Temporal Variation of the Profile and Concentrations of Paralytic Shellfish Toxins and Tetrodotoxin in the Scallop, Patinopecten yessoensis, Cultured in a Bay of East Japan
Abstract
1. Introduction
2. Results
2.1. Optimization of HILIC-MS/MS Conditions for Simultaneous Analysis of PSTs and TTX
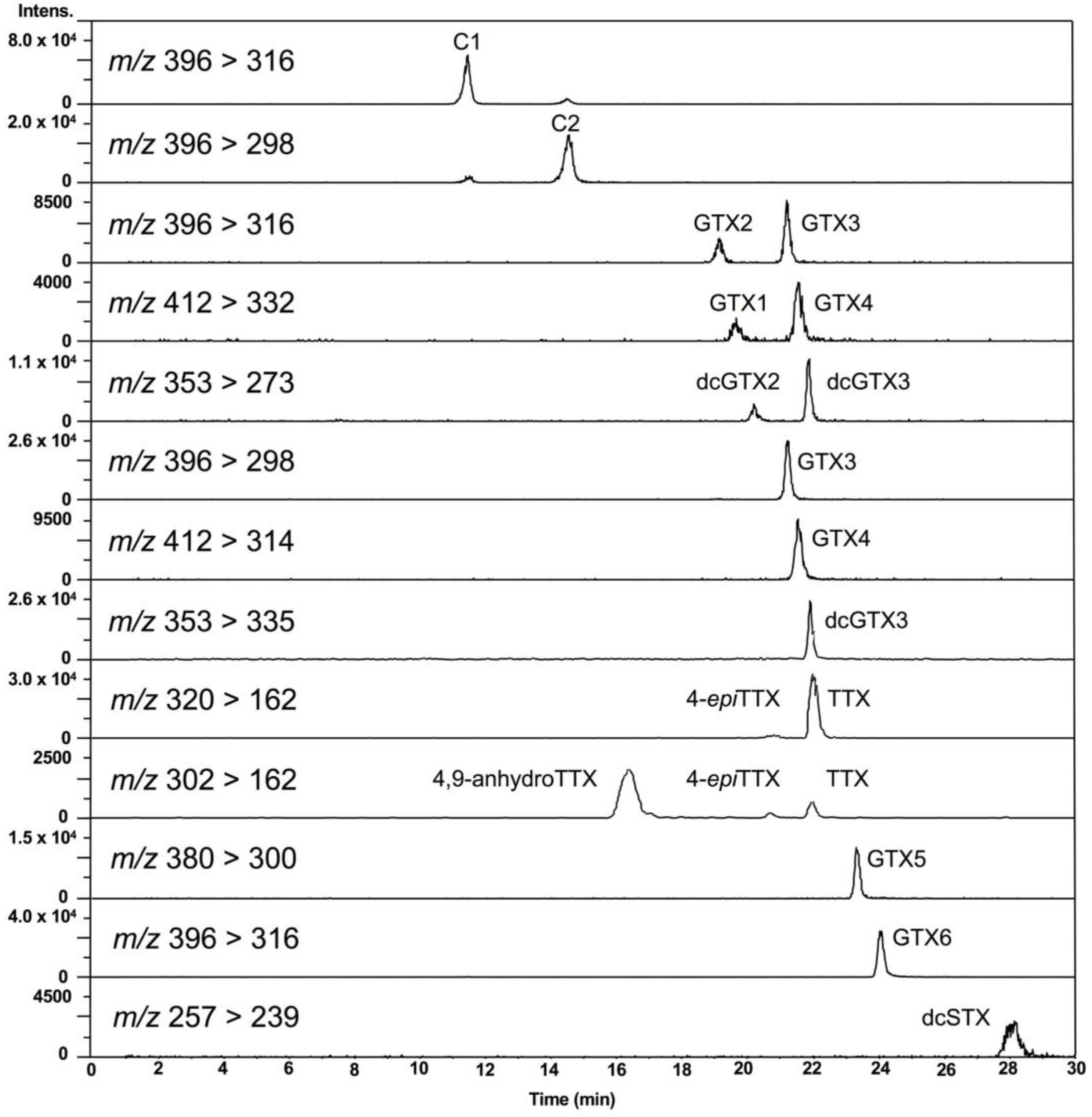
2.2. Analysis of PSTs and TTX in Scallops Using HILIC-MS/MS
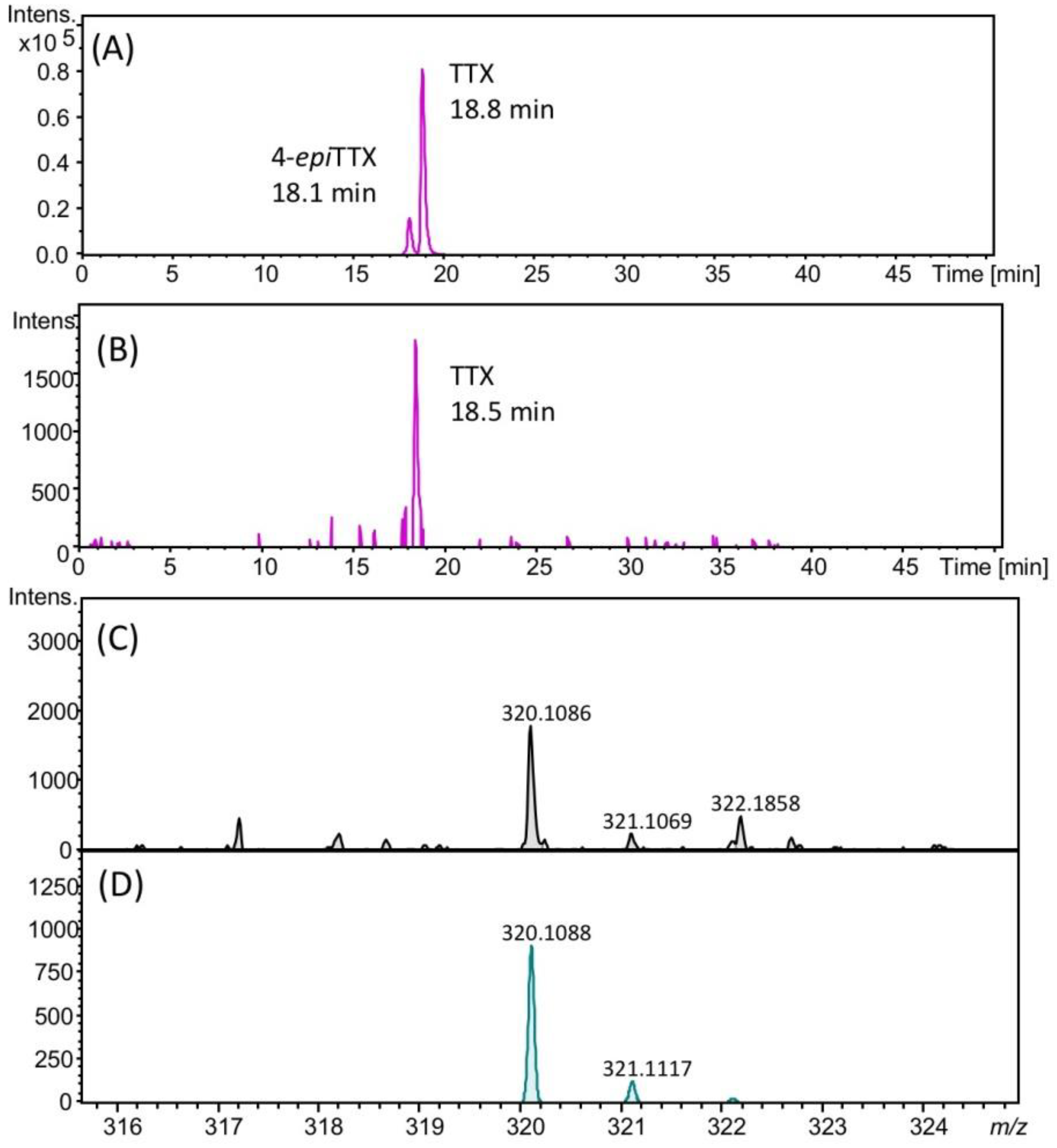
2.3. Identification of TTX in Scallops Using HR-LC-MS and HR-LC-MS/MS
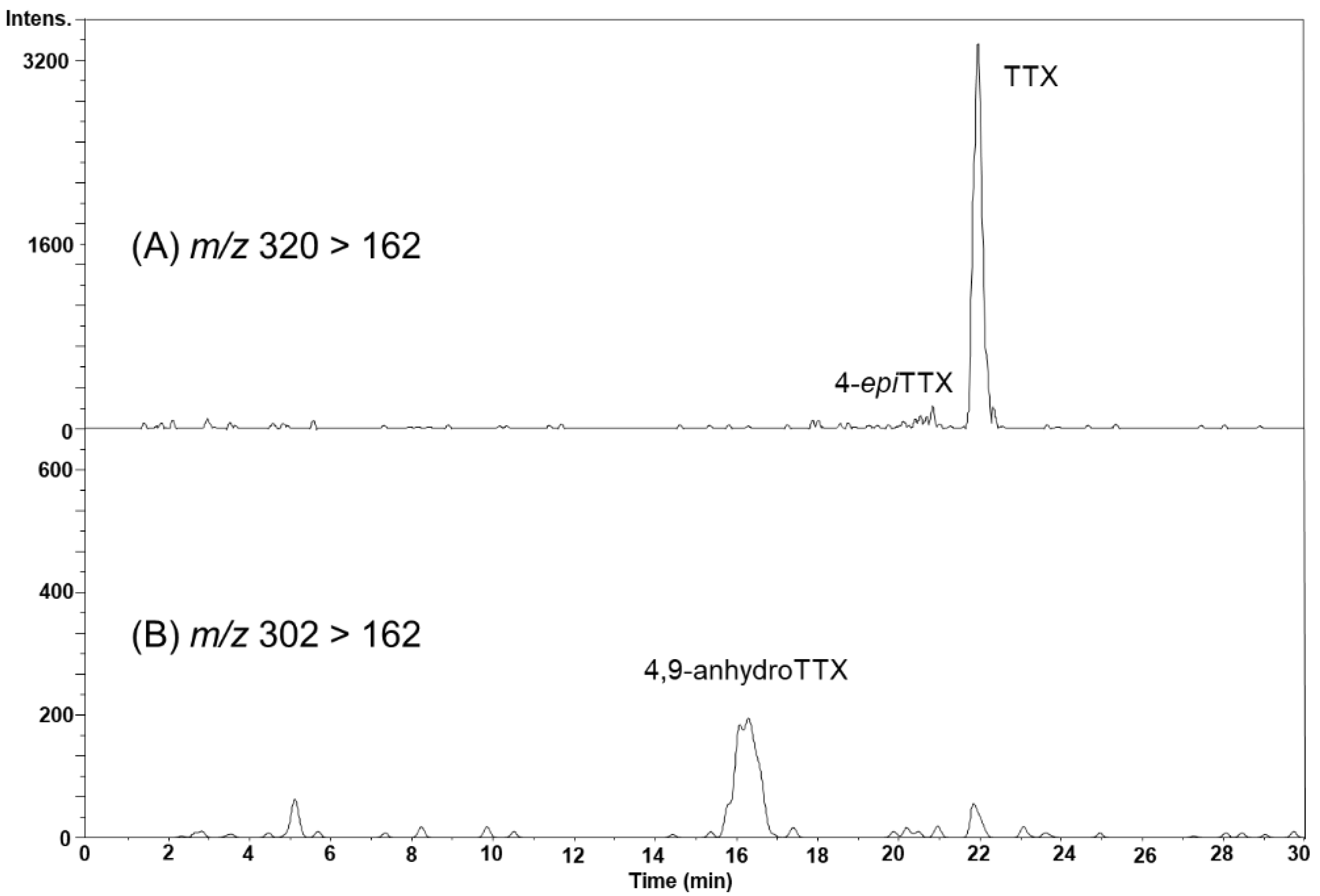
2.4. TTX Analogues in Scallops
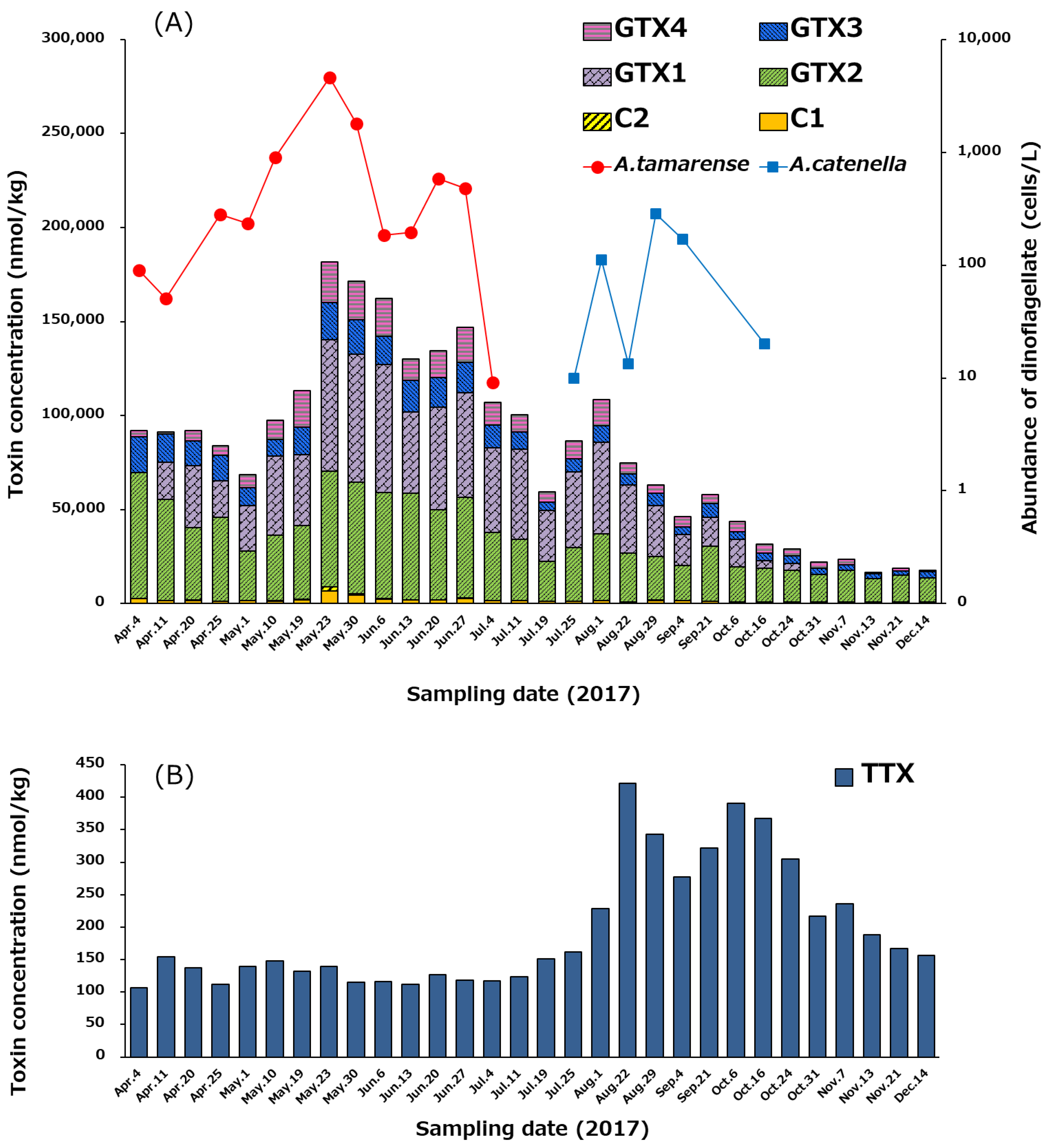
