Chemistry and Biology of Siderophores from Marine Microbes
Abstract
1. Introduction
2. Diversity of Siderophores from Marine Microorganisms
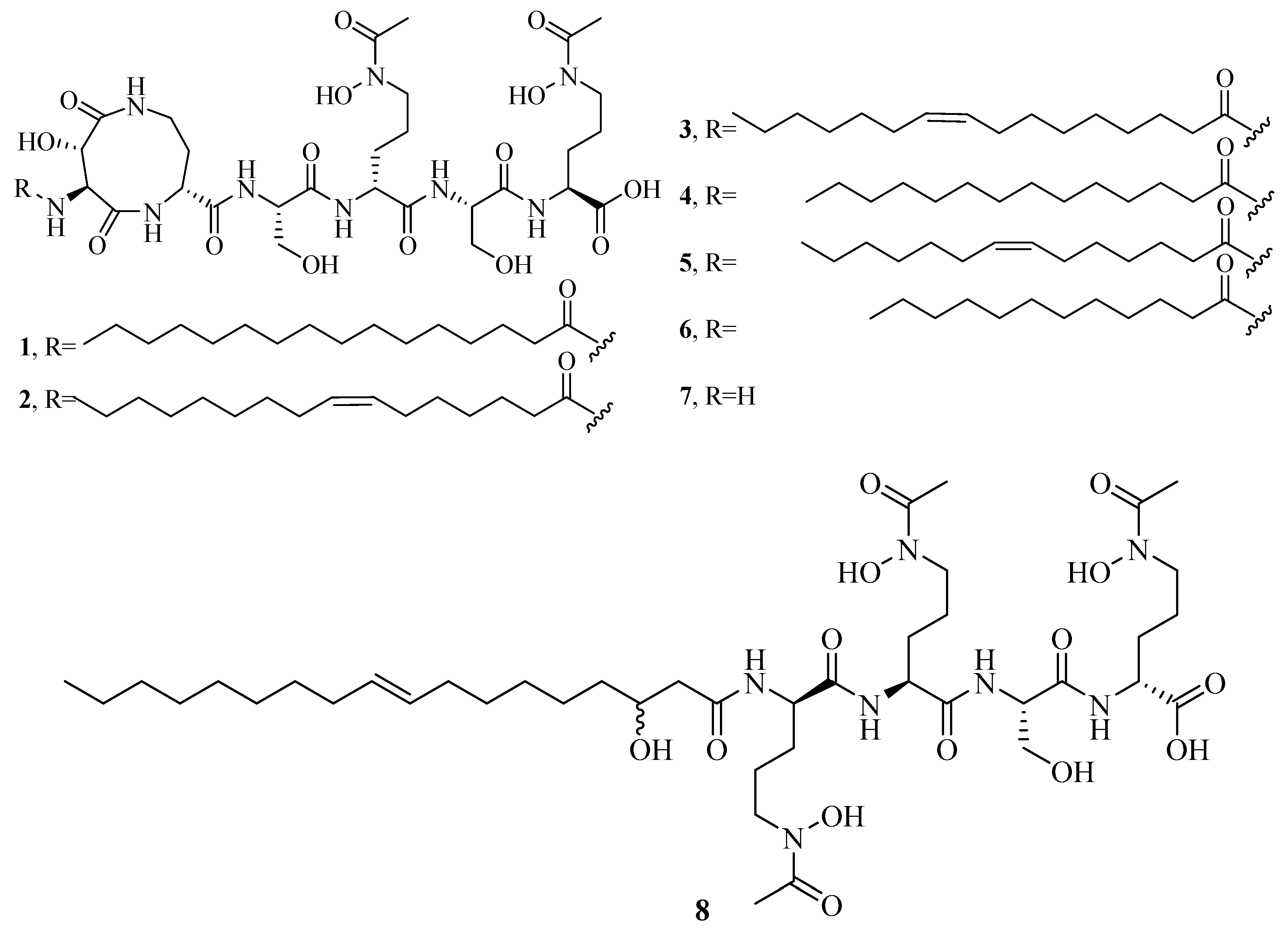
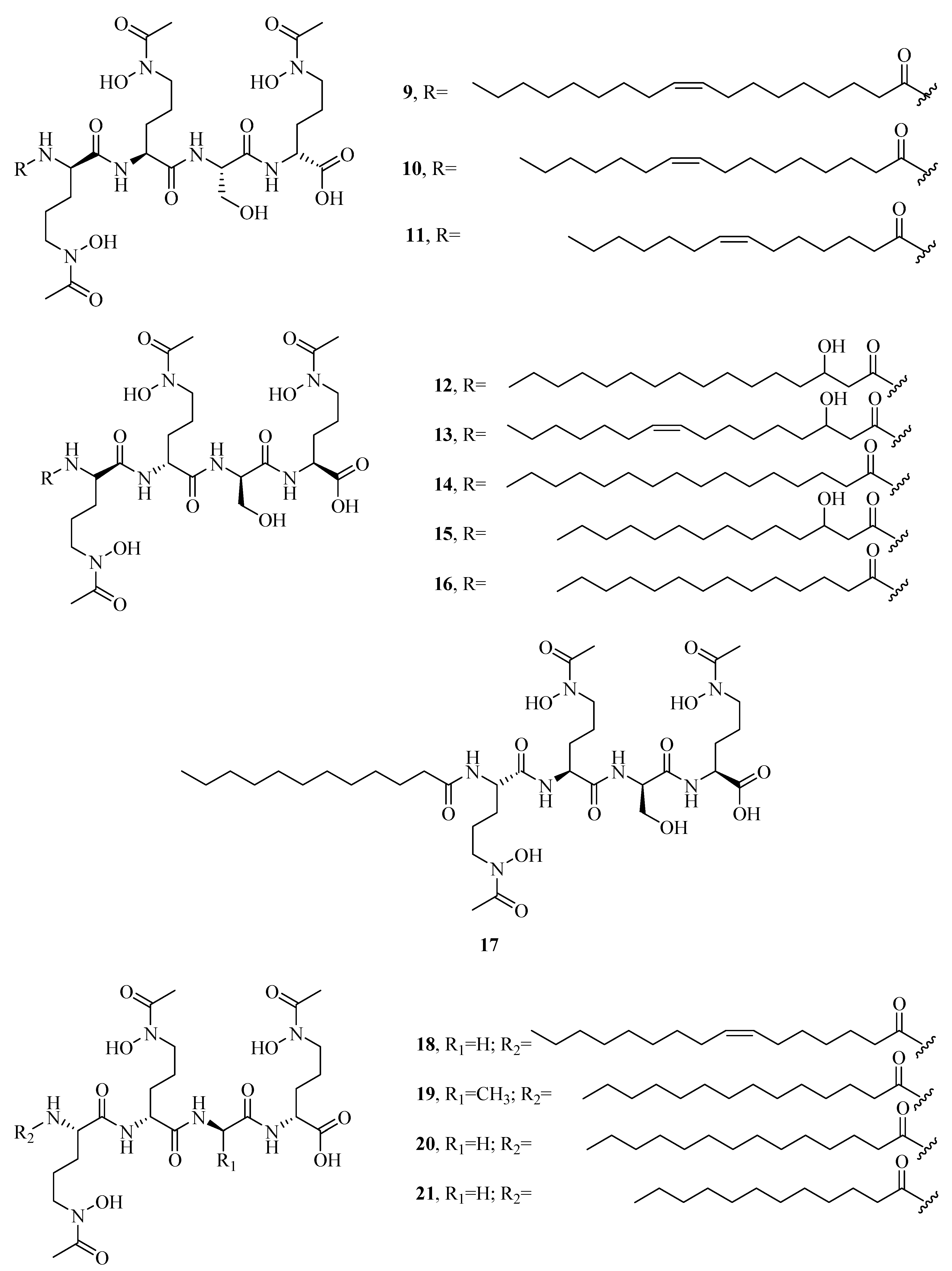
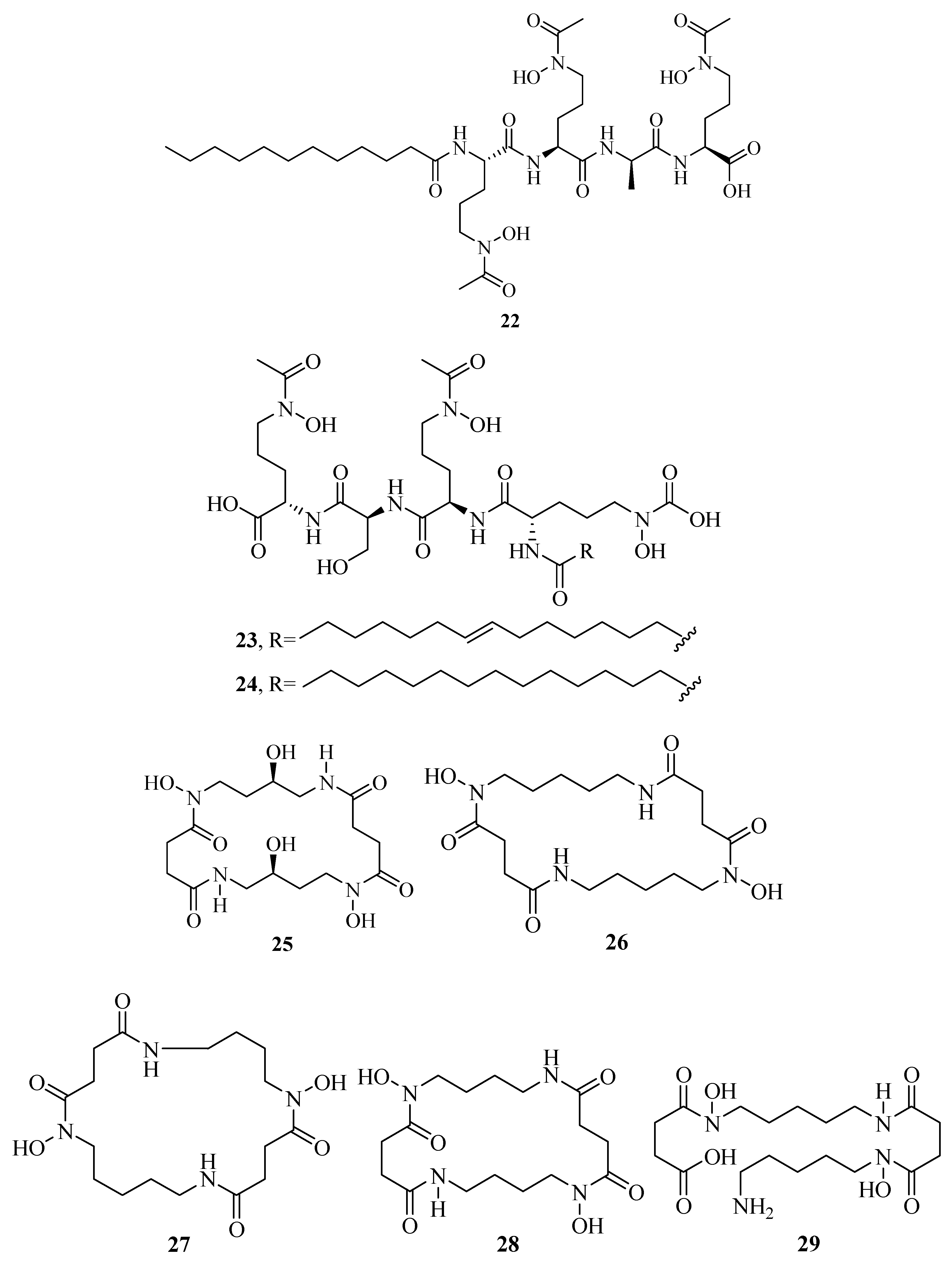
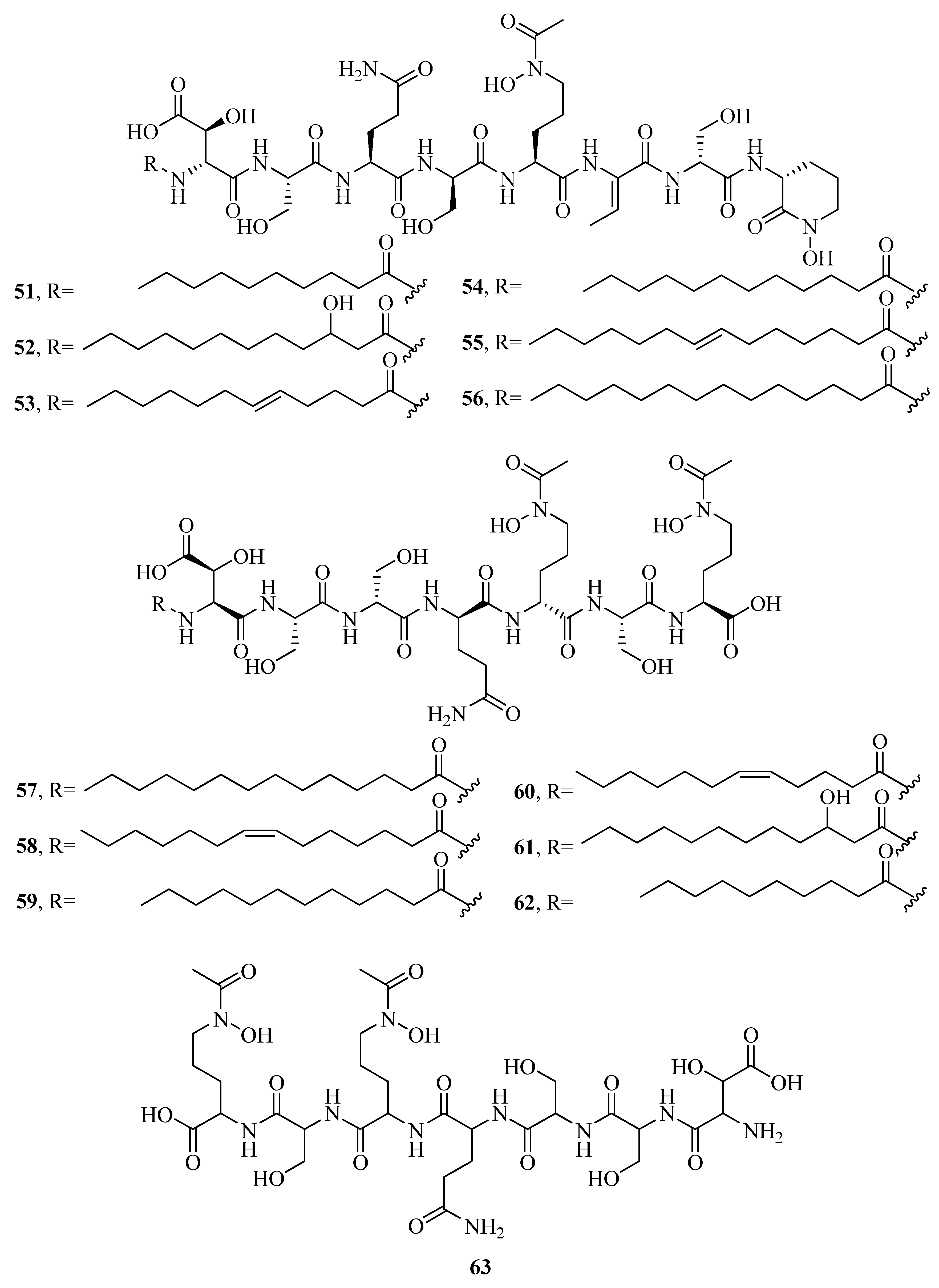
2.1. Hydroxamate-Type Siderophores
2.2. α-Hydroxycarboxylates
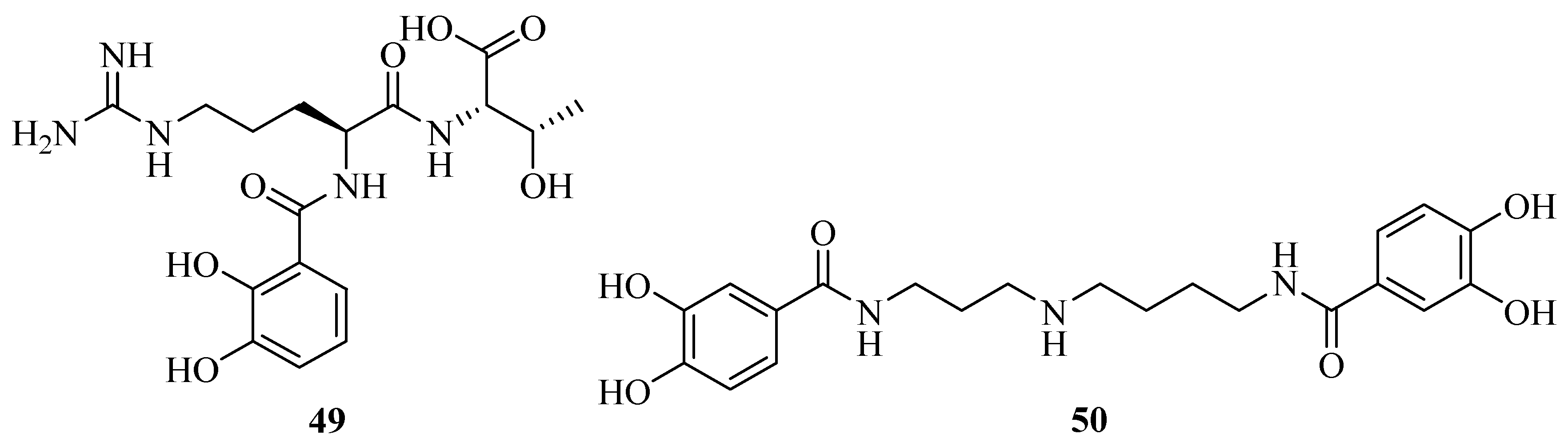
2.3. Catecholates
2.4. Mixed Hydroxamates/α-Hydroxycarboxylates
2.5. Mixed α-Hydroxycarboxylates/Catecholates
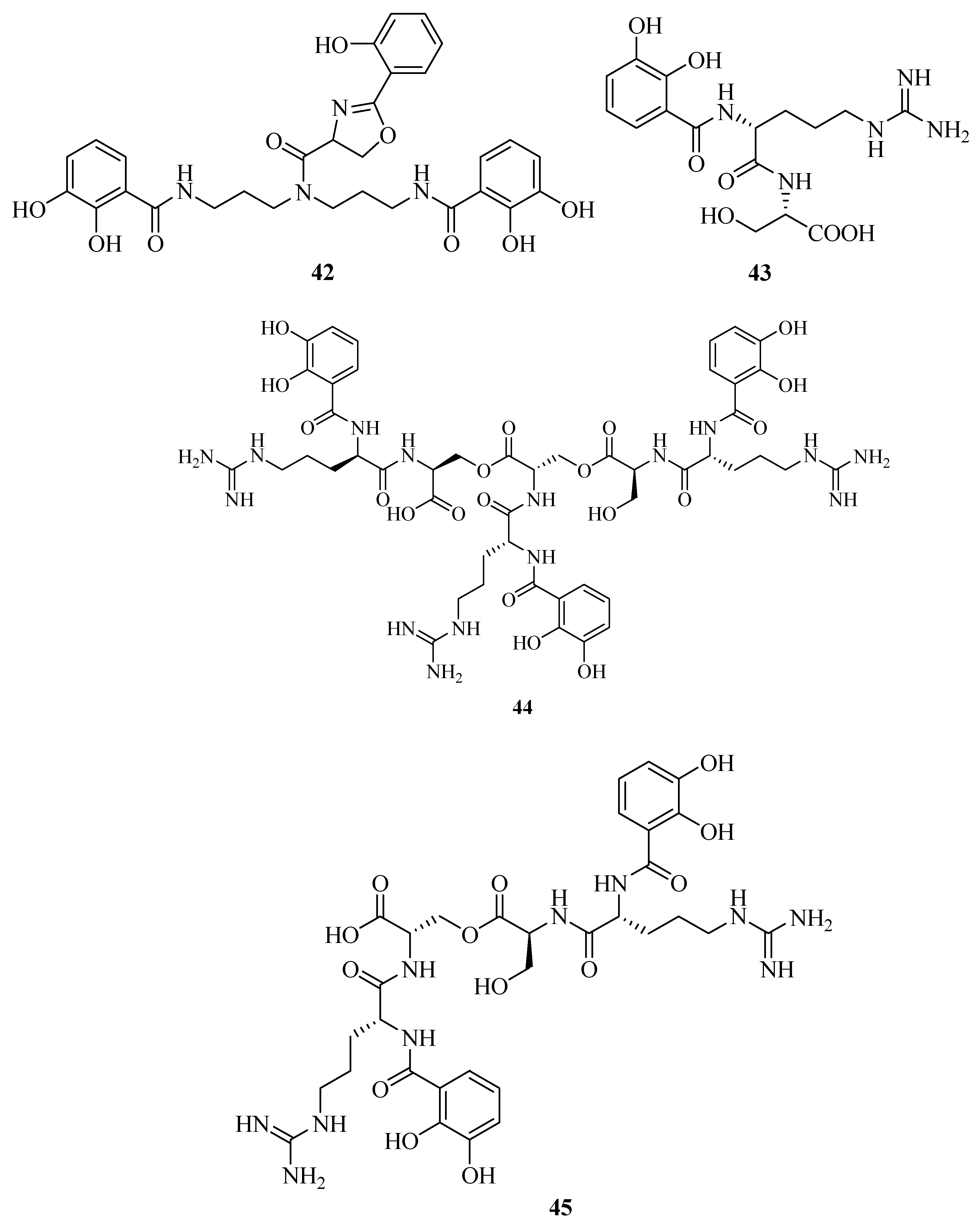
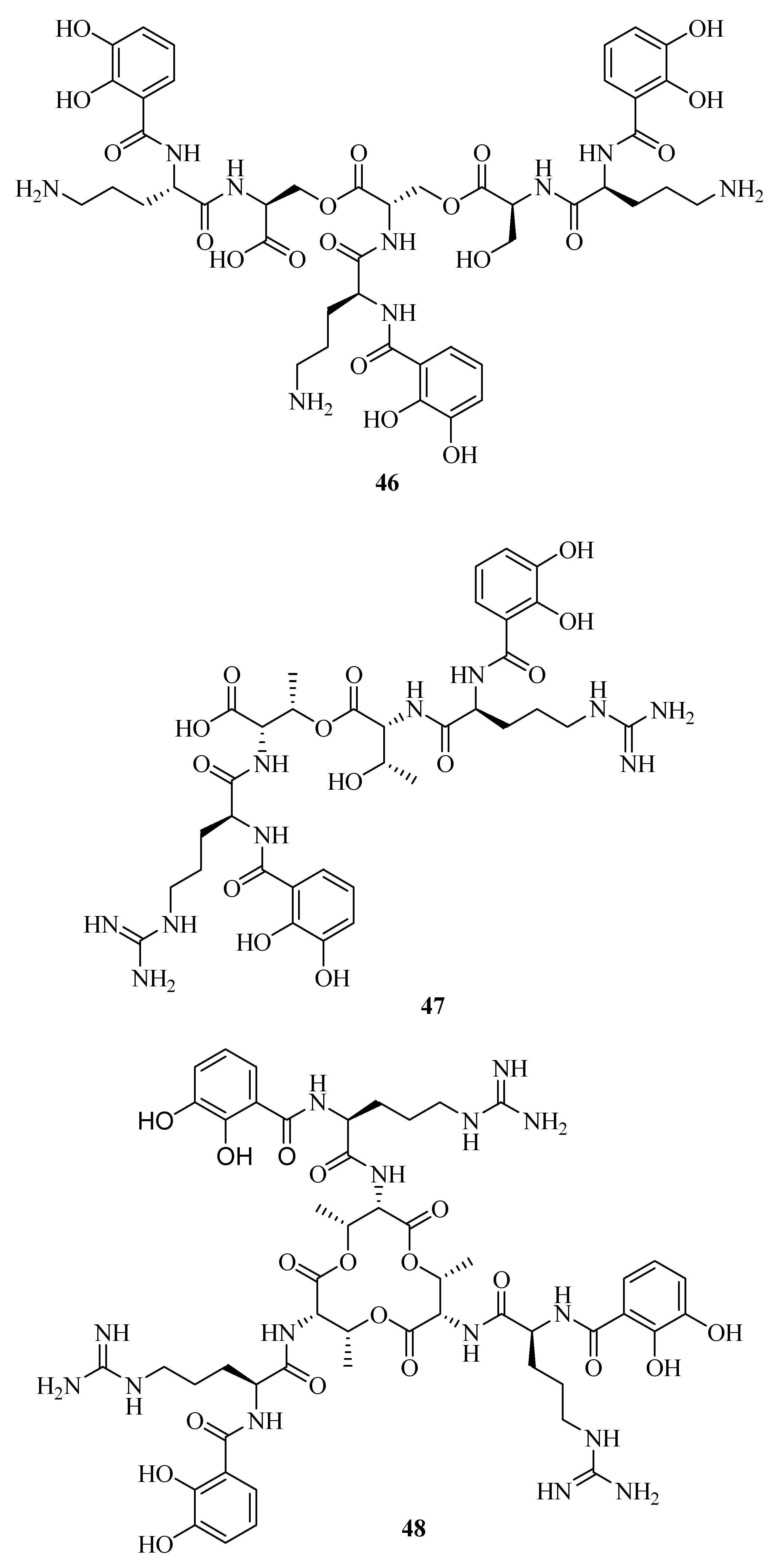
2.6. Mixed Hydroxamates/Catecholates
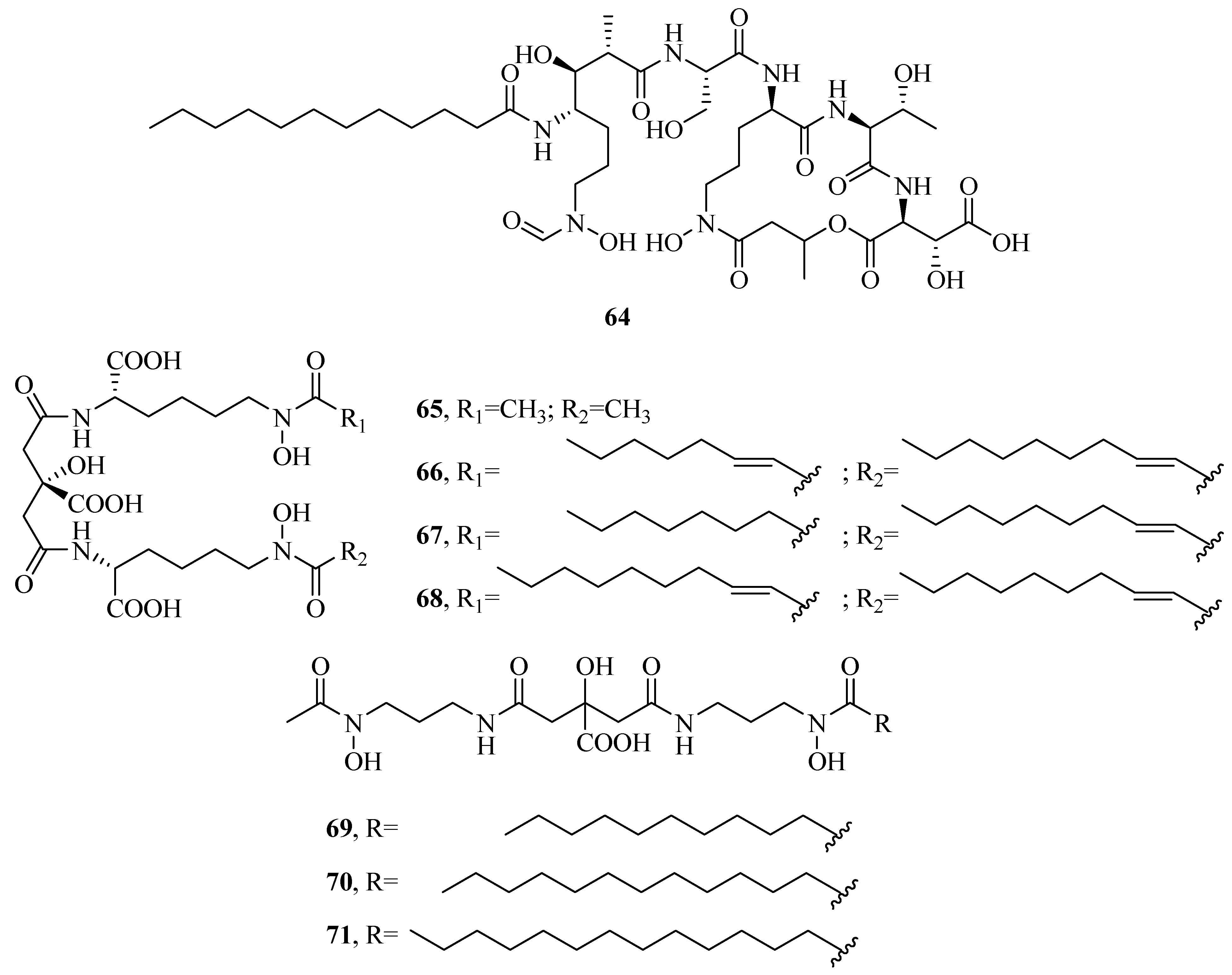
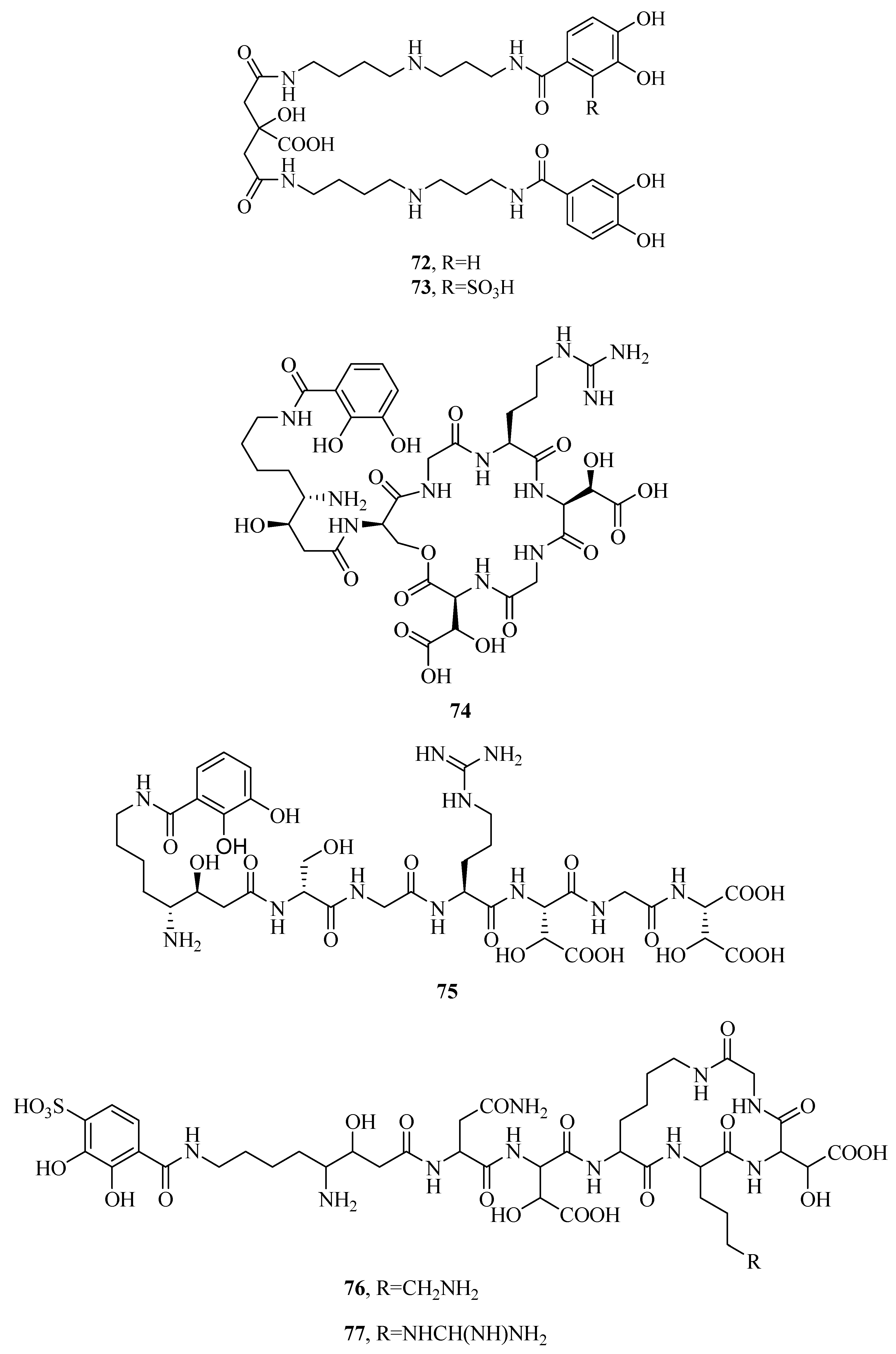
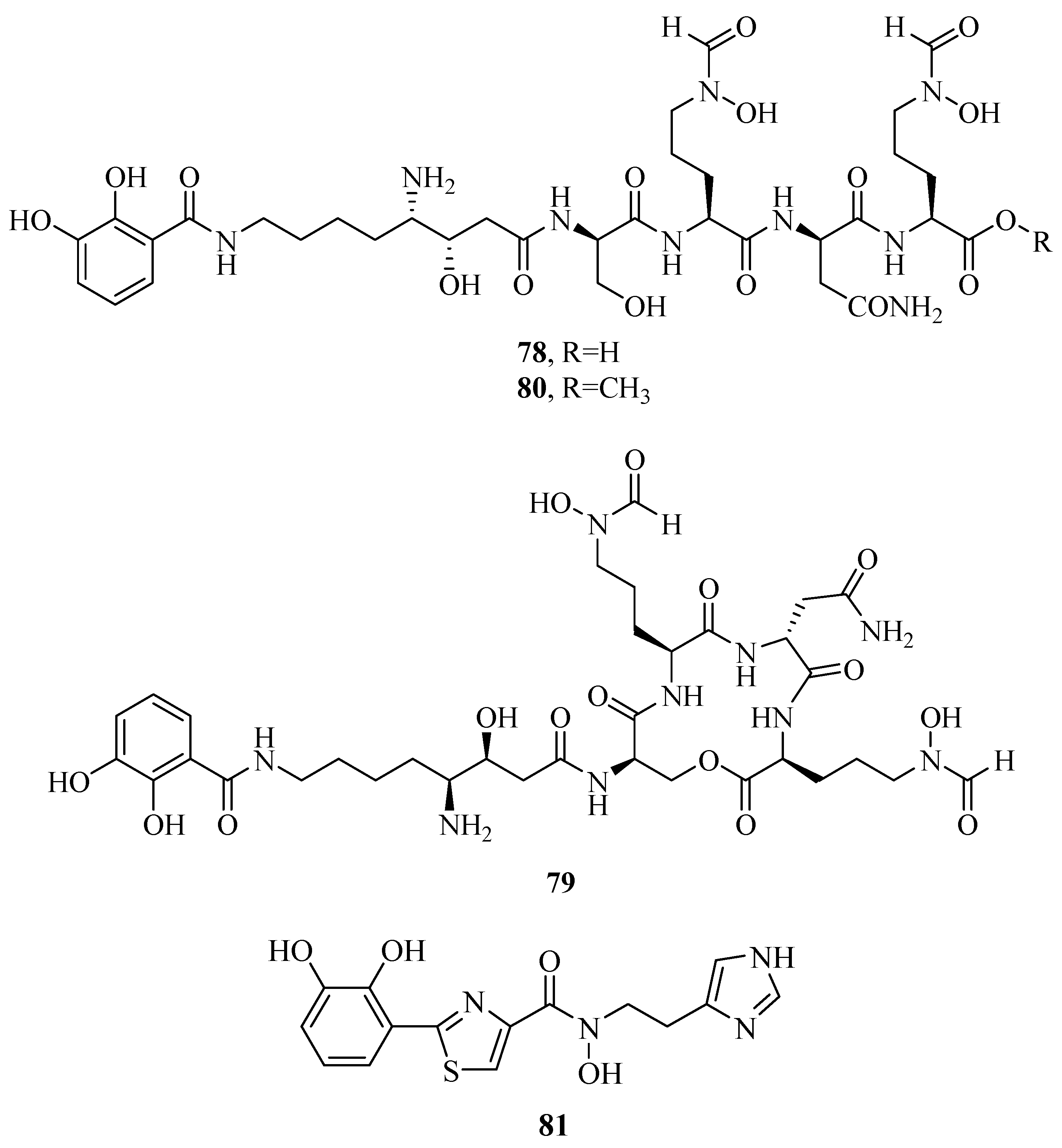
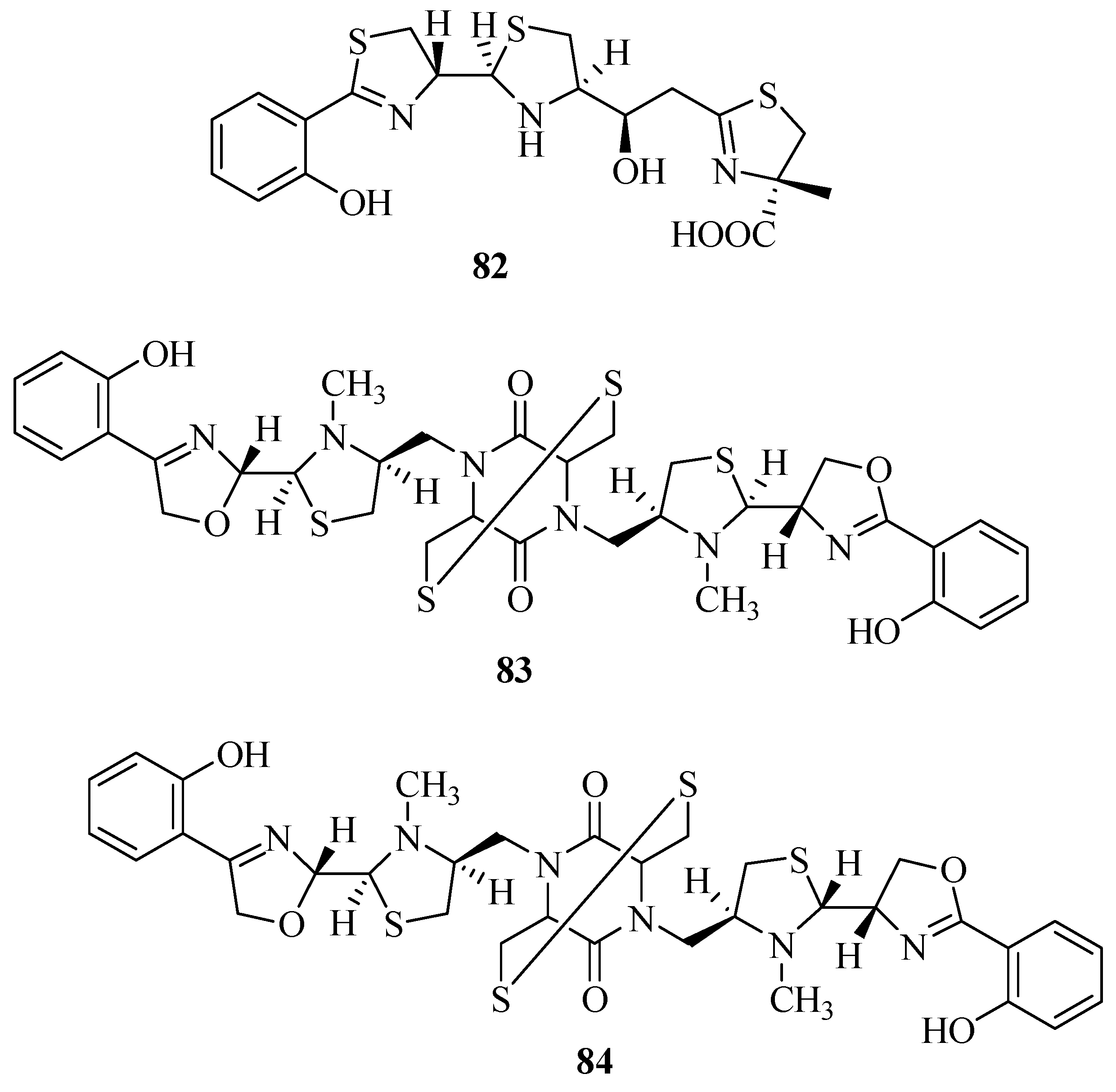
2.7. Other Types of Siderophores
| Compd. | Name | PubChem CID | PubChem Database | Ref. |
|---|---|---|---|---|
| 1 | Marinobactin A | – | – | [22] |
| 2 | Marinobactin B | – | – | [22] |
| 3 | Marinobactin C | – | – | [22] |
| 4 | Marinobactin D | – | – | [22] |
| 5 | Marinobactin E | – | – | [22] |
| 6 | Marinobactin F | – | – | [22] |
| 7 | Marinobactin HG | – | – | [23] |
| 8 | Amphibactin | – | – | [24] |
| 9 | Amphibactin | – | – | [24] |
| 10 | Amphibactin | – | – | [24] |
| 11 | Amphibactin | – | – | [24] |
| 12 | Amphibactin | – | – | [24] |
| 13 | Amphibactin | – | – | [24] |
| 14 | Amphibactin | – | – | [24] |
| 15 | Amphibactin | – | – | [24] |
| 16 | Amphibactin | – | – | [24] |
| 17 | Amphibactin | – | – | [24] |
| 18 | Moanachelin | – | – | [25] |
| 19 | Moanachelin | – | – | [25] |
| 20 | Moanachelin | – | – | [25] |
| 21 | Moanachelin | 122223347 | https://pubchem.ncbi.nlm.nih.gov/compound/122223347 | [25] |
| 22 | Moanachelin | – | – | [25] |
| 23 | Amphibactin U | – | – | [26] |
| 24 | Amphibactin V | – | – | [26] |
| 25 | Alcaligin | – | – | [27] |
| 26 | Bisucaberin | – | – | [28] |
| 27 | Avaroferrin | – | – | [29] |
| 28 | Putrebactin | – | – | [30] |
| 29 | Bisucaberin B | – | – | [34] |
| 30 | Thalassosamide | – | – | [35] |
| 31 | Fradiamine A | 129008905 | https://pubchem.ncbi.nlm.nih.gov/compound/129008905 | [36] |
| 32 | Fradiamine B | 60151746 | https://pubchem.ncbi.nlm.nih.gov/compound/60151746 | [36] |
| 33 | Albisporachelin | – | – | [37] |
| 34 | Desferrioxamine A1 | – | – | [38] |
| 35 | Desferrioxamine A2 | – | – | [38] |
| 36 | Desferrioxamine B | – | – | [38] |
| 37 | Desferrioxamine D1 | – | – | [38] |
| 38 | Desferrioxamine D2 | – | – | [38] |
| 39 | Desferrioxamine E | – | – | [38] |
| 40 | Desferrioxamine N | – | – | [38] |
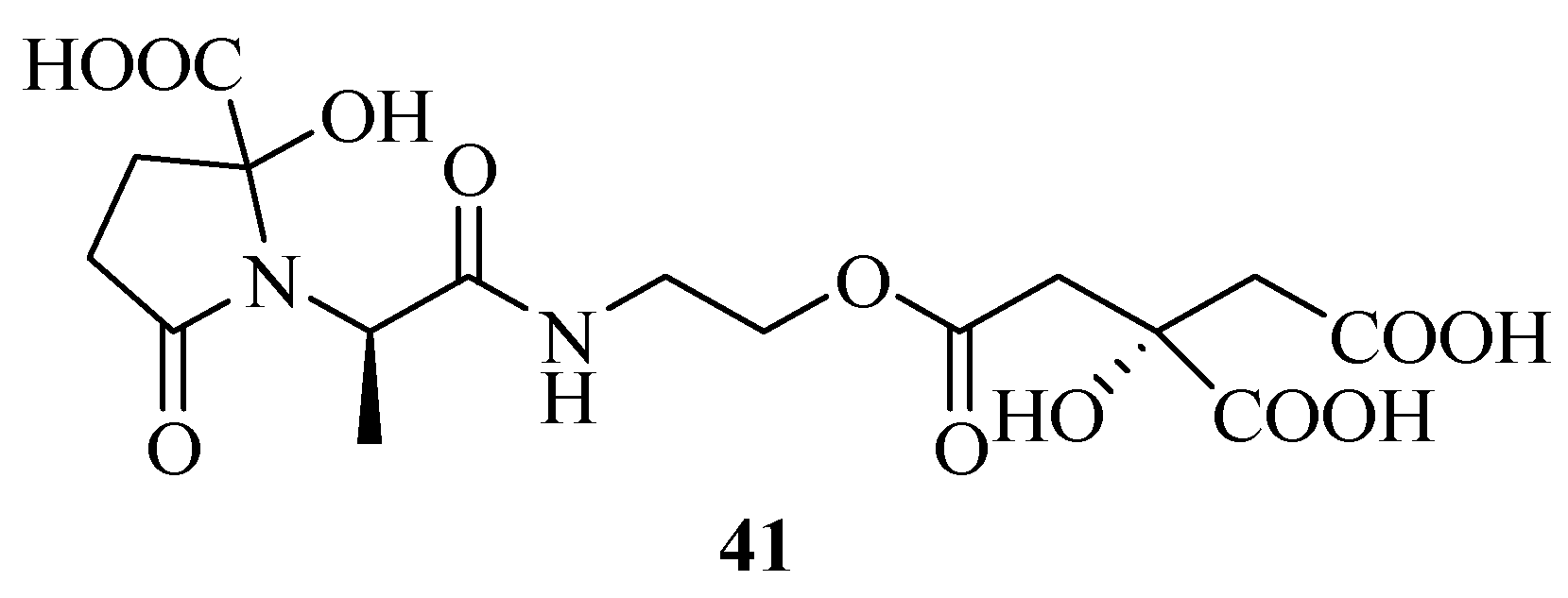
| 41 | Vibrioferrin | 11102119 | https://pubchem.ncbi.nlm.nih.gov/compound/11102119 | [39] |
| 42 | Nigribactin | – | – | [40] |
| 43 | Vanchrobactin | – | – | [45] |
| 44 | Trivanchrobactin | – | – | [45] |
| 45 | Divanchrobactin | – | – | [45] |
| 46 | Turnerbactin | [46] | ||
| 47 | Dibenarthin | – | – | [47] |
| 48 | Streptobactin | – | – | [47] |
| 49 | Tribenarthin | – | – | [47] |
| 50 | Pistillarin | – | – | [48] |
| 51 | Loihichelin A | 101476230 | https://pubchem.ncbi.nlm.nih.gov/compound/101476230 | [51] |
| 52 | Loihichelin B | 101476231 | https://pubchem.ncbi.nlm.nih.gov/compound/101476231 | [51] |
| 53 | Loihichelin C | 101476232 | https://pubchem.ncbi.nlm.nih.gov/compound/101476232 | [51] |
| 54 | Loihichelin D | 101476233 | https://pubchem.ncbi.nlm.nih.gov/compound/101476233 | [51] |
| 55 | Loihichelin E | 101476234 | https://pubchem.ncbi.nlm.nih.gov/compound/101476234 | [51] |
