Two New Neo-debromoaplysiatoxins—A Pair of Stereoisomers Exhibiting Potent Kv1.5 Ion Channel Inhibition Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of the New Compounds
2.2. Biological Activities of the Isolated Compounds
2.3. Molecular Docking Analysis of the Isolated Compounds
3. Experimental Section
3.1. General Experimental Procedures
3.2. Materials
3.3. Extraction and Isolation
3.4. ECD Calculations
3.5. NMR Calculations
3.6. Measurement of Ion Channel Inhibition Activity
3.7. Molecular Modeling and Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yanagita, R.C.; Kamachi, H.; Kikumori, M.; Tokuda, H.; Suzuki, N.; Suenaga, K.; Nagai, H.; Irie, K. Effects of the methoxy group in the side chain of debromoaplysiatoxin on its tumor-promoting and anti-proliferative activities. Bioorg. Med. Chem. Lett. 2013, 23, 4319–4323. [Google Scholar] [CrossRef] [PubMed]
- Kikumori, M.; Yanagita, R.C.; Tokuda, H.; Suzuki, N.; Nagai, H.; Suenaga, K.; Irie, K. Structure-activity studies on the spiroketal moiety of a simplified analogue of debromoaplysiatoxin with antiproliferative activity. J. Med. Chem. 2012, 55, 5614–5626. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Fujiki, H.; Tahira, T.; Cheuk, C.; Moore, R.E.; Sugimura, T. Estimation of tumor promoting activity and structure-function relationships of aplysiatoxins. Carcinogenesis 1984, 5, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Mynderse, J.S.; Moore, R.E.; Kashiwagi, M.; Norton, T.R. Antileukemia activity in the Osillatoriaceae: Isolation of Debromoaplysiatoxin from Lyngbya. Science 1977, 196, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Kaur, P.; Leong, S.T.; Tan, L.T.; Prinsep, M.R.; Chu, J.J. Anti-Chikungunya viral activities of aplysiatoxin-related compounds from the marine