Anti-Aging Effect of Agar Oligosaccharide on Male Drosophila melanogaster and Its Preliminary Mechanism
Abstract
1. Introduction
2. Results
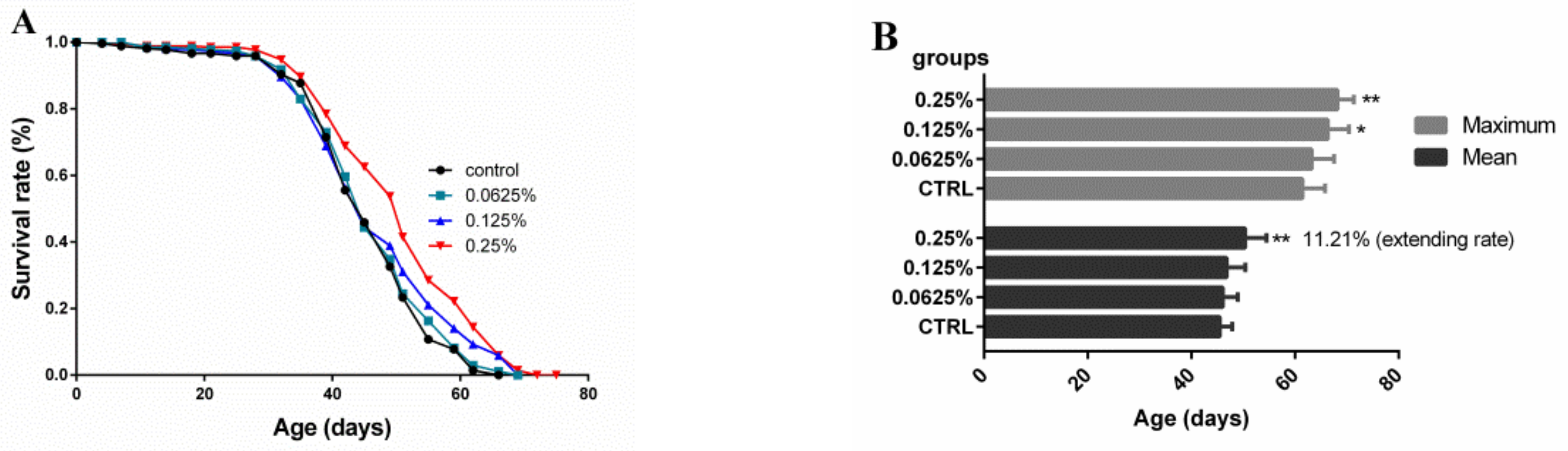
2.1. Effect of AOS on the Lifespan of Drosophila
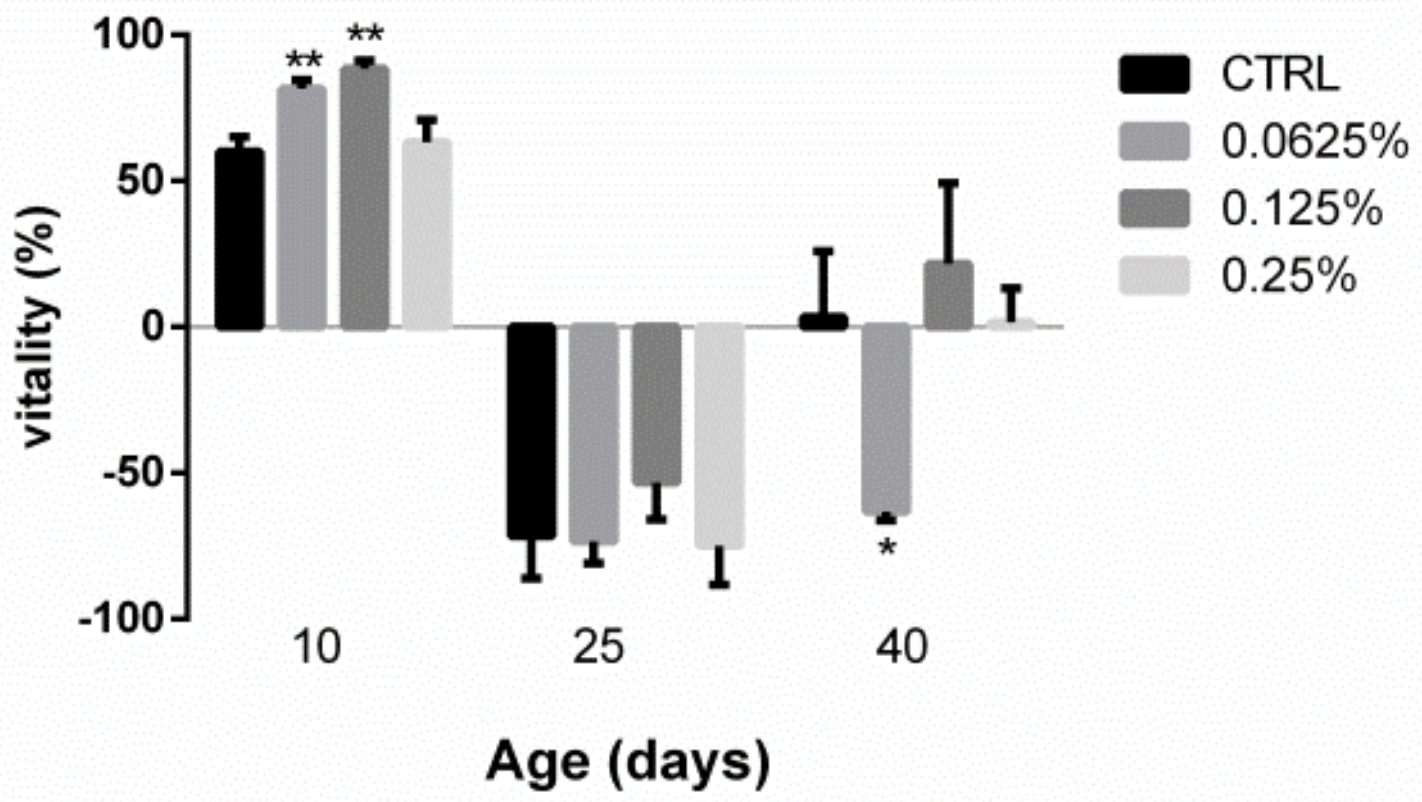
2.2. AOS Improved the Vitality of Juvenile Fruit Flies
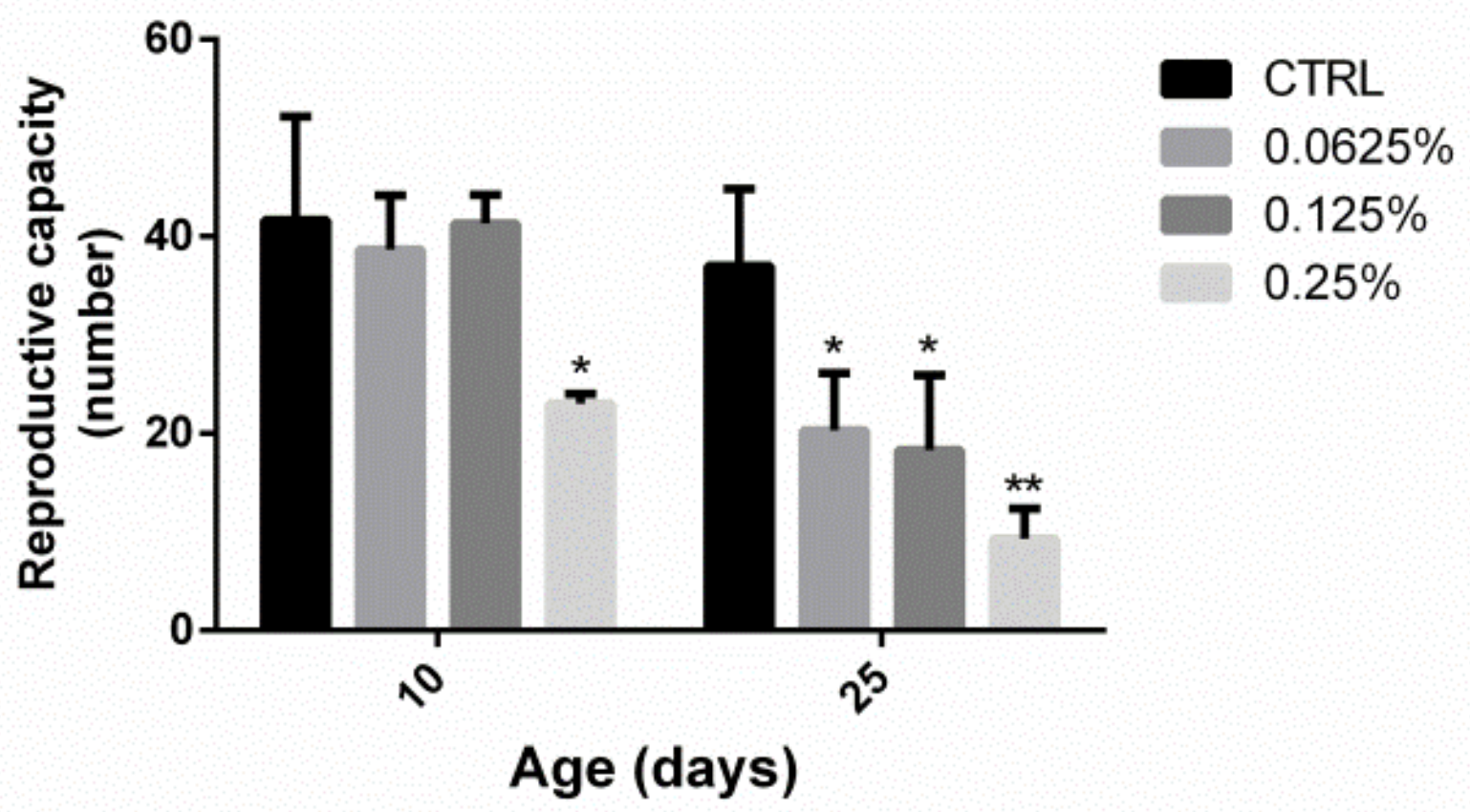
2.3. AOS Reduced the Reproductive Capacity of Drosophila
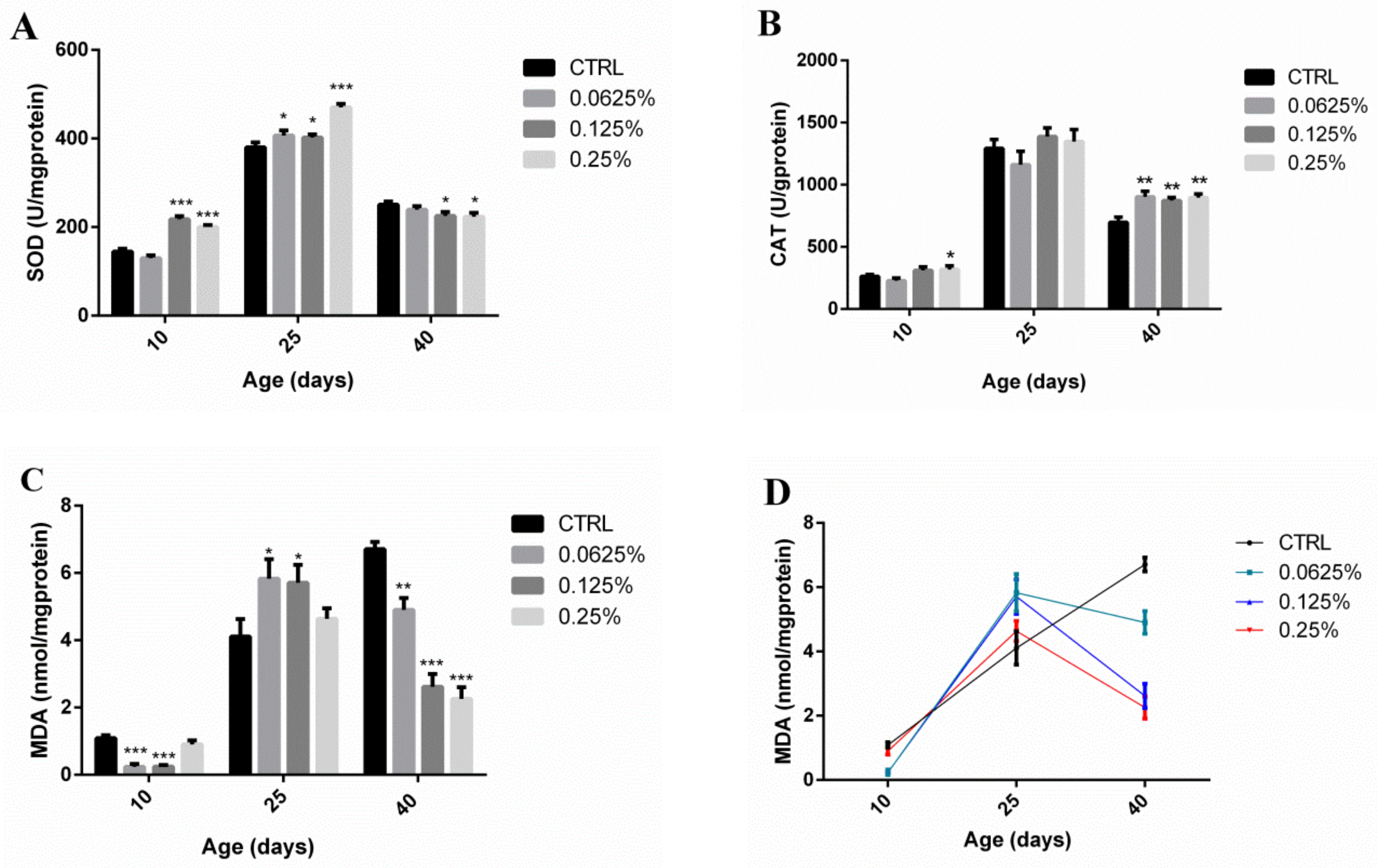
2.4. AOS Improved the Antioxidant Capacity of Drosophila
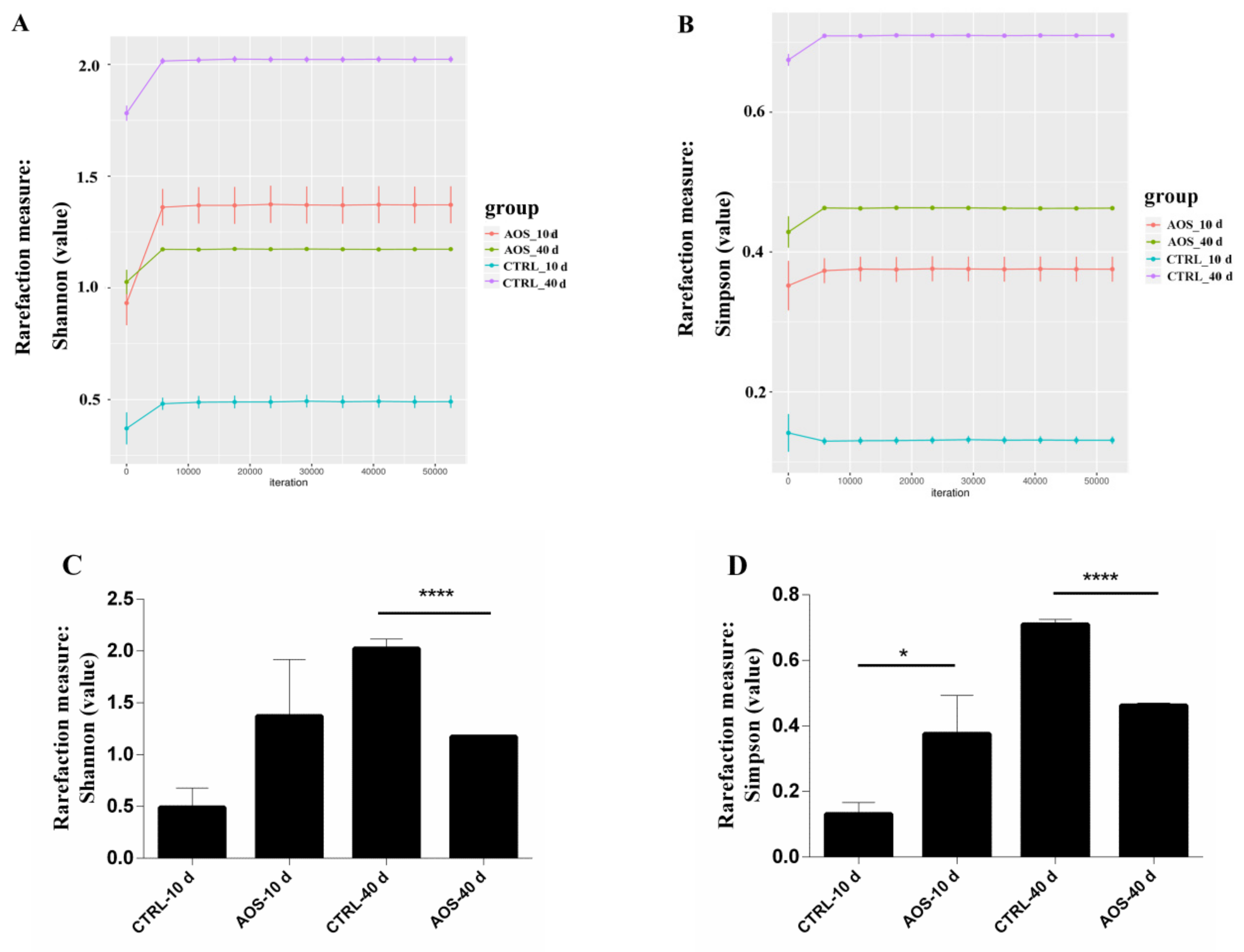
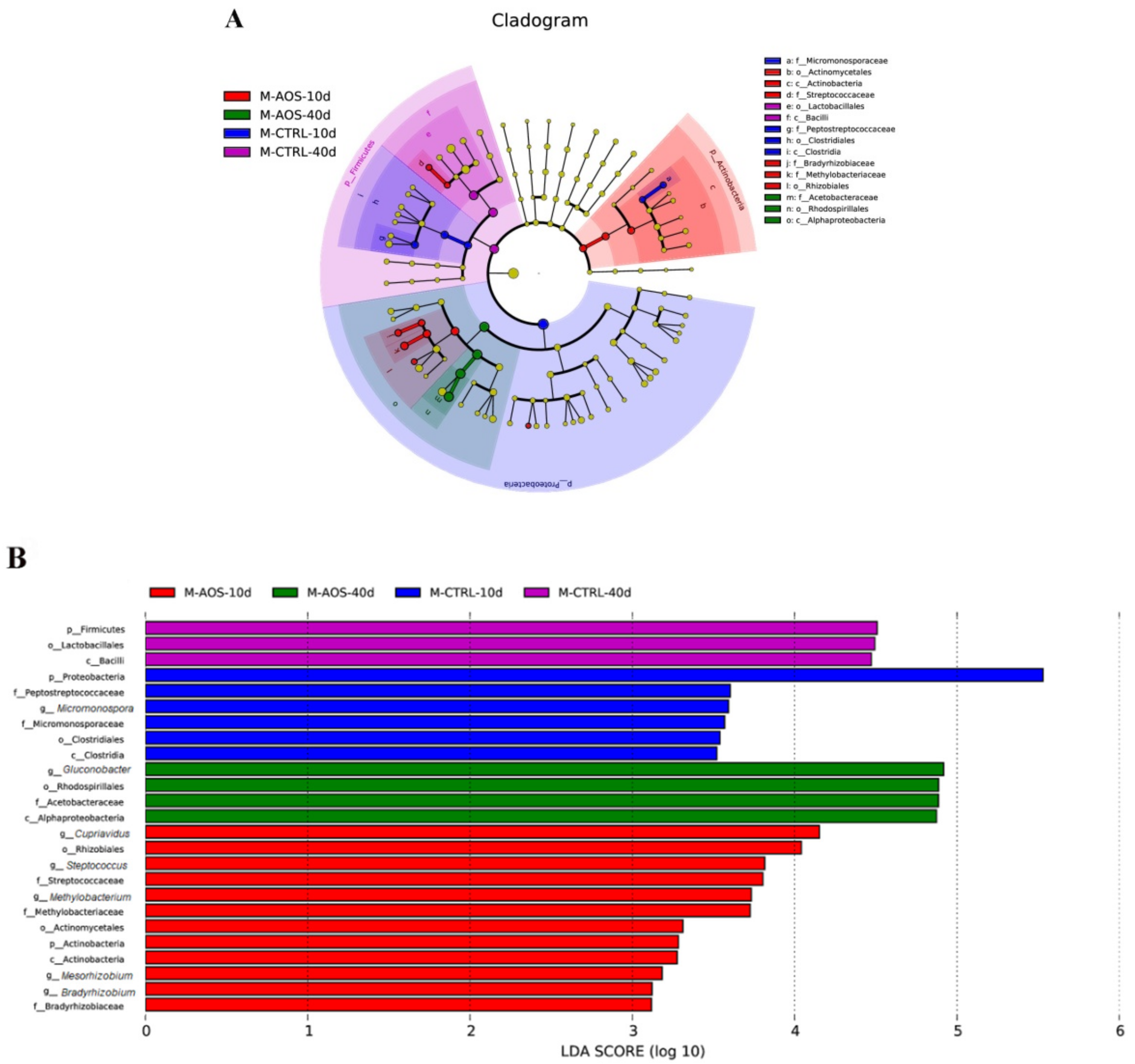
2.5. Analysis of the Diversity of the Midgut Microflora in Old Flies by 16S rDNA Sequencing
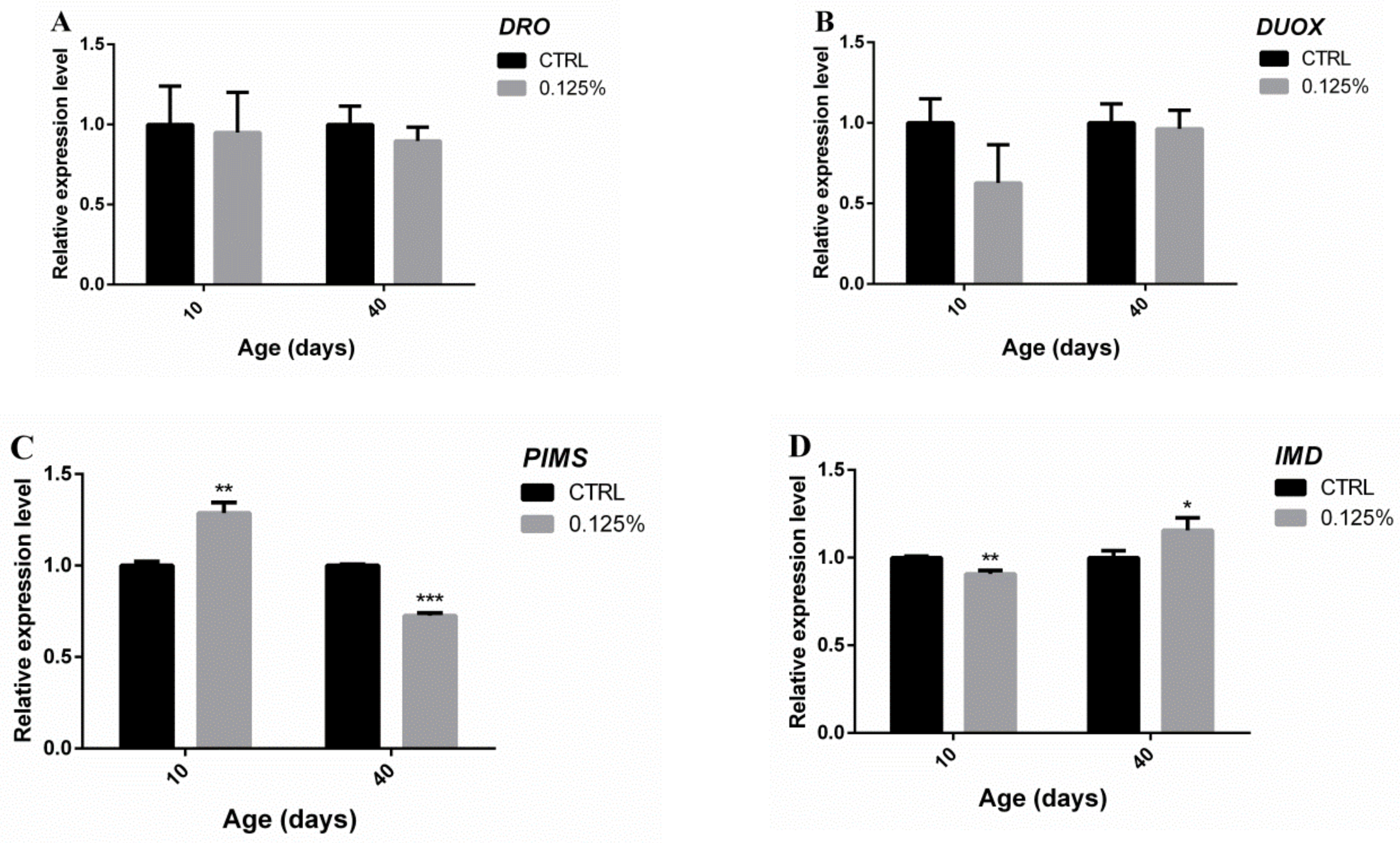
2.6. Immune Defense-Related Gene Expression in Senile Flies Fed Diets Containing AOS
3. Discussion
4. Materials and Methods
4.1. Animals, Diets and Sample Preparation
4.2. Lifespan Assay [49,50]
4.3. Climbing Ability Assay [51]
4.4. Reproduction Assay [9]
4.5. Antioxidation Assay
4.6. 16S rDNA Sequencing
4.7. Quantitative RT-PCR Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Antonio, J.E.; Eva, G.O.; María, D.T.; Pilar, R. Antioxidant and prebiotic effects of dietary fiber co-travelers from sugar Kombu in healthy rats. J. Appl. Phycol. 2013, 25, 503–512. [Google Scholar]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Araki, C. Some recent studies on the polysaccharides of agarophytes. Proc. Int. Seaweed Symp. 1966, 5, 3–17. [Google Scholar]