| 56 | Loihichelin F | 101476235 | https://pubchem.ncbi.nlm.nih.gov/compound/101476235 | [51] |
| 57 | Aquachelin A | – | – | [22] |
| 58 | Aquachelin B | – | – | [22] |
| 59 | Aquachelin C | – | – | [22] |
| 60 | Aquachelin D | – | – | [22] |
| 61 | Aquachelin I | – | – | [23] |
| 62 | Aquachelin J | – | – | [52] |
| 63 | Aquachelin HG | – | – | [29] |
| 64 | Imaqobactin | – | – | [53] |
| 65 | Aerobactin | – | – | [54] |
| 66 | Ochrobactin A | – | – | [56] |
| 67 | Ochrobactin B | – | – | [56] |
| 68 | Ochrobactin C | – | – | [56] |
| 69 | Synechobactin | 122377042 | https://pubchem.ncbi.nlm.nih.gov/compound/122377042 | [57] |
| 70 | Synechobactin | 122377043 | https://pubchem.ncbi.nlm.nih.gov/compound/122377043 | [57] |
| 71 | Synechobactin | 122377044 | https://pubchem.ncbi.nlm.nih.gov/compound/122377044 | [57] |
| 72 | Petrobactin | – | – | [58] |
| 73 | Petrobactin sulfonate | – | – | [59] |
| 74 | Alterobactin A | – | – | [61] |
| 75 | Alterobactin B | 101775921 | https://pubchem.ncbi.nlm.nih.gov/compound/101775921 | [62] |
| 76 | Pseudoalterobactin A | 11434714 | https://pubchem.ncbi.nlm.nih.gov/compound/11434714 | [63] |
| 77 | Pseudoalterobactin B | 11788080 | https://pubchem.ncbi.nlm.nih.gov/compound/11788080 | [63] |
| 78 | Lystabactin A | – | – | [64] |
| 79 | Lystabactin B | – | – | [64] |
| 80 | Lystabactin C | – | – | [64] |
| 81 | Anguibactin | – | – | [42,45] |
| 82 | piscibactin | 136754132 | https://pubchem.ncbi.nlm.nih.gov/compound/136754132 | [65,66,67] |
| 83 | Tetroazolemycin A | – | – | [68] |
| 84 | Tetroazolemycin B | – | – | [68] |
3. Biosynthesis of Siderophores from Marine Microorganisms
3.1. NRPS-Mediated Siderophore Biosynthetic Pathway
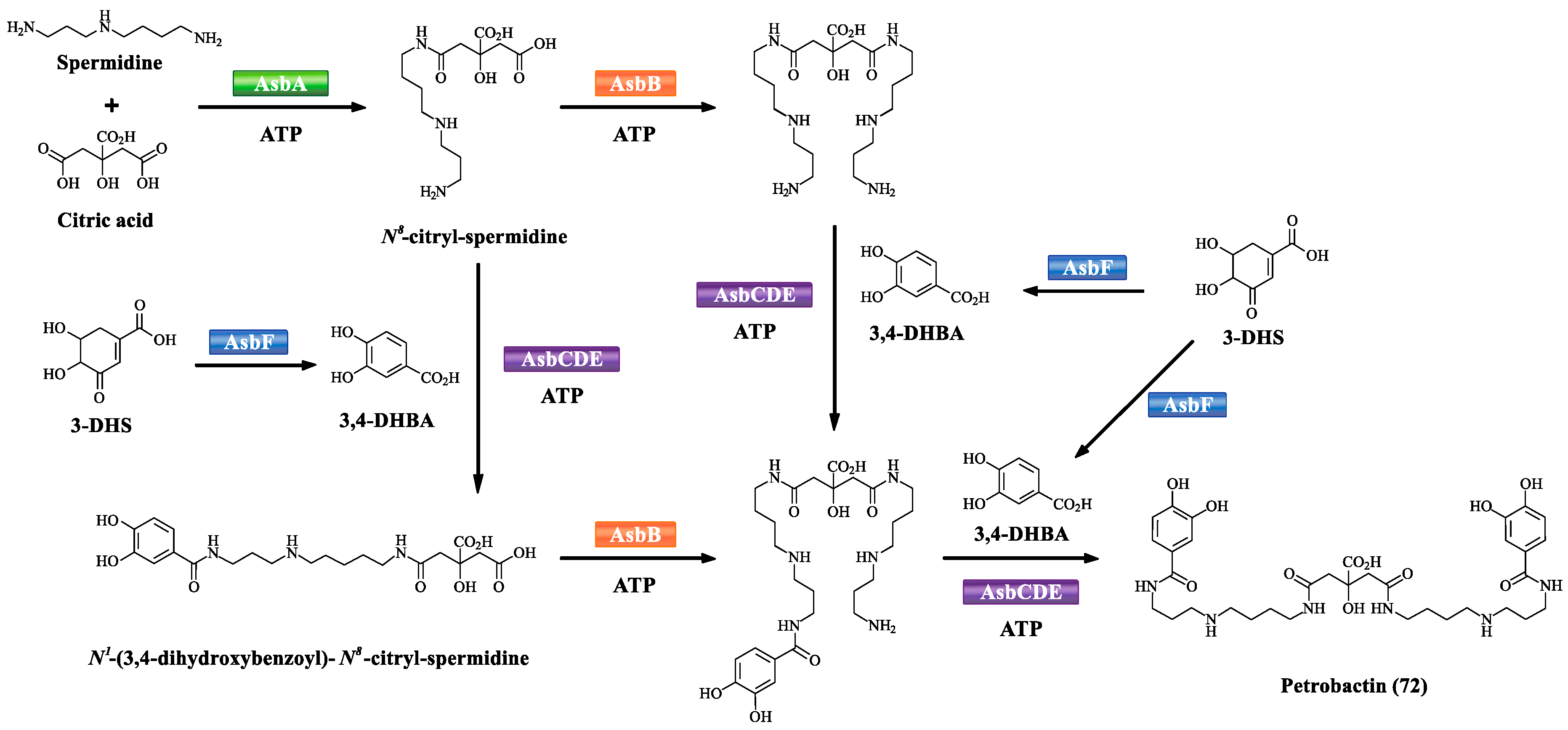
3.2. NRPS-Independent Siderophore Biosynthetic Pathway
4. Synthesis and Study of Siderophores from Marine Microorganisms
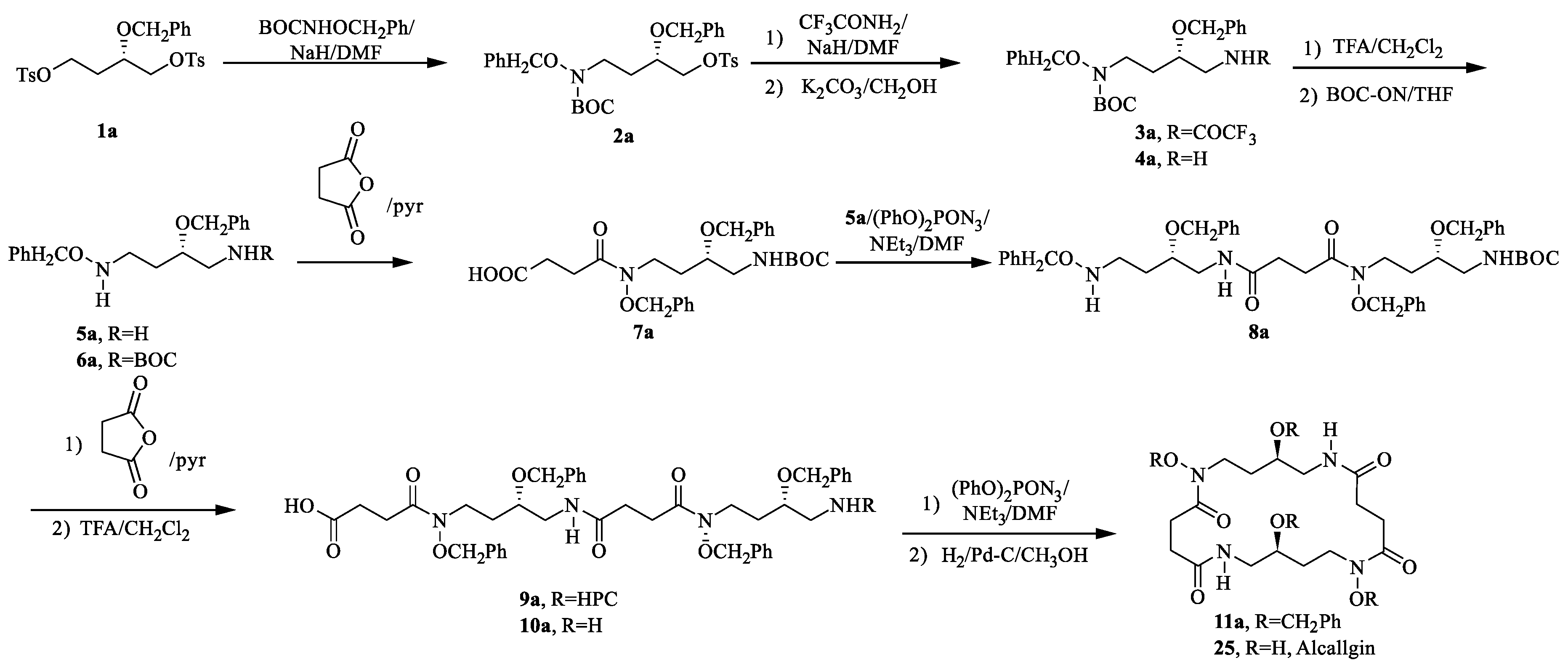
4.1. Alcaligin
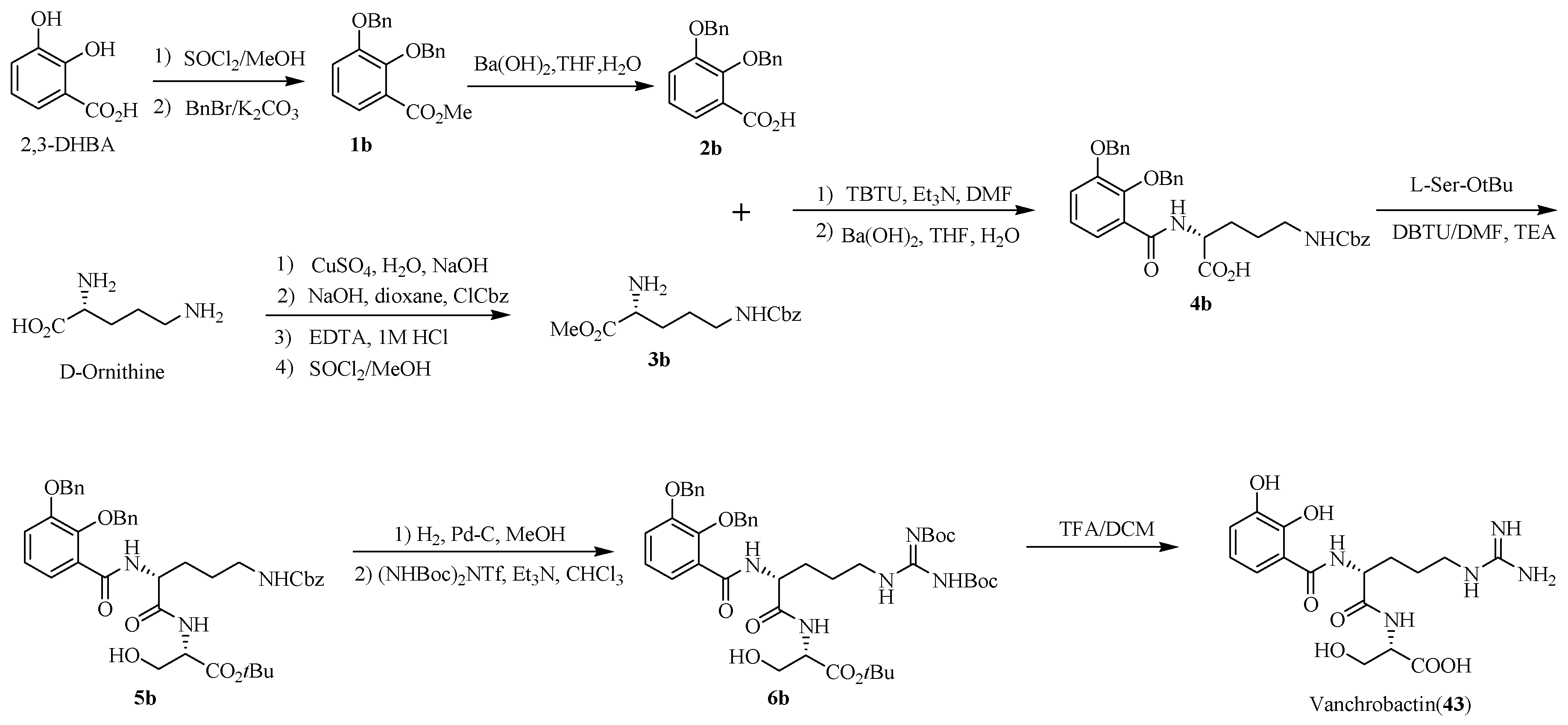
4.2. Vanchrobactin
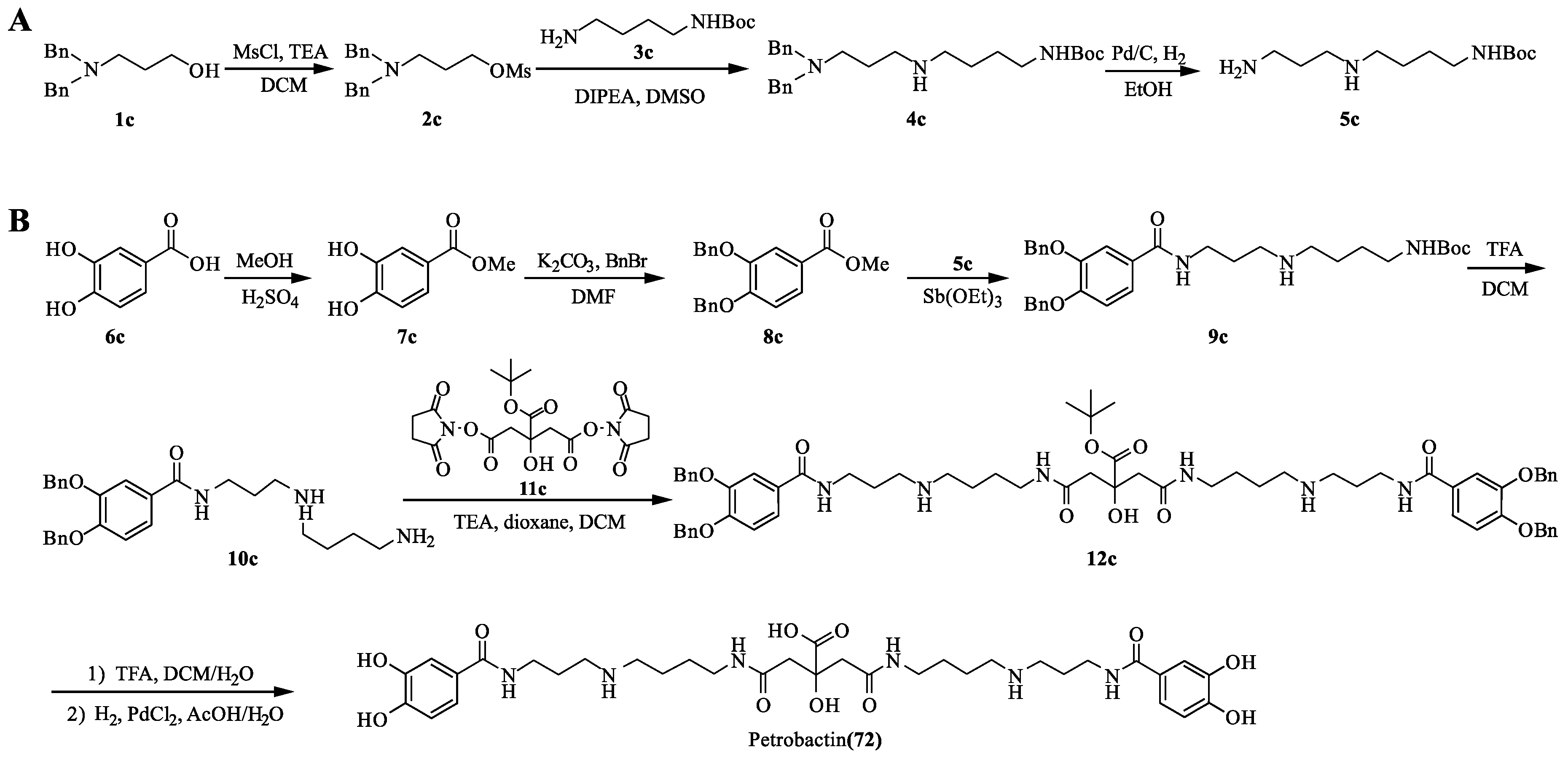
4.3. Petrobactin
5. Conclusions and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, J.W.; Wang, B.X.; Lu, Y.J.; Guo, Y.Q.; Sun, J.D.; Wei, B.; Zhang, H.W.; Wang, H. Quorum sensing inhibitors from marine microorganisms and their synthetic derivatives. Mar. Drugs 2019, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ashforth, E.; Ren, B.; Song, F.; Dai, H.; Liu, M.; Wang, J.; Xie, Q.; Zhang, L. Bioprospecting microbial natural product libraries from the marine environment for drug discovery. J. Antibiot. 2010, 63, 415–422. [Google Scholar] [PubMed]
- Chen, J.W.; Wu, Q.H.; Rowley, D.C.; Al-Kareef, A.M.; Wang, H. Anticancer agent-based marine natural products and related compounds. J. Asian Nat. Prod. Res. 2015, 17, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, M.; Buck, K.N. The organic complexation of iron in the marine environment: A review. Front. Microbiol. 2012, 69, 117. [Google Scholar] [CrossRef] [PubMed]
- Vraspir, J.M.; Butler, A. Chemistry of marine ligands and siderophores. Ann. Rev. Mar. Sci. 2009, 1, 43–63. [Google Scholar] [PubMed]
- Butler, A. Marine siderophores and microbial iron mobilization. Biometals 2005, 18, 369–374. [Google Scholar]
- Paul, A.; Dubey, R. Characterization of protein involved in nitrogen fixation and estimation of cofactor. Intl. J. Adv. Biotech. Res. 2014, 5, 582–597. [Google Scholar]
- Wencewicz, T.A.; Long, T.E.; Mollmann, U.; Miller, M.J. Trihydroxamate siderophore-fluoroquinolone conjugates are selective sideromycin antibiotics that target Staphylococcus aureus. Bioconjugate Chem. 2013, 24, 473–486. [Google Scholar] [CrossRef]