cyanobacterium Trichodesmium erythraeum. Mar. Drugs 2014, 12, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Kikumori, M.; Yanagita, R.C.; Tokuda, H.; Suenaga, K.; Nagai, H.; Irie, K. Structural optimization of 10-methyl-aplog-1, a simplified analog of debromoaplysiatoxin, as an anticancer lead. Biosci. Biotechnol. Biochem. 2016, 80, 221–231. [Google Scholar] [CrossRef]
- Kato, Y.; Scheuer, P.J. ChemInform Abstract: Aplysiatoxin and debromoaplysiatoxin, constituents of the marine mollusk stylocheilus longicauda (quoy and gaimard, 1824). J. Am. Chem. Soc. 1974, 5, 2245–2246. [Google Scholar] [CrossRef]
- Watson, M. Midgut gland toxins of Hawaiian sea hares-I, Isolation and preliminary toxicological observations. Toxicon 1973, 11, 259–267. [Google Scholar] [CrossRef]
- Luesch, H.; Harrigan, G.; Goetz, G.; Horgen, F.D. The cyanobacterial origin of potent anticancer agents originally isolated from sea hares. Curr. Med. Chem. 2002, 9, 1791–1806. [Google Scholar] [CrossRef]
- Han, B.N.; Liang, T.T.; Keen, L.J.; Fan, T.T.; Lin, H.W. Two Marine Cyanobacterial Aplysiatoxin Polyketides, Neo-debromoaplysiatoxin A and B, with K+ Channel Inhibition Activity. Org. Lett. 2018, 20, 578–581. [Google Scholar] [CrossRef]
- Tang, Y.H.; Liang, T.T.; Fan, T.T.; Keen, L.J.; Zhang, X.D.; Xu, L.; Zhao, Q.; Zeng, R.; Han, B.N. Neo-debromoaplysiatoxin C, with new structural rearrangement, derived from debromoaplysiatoxin. Nat. Prod. Res. 2019, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.H.; Wu, J.; Fan, T.T.; Zhang, H.H.; Gong, X.X.; Cao, Z.Y.; Zhang, J.; Lin, H.W.; Han, B.N. Chemical and biological study of aplysiatoxin derivatives showing inhibition of potassium channel Kv1.5. RSC Adv. 2019, 9, 7594. [Google Scholar] [CrossRef]