- Ramnani, P.; Chitarrari, R.; Tuohy, K.; Grant, J.; Hotchkiss, S.; Philp, K.; Campbell, R.; Gill, C.; Rowland, I. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe 2012, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Kan, J.; Hu, Z.; Liu, Y.; Du, H.; Pang, G.C.; Cheong, K.L. Quantification of neoagaro-oligosaccharide production through enzymatic hydrolysis and its anti-oxidant activities. Molecules 2018, 23, 1354. [Google Scholar] [CrossRef]
- Wang, W.; Liu, P.; Hao, C.; Wu, L.; Wan, W.; Mao, X. Neoagaro-oligosaccharide monomers inhibit inflammation in LPS-stimulated macrophages through suppression of MAPK and NF-κB pathways. Sci. Rep. 2017, 7, 44252. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, E.J.; Yu, S.; Kim, K.H. Different levels of skin whitening activity among 3,6-anhydro-l-galactose, agarooligosaccharides, and neoagarooligosaccharides. Mar. Drugs 2017, 15, 321. [Google Scholar] [CrossRef]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Ushiroda, C.; Ohnogi, H.; Kudo, Y.; Yasui, M.; Inui, S.; et al. Protective effect of agaro-oligosaccharides on gut dysbiosis and colon tumorigenesis in high-fat diet-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G367–G375. [Google Scholar] [CrossRef]
- Zou, Y.X.; Ruan, M.H.; Luan, J.; Feng, X.; Chu, Z.Y. Anti-aging effect of riboflavin via endogenous antioxidant in fruit fly Drosophila melanogaster. J. Nutr. Health Aging 2016, 21, 1–6. [Google Scholar] [CrossRef]
- Gilbert, M.J.H.; Zerulla, T.C.; Tierney, K.B. Zebrafish (Danio rerio) as a model for the study of aging and exercise: Physical ability and trainability decrease with age. Exp. Gerontol. 2014, 50, 106–113. [Google Scholar] [CrossRef]