- Schalk, I.J. A trojan-horse strategy including a bacterial suicide action for the efficient use of a specific Gram-positive antibiotic on Gram-negative bacteria. J. Med. Chem. 2018, 61, 3842–3844. [Google Scholar]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Page, M.G. Siderophore conjugates. Ann. N. Y. Acad. Sci. 2013, 1277, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Souto, A.; Montaos, M.A.; Balado, M.; Osorio, C.R.; Rodríguez, J.; Lemos, M.L.; Jiménez, C. Synthesis and antibacterial activity of conjugates between norfloxacin and analogues of the siderophore vanchrobactin. Bioorg. Med. Chem. 2013, 21, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Wencewicz, T.A.; Miller, M.J. Biscatecholate-monohydroxamate mixed ligand siderophore- carbacephalosporin conjugates are selective sideromycin antibiotics that target Acinetobacter baumannii. J. Med. Chem. 2013, 56, 4044–4052. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Hatef, A.; ul-haq, M.I.; Nair, N.; Unniappan, S.; Kizhakkedathu, J.N. Clinically approved iron chelators influence zebrafish mortality, hatching morphology and cardiac function. PloS ONE 2014, 9, e109880. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.L.; Kamino, K. Bacterial response to siderophore and quorum-sensing chemical signals in the seawater microbial community. Bmc Microbiol. 2001, 1, 1–11. [Google Scholar] [CrossRef]
- Popat, R.; Harrison, F.; Da, S.A.; Easton, S.A.; Mcnally, L.; Williams, P.; Diggle, S.P. Environmental modification via a quorum sensing molecule influences the social landscape of siderophore production. P. Roy. Soc. B Biol. Sci. 2017, 284, 20170200. [Google Scholar] [CrossRef]
- Lamont, I.L.; Beare, P.A.; Urs, O.; Vasil, A.I.; Vasil, M.L. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2002, 99, 7072–7077. [Google Scholar] [CrossRef]
- Baakza, A.; Vala, A.; Dave, B.; Dube, H. A comparative study of siderophore production by fungi from marine and terrestrial habitats. J. Exp. Mar. Biol. Ecol. 2004, 311, 1–9. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: a review. Environ. Sci. Pollut. Res. Int. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- Moriah, S.; Alison, B. Microbial iron acquisition: marine and terrestrial siderophores. Chem. Rev. 2009, 109, 4580–4595. [Google Scholar]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator-siderophore: a review. Microbiol. Res. 2018, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, J.M.; Akira, I.; Yusai, I.; Alison, B. Microbial tailoring of acyl peptidic siderophores. Biochemistry 2014, 53, 2624–2631. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.S.; Zhang, G.P.; Holt, P.D.; Jung, H.T.; Carrano, C.J.; Haygood, M.G.; Butler, A. Self-assembling amphiphilic siderophores from marine bacteria. Science 2000, 287, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.S.; Carter-Franklin, J.N.; Mann, E.L.; Martin, J.D.; Haygood, M.G.; Butler, A. Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proc. Natl. Acad. Sci. USA 2003, 100, 3754–3759. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, J.M.; Butler, A. Amino acid variability in the peptide composition of a suite of amphiphilic peptide siderophores from an open ocean Vibrio species. J. Biol. Inorg. Chem. 2013, 18, 489–497. [Google Scholar] [CrossRef]