- Moore, R.E.; Blackman, A.J.; Cheuk, C.E.; Mynderse, J.S. Absolute Stereochemistries of the Aplysiatoxins and Oscillatoxin A. J. Org. Chem. 1984, 15, 2482–2489. [Google Scholar] [CrossRef]
- Entzeroth, M.; Blackman, A.J.; Mynderse, J.S.; Moore, R.E. Structures and stereochemistries of oscillatoxin B, 31-noroscillatoxin B, oscillatoxin D, and 30-methyloscillatoxin D. J. Org. Chem. 1985, 50, 1255–1259. [Google Scholar] [CrossRef]
- Nagai, H.; Sato, S.; Iida, K.; Hayashi, K.; Kawaguchi, M.; Uchida, H.; Satake, M. Oscillatoxin I: A New Aplysiatoxin Derivative, from a Marine Cyanobacterium. Toxins 2019, 11, 366. [Google Scholar] [CrossRef]
- Chlipala, G.E.; Huu Tri, P.; Van, H.N.; Aleksej, K.; Hee, S.S.; Doel, S.; Jimmy, O. Nhatrangins A and B, aplysiatoxin-related metabolites from the marine cyanobacterium Lyngbya majuscula from Vietnam. J. Nat. Prod. 2010, 73, 784–787. [Google Scholar] [CrossRef]
- Dorian, P. Antiarrhythmic drug therapy of atrial fibrillation: Focus on new agents. J. Cardiovasc. Pharmacol. Ther. 2003, 8, S27–S31. [Google Scholar] [CrossRef]
- Bhuyan, R.; Seal, A. Dynamics and modulation studies of human voltage gated Kv1.5 channel. J. Biomol. Struct. Dyn. 2016, 35, 380–398. [Google Scholar] [CrossRef]
- Schumacher, S.M.; McEwen, D.P.; Zhang, L.; Arendt, K.L.; Van Genderen, K.M.; Martens, J.R. Antiarrhythmic Drug-Induced Internalization of the Atrial-Specific K+ Channel Kv1.5. Circ. Res. 2009, 104, 1390–1398. [Google Scholar] [CrossRef]
- Trotter, B.W.; Bilodeau, M.T. Kv1.5 Blockers for the Treatment of Atrial Fibrillation: Approaches to Optimization of Potency and Selectivity and Translation to In Vivo Pharmacology. Curr. Top. Med. Chem 2009, 9, 436–451. [Google Scholar]