- Harman, D. Free radical theory of aging: Alzheimer’s disease pathogenesis. AGE 1995, 18, 97–119. [Google Scholar] [CrossRef]
- Abdollahi, M.; Moridani, M.Y.; Aruoma, O.; Mostafalou, S. Oxidative stress in aging. Oxid. Med. Cell Longev. 2014, 2014, 876834. [Google Scholar] [CrossRef]
- Li, Y.M.; Chan, H.Y.E.; Yu, H.; Zhen, Y.C. Green tea catechins upregulate superoxide dismutase and catalase in fruit flies. Mol. Nutr. Food Res. 2010, 51, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Yu, L.E.; Ong, C.N.; Bay, B.H.; Baeg, G.H. The use of Drosophila melanogaster as a model organism to study immune-nanotoxicity. Nanotoxicology 2018, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Staats, S.; Wagner, A.E.; Lüersen, K.; Künstner, A.; Rimbach, G. Dietary ursolic acid improves health span and life span in male Drosophila melanogaster. BioFactors 2019, 45, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Sun, H.; Yang, W.; Ma, S. The effect of inulin on lifespan, related gene expression and gut microbiota in InR(p5545)/TM3 mutant Drosophila melanogaster: A Preliminary Study. Nutrients 2019, 11, 636. [Google Scholar] [CrossRef]
- Clark, R.I.; Salazar, A.; Yamada, R.; Fitz-Gibbon, S.; Morselli, M.; Alcaraz, J.; Rana, A.; Rera, M.; Pellegrini, M.; Ja, W.W. Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep. 2015, 12, 1656–1667. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Cousin, F.J.; Lynch, D.B.; Menon, R.; Brulc, J.; Brown, J.R.-M.; O’Herlihy, E.; Butto, L.F.; Power, K.; Jeffery, I.B. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome 2019, 7, 39. [Google Scholar] [CrossRef]
