Antimicrobial Sesquiterpenoid Derivatives and Monoterpenoids from the Deep-Sea Sediment-Derived Fungus Aspergillus versicolor SD-330
Abstract
:1. Introduction
2. Results and Discussion
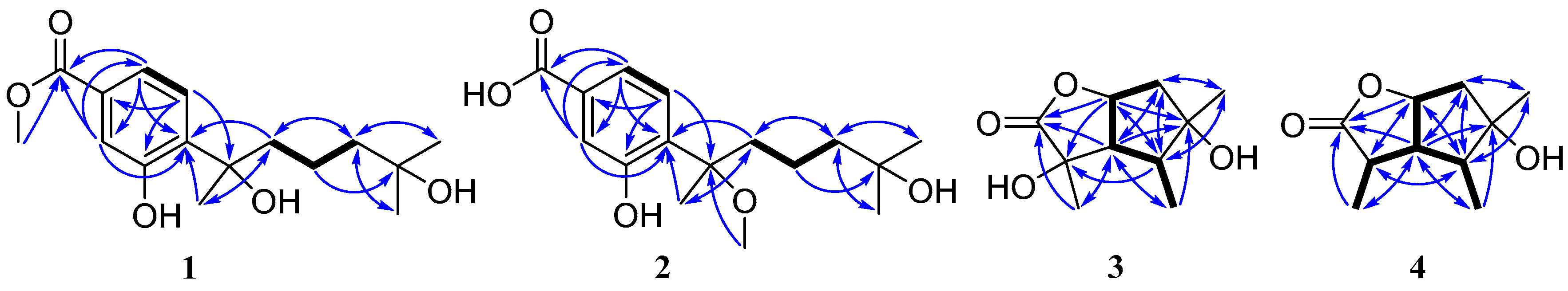
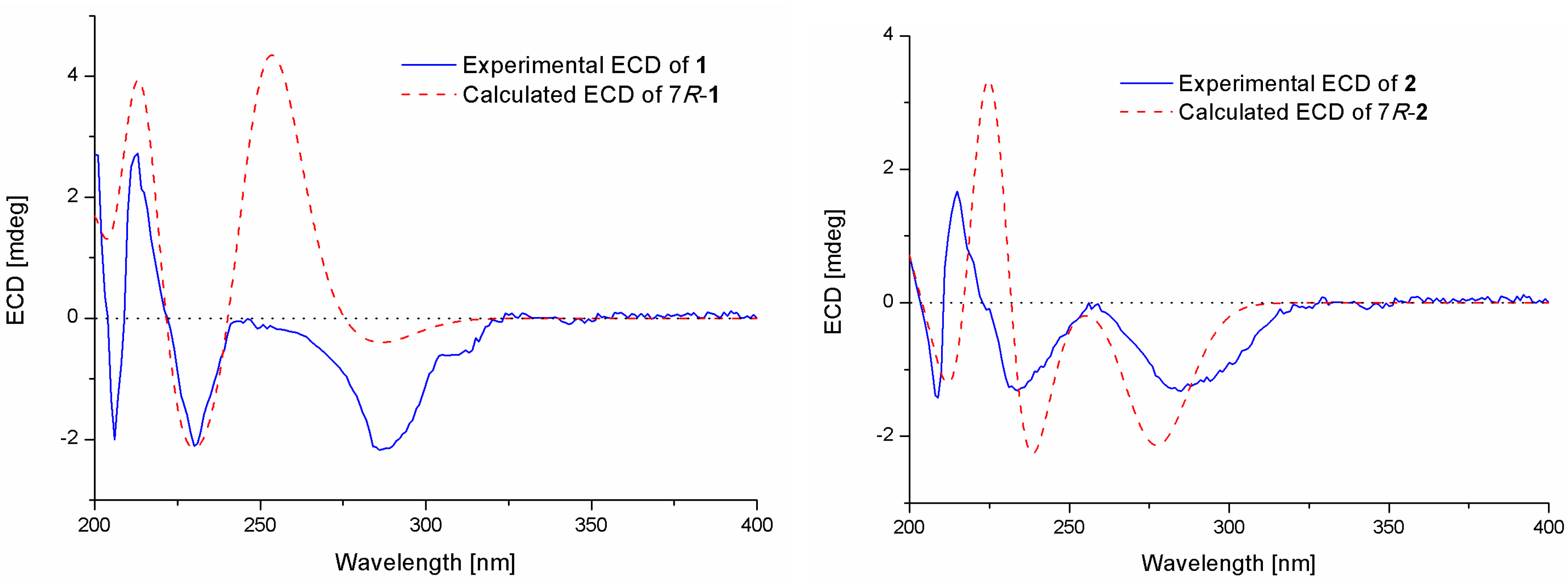
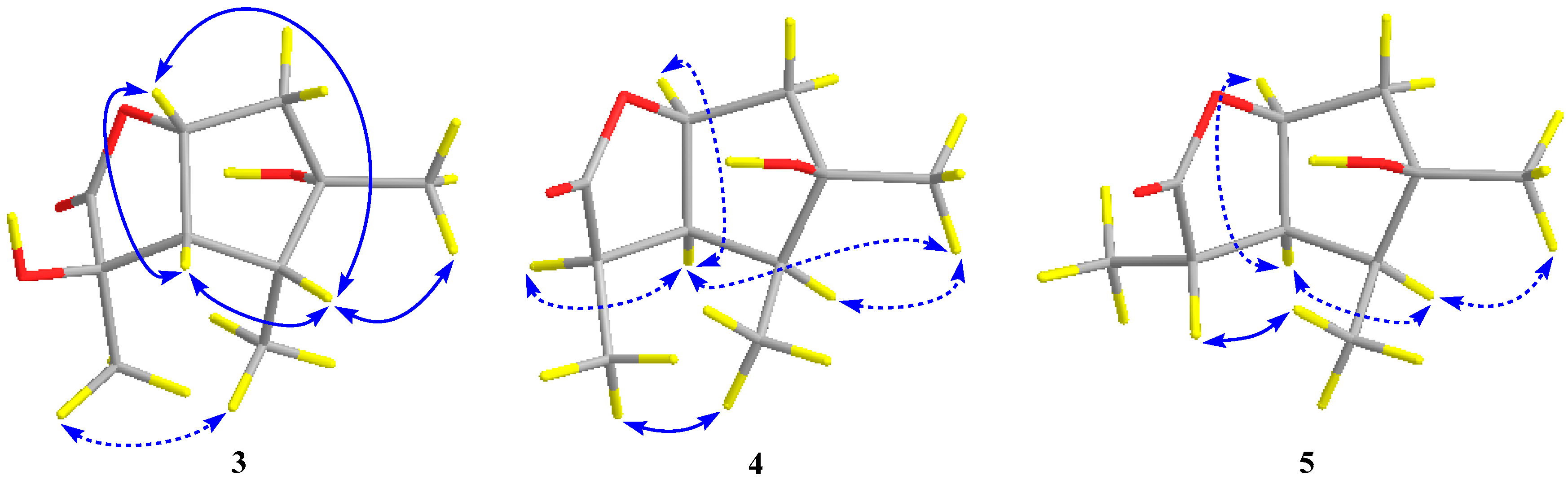
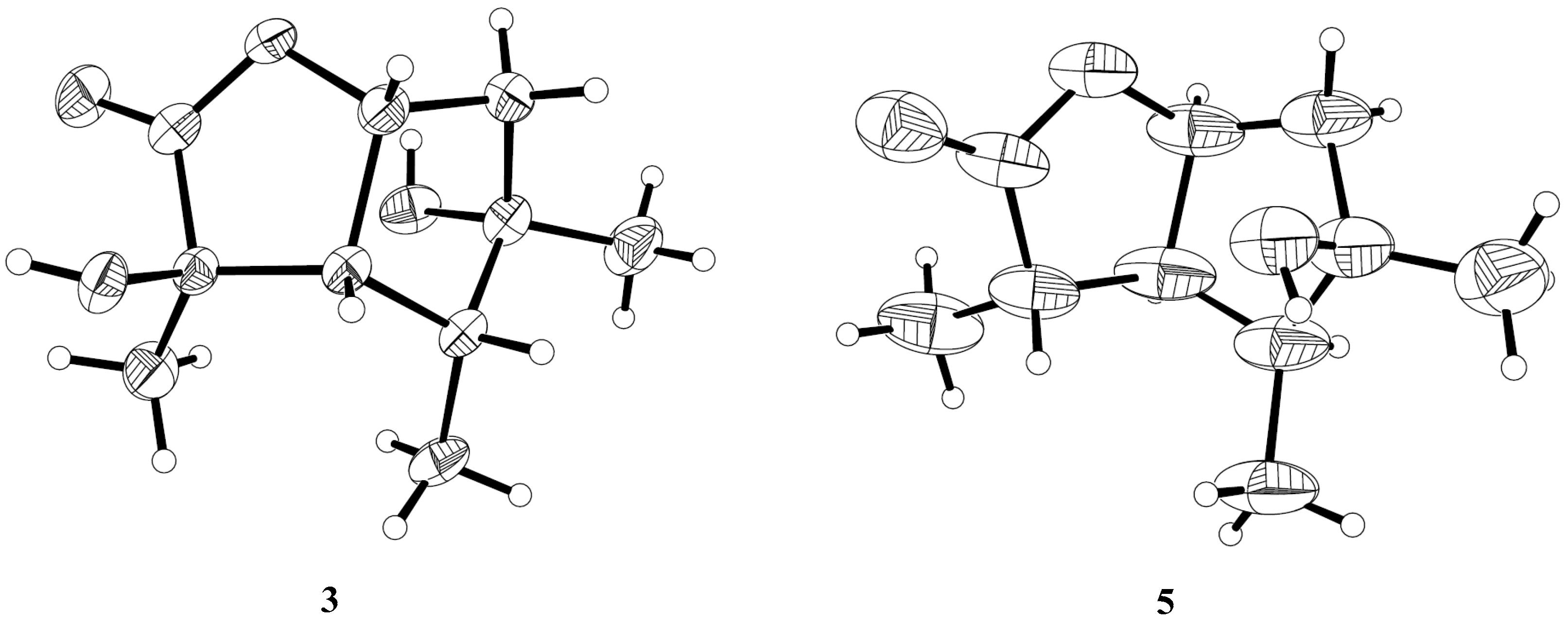
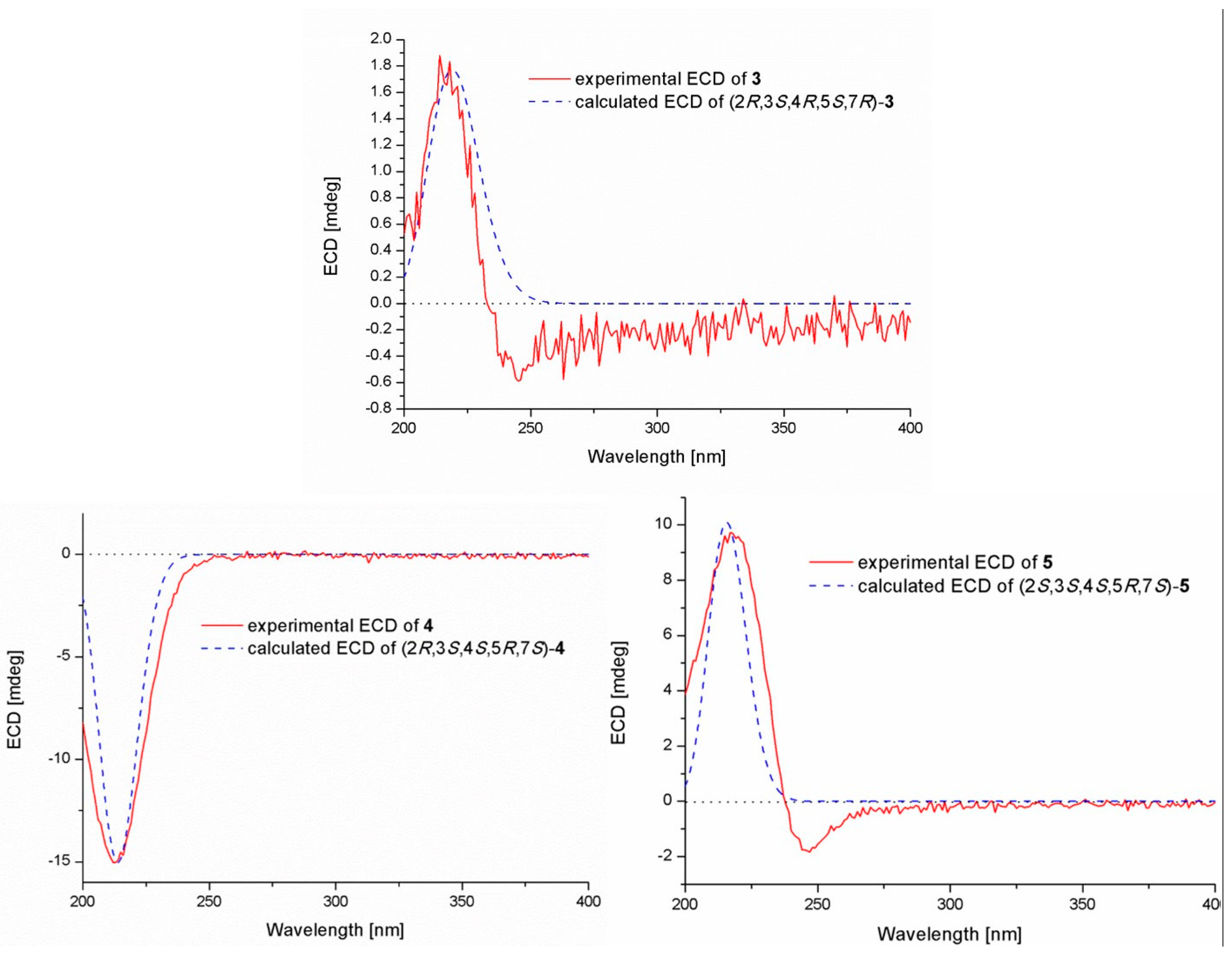
2.1. Structure Elucidation of the Compounds 1–5
2.2. Biological Activities of the Isolated Compounds
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation
3.4. Extraction and Isolation
3.5. Antimicrobial Assays
3.6. X-ray Crystallographic Analysis
3.7. Computational Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Note
- Cheng, Z.; Lou, L.; Liu, D.; Li, X.; Proksch, P.; Yin, S.; Lin, W. Versiquinazolines A−K, fumiquinazoline-type alkaloids from the gorgonian-derived fungus Aspergillus versicolor LZD-14-1. J. Nat. Prod. 2016, 79, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.-Y.; Liu, X.-H.; Miao, F.-P.; Qiao, M.-F. Aspeverin, a new alkaloid from an algicolous strain of Aspergillus versicolor. Org. Lett. 2013, 15, 2327–2329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Miao, M.-M.; Du, G.; Li, X.-N.; Shang, S.-Z.; Zhao, W.; Liu, Z.-H.; Yang, G.-Y.; Che, C.-T.; Hu, Q.-F.; et al. Aspergillines A−E, highly oxygenated hexacyclic indole−tetrahydrofuran−tetramic acid derivatives from Aspergillus versicolor. Org. Lett. 2014, 16, 5016–5019. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, M.; Hao, H.; Lu, C. Avertoxins A−D, prenyl asteltoxin derivatives from Aspergillus versicolor Y10, an endophytic fungus of Huperzia serrata. J. Nat. Prod. 2015, 78, 3067–3070. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, W.; Huang, X.; Tian, X.; Liao, S.; Yang, B.; Wang, F.; Zhou, X.; Liu, Y. Antifungal new oxepine-containing alkaloids and xanthones from the deep-sea-derived fungus Aspergillus versicolor SCSIO 05879. J. Agric. Food Chem. 2016, 64, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wu, G.; Sun, Z.; Zhang, X.; Che, Q.; Gu, Q.; Zhu, T.; Li, D.; Zhang, G. Cytotoxic tetrahydroxanthone dimers from the mangrove-associated fungus Aspergillus versicolor HDN1009. Mar. Drugs 2018, 16, 335. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Y.; Li, T.; Zhang, Z.; Ding, M.; Long, Y.; She, Z. 3-Arylisoindolinone and sesquiterpene derivatives from the mangrove endophytic fungi Aspergillus versicolor SYSU-SKS025. Fitoterapia 2018, 124, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Morinaga, H.; Masuoka, C.; Ikeda, T.; Okawa, M.; Kinjo, J.; Nohara, T. New bisabolane-type sesquiterpenes from the aerial parts of Lippia dulcis. Chem. Pharm. Bull. 2005, 53, 1175–1177. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, T.-H.; Bastow, K.F.; Lee, K.-H.; Chen, D.-F. Altaicalarins A-D, cytotoxic bisabolane sesquiterpenes from Ligularia altaica. J. Nat. Prod. 2010, 73, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Ogasawara, K. A new stereocontrolled route to (+)-curcuphenol, a phenolic sesquiterpene from the marine sponge Didiscus flavus. Tetrahedron: Asymmetry 1998, 9, 2215–2217. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hodges, T.W.; Rajbhandari, I.; Gerwick, W.H.; Hamann, M.T.; Nagle, D.G. Marine natural products as novel antioxidant prototypes. J. Nat. Prod. 2003, 66, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Franzblau, S.G.; Zhang, F.; Hamann, M.T. Novel sesquiterpenes and a lactone from the Jamaican sponge Myrmekioderma styx. Tetrahedron Lett. 2002, 43, 9699–9702. [Google Scholar] [CrossRef]
- Almedia, C.; Elsaedi, S.; Kehraus, S.; Koenig, G.M. Novel bisabolene sequiterpenes from the marine-derived fungus Verticillium tenerum. Nat. Prod. Commun. 2010, 5, 507–510. [Google Scholar]
- Wei, M.-Y.; Wang, C.-Y.; Liu, Q.-A.; Shao, C.-L.; She, Z.-G.; Lin, Y.-C. Five sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from a gorgonian Dichotella gemmacea. Mar. Drugs 2010, 8, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Trisuwan, K.; Rukachaisirikul, V.; Kaewpet, M.; Phongpaichit, S.; Hutadilok-Towatana, N.; Preedanon, S.; Sakayaroj, J. Sesquiterpene and xanthone derivatives from the sea fan-derived fungus Aspergillus sydowii PSU-F154. J. Nat. Prod. 2011, 74, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Li, X.-M.; Xu, G.-M.; Zhang, P.; Wang, B.-G. Antimicrobial phenolic bisabolanes and related derivatives from Penicillium aculeatum SD-321, a deep sea sediment-derived fungus. J. Nat. Prod. 2015, 78, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, Y.; Shao, C.-L.; Yang, R.-Y.; Zheng, C.-J.; Chen, Y.-Y.; Fu, X.-M.; Qian, P.-Y.; She, Z.