2.5. Temporal Variation of PSTs and TTX in Scallops, and the Cell Densities of Alexandrium tamarense and A. catenella
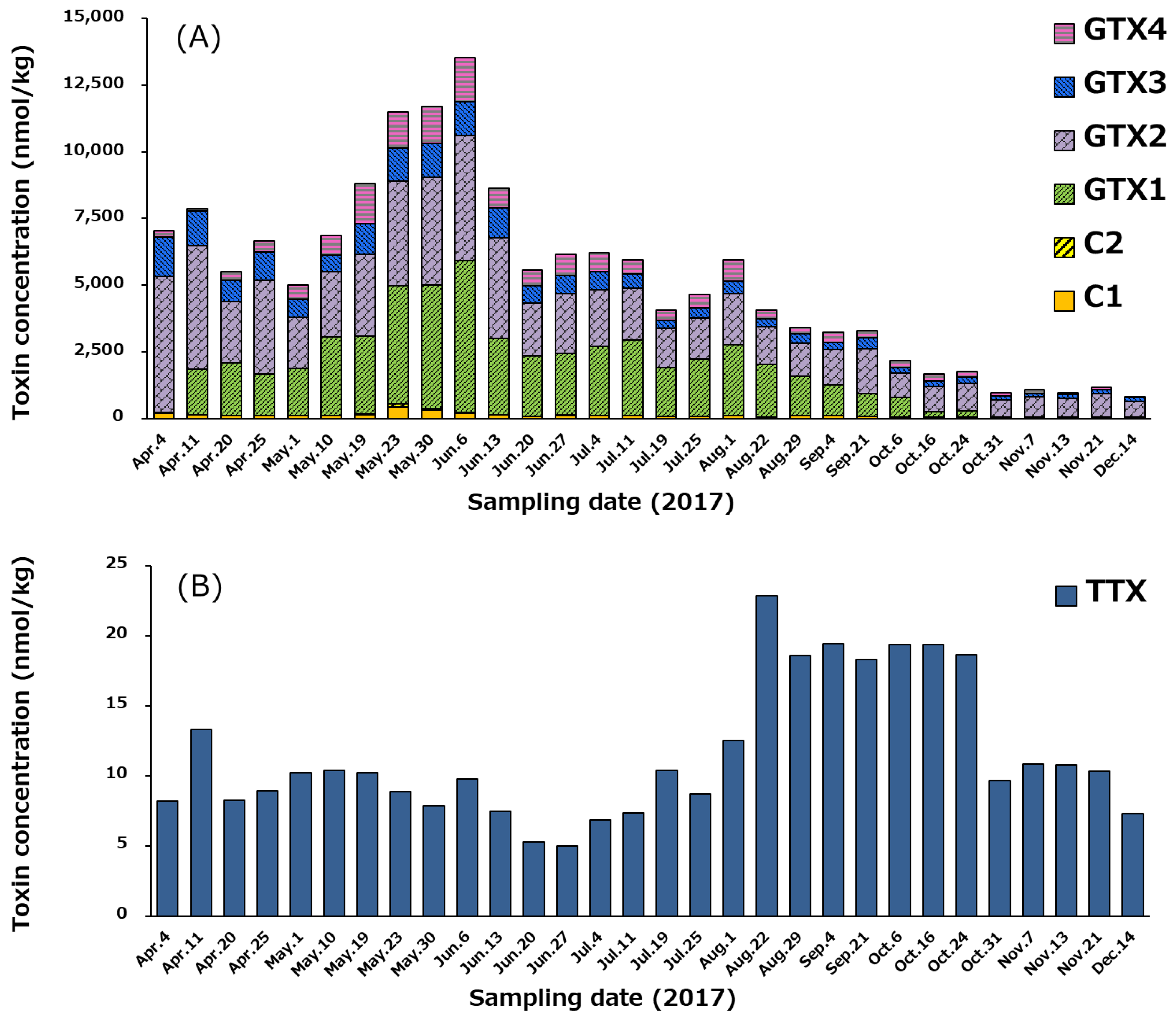
2.6. Concentration of PSTs and TTX in Whole Edible Tissues of Scallops
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Validation of the HILIC-MS/MS Method
4.3. Scallop Samples
4.4. Cell counts of A. tamarense and A. catenella
4.5. Toxin Extraction and Clean-Up
4.6. Estimation of Toxin Concentration in the Whole Edible Tissue
4.7. LC-MS/MS for Simultaneous Analysis of PSTs and TTXs in Scallops
4.8. HR-MS/MS Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Llewellyn, L.E. