|---|---|---|---|---|
| Canned by-product | Tuna | Oils (volatiles) | Temperature ≥ 40 °C Pressure ≥ 25 MPa CO2 flow ≥ 10 kg/h Extraction time: 3 h | Extracted oils showed better conditions, quality (type of compounds and indicators of lipid oxidation) and yield. | [78] | |
| Caviar, fillet and viscera | Carp (Cyprinus carpio L.) | Oil (MUFA and PUFA) | Temperature: 40, 50 and 60 °C Pressure: 200, 300, 350 and 400 bar CO2 flow: 0.194 kg/h Extraction time: 180 min | Omega-enriched fish oils (DHA and EPA). High yields, above 50 g/100 g in viscera, which are similar to those obtained with petroleum ether. | [79] | |
| Fish meal | n.a. 1 | Oil (MUFA and PUFA) | Temperature: 25–80 °C Pressure: 10–40 MPa CO2 flow with ethanol: 9.5 g/min | High reductions of fat (90%). Extract with a lighter colour due to astaxanthin extraction. | [77] | |
| Head | Thunnus tonggol | Fatty acid (omega 3 and omega 6) | Temperature: 65 °C Pressure: 40 MPa CO2 flow with ethanol: 3 mL/min Extraction time: 2 h | SC-CO2 (co-solvent) is a good technique to extract omega3/6 after fractionations of oil. | [80] | |
| PUFA | Temperature: 65 °C Pressure: 40 MPa CO2 flow with ethanol: 2.4 mL/min Ethanol flow: 0.6 mL/min Extraction time: 120 min | Good quality of extracted PUFA-rich fraction, even 60 days after storage. | [81] | |||
| Heads and tails | Sardine | DHA and EPA | Temperature: 75 °C Pressure: 300 bar CO2 flow: 2.5 mL/min Extraction time: 45 min | Increase of the extraction yields: DHA (59%), EPA (28%). | [44] | |
| Liver | Tuna | Fatty acids | Step of freeze-drying (12h) Temperature: 40 °C Pressure: 35 MPa Continuous CO2 flow: 3mL/min (at 20 °C) Extraction time: 4h | High quality and excellent yield obtained 98.45%. | [82] | |
| Muscle | Mackerel | Vitamins | Temperature: 45 °C Pressure: 15–25 MPa CO2 flow: 27 g/min Extraction time: 2 h | High extraction of vitamins A, D2, D3 and α-tocopherol | [83] | |
| Muscle, bone and skin | Salmon | Oil (PUFA) | Temperature: 45 °C Pressure: 250 bar CO2 Flow: 27g/min Extraction time: 3 h | Premium quality oil of physical, biochemical and biological properties. Yield 76.12 %–86.99%. | [84] | |
| Muscle | Mackerel | Oil (EPA and DHA) | Temperature: 45 °C Pressure: 15–25 MPa CO2 flow: 27 g/min Extraction time: 2 h | The extracted oil presented significant contents of PUFAs (EPA, DHA). Higher stability compared with n-hexane extracted oil. | [83] | |
| Off-cuts | Hake (Merluccius capensis– Merluccius paradoxus) | Oil (omega-3 fatty acids) | Temperature: 313 K Pressure: 25 MPa CO2 flow: 880 kg/m3 | PUFA extraction. Reduction of fish oil oxidation. Reduction of certain impurities. Co-extraction of some endogenous volatile compounds. | [85] | |
| Orange roughy (Hoplostethus atlanticus) | ||||||
| Salmon (Salmo salar) | ||||||
| Liver | Jumbo squid (Dosidicus gigas) | |||||
| Skin | Mackerel (Rastrelliger kanagurta) | Oil (PUFA) | Temperature: 45–75 °C Pressure: 20–35 MPa | Continuous: Pressurized (5 min, CO2 flow 2 mL/min | Yield very close to those obtained with the Soxhlet technique. | [86] |
| Co-solvent technique: CO2 and ethanol (80%–20% at 2 mL/min) for 6 h | PUFA constituents of co-solvent, soaking and pressure swing techniques were similar to the Soxhlet method. | |||||
| Soaking: Samples soaked with pure CO2 for 10 h then extracted for 6 h | The largest recoveries of PUFA, especially the ω-3 family, were achieved from the soaking and pressure swing techniques at 35 MPa and 75 °C. | |||||
| Pressure swing: Samples pressurized (CO2) (2 h, extracted 3 h | ||||||