- Tamargo, J.; Caballero, R.; Gomez, R.; Delpon, E. IKur/Kv1.5 channel blockers for the treatment of atrial fibrillation. Expert Opin. Investig. Drugs 2009, 18, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Brendel, J.; Peukert, S. Blockers of the Kv1.5 channel for the treatment of atrial arrhythmias. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2003, 1, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Aunes-Jansson, M.; Edvardsson, N.; Stridh, M.; Sornmo, L.; Frison, L.; Berggren, A. Decrease of the atrial fibrillatory rate, increased organization of the atrial rhythm and termination of atrial fibrillation by AZD7009. J. Electrocardiol. 2013, 46, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Christ, T.; Wettwer, E.; Voigt, N.; Hala, O.; Radicke, S.; Matschke, K.; Varro, A.; Dobrev, D.; Ravens, U. Pathology-specific effects of the IKur/Ito/IK,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br. J. Pharmacol. 2010, 154, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Eldstrom, J.; Wang, Z.; Xu, H. The molecular basis of high-affinity binding of the antiarrhythmic compound vernakalant (RSD1235) to Kv1.5 channels. Mol. Pharmacol. 2007, 72, 1522–1534. [Google Scholar] [CrossRef]
- Fujii, M.; Hayashi, K.; Ohya, S.; Yamamura, H.; Imaizumi, Y. New Screening System for Selective Blockers of Voltage-Gated K+ Channels Using Recombinant Cell Lines Dying Upon Single Action Potential. J. Pharmacol. Sci. 2013, 123, 147–158. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, W.; Sun, H.Y.; Qin, G.W.; Wang, H.B.; Wang, P.W.; Yalamanchili, H.K.; Wang, J.W.; Tse, H.F.; Lau, C.P.; et al. Acacetin causes a frequency- and use-dependent blockade of hKv1.5 channels by binding to the S6 domain. J. Mol. Cell. Cardiol. 2011, 51, 966–973. [Google Scholar] [CrossRef]