- Walker, L.R.; Tfaily, M.M.; Shaw, J.B.; Hess, N.J.; Paša-Tolić, L.; Koppenaal, D.W. Unambiguous identification and discovery of bacterial siderophores by direct injection 21 Tesla Fourier transform ion cyclotron resonance mass spectrometry. Metallomics 2017, 9, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Gilis, A.; Khan, M.A.; Cornelis, P.; Meyer, J.M.; Mergeay, M.; Lelie, D.V.D. Siderophore-mediated iron uptake in Alcaligenes eutrophus CH34 and identification of aleB encoding the ferric iron-alcaligin E receptor. J. Bacterial. 1996, 178, 5499–5507. [Google Scholar] [CrossRef]
- Takahashi, A.; Nakamura, H.; Kameyama, T.; Kurasawa, S.; Naganawa, H.; Okami, Y.; Takeuchi, T.; Umezawa, H.; Iitaka, Y. Bisucaberin, a new siderophore, sensitizing tumor cells to macrophage-mediated cytolysis. II. Physico-chemical properties and structure determination. J. Antibiot. 1987, 40, 1671–1676. [Google Scholar] [CrossRef]
- Fujita, M.J.; Sakai, R. Production of avaroferrin and putrebactin by heterologous expression of a deep-sea metagenomic DNA. Mar. Drugs 2014, 12, 4799–4809. [Google Scholar] [CrossRef]
- Nadia, K.; Simon, A.; Song, L.J.; Daniel, O.C.; Gregory, L.C. Identification of a gene cluster that directs putrebactin biosynthesis in Shewanella species: PubC catalyzes cyclodimerization of N-hydroxy-N-succinylputrescine. J. Am. Chem. Soc. 2008, 130, 10458–10459. [Google Scholar]
- Moore, C.H.; Foster, L.; Gerbig, D.G.; Dyer, D.W.; Gibson, B.W. Identification of alcaligin as the siderophore produced by Bordetella pertussis and B. bronchiseptica. J. Bacteriol. 1995, 177, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, T.; Takahashi, A.; Kurasawa, S.; Ishizuka, M.; Okami, Y.; Takeuchi, T.; Umezawa, H. Bisucaberin, a new siderophore, sensitizing tumor cells to macrophage-mediated cytolysis. I. Taxonomy of the producing organism, isolation and biological properties. J. Antibiot. 1987, 40, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hayashi, K.I.; Ohmoto, H. Dissolution of iron hydroxides by marine bacterial siderophore. Chem. Geol. 2002, 184, 1–9. [Google Scholar] [CrossRef]
- Masaki, J.F.; Koji, N.; Ryuichi, S. Bisucaberin B, a linear hydroxamate class siderophore from the marine bacterium Tenacibaculum mesophilum. Molecules 2013, 18, 3917–3926. [Google Scholar]
- Zhang, F.; Barns, K.; Hoffmann, F.M.; Braun, D.R.; Andes, D.R.; Bugni, T.S. Thalassosamide, a siderophore discovered from the marine-derived bacterium, Thalassospira profundimaris. J. Nat. Prod. 2017, 80, 2551–2555. [Google Scholar] [CrossRef] [PubMed]
- Takehana, Y.; Umekita, M.; Hatano, M.; Kato, C.; Sawa, R.; Igarashi, M. Fradiamine A, a new siderophore from the deep-sea actinomycete Streptomyces fradiae MM456M-mF7. J. Antibiot. 2017, 70, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Deering, R.W.; Zhang, G.; Wang, B.; Li, X.; Sun, J.; Chen, J.; Zhang, H.; Rowley, D.C.; Wang, H. Albisporachelin, a new hydroxamate type siderophore from the deep ocean sediment-derived actinomycete Amycolatopsis albispora WP1T. Mar. Drugs 2018, 16, 199. [Google Scholar] [CrossRef] [PubMed]
- Ejje, N.; Soe, C.Z.; Gu, J.; Codd, R. The variable hydroxamic acid siderophore metabolome of the marine actinomycete Salinispora tropica CNB-440. Metallomics 2013, 5, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Green, D.H.; Küpper, F.C.; Carrano, C.J. Vibrioferrin, an unusual marine siderophore: iron binding, photochemistry, and biological implications. Inorg. Chem. 2009, 48, 11451–11458. [Google Scholar] [CrossRef]
- Nielsen, A.; Mansson, M.; Wietz, M.; Varming, A.N.; Phipps, R.K.; Larsen, T.O.; Gram, L.; Ingmer, H. Nigribactin, a novel siderophore from Vibrio nigripulchritudo, modulates Staphylococcus aureus virulence gene expression. Mar. Drugs 2012, 10, 2584–2589. [Google Scholar] [CrossRef]