- Hu, B.; Gong, Q.; Wang, Y.; Ma, Y.; Li, J.; Yu, W. Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe 2006, 12, 260–266. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.S.; Shon, Y.H. Suppression of metastasis of human breast cancer cells by chitosan oligosaccharides. J. Microbiol. Biotechnol. 2009, 19, 629–633. [Google Scholar] [PubMed]
- Jing, B.; Cheng, G.; Li, J. Inhibition of liver tumor cell metastasis by partially acetylated chitosan oligosaccharide on a tumor-vessel microsystem. Mar. Drugs 2019, 17, 415. [Google Scholar] [CrossRef]
- Ai, L.; Chung, Y.C.; Lin, S.Y.; Jeng, K.C.G.; Lai, P.F.H.; Xiong, Z.Q.; Wang, G. Carrageenan polysaccharides and oligosaccharides with distinct immunomodulatory activities in murine microglia BV-2 cells. Int. J. Biol. Macromol. 2018, 120, 633–640. [Google Scholar] [CrossRef]
- Han, Z.L.; Yang, M.; Fu, X.D.; Chen, M.; Su, Q.; Zhao, Y.H. Evaluation of prebiotic potential of three marine algae oligosaccharides from enzymatic hydrolysis. Mar. Drugs 2019, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Dai, J. Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Cancer Lett. 2006, 243, 228–234. [Google Scholar] [CrossRef]
- Michael, C.; Anthony, B. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar]
- Hopkins, M.J.; Macfarlane, G.T. Nondigestible oligosaccharides enhance bacterial colonization resistance against Clostridium difficile in vitro. Appl. Environ. Microbiol. 2003, 69, 1920–1927. [Google Scholar] [CrossRef]