- Han, B.N.; Mcphail, K.L.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Isolation and Structure of Five Lyngbyabellin Derivatives from a Papua New Guinea Collection of the Marine Cyanobacterium Lyngbya majuscula. Cheminform 2005, 61, 11723–11729. [Google Scholar]
- Smith, S.G.; Goodman, J.M. Assigning the stereochemistry of pairs of diastereoisomers using GIAO NMR shift calculation. J. Org. Chem. 2009, 74, 4597. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Tarazona, G.; Benedit, G.; Fernández, R.; Pérez, M.; Rodríguez, J.; Jiménez, C.; Cuevas, C. Can stereoclusters separated by two methylene groups be related by DFT studies? The case of the cytotoxic meroditerpenes halioxepines. J. Nat. Prod. 2018, 81, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Zhu, R.X.; Li, G.; Wang, N.N.; Lou, H.X. Secondary metabolites from the endolichenic fungus Ophiosphaerella korrae. RSC Adv. 2019, 9, 4140–4149. [Google Scholar] [CrossRef]
- Nagai, H.; Watanabe, M.; Sato, S.; Kawaguchi, M.; Xiao, Y.Y.; Hayashi, K.; Watanabe, R.; Uchida, H.; Satake, M. New aplysiatoxin derivatives from the Okinawan cyanobacterium Moorea producens. Tetrahedron 2019, 75, 2486–2494. [Google Scholar] [CrossRef]
- Li, X.C.; Ferreira, D.; Ding, Y. Determination of Absolute Configuration of Natural Products: Theoretical Calculation of Electronic Circular Dichroism as a Tool. Curr. Org. Chem. 2010, 14, 1678–1697. [Google Scholar] [CrossRef]
- Jeong, I.; Yoon, S.H.; Hahn, S.J. Effects of dapoxetine on cloned Kv1.5 channels expressed in CHO cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 707–716. [Google Scholar] [CrossRef]
- Yu, J.; Park, M.H.; Jo, S.H. Inhibitory effects of cortisone and hydrocortisone on human Kv1.5 channel currents. Eur. J. Pharmacol. 2015, 746, 158–166. [Google Scholar] [CrossRef]