-G.; Voogd, N.J.; et al. Antibacterial bisabolane-type sesquiterpenoids from the sponge-serived fungus Aspergillus sp. Mar. Drugs 2012, 10, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, T.; Nagayama, K.; Hatsuda, Y. Two new metabolites, sydonic acid and hydroxysydonic acid, from Aspergillus sydowi. Agric. Biol. Chem. 1978, 42, 37–40. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Tan, M.-H.; Liu, C.-X.; Lv, M.-M.; Deng, Z.-S.; Cao, F.; Zou, K.; Proksch, P. Aspergoterpenins A–D: Four new antimicrobial bisabolane sesquiterpenoid derivatives from an endophytic fungus Aspergillus versicolor. Molecules 2018, 23, 1291. [Google Scholar] [CrossRef] [PubMed]
- Mülhaupt, T.; Kaspar, H.; Otto, S.; Reichert, M.; Bringmann, G.; Lindel, T. Isolation, structural elucidation, and synthesis of curcutetraol. Eur. J. Org. Chem. 2005, 2, 334–341. [Google Scholar] [CrossRef]
- Liu, S.; Dai, H.-F.; Heering, C.; Janiak, C.; Lin, W.-H.; Liu, Z.; Proksch, P. Inducing new secondary metabolites through co-cultivation of the fungus Pestalotiopsis sp. with the bacterium Bacillus subtilis. Tetrahedron Lett. 2017, 58, 257–261. [Google Scholar] [CrossRef]
- Hamasaki, T.; Sato, Y.; Hatsuda, Y.; Tanabe, M.; Cary, L.-W. Sydowic acid, a new metabolite from Aspergillus sydowi. Tetrahedron Lett. 1975, 16, 659–660. [Google Scholar] [CrossRef]
- Li, X.-B.; Zhou, Y.-H.; Zhu, R.-X.; Chang, W.-Q.; Yuan, H.-Q.; Gao, W.; Zhang, L.-L.; Zhao, Z.-T.; Lou, H.-X. Identification and biological evaluation of secondary metabolites from the endolichenic fungus Aspergillus versicolor. Chem. Biodivers. 