- Flatt, T.; Min, K.J.; D’Alterio, C.; Villacuesta, E.; Cumbers, J.; Lehmann, R.; Jones, D.L.; Tatar, M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl. Acad. Sci. USA 2008, 105, 6368–6373. [Google Scholar] [CrossRef]
- Wan, Q.L.; Shi, X.; Liu, J.; Ding, A.J.; Pu, Y.Z.; Li, Z.; Wu, G.S.; Luo, H.R. Metabolomic signature associated with reproduction-regulated aging in Caenorhabditis elegans. Aging 2017, 9, 447–474. [Google Scholar] [CrossRef]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, R.V.; Malcher, N.S.; Amado, L.L.; Coleman, M.D.; Dos Santos, D.C.; Borges, R.S.; Valente, S.A.; Valente, V.C.; Monteiro, M.C. In vitro protective effect and antioxidant mechanism of resveratrol induced by dapsone hydroxylamine in human cells. PLoS ONE 2015, 10, e0134768. [Google Scholar] [CrossRef] [PubMed]
- Orr, W.C.; Sohal, R.S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 1994, 263, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Jiang, B.; Luo, X. Gut microbiota influences Alzheimer’s disease pathogenesis by regulating acetate in Drosophila model. Future Microbiol. 2018, 13, 1117–1128. [Google Scholar]
- Duguma, D.; Rugman-Jones, P.; Kaufman, M.G.; Hall, M.W.; Neufeld, J.D.; Stouthamer, R.; Walton, W.E. Bacterial communities associated with culex mosquito larvae and two emergent aquatic plants of bioremediation importance. PLoS ONE 2013, 8, e72522. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Liu, S.; Huang, J.; Zhai, Z.; He, C.; Ding, J.; Wang, J.; Wang, H.; Fan, W.; et al. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS ONE 2015, 10, e0117441. [Google Scholar] [CrossRef]