- Andersen, M.N.; Skibsbye, L.; Tang, C.; Petersen, F.; MacAulay, N.; Rasmussen, H.B.; Jespersen, T. PKC and AMPK regulation of Kv1.5 potassium channels. Channels 2015, 9, 121–128. [Google Scholar] [CrossRef]
- Fujiki, H.; Tanaka, Y.; Miyake, R.; Kikkawa, U.; Nishizuka, Y.; Sugimura, T. Activation of calcium-activated, phospholipid-dependent protein kinase (protein kinase C) by new classes of tumor promoters: Teleocidin and debromoaplysiatoxin. Biochem. Biophys. Res. Commun. 1984, 120, 339–343. [Google Scholar] [CrossRef]
- Ashida., Y.; Yanagita, R.C.; Takahashi, C.; Kawanami, Y.; Irie, K. Binding mode prediction of aplysiatoxin, a potent agonist of protein kinase C, through molecular simulation and structure–activity study on simplified analogs of the receptor-recognition domain. Bioorg. Med. Chem. 2016, 24, 4218–4227. [Google Scholar] [CrossRef]
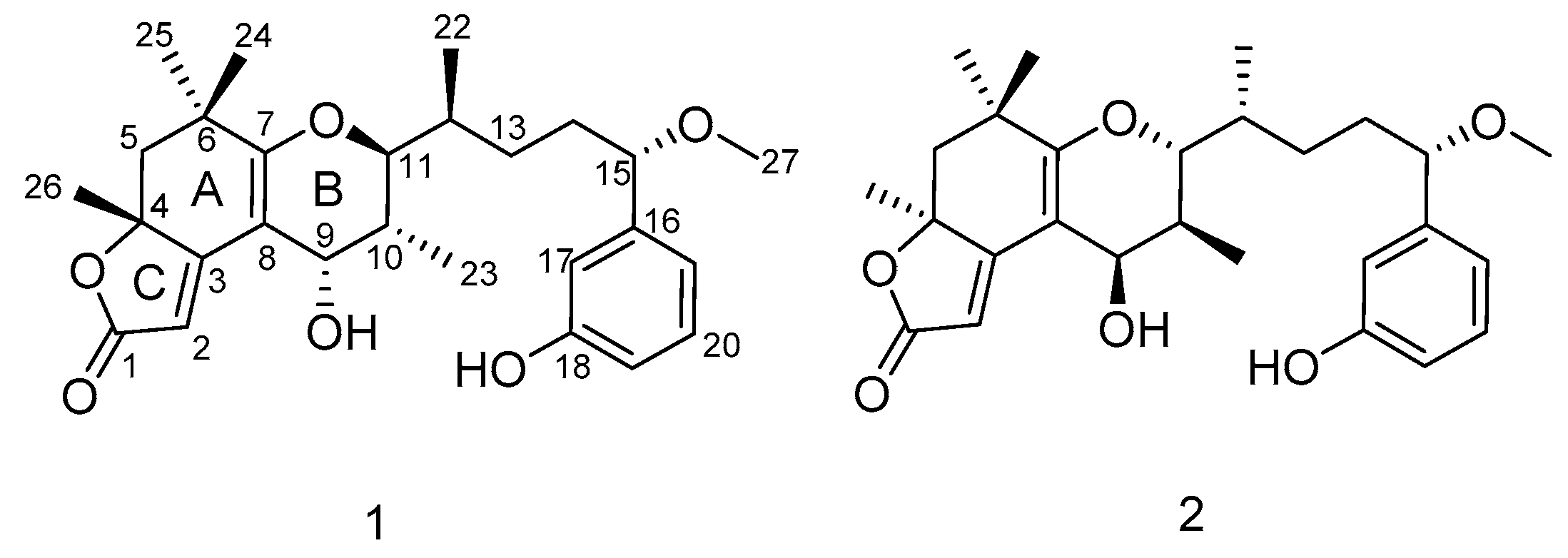

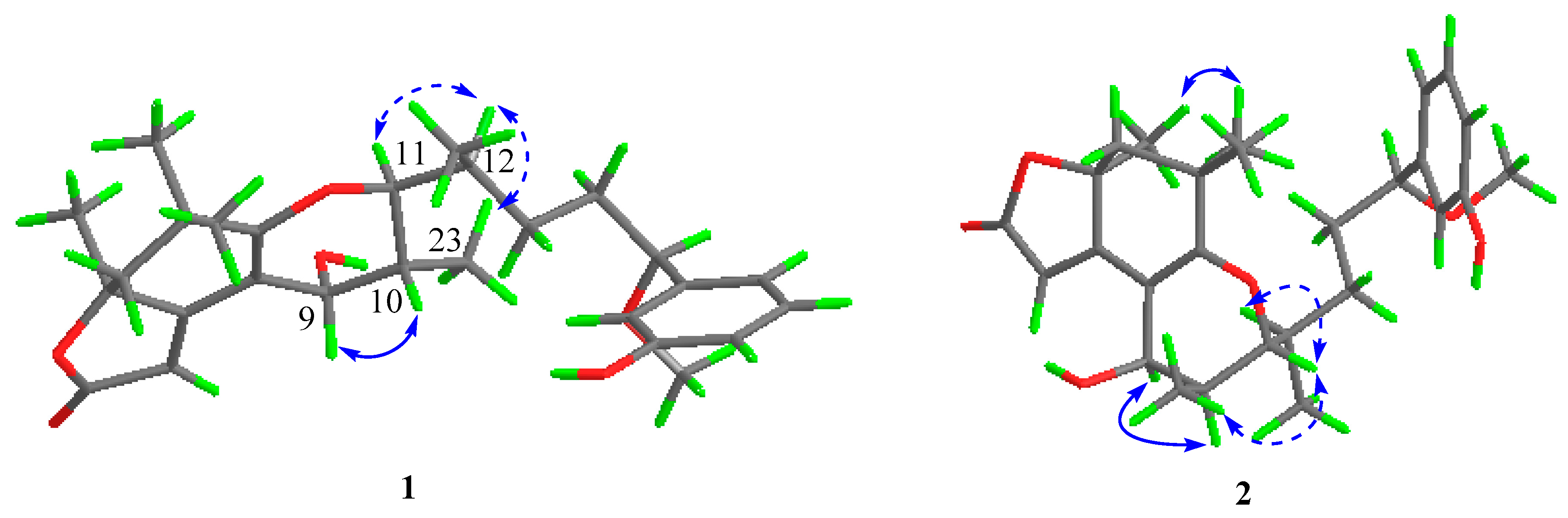



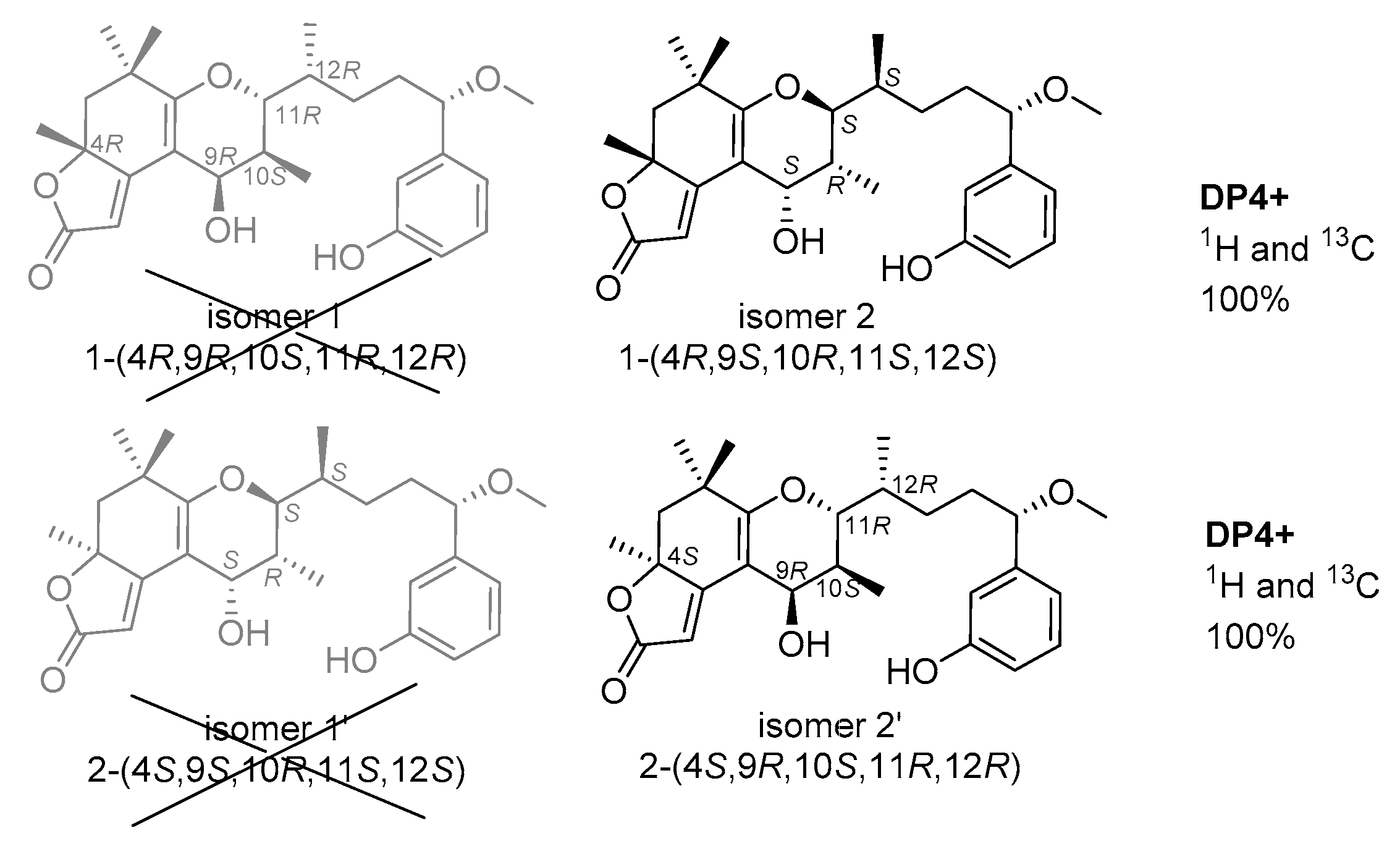
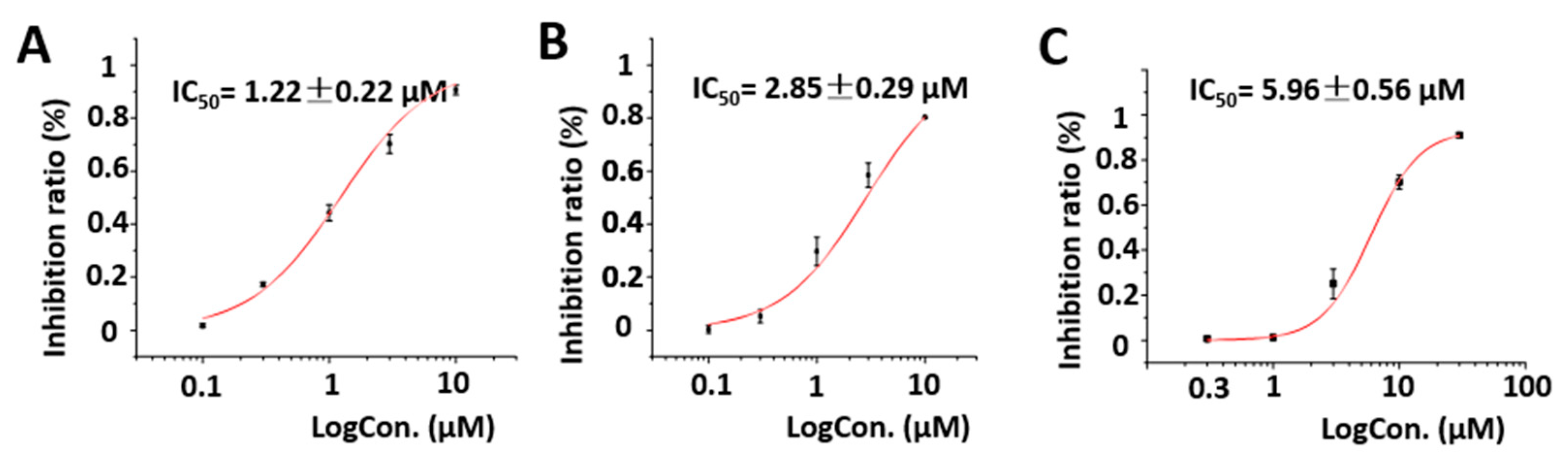

| Pos. | 1 | 2 | ||
|---|---|---|---|---|
| a δH (J in Hz) | b δC | a δH (J in Hz) | b δC | |
| 1 | 176.3, C | 176.4, C | ||
| 2 | 5.57, S | 103.1, CH | 5.66, S | 104.7, CH |
| 3 | 174.2, C | 174.5, C | ||
| 4 | 84.9, C | 84.7, C | ||
| 5 | a 2.16, d (13.0) b 1.71, d (13.0) | 47.1, CH2 | a 2.14, d (13.0) b 1.78, d (13.0) | 47.5, CH2 |
| 6 | 37.5, C | 37.7, C | ||
| 7 | 167.4, C | 167.1, C | ||
| 8 | 105.7, C | 104.7, C | ||
| 9 | 4.26, d (3.6) | 64.5, CH | 4.05, d (3.1) | 66.8, CH |
| 10 | 1.75, m | 36.1, CH | 1.88, m | 35.6, CH |
| 11 | 3.87, dd (11.1, 2.0) | 79.0, CH | 3.95, dd (11.4, 2.1) | 79.4, CH |
| 12 | 1.79, m | 34.3, CH | 1.75, m | 34.1, CH |
| 13 | a 1.54, m b 1.46, m | 31.4, CH2 | a 1.56, m b 1.47, m | 31.4, CH2 |
| 14 | a 1.86, m b 1.73, m | 36.7, CH2 | a 1.83, m b 1.73, m | 36.7, CH2 |
| 15 | 4.08, t (6.7) | 85.7, CH | 4.07, t (6.7) | 85.7, CH |