| Viscera | Squid (Todarodes pacificus) | Enzymes | Temperature: 35–45 °C Pressure: 15–25 MPa CO2 flow: 22 g/min Extraction time: 2.5 h | Thermal stability of enzymes was slightly higher than n-hexane-treated squid viscera. Denaturation of proteins did not occur. | [87] | |
| Amino acids | SFE: Temperature: 35–45 °C Pressure: 15–25 MPa CO2 flow: 22 g/min Extraction time: 2.5 h | SWH: Temperature: 180–280 °C Pressure: 0.101–6.41 MPa Extraction time: 5 min | Positive effects of the use of SFE as a pretreatment method. Amino acids were 1.5 times higher than those obtained in non-deoiled samples. | [88] | ||
| Lecithin | Temperature: 35–45 °C Pressure: 15–25 MPa CO2 flow: 22 g/min Extraction time: 2.5 h | Extraction yield was higher at the highest temperature and pressure (0.34 g/g squid viscera at 45 °C and 25 MPa). Lecithin that was isolated had in its composition some polyunsaturated fatty acids (EPA and DHA) with a high oxidative stability. | [89] | |||
| Common carp (Cyprinus carpio L.) | PUFA | Temperature: 40, 50 and 60 °C Pressure: 200, 300, 350 and 400 bar CO2 mass flow: 0.194, 0.277 and 0.354 kg/h Extraction time: 30, 60, 120 and 180 min | Adequate for the isolation of bioactive components. Positive impact on the total yield and extraction time. | [90] | ||
| By-Product | Source | Bioactive Compound | SC-CO2 Conditions | Outcomes | Ref. |
|---|---|---|---|---|---|
| Head, shells and tails | Brazilian redspotted shrimp (Farfantepenaeus paulensis) | Lipids and carotenoids | Temperature: 50 °C Pressure: 30 MPa CO2 flow: 4.2 × 10−5 kg/s Extraction time: 20 min Solvent for compounds recovery: n-hexane | Increase extraction yield: Astaxanthin (36%) | [95] |
| Temperature: 50 °C Pressure: 30 MPa CO2 flow with ethanol: 8.3 × 10−5 kg/s Ethanol flow: 4.4 × 10−6 kg/s Extraction time: 200 min Solvent for compounds recovery: n-hexane | Increase extraction yield: Astaxanthin (57.9%) | ||||
| Temperature: 43 °C Pressure: 370 bar CO2 flow: 1.5 L/min Extraction time: 200 min Solvent for compounds recovery: n-hexane | Increase extraction yield: Astaxanthin (39%) | [96] | |||
| Northern shrimp (Pandalus borealis Kreyer) | PUFA | Temperature: 40 °C Pressure: 35 MPa CO2 flow: 3-5 L/min Extraction time: 90 min | Lower yields (137 mg oil/g) than those obtained in organic solvent extraction. Higher contents of total fatty acid content (795 mg/g), DHA (8%), EPA (7.8%). | [94] | |
| Liver | Rock lobsters (Jasus edwardsii) | PUFA and vitamins | Temperature: 50 °C Pressure: 35 MPa Continuous CO2 flow: 0.434 kg/h Extraction time: 4h | Enrichment in PUFAs (DHA, EPA) vs. Soxhlet extraction. Reduction in the amounts of toxic heavy metals. | [97] |
| Shell | Crawfish | Pigments | Temperature: 50–70 °C Pressure: 13.8-31.0 MPa CO2 flow: 1.0–1.5 L/min Co-solvent: 10% ethanol | Increase extraction yield: Astaxanthin (197.6 mg/kg) | [98] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Mar. Drugs 2019, 17, 689. https://doi.org/10.3390/md17120689
Al Khawli F, Pateiro M, Domínguez R, Lorenzo JM, Gullón P, Kousoulaki K, Ferrer E, Berrada H, Barba FJ. Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Marine Drugs. 2019; 17(12):689. https://doi.org/10.3390/md17120689
Chicago/Turabian StyleAl Khawli, Fadila, Mirian Pateiro, Rubén Domínguez, José M. Lorenzo, Patricia Gullón, Katerina Kousoulaki, Emilia Ferrer, Houda Berrada, and Francisco J. Barba. 2019. "Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products" Marine Drugs 17, no. 12: 689. https://doi.org/10.3390/md17120689
APA StyleAl Khawli, F., Pateiro, M., Domínguez, R., Lorenzo, J. M., Gullón, P., Kousoulaki, K., Ferrer, E., Berrada, H., & Barba, F. J. (2019). Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Marine Drugs, 17(12), 689. https://doi.org/10.3390/md17120689