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat. Prod. Rep. 2006, 23, 200–222. [Google Scholar] [CrossRef] [PubMed]
- Thottumkara, A.P.; Parsons, W.H.; Du Bois, J. Saxitoxin. Angew. Chem. Int. Ed. 2014, 53, 5760–5784. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomenon. Pharmacol. Rev. 1966, 18, 997–1049. [Google Scholar] [PubMed]
- Carmichael, W.W.; Evans, W.R.; Yin, Q.Q.; Bell, P.; Moczydlowski, E. Evidence for paralytic shellfish poisons in the freshwater cyanobacterium Lyngbya wollei (Farlow ex Gomont) comb. nov. Appl. Environ. Microbiol. 1997, 63, 3104–3110. [Google Scholar]
- Kellmann, R.; Mihali, T.K.; Young, J.J.; Pickford, R.; Pomati, F.; Neilan, B.A. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 2008, 74, 4044–4053. [Google Scholar] [CrossRef]
- Codex. Standard for live and raw bivalve molluscs. In CODEX, CODEX STAN; FAO/WHO: Rome, Italy, 2008; pp. 292–2008. [Google Scholar]
- EFSA. Scientific opinion of the panel on contaminants in the food chain, Marine biotoxins in shellfish–saxitoxin group. EFSA J. 2009, 1019, 1–76. [Google Scholar]
- U.S. Food and Drug Administration. National Shellfish Sanitation Program Guide for the Control of Molluscan Shellfish 2017 Revision. Available online: https://www.fda.gov/media/117080/download (accessed on 23 September 2019).