2015, 12, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Murakami, T.; Miyanishi, J.; Tanaka, K.; Takada, N.; Hashimoto, M. Isolation and absolute stereochemistry of optically active sydonic acid from Glonium sp. (Hysterales, Aascomycota). Biosci. Biotechnol. Biochem. 2009, 73, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.-M.; Teuscher, F.; Li, D.-L.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B.-G. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Liu, X.; Guo, H.; Ren, B.; Chen, C.; Piggott, A.M.; Yu, K.; Gao, H.; Wang, Q.; Liu, M.; et al. Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Org. Lett. 2012, 14, 4770–4773. [Google Scholar] [CrossRef] [PubMed]
- Crystallographic data of compounds 3 and 5 have been deposited in the Cambridge Crystallographic Data Centre as CCDC 1940573 and 1940570, respectively. Those data can be obtained free of charge via http://www.ccdc.cam.ac.uk/data request/cif (or from the CCDC, 12 Union Road, Cambridge CB21EZ, U.K.; fax: +44-1223-336-033; e-mail: deposit@ccdc.cam.ac.uk).
- Sheldrick, G.M. SADABS, Software for Empirical Absorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXTL, Structure Determination Software Programs; Bruker Analytical X-ray System Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97 and SHELXS-97, Program for X-ray Crystal Structure Solution and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]

| No. | 1 | 2 | ||
|---|---|---|---|---|
| δH (Mult, J in Hz) a | δC, Type b | δH (Mult, J in Hz) a | δC, Type b | |
| 1 | - | 155.0, qC | - | 154.4, qC |
| 2 | - | 138.6, qC | - | 134.2, qC |
| 3 | 7.44, d (8.1) | 127.1, CH | 7.30, d (8.0) | 127.3, CH |
| 4 | 7.34, dd (8.1, 1.6) | 119.2, CH | 7.35, d (8.0) | 119.7, CH |