- Soengas, R.G.; Anta, C.; Espada, A.; Paz, V.; Ares, I.R.; Balado, M.; Rodríguez, J.; Lemos, M.L.; Jiménez, C. Structural characterization of vanchrobactin, a new catechol siderophore produced by the fish pathogen Vibrio anguillarum serotype O2. Tetrahedron Lett. 2006, 47, 7113–7116. [Google Scholar] [CrossRef]
- Balado, M.; Osorio, C.R.; Lemos, M.L. Biosynthetic and regulatory elements involved in the production of the siderophore vanchrobactin in Vibrio anguillarum. Microbiol. 2008, 154, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Balado, M.; Osorio, C.R.; Lemos, M.L. A gene cluster involved in the biosynthesis of vanchrobactin, a chromosome-encoded siderophore produced by Vibrio anguillarum. Microbiol. 2006, 152, 3517–3528. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, E.; Brandariz, I.; Jiménez, C.; Soengas, R.G. Iron(III) complexation by vanchrobactin, a siderophore of the bacterial fish pathogen Vibrio anguillarum. Metallomics 2011, 3, 521–528. [Google Scholar] [CrossRef]
- Moriah, S.; Andrew, H.; John, B.; Murray, M.; Margo, H.; Alison, B. Vanchrobactin and anguibactin siderophores produced by Vibrio sp. DS40M4. J. Nat. Prod. 2010, 73, 1038–1043. [Google Scholar]
- Han, A.W.; Sandy, M.; Fishman, B.; Trindade-Silva, A.E.; Soares, C.A.G.; Distel, D.L.; Butler, A.; Haygood, M.G. Turnerbactin, a novel triscatecholate siderophore from the shipworm endosymbiont Teredinibacter turnerae T7901. PLoS ONE 2013, 8, e76151. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Kanoh, K.; Jang, J.H.; Adachi, K.; Matsuda, S.; Miki, O.; Kato, T.; Shizuri, Y. Streptobactin, a tricatechol-type siderophore from marine-derived Streptomyces sp. YM5-799. J. Nat. Prod. 2011, 74, 2371–2376. [Google Scholar] [CrossRef]
- Capon, R.J.; Stewart, M.; Ratnayake, R.; Lacey, E.; Gill, J.H. Citromycetins and Bilains A-C: new aromatic polyketides and diketopiperazines from Australian marine-derived and terrestrial Penicillium spp. J. Nat. Prod. 2007, 70, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Ki, D.W.; Kim, S.E.; Yeom, J.H.; Kim, Y.S.; Yun, B.S. Pistillarin salt, a dicatecholspermidine family member from Gomphus floccosus, inhibits DNA single strand breakage by the fenton reaction. J. Korean Soc. Appl. Biol. 2011, 54, 312–315. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Homann, V.V.; Sandy, M.; Tincu, J.A.; Templeton, A.S.; Tebo, B.M.; Butler, A. Loihichelins A-F, a suite of amphiphilic siderophores produced by the marine bacterium Halomonas LOB-5. J. Nat. Prod. 2009, 72, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Vraspir, J.M.; Holt, P.D.; Butler, A. Identification of new members within suites of amphiphilic marine siderophores. Biometals 2011, 24, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Andrew, W.; Nicholas, G.; Logan, W.; Hebelin, C.; Brad, H.; Douglas, H.; Russell, G. Isolation of imaqobactin, an amphiphilic siderophore from the Arctic marine bacterium Variovorax Species RKJM285. J. Nat. Prod. 2018, 81, 858–865. [Google Scholar]
- Haygood, M.G.; Holt, P.D.; Butler, A. Aerobactin production by a planktonic marine Vibrio sp. Limnol. Oceanogr. 1993, 38, 1091–1097. [Google Scholar] [CrossRef]
- Fuse, H.; Murakami, K.; Takimura, O.; Kamimura, K.; Yamaoka, Y. Phylogenetic analysis of marine environmental strains of Vibrio that produce aerobactin. J. Mar. Biotechnol. 1998, 6, 76–79. [Google Scholar]
- Martin, J.D.; Ito, Y.; Homann, V.V.; Haygood, M.G.; Butler, A. Structure and membrane affinity of new amphiphilic siderophores produced by Ochrobactrum sp. SP18. J. Biol. Inorg. Chem. 2006, 11, 633–641. [Google Scholar] [CrossRef]