- Kao, C.Y.; Nishiyama, A. Actions of saxitoxin on peripheral neuromuscular systems. J. Physiol. 1965, 180, 50–66. [Google Scholar]
- Shen, H.; Liu, D.; Wu, K.; Lei, J.; Yan, N. Structures of human Nav1.7 channel in complex with auxiliary subunits and animal toxins. Science 2019, 363, 1303–1308. [Google Scholar] [CrossRef]
- Yokoo, A. Chemical studies on tetrodotoxin Rept. III. Isolation of spheroidine. J. Chem. Soc. Jpn. 1950, 71, 591–592. [Google Scholar]
- Sheumack, D.D.; Howden, M.E.H.; Spence, I.; Quinn, R.J. Maculotoxin: A neurotoxin from the venom glands of the octopus Hapalochlaena maculosa identified as tetrodotoxin. Science 1978, 199, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, D.; Oshima, Y.; Yasumoto, T.; Alcala, A.C.; Alcala, L.C. Tetrodotoxin and paralytic shellfish toxins in Philippine crabs. Agric. Biol. Chem. 1986, 50, 593–598. [Google Scholar]
- Maruyama, J.; Noguchi, T.; Narita, H.; Jeon, J.K.; Otsuka, M.; Hashimoto, K. Occurrence of tetrodotoxin in a starfish, Astropecten scoparius. Agric. Biol. Chem. 1985, 49, 3069–3070. [Google Scholar]
- Buchwald, H.D.; Durham, L.; Fischer, H.G.; Harada, R.; Mosher, H.S.; Kao, C.Y.; Fuhrman, F.A. Identity of tarichatoxin and tetrodotoxin. Science 1964, 143, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Brown, G.B.; Mosher, H.S.; Fuhrman, F.A. Tetrodotoxin: Occurrence in atelopid frogs of Costa Rica. Science 1975, 189, 151–152. [Google Scholar] [CrossRef]
- Nakamura, M.; Oshima, Y.; Yasumoto, T. Occurrence of saxitoxin in puffer fish. Toxicon 1984, 22, 381–385. [Google Scholar] [CrossRef]
- Nakashima, K.; Arakawa, O.; Taniyama, S.; Nonaka, M.; Takatani, T.; Yamamori, K.; Fuchi, Y.; Noguchi, T. Occurrence of saxitoxins as a major toxin in the ovary of a marine puffer Arothron firmamentum. Toxicon 2004, 43, 207–212. [Google Scholar] [CrossRef]
- Yasumoto, T.; Yasumura, D.; Yotsu, M.; Michishita, T.; Endo, A.; Kotaki, Y. Bacterial production of tetrodotoxin and anhydrotetrodotoxin. Agric. Biol. Chem. 1986, 50, 793–795. [Google Scholar]
- Noguchi, T.; Jeon, J.K.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Shida, Y.; Hashimoto, K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of axantihd crab, Atergatis floridus. J. Biochem. 1986, 99, 311–314. [Google Scholar] [CrossRef]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef]
- Jang, J.; Lee, J.S.; Yotsu-Yamashita, M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackish water puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from southeast Asia. Mar. Drugs 2010, 8, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M.; Jang, J.H.; Cho, Y.; Konoki, K. Optimization of simultaneous analysis of tetrodotoxin, 4-epitetrodotoxin, 4,9-anhydrotetrodotoxin, and 5,6,11-trideoxytetrodotoxin by hydrophilic interaction liquid chromatography–tandem mass spectrometry. Forensic Toxicol. 2011, 29, 61–64. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Abe, Y.; Kudo, Y.; Ritson-Williams, R.; Paul, V.J.; Konoki, K.; Cho, Y.; Adachi, M.; Imazu, T.; Nishikawa, T.; et al. First identification of 5,11-dideoxytetrodotoxin in marine animals, and characterization of major fragment ions of tetrodotoxin and its analogs by high resolution ESI-MS/MS. Mar. Drugs 2013, 11, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Puilingi, C.G.; Kudo, Y.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Tetrodotoxin and its analogues in the pufferfish Arothron hispidus and A. nigropunctatus from the Solomon Islands: A comparison of their toxin profiles with the same species from Okinawa, Japan. Toxins 2015, 7, 3436–3454. [Google Scholar] [CrossRef]
- Kudo, Y.; Yasumoto, T.; Mebs, D.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Cyclic guanidine compounds from newts support the hypothesis that tetrodotoxin is derived from monoterpene. Angew. Chem. Int. Ed. 2016, 55, 8728–8731. [Google Scholar] [CrossRef]