| 5 | - | 128.7, qC | - | 132.2, qC |
| 6 | 7.31, d (1.6) | 116.4, CH | 7.36, s | 116.9, CH |
| 7 | - | 74.6, qC | - | 79.5, qC |
| 8a | 1.93, dt (13.0, 4.4) | 41.8, CH2 | 1.87, t (11.4) | 38.4, CH2 |
| 8b | 1.65, dt (13.0, 4.0) | - | 1.76, t (11.4) | - |
| 9a | 1.30, m | 18.5, CH2 | 1.19, m | 18.2, CH2 |
| 9b | 1.02, m | - | 1.03, m | - |
| 10 | 1.23, m | 44.0, CH2 | 1.22, t (12.3) | 43.8, CH2 |
| 11 | - | 68.7, qC | - | 68.6, qC |
| 12 | 0.97, s | 29.3, CH3 | 0.97, s | 29.2, CH3 |
| 13 | 0.95, s | 29.2, CH3 | 0.97, s | 29.2, CH3 |
| 14 | 1.50, s | 28.1, CH3 | 1.54, s | 22.2, CH3 |
| 15 | - | 166.2, qC | - | 167.9, qC |
| 15-COOCH3 | 3.80, s | 51.8, CH3 | - | - |
| 7-OCH3 | - | - | 3.15, s | 49.4, CH3 |
| No. | 3 | 4 | ||
|---|---|---|---|---|
| δH (Mult, J in Hz) a | δC, Type b | δH (Mult, J in Hz) a | δC, Type b | |
| 1 | - | 178.1, qC | - | 179.5, qC |
| 2 | - | 75.6, qC | 2.89, m | 38.2, CH |
| 3 | 2.72, dd (8.9, 7.3) | 53.9, CH | 2.99, m | 44.3, CH |
| 4 | 1.84, m | 46.0, CH | 1.78, m | 46.0, CH |
| 5 | - | 77.5, qC | - | 77.8, qC |
| 6α | 1.91, d (14.3) | 45.5, CH2 | 1.92, d (14.3) | 45.7, CH2 |
| 6β | 1.73, dd (14.3, 6.5) | - | 1.81, dd (14.3, 6.9) | - |
| 7 | 4.93, t (6.8) | 80.3, CH | 4.80, t (6.7) | 81.0, CH |
| 8 | 1.39, s | 21.6, CH3 | 1.22, d (7.3) | 12.3, CH3 |
| 9 | 1.02, d (7.5) | 9.7, CH3 | 0.98, d (7.5) | 10.2, CH3 |
| 10 | 1.08, s | 24.8, CH3 | 1.11, s | 26.0, CH3 |
| Strains | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Positive Control |
|---|---|---|---|---|---|---|---|---|---|---|
| Escherichia colib | 4.0 | 2.0 | – | – | – | 1.0 | – | 32 | 16 | 2.0 |