- Boiteau, R.M.; Repeta, D.J. An extended siderophore suite from Synechococcus sp. PCC 7002 revealed by LC-ICPMS-ESIMS. Metallomics 2015, 7, 877–884. [Google Scholar] [CrossRef]
- Barbeau, K.; Zhang, G.; Live, D.H.; Butler, A. Petrobactin, a photoreactive siderophore produced by the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus. J. Am. Chem. Soc. 2002, 33, 378–379. [Google Scholar] [CrossRef]
- Hickford, S.J.; Kupper, F.G.; Carrano, C.J.; Blunt, J.W.; Butler, A. Petrobactin sulfonate, a new siderophore produced by the marine bacterium Marinobacter hydrocarbonoclasticus. J. Nat. Prod. 2004, 67, 1897–1899. [Google Scholar] [CrossRef]
- Lee, J.Y.; Janes, B.K.; Passalacqua, K.D.; Pfleger, B.F.; Bergman, N.H.; Liu, H.; Håkansson, K.; Somu, R.V.; Aldrich, C.C.; Cendrowski, S.; et al. Biosynthetic analysis of the petrobactin siderophore pathway from Bacillus anthracis. J. Bacteriol. 2007, 189, 1698–1710. [Google Scholar] [CrossRef]
- Holt, P.D.; Reid, R.R.; Lewis, B.L.; Luther, G.W.; Alison, B. Iron(III) coordination chemistry of alterobactin A: a siderophore from the marine bacterium Alteromonas luteoviolacea. Inorg. Chem. 2005, 44, 7671–7677. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.T.; Livet, D.H.; Faulkner, D.J.; Butler, A. A siderophore from a marine bacterium with an exceptional ferric ion affinity constant. Nature 1993, 366, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Kaneo, K.; Kei, K.; Guan, L.; Kyoko, A.; Yoshikazu, S. Pseudoalterobactin A and B, new siderophores excreted by marine bacterium Pseudoalteromonas sp. KP20-4. J. Antibiot. 2004, 35, 871–875. [Google Scholar]
- Hannah, K.; Alison, B. Isolation, structure elucidation, and iron-binding properties of lystabactins, siderophores isolated from a marine Pseudoalteromonas sp. J. Nat. Prod. 2013, 76, 648–654. [Google Scholar]
- Souto, A.; Montaos, M.A.; Rivas, A.J.; Balado, M.; Osorio, C.R.; Rodríguez, J.; Lemos, M.L.; Jiménez, C. Structure and biosynthetic assembly of piscibactin, a siderophore from Photobacterium damselae subsp. piscicida, predicted from genome analysis. Eur. J. Org. Chem. 2012, 29, 5693–5700. [Google Scholar]
- Balado, M.; Benzekri, H.; Labella, A.M.; Claros, M.G.; Manchado, M.; Borrego, J.J.; Osorio, C.R.; Lemos, M.L. Genomic analysis of the marine fish pathogen Photobacterium damselae subsp. piscicida: Insertion sequences proliferation is associated with chromosomal reorganisations and rampant gene decay. Infect. Genet. Evol. 2017, 54, 221–229. [Google Scholar] [PubMed]
- Balado, M.; Lages, M.A.; Fuentes-Monteverde, J.C.; Martinez-MatamorosJaime, D.; Rodriguez, J.; Jiménez, C.; Lemos, M.L. The siderophore piscibactin is a relevant virulence factor for vibrio anguillarum favored at low temperatures. Front. Microbiol. 2018, 9, 1766–1781. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shang, F.; Xi, L.; Huang, Y. Tetroazolemycins A and B, two new oxazole-thiazole siderophores from deep-sea Streptomyces olivaceus FXJ8.012. Mar. Drugs 2013, 11, 1524–1533. [Google Scholar] [CrossRef]
- Kadi, N.; Oves-Costales, D.; Barona-Gomez, F.; Challis, G.L. A new family of ATP-dependent oligomerization-macrocyclization biocatalysts. Nat. Chem. Biol. 2007, 3, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.J.; Kimura, N.; Yokose, H.; Otsuka, M. Heterologous production of bisucaberin using a biosynthetic gene cluster cloned from a deep sea metagenome. Mol. Biosyst. 2012, 8, 482–485. [Google Scholar] [CrossRef]