- Ueyama, N.; Sugimoto, K.; Kudo, Y.; Onodera, K.; Cho, Y.; Konoki, K.; Nishikawa, T.; Yotsu-Yamashita, M. Spiro bicyclic guanidino compounds from pufferfish, possible biosynthetic intermediates of tetrodotoxin in marine environments. Chem. Eur. J. 2018, 24, 7250–7258. [Google Scholar] [CrossRef]
- Kudo, Y.; Yotsu-Yamashita, M. Isolation and biological activity of 8-epitetrodotoxin and the structure of a possible biosynthetic shunt product of tetrodotoxin, Cep-226A, from the newt, Cynops ensicauda popei. J. Nat. Prod. 2019, 82, 1656–1663. [Google Scholar] [CrossRef]
- McNabb, P.S.; Taylor, D.I.; Ogilvie, S.C.; Wilkinson, L.; Anderson, A.; Hamon, D.; Wood, S.A.; Peake, B.M. First detection of tetrodotoxin in the bivalve Paphies australis by liquid chromatography coupled to triple quadrupole mass spectrometry with and without precolumn reaction. J. AOAC Int. 2014, 97, 325–333. [Google Scholar] [CrossRef]
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the pufferfish toxin tetrodotoxin in European bivalves, England, 2013 to 2014. Eurosurveillance 2015, 20, 2–8. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Coates, L.; Bickerstaff, L.; Milligam, S.; O’Neill, A.; Faulkner, D.; McEneny, H.; Baker-Austin, C.; Lees, D.N.; et al. Detection of tetrodotoxin shellfish poisoning (TSP) toxins and causative factors in bivalve molluscs from the UK. Mar. Drugs 2017, 15, 277. [Google Scholar] [CrossRef]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazacharious, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First detection of Tetrodotoxin in Greek Shellfish by UPLC-MS/MS potentially linked to the presence of the dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef] [PubMed]
- Gerssen, A.; Bovee, T.; Klijnstra, M.; Poelman, M.; Portier, L.; Hoogenboom, R. First report on the occurrence of tetrodotoxins in bivalve mollusks in the Netherlands. Toxins 2018, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aversano, C.; Tartaglione, L.; Polito, G.; Dean, K.; Giacobbe, M.; Casabianca, S.; Capellacci, S.; Penna, A.; Turner, A.D. First detection of tetrodotoxin and high levels of paralytic shellfish poisoning toxins in shellfish from Sicily (Italy) by three different analytical methods. Chemosphere 2019, 215, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, X.; Li, L.; Chen, S.; Yan, Z.; Gao, X.; Chang, J. Analysis and evaluation of tetrodotoxin in coastal aquatic products of Zhejiang Province. J. Coast. Res. 2018, 83, 380–385. [Google Scholar] [CrossRef]
- Biessy, L.; Boundy, M.J.; Smith, K.F.; Harwood, D.T.; Hawes, I.; Wood, S.A. Tetrodotoxin in marine bivalves and edible gastropods: A mini-review. Chemosphere 2019, 236, 124404. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Alfonso, A.; Alonso, E.; Rubiolo, J.A.; Roel, M.; Vlamis, A.; Katikou, P.; Jackson, S.A.; Menon, M.L.; Dobson, A.; et al. The association of bacterial C9-based TTX-like compounds with Prorocentrum minimum opens new uncertainties about shellfish seafood safety. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, e04752. [Google Scholar]
- Rodrigues, S.M.; Pinto, E.P.; Oliveira, P.; Pedro, S.; Costa, P.R. Evaluation of the occurrence of tetrodotoxin in bivalve mollusks from the Portuguese coast. J. Mar. Sci. Eng. 2019, 7, 232. [Google Scholar] [CrossRef]
- Guardone, L.; Maneschi, A.; Meucci, V.; Gasperetti, L.; Nucera, D.; Armani, A. A global retrospective study on human cases of tetrodotoxin (TTX) poisoning after seafood consumption. Food Rev. Int. 2019, 35, 1–23. [Google Scholar] [CrossRef]
- Pinto, E.P.; Rodrigues, S.M.; Gouveia, N.; Timóteo, V.; Costa, P.R. Tetrodotoxin and saxitoxin in two native species of puffer fish, Sphoeroides marmoratus and Lagocephalus lagocephalus, from NE Atlantic Ocean (Madeira Island, Portugal). Mar. Environ. Res. 2019, 151, 104780. [Google Scholar] [CrossRef]