| Aeromonas hydrophiliab | – | 8.0 | 16 | – | – | 4.0 | 16 | 32 | 8.0 | 2.0 |
| Edwardsiella tardab | 8.0 | 4.0 | – | 32 | – | 4.0 | 32 | – | 8.0 | 2.0 |
| Pseudomonas aeruginosab | 8.0 | 32 | – | – | – | 8.0 | – | – | – | 2.0 |
| Vibrio anguillarumb | – | 4.0 | 32 | – | 32 | 4.0 | 32 | 32 | 32 | 0.5 |
| Vibrio harveyib | 8.0 | 8.0 | – | 32 | 32 | 4.0 | 16 | 32 | 16 | 4.0 |
| Vibrio parahaemolyticusb | 8.0 | 8.0 | 16 | – | – | 8.0 | – | – | 32 | 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-D.; Li, X.-M.; Yin, X.-L.; Li, X.; Wang, B.-G. Antimicrobial Sesquiterpenoid Derivatives and Monoterpenoids from the Deep-Sea Sediment-Derived Fungus Aspergillus versicolor SD-330. Mar. Drugs 2019, 17, 563. https://doi.org/10.3390/md17100563
Li X-D, Li X-M, Yin X-L, Li X, Wang B-G. Antimicrobial Sesquiterpenoid Derivatives and Monoterpenoids from the Deep-Sea Sediment-Derived Fungus Aspergillus versicolor SD-330. Marine Drugs. 2019; 17(10):563. https://doi.org/10.3390/md17100563
Chicago/Turabian StyleLi, Xiao-Dong, Xiao-Ming Li, Xiu-Li Yin, Xin Li, and Bin-Gui Wang. 2019. "Antimicrobial Sesquiterpenoid Derivatives and Monoterpenoids from the Deep-Sea Sediment-Derived Fungus Aspergillus versicolor SD-330" Marine Drugs 17, no. 10: 563. https://doi.org/10.3390/md17100563
APA StyleLi, X.-D., Li, X.-M., Yin, X.-L., Li, X., & Wang, B.-G. (2019). Antimicrobial Sesquiterpenoid Derivatives and Monoterpenoids from the Deep-Sea Sediment-Derived Fungus Aspergillus versicolor SD-330. Marine Drugs, 17(10), 563. https://doi.org/10.3390/md17100563