- Fujita, M.J.; Nobutada, K.; Atsushi, S.; Yoichi, I.; Tomohiro, H.; Masami, O. Cloning and heterologous expression of the vibrioferrin biosynthetic gene cluster from a marine metagenomic library. Biosci. Biotech. Bioch. 2011, 75, 2283–2287. [Google Scholar] [CrossRef] [PubMed]
- Naka, H.; Liu, M.; Actis, L.A.; Crosa, J.H. Plasmid- and chromosome-encoded siderophore anguibactin systems found in marine vibrios: biosynthesis, transport and evolution. Biometals 2013, 26, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Alice, A.F.; Lopez, C.S.; Crosa, J.H. Plasmid- and chromosome-encoded redundant and specific functions are involved in biosynthesis of the siderophore anguibactin in Vibrio anguillarum 775: a case of chance and necessity? J. Bacteriol. 2005, 187, 2209–2214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Q.; Ma, Y.; Zhou, L.; Zhang, Y. Gene cloning, expression and functional characterization of a phosphopantetheinyl transferase from Vibrio anguillarum serotype O1. Arch. Microbiol. 2005, 183, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.D.; Stork, M.; Crosa, J.H. Genetic and biochemical analyses of chromosome and plasmid gene homologues encoding ICL and ArCP domains in Vibrio anguillarum strain 775. Biometals 2011, 24, 629–643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Welch, T.J.; Chai, S.; Crosa, J.H. The overlapping angB and angG genes are encoded within the trans-acting factor region of the virulence plasmid in Vibrio anguillarum: essential role in siderophore biosynthesis. J. Bacteriol. 2000, 182, 6762–6773. [Google Scholar] [CrossRef] [PubMed]
- Naka, H.; Lopez, C.S.; Crosa, J.H. Reactivation of the vanchrobactin siderophore system of Vibrio anguillarum by removal of a chromosomal insertion sequence originated in plasmid pJM1 encoding the anguibactin siderophore system. Environ. Microbiol. 2008, 10, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.D.; Poppelaars, S.; Stork, M.; Nagasawa, M.; Tolmasky, M.E.; Crosa, J.H. A nonribosomal peptide synthetase with a novel domain organization is essential for siderophore biosynthesis in Vibrio anguillarum. J. Bacteriol. 2004, 186, 7327–7336. [Google Scholar] [CrossRef]
- Tolmasky, M.E.; Actis, L.A.; Crosa, J.H. A histidine decarboxylase gene encoded by the Vibrio anguillarum plasmid pJM1 is essential for virulence: histamine is a precursor in the biosynthesis of anguibactin. Mol. Microbiol. 1995, 15, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, G.; Muckenthaler, M.; Galy, B.; Hentze, M.W.; Pantopoulos, K. Iron-mediated degradation of Irp2, an unexpected pathway involving a 2-oxoglutarate-dependent oxygenase activity. Mol. Cell. Biol. 2004, 24, 954–965. [Google Scholar] [CrossRef]
- Macheroux, P.; Plattner, H.J.; Romaguera, A.; Diekmann, H. FAD and substrate analogs as probes for lysine N6-hydroxylase from Escherichia coli EN 222. Eur. J. Biochem. 1993, 213, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Coy, M.; Paw, B.H.; Bindereif, A.; Neilands, J.B. Isolation and properties of N epsilon-hydroxylysine: acetyl coenzyme A N epsilon-transacetylase from Escherichia coli pABN11. Biochemistry 1986, 25, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.C.; Alexander, E.; Rice, M.R.; Drake, E.J.; Mydy, L.S.; Aldrich, C.C.; Gulick, A.M. Structural and functional delineation of aerobactin biosynthesis in hypervirulent Klebsiella pneumoniae. J. Biol. Chem. 2018, 293, 7841–7852. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, B.F.; Kim, Y.; Nusca, T.D.; Maltseva, N.; Lee, J.Y.; Scaglione, J.B.; Janes, B.K.; Anderson, E.C.; Bergman, N.H.; Hanna, P.C.; et al. Structural and functional analysis of AsbF: origin of the stealth 3,4-dihydroxybenzoic acid subunit for petrobactin biosynthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 17133–17138. [Google Scholar] [CrossRef] [PubMed]