- Blanco, L.; Lago, J.; González, V.; Paz, B.; Rambla-Alegre, M.; Cabado, A.G. Occurrence of tetrodotoxin in bivalves and gastropods from harvesting areas and other natural spaces in Spain. Toxins 2019, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Sato, S.; Ogata, T. Alexandrium tamarense as a source of tetrodotoxin in the scallop Patinopecten yessoensis. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: New York, NY, USA, 1993; pp. 401–406. [Google Scholar]
- Kodama, M.; Sato, S.; Sakamoto, S.; Ogata, T. Occurrence of tetrodotoxin in Alexandirum tamarense, a causative dinoflagellate of paralytic shellfish poisoning. Toxicon 1996, 34, 1101–1105. [Google Scholar] [CrossRef]
- Taniyama, S.; Isami, Y.; Matsumoto, T.; Nagashima, Y.; Takatani, T.; Arakawa, O. Toxicity and toxin profile of tetrodotoxin detected in the scavenging gastropod Nassarius (Alectrion) glans “Kinshibai”. J. Food Hyg. Soc. Jpn. 2009, 50, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aversano, C.; Hess, P.; Quilliam, M.A. Hydrophilic interaction liquid chromatography-mass spectrometry for the analysis of paralytic shellfish poisoning (PSP) toxins. J. Chromatogr. A 2005, 1081, 190–201. [Google Scholar] [CrossRef]
- Rodriguez, I.; Alfonso, A.; Gonzalez-Jartin, J.M.; Vieytes, M.R.; Botana, L.M. A single run UPLC-MS/MS method for detection of all EU-regulated marine toxins. Talanta 2018, 189, 622–628. [Google Scholar] [CrossRef]
- Rey, V.; Botana, A.M.; Antelo, A.; Alvarez, M.; Botana, L.M. Rapid analysis of paralytic shellfish toxins and tetrodotoxins by liquid chromatography-tandem mass spectrometry using a porous graphitic carbon column. Food Chem. 2018, 269, 166–172. [Google Scholar] [CrossRef]
- Turner, A.D.; McNabb, P.S.; Harwood, D.T.; Selwood, A.I.; Boundy, M.J. Single-laboratory validation of a multitoxin Ultra-Performance LC-hydrophilic interaction LC-MS/MS method for quantitation of paralytic shellfish toxins in bivalve shellfish. J. AOAC Int. 2015, 98, 609–621. [Google Scholar] [CrossRef]
- Turner, A.D.; Boundy, M.J.; Dhanji-Rapkova, M. Development and single-laboratory validation of a liquid chromatography tandem mass spectrometry method for quantitation of Tetrodotoxin in mussels and oysters. J. AOAC Int. 2017, 100, 1–14. [Google Scholar] [CrossRef]
- Thomas, K.M.; Beach, D.G.; Reeves, K.L.; Gibbs, R.S.; Kerrin, E.S.; McCarron, P.; Quilliam, M.A. Hydrophilic interaction liquid chromatography-tandem mass spectrometry for quantitation of paralytic shellfish toxins: Validation and application to reference materials. Anal. Bioanal. Chem. 2017, 409, 5675–5687. [Google Scholar] [CrossRef]
- Minowa, T.; Cho, Y.; Oshima, Y.; Konoki, K.; Yotsu-Yamashita, M. Identification of a novel saxitoxin analogue, 12β-deoxygonyautoxin 3, in the cyanobacterium, Anabaena circinalis (TA04). Toxins 2019, 11, 539. [Google Scholar] [CrossRef]
- Cho, Y.; Tsuchiya, S.; Omura, T.; Koike, K.; Oikawa, H.; Konoki, K.; Oshima, Y.; Yotsu-Yamashita, M. Metabolomic study of saxitoxin analogues and biosynthetic intermediates in dinoflagellates using 15N-labelled sodium nitrate as a nitrogen source. Sci. Rep. 2019, 9, 3460. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aversano, C.; Walter, J.A.; Burton, I.W.; Stirling, D.J.; Fattorusso, E.; Quilliam, M.A. Isolation and structure elucidation of new and unusual saxitoxin analogues from mussels. J. Nat. Prod. 2008, 71, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Ma, J.; Cao, J.; Wang, Q.; Yu, R.; Thomas, K.; Quilliam, M.A. Analysis of paralytic shellfish toxins and their metabolites in shellfish from the north yellow sea of china. Food Addit. Contam. 2012, 29, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Vale, P. Metabolites of saxitoxin analogues in bivalves contaminated by Gymnodinium catenatum. Toxicon 2010, 55, 162–165. [Google Scholar] [CrossRef]
- Act on the Prohibition of Chemical Weapons and Control, of Specific Chemicals. Available online: https://www.meti.go.jp/policy/chemical_management/english/measures.html#kahei (accessed on 20 November 2019).