- Staats, S.; Luersen, K.; Wagner, A.E.; Rimbach, G. Drosophila melanogaster as a versatile model organism in food and nutrition research. J. Agr. Food Chem. 2018, 66, 3737–3753. [Google Scholar] [CrossRef]
- Douglas, A.E. The Drosophila model for microbiome research. Lab. Anim. 2018, 47, 157–164. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Kim, S.-H.; Lee, H.-Y.; Bai, J.Y.; Nam, Y.-D.; Bae, J.-W.; Lee, D.G.; Shin, S.C.; Ha, E.-M.; Lee, W.-J. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 2008, 319, 777–782. [Google Scholar] [CrossRef]
- Routy, B.; Chatelier, E.L.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2017, 359, 91. [Google Scholar] [CrossRef] [PubMed]
- Gerrit, L.; Ingo, Z.; Tetsushi, M.; Pilar, C.; Jonas, S.; Haruko, T.; Michael, H.; Gregg, R. Antimicrobial peptides extend lifespan in Drosophila. PLoS ONE 2017, 12, e0176689. [Google Scholar]
- Lee, K.-A.; Cho, K.-C.; Kim, B.; Jang, I.-H.; Nam, K.; Kwon, Y.E.; Kim, M.; Hyeon, D.Y.; Hwang, D.; Seol, J.-H. Inflammation-modulated metabolic reprogramming is required for duox-dependent gut immunity in Drosophila. Cell Host Microbe 2018, 23, 338–352.e335. [Google Scholar] [CrossRef]
- Petkau, K.; Ferguson, M.; Guntermann, S.; Foley, E. Constitutive immune activity promotes tumorigenesis in Drosophila intestinal progenitor cells. Cell Rep. 2017, 20, 1784–1793. [Google Scholar] [CrossRef]