- Ovescostales, D.; Kadi, N.; Fogg, M.J.; Song, L.; Wilson, K.S.; Challis, G.L. Petrobactin biosynthesis: AsbB catalyzes condensation of spermidine with N8-citryl-spermidine and its N1-(3,4-dihydroxybenzoyl) derivative. Chem. Commun. 2008, 66, 4034–4036. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, R.J.; Mcmanis, J.S.; Perumal, P.T.; Algee, S.E. The total synthesis of alcaligin. J. Org. Chem. 1991, 56, 5560–5563. [Google Scholar] [CrossRef]
- Soengas, R.G.; Anta, C.; Espada, A.; Nieto, R.M.; Larrosa, M.; Rodríguez, J.; Jiménez, C. Vanchrobactin: absolute configuration and total synthesis. Tetrahedron Lett. 2007, 48, 3021–3024. [Google Scholar] [CrossRef]
- Soengas, R.G.; Larrosa, M.; Balado, M.; Rodriguez, J.; Lemos, M.L.; Jimenez, C. Synthesis and biological activity of analogues of vanchrobactin, a siderophore from Vibrio anguillarum serotype O2. Org. Biomol. Chem. 2008, 6, 1278–1287. [Google Scholar] [CrossRef]
- Pandey, R.K.; Jarvis, G.G.; Low, P.S. Efficient synthesis of the siderophore petrobactin via antimoy triethoxide mediated coupling. Tetrahedron Lett. 2012, 53, 1627–1629. [Google Scholar] [CrossRef]
- Bugdahn, N.; Oberthür, M. Syntheses and iron binding affinities of the Bacillus anthracis siderophore petrobactin and sidechain-modified analogues. Eur. J. Org. Chem. 2014, 426–435. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed]

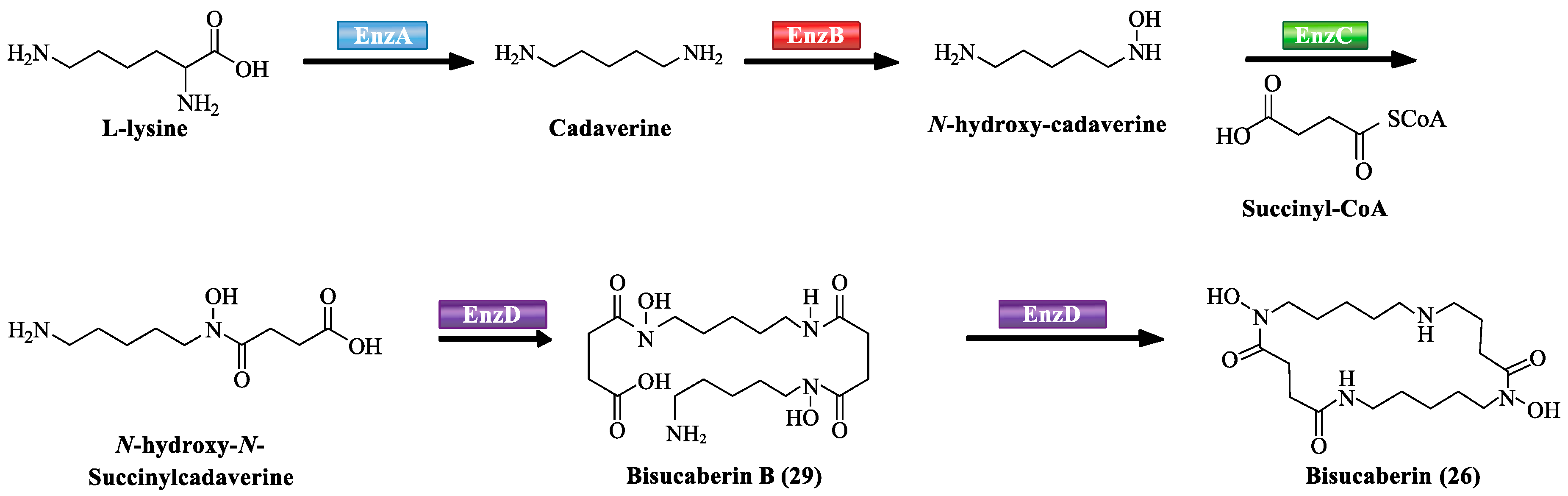
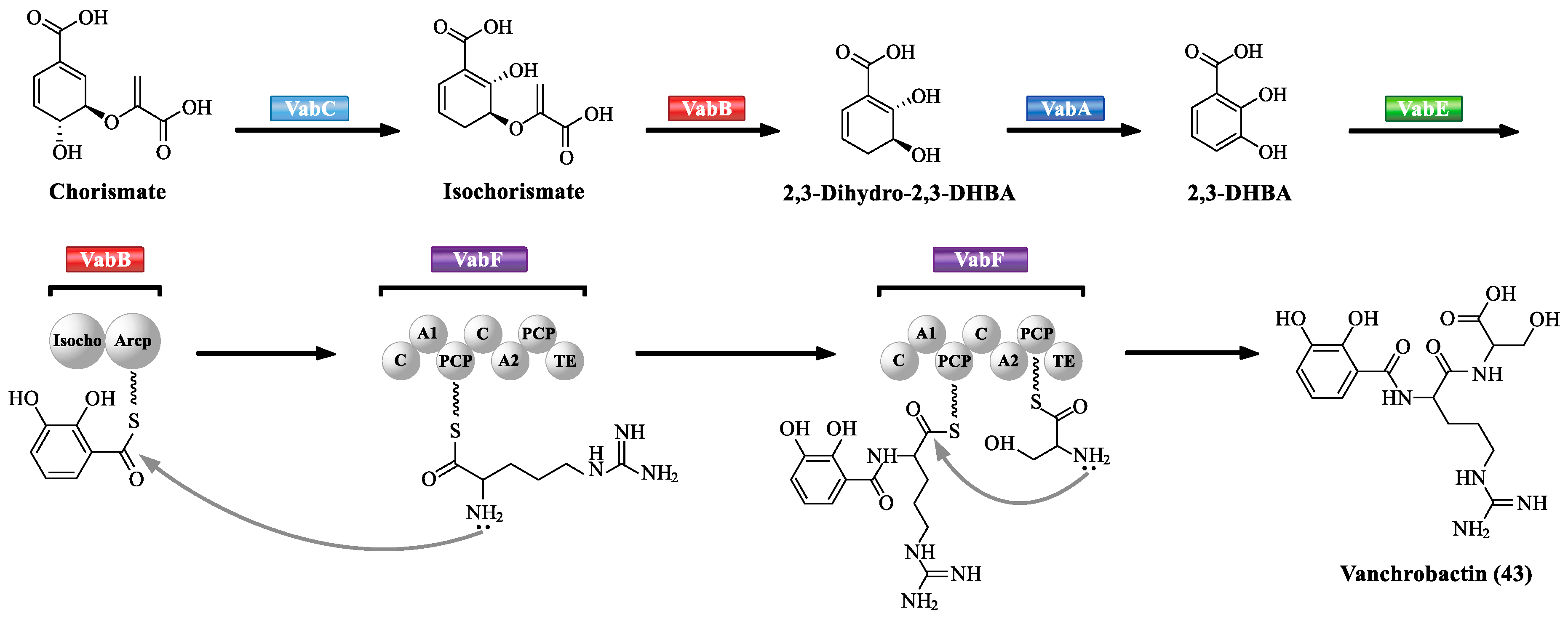
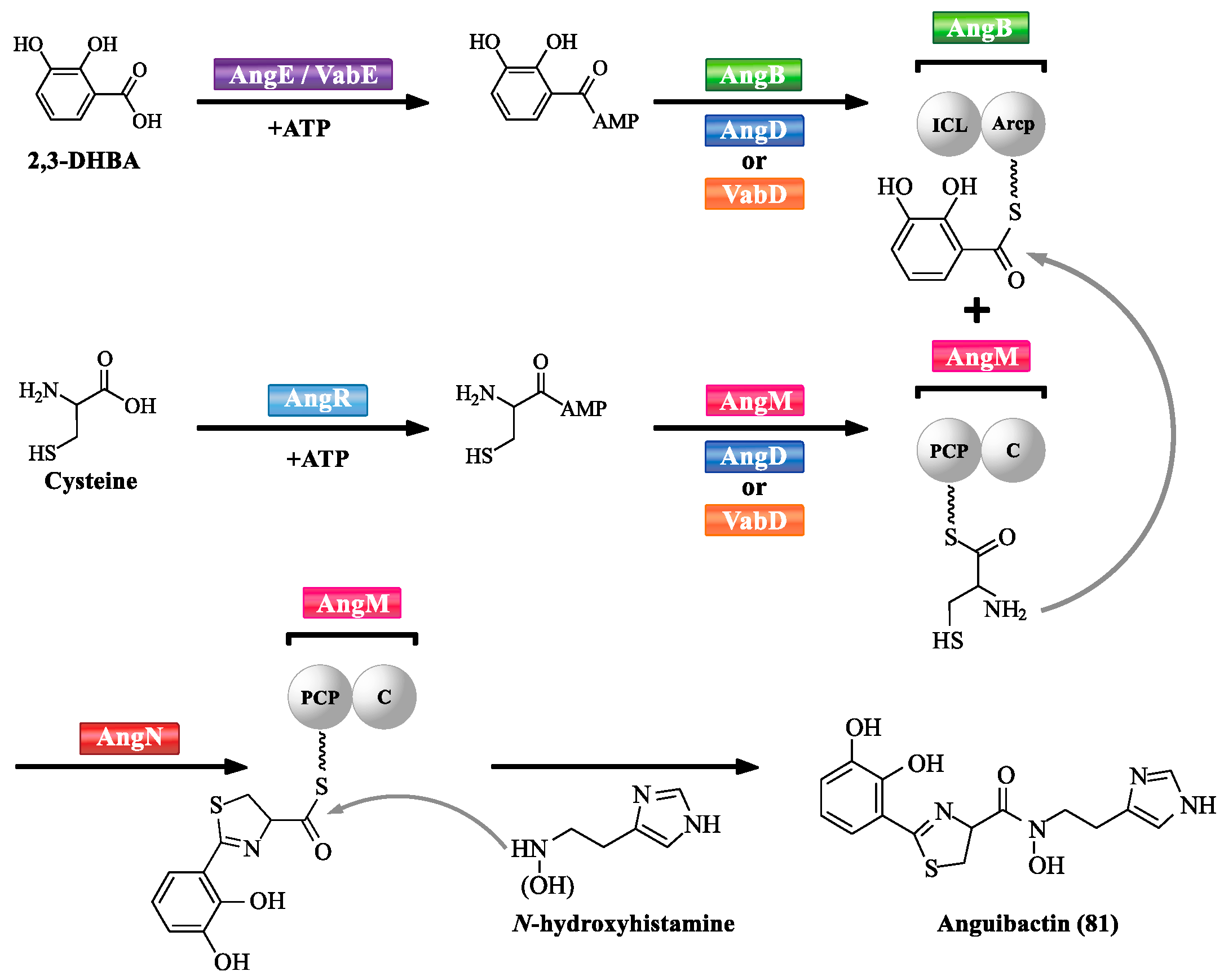
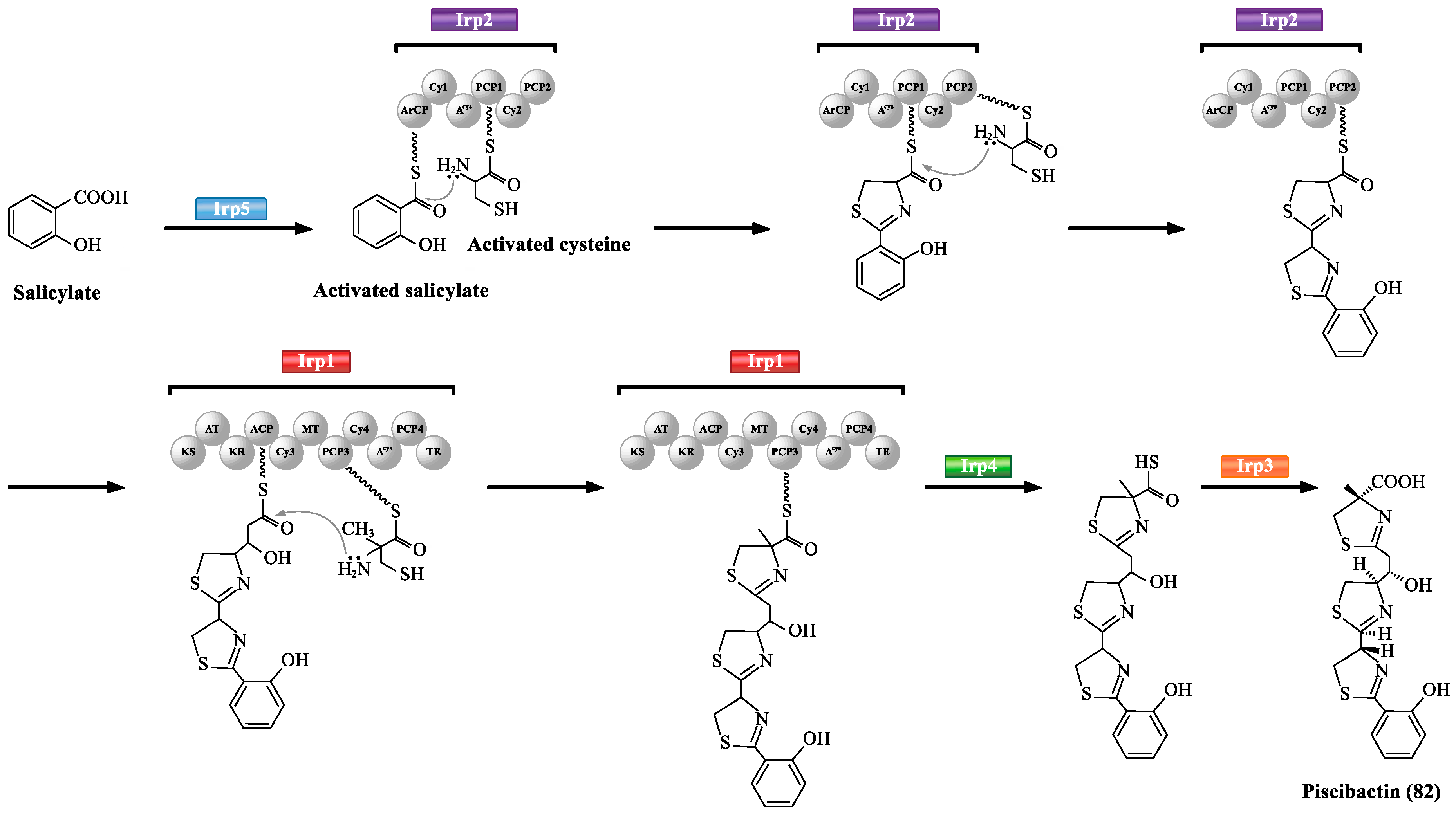

| Abbreviation | Full Name |
|---|---|
| CAS | Chrome azurol sulfonate |
| ED50 | Median effective dose |
| IC50 | Hablalf maximal inhibition concentration |
| NRPSs | Nonribosomal peptide synthetases |
| HSC | N-hydroxy-N-succinylcadaverine |
| HSDs | N-hydroxy-N-succinyl diamines |
| ArCP | Aryl carrier protein |
| Cy | Cyclisation |
| PCP | Peptidyl carrier protein |
| NIS | NRPS-independent siderophore |
| hLys | N6-hydroxy-l-lysine |
| ahLys | N6-acetyl-N6-hydroxy-l-lysine |
| 3-DHS | 3-dehydroshikimate |
| 3,4-DHBA | 3,4-dihydroxybenzoic acid |
| 2,3-DHBA | 2,3-dihydroxybenzoic acid |
| Enzymes | EC Numbers |
|---|---|
| EnzA | 4.1.1.7 |
| VabC | 3.1.22.4 |
| VabD | 2.7.8.7 |
| VabF | 3.2.1.55 |
| Irp1 | 2.3.2.27 4.2.1.3 |
| Irp2 | 2.3.2.27 |
| Irp3 | 2.3.2.27 |
| Irp4 | 2.3.2.27 |
| AngB | 3.3.2.1 |
| AngE | 1.14.14.148 |
| VabD | 2.7.8.7 |
| IucA | 6.3.2.38 |
| IucC | 6.3.2.39 |
| IucD | 1.14.13.59 |
| AsbF | 4.2.1.118 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Guo, Y.; Lu, Y.; Wang, B.; Sun, J.; Zhang, H.; Wang, H. Chemistry and Biology of Siderophores from Marine Microbes. Mar. Drugs 2019, 17, 562. https://doi.org/10.3390/md17100562
Chen J, Guo Y, Lu Y, Wang B, Sun J, Zhang H, Wang H. Chemistry and Biology of Siderophores from Marine Microbes. Marine Drugs. 2019; 17(10):562. https://doi.org/10.3390/md17100562
Chicago/Turabian StyleChen, Jianwei, Yuqi Guo, Yaojia Lu, Bixia Wang, Jiadong Sun, Huawei Zhang, and Hong Wang. 2019. "Chemistry and Biology of Siderophores from Marine Microbes" Marine Drugs 17, no. 10: 562. https://doi.org/10.3390/md17100562
APA StyleChen, J., Guo, Y., Lu, Y., Wang, B., Sun, J., Zhang, H., & Wang, H. (2019). Chemistry and Biology of Siderophores from Marine Microbes. Marine Drugs, 17(10), 562. https://doi.org/10.3390/md17100562