| 16 | 144.9, C | 145.9, C | ||
| 17 | 6.74, t (2.0) | 114.3, CH | 6.73, t (2.0) | 114.3, CH |
| 18 | 158.8, C | 158.8, C | ||
| 19 | 6.71, ddd (8.0, 2.5, 1.0) | 115.6, CH | 6.70, ddd (8.0, 2.5, 1.2) | 115.6, CH |
| 20 | 7.16, t (7.7) | 130.4, CH | 7.15, t (7.8) | 130.4, CH |
| 21 | 6.76, dt (7.7, 1.3) | 119.2, CH | 6.75, dt (7.7, 1.2) | 119.1, CH |
| 22 | 0.90, d (6.8) | 13.0, CH3 | 0.89, d (6.8) | 13.1, CH3 |
| 23 | 1.03, d (7.0) | 11.8, CH3 | 0.99, d (6.9) | 12.3, CH3 |
| 24 | 1.15, s | 32.0, CH3 | 1.10, s | 32.1, CH3 |
| 25 | 1.23, s | 26.8, CH3 | 1.29, s | 26.4, CH3 |
| 26 | 1.56, s | 28.0, CH3 | 1.52, s | 28.3, CH3 |
| 15-OCH3 | 3.21, s | 56.8, CH3 | 3.21, s | 56.8, CH3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, T.-T.; Zhang, H.-H.; Tang, Y.-H.; Zhang, F.-Z.; Han, B.-N. Two New Neo-debromoaplysiatoxins—A Pair of Stereoisomers Exhibiting Potent Kv1.5 Ion Channel Inhibition Activities. Mar. Drugs 2019, 17, 652. https://doi.org/10.3390/md17120652
Fan T-T, Zhang H-H, Tang Y-H, Zhang F-Z, Han B-N. Two New Neo-debromoaplysiatoxins—A Pair of Stereoisomers Exhibiting Potent Kv1.5 Ion Channel Inhibition Activities. Marine Drugs. 2019; 17(12):652. https://doi.org/10.3390/md17120652
Chicago/Turabian StyleFan, Ting-Ting, Hui-Hui Zhang, Yang-Hua Tang, Fan-Zhong Zhang, and Bing-Nan Han. 2019. "Two New Neo-debromoaplysiatoxins—A Pair of Stereoisomers Exhibiting Potent Kv1.5 Ion Channel Inhibition Activities" Marine Drugs 17, no. 12: 652. https://doi.org/10.3390/md17120652
APA StyleFan, T.-T., Zhang, H.-H., Tang, Y.-H., Zhang, F.-Z., & Han, B.-N. (2019). Two New Neo-debromoaplysiatoxins—A Pair of Stereoisomers Exhibiting Potent Kv1.5 Ion Channel Inhibition Activities. Marine Drugs, 17(12), 652. https://doi.org/10.3390/md17120652





