UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs)
Abstract
1. Introduction
2. Results