- FAO/WHO. Technical paper on toxicity equivalency factors for marine biotoxins. In Toxicity Equivalence Factors for Marine Biotoxins Associated with Bivalve Molluscs; FAO/WHO: Rome, Italy, 2016; Available online: http://www.fao.org/3/a-i5970e.pdf (accessed on 20 November 2019).
- Shimizu, Y.; Yoshioka, M. Transformation of paralytic shellfish toxins as demonstrated in scallop homogenates. Science 1981, 212, 547–549. [Google Scholar] [CrossRef]
- Oshima, Y.; Sugino, K.; Itakura, H.; Hirota, M.; Yasumoto, T. Comparative studies on paralytic shellfish toxin profile of dinoflagellates and bivalves. In Toxic Marine Phytoplankton; Granèli, E., Sundstrom, B., Edler, L., Anderson, D., Eds.; Elsevier: New York, NY, USA, 1990; pp. 391–396. [Google Scholar]
- Suzuki, H.; Machii, K. Comparison of toxicity between saxitoxin and decarbamoyl saxitoxin in the mouse bioassay for paralytic shellfish poisoning toxins. J. Vet. Med. Sci. 2014, 76, 1523–1525. [Google Scholar] [CrossRef]
- Oshima, Y. Postcolumn derivatization liquid chromatographic method for paralytic shellfish toxins. J. AOAC Int. 1995, 78, 528–532. [Google Scholar]
- AOAC official method 959.08. Paralytic shellfish poison. Biological method. In Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 2005; pp. 79–82. [Google Scholar]
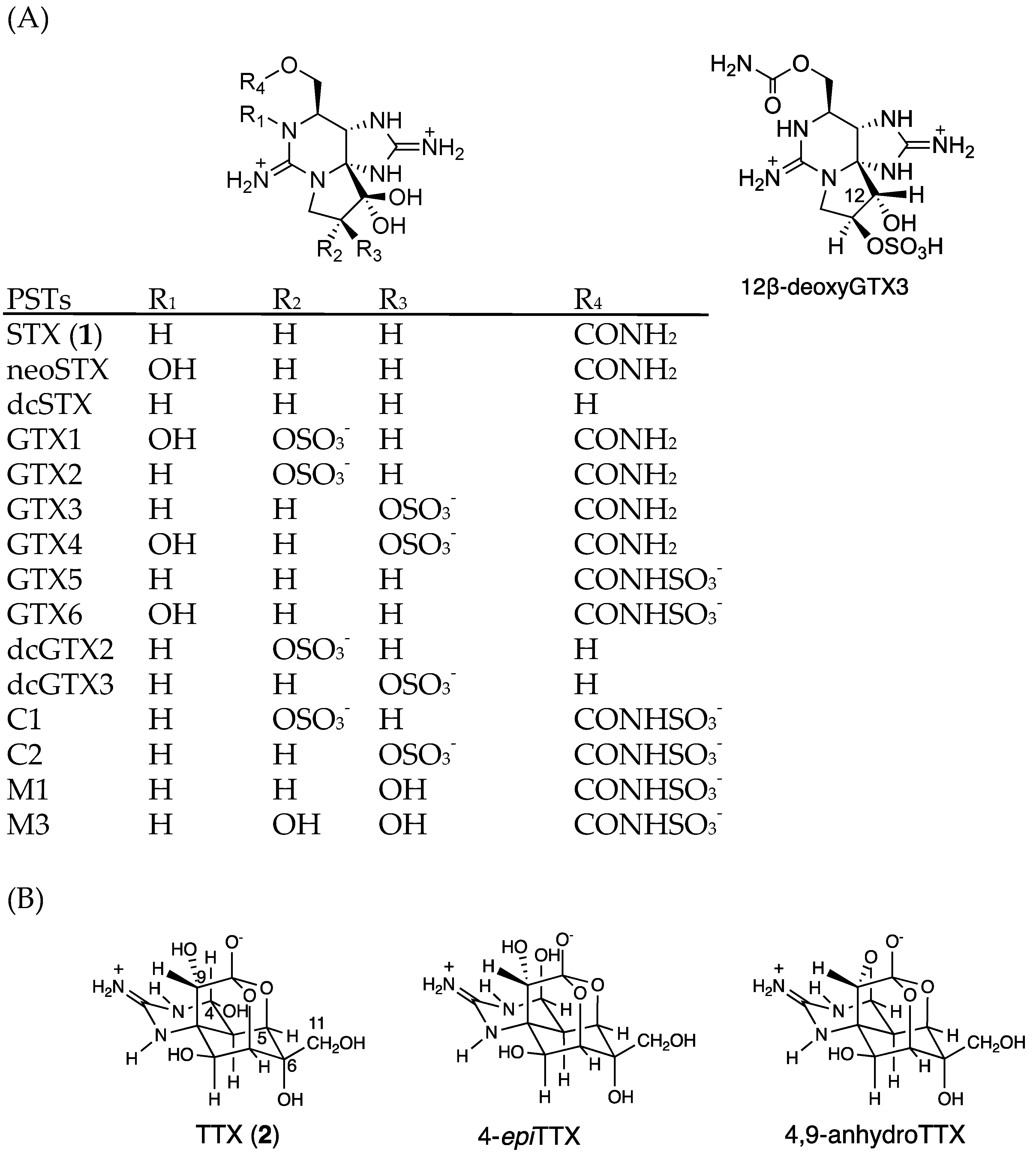


| Analyte | Precursor Ion | Product Ion | DP (V) | CE (V) | Standard LOD | Standard LOQ | Sample LOD | Sample LOQ |
|---|---|---|---|---|---|---|---|---|
| (m/z) | (m/z) | (nmol/L) | (nmol/L) | (nmol/kg) | (nmol/kg) | |||
| C1 | 396 | 316 | 46 | 17 | 8 | 17 | 105 | 210 |
| C2 | 396 | 298 | 66 | 23 | 6 | 11 | 79 | 131 |
| GTX1 | 412 | 314 | 65 | 19 | 221 | 440 | 2765 | 5500 |
| 412 | 332 | 65 | 19 | 255 | 510 | 3190 | 6381 | |
| GTX2 | 396 | 316 | 46 | 17 | 167 | 334 | 2086 | 4173 |
| GTX3 | 396 | 298 | 56 | 23 | 13 | 25 | 158 | 316 |
| 396 | 316 | 56 | 17 | 25 | 51 | 316 | 632 | |
| GTX4 | 412 | 394 | 65 | 17 | 24 | 49 | 304 | 608 |
| 412 | 314 | 65 | 23 | 17 | 24 | 213 | 304 | |
| GTX5 | 380 | 300 | 46 | 19 | 11 | 20 | 132 | 244 |
| 380 | 204 | 46 | 39 | 29 | 58 | 362 | 725 | |
| GTX6 | 396 | 316 | 46 | 19 | 33 | 96 | 411 | 1201 |
| 396 | 298 | 46 | 39 | 91 | 182 | 1138 | 2276 | |
| dcGTX2 | 353 | 273 | 41 | 19 | 99 | 199 | 1242 | 2484 |
| dcGTX3 | 353 | 335 | 41 | 17 | 17 | 37 | 213 | 461 |
| dcSTX | 257 | 239 | 85 | 21 | 252 | 507 | 3152 | 6341 |
| 257 | 180 | 95 | 29 | 109 | 122 | 1367 | 1519 | |
| TTX, 4-epiTTX | 320 | 162 | 80 | 51 | 1 | 2 | 16 | 30 |
| 4,9-anhydroTTX | 302 | 162 | 80 | 41 | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Numano, S.; Kudo, Y.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Temporal Variation of the Profile and Concentrations of Paralytic Shellfish Toxins and Tetrodotoxin in the Scallop, Patinopecten yessoensis, Cultured in a Bay of East Japan. Mar. Drugs 2019, 17, 653. https://doi.org/10.3390/md17120653
Numano S, Kudo Y, Cho Y, Konoki K, Yotsu-Yamashita M. Temporal Variation of the Profile and Concentrations of Paralytic Shellfish Toxins and Tetrodotoxin in the Scallop, Patinopecten yessoensis, Cultured in a Bay of East Japan. Marine Drugs. 2019; 17(12):653. https://doi.org/10.3390/md17120653
Chicago/Turabian StyleNumano, Satoshi, Yuta Kudo, Yuko Cho, Keiichi Konoki, and Mari Yotsu-Yamashita. 2019. "Temporal Variation of the Profile and Concentrations of Paralytic Shellfish Toxins and Tetrodotoxin in the Scallop, Patinopecten yessoensis, Cultured in a Bay of East Japan" Marine Drugs 17, no. 12: 653. https://doi.org/10.3390/md17120653
APA StyleNumano, S., Kudo, Y., Cho, Y., Konoki, K., & Yotsu-Yamashita, M. (2019). Temporal Variation of the Profile and Concentrations of Paralytic Shellfish Toxins and Tetrodotoxin in the Scallop, Patinopecten yessoensis, Cultured in a Bay of East Japan. Marine Drugs, 17(12), 653. https://doi.org/10.3390/md17120653