- Kleino, A.; Ramia, N.F.; Bozkurt, G.; Shen, Y.; Nailwal, H.; Huang, J.; Napetschnig, J.; Gangloff, M.; Chan, F.K.; Wu, H.; et al. Peptidoglycan-sensing receptors trigger the formation of functional amyloids of the adaptor protein imd to initiate Drosophila NF-kappaB signaling. Immunity 2017, 47, 635–647.e636. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, O.; Kellenberger, C.; Kemp, C.; Troxler, L.; Pelte, N.; Boutros, M.; Marques, J.T.; Daeffler, L.; Hoffmann, J.A.; Roussel, A. Cytokine Diedel and a viral homologue suppress the IMD pathway in Drosophila. Proc. Natl. Acad. Sci. USA 2016, 113, 698. [Google Scholar] [CrossRef]
- Buchon, N.; Broderick, N.A.; Chakrabarti, S.; Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009, 23, 2333. [Google Scholar] [CrossRef]
- Liu, N.; Fu, X.; Duan, D.; Xu, J.; Gao, X.; Zhao, L. Antioxidant activities of agaro-oligosaccharides in aaph-induced Zebrafish model. Sci. Tech. Food Ind. 2019, 40, 292–297. [Google Scholar]
- Wang, C.; Wheeler, C.T.; Alberico, T.; Sun, X.; Seeberger, J.; Laslo, M.; Spangler, E.; Kern, B.; Cabo, R.; Zou, S. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age 2013, 35, 69–81. [Google Scholar] [CrossRef]
- Shen, L.R.; Xiao, F.; Yuan, P.; Chen, Y.; Gao, Q.-K.; Parnell, L.D. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age 2013, 35, 1133–1142. [Google Scholar] [CrossRef]
- Coulom, H. Chronic exposure to rotenone models sporadic parkinson’s disease in Drosophila melanogaster. J. Neurosci. 2004, 24, 10993. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Prakash, S. A novel polyphenolic prebiotic and probiotic formulation have synergistic effects on the gut microbiota influencing Drosophila melanogaster physiology. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1–15. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Sequence 5′-3′ | Annealing Temp |
|---|---|---|
| Duox | F:CAGTTTCGAACGAACTCTTGG R:CATGGTTTTCCAATTATGCAGATTC | 54 °C |
| IMD | F:AAAACGGTCAGCATTGTGGC R:GTCCTTCCGACATGCCAAGA | 52 °C |
| Drosocin | F:GCACAATGAAGTTCACCATCGT R:CCACACCCATGGCAAAAAC | 56 °C |
| PIMS | F:ATCGTTTTCCTGCTGCTTGC R:ATTACTTGCAGTTGCCCGGA | 59 °C |
| Rp49 | F:AGGGTATCGACAACAGAGTG R:CACCAGGAACTTCTTGAATC | 52 °C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Yang, K.; Wang, Y.; Dai, X. Anti-Aging Effect of Agar Oligosaccharide on Male Drosophila melanogaster and Its Preliminary Mechanism. Mar. Drugs 2019, 17, 632. https://doi.org/10.3390/md17110632
Ma C, Yang K, Wang Y, Dai X. Anti-Aging Effect of Agar Oligosaccharide on Male Drosophila melanogaster and Its Preliminary Mechanism. Marine Drugs. 2019; 17(11):632. https://doi.org/10.3390/md17110632
Chicago/Turabian StyleMa, Chao, Kun Yang, Yifan Wang, and Xianjun Dai. 2019. "Anti-Aging Effect of Agar Oligosaccharide on Male Drosophila melanogaster and Its Preliminary Mechanism" Marine Drugs 17, no. 11: 632. https://doi.org/10.3390/md17110632
APA StyleMa, C., Yang, K., Wang, Y., & Dai, X. (2019). Anti-Aging Effect of Agar Oligosaccharide on Male Drosophila melanogaster and Its Preliminary Mechanism. Marine Drugs, 17(11), 632. https://doi.org/10.3390/md17110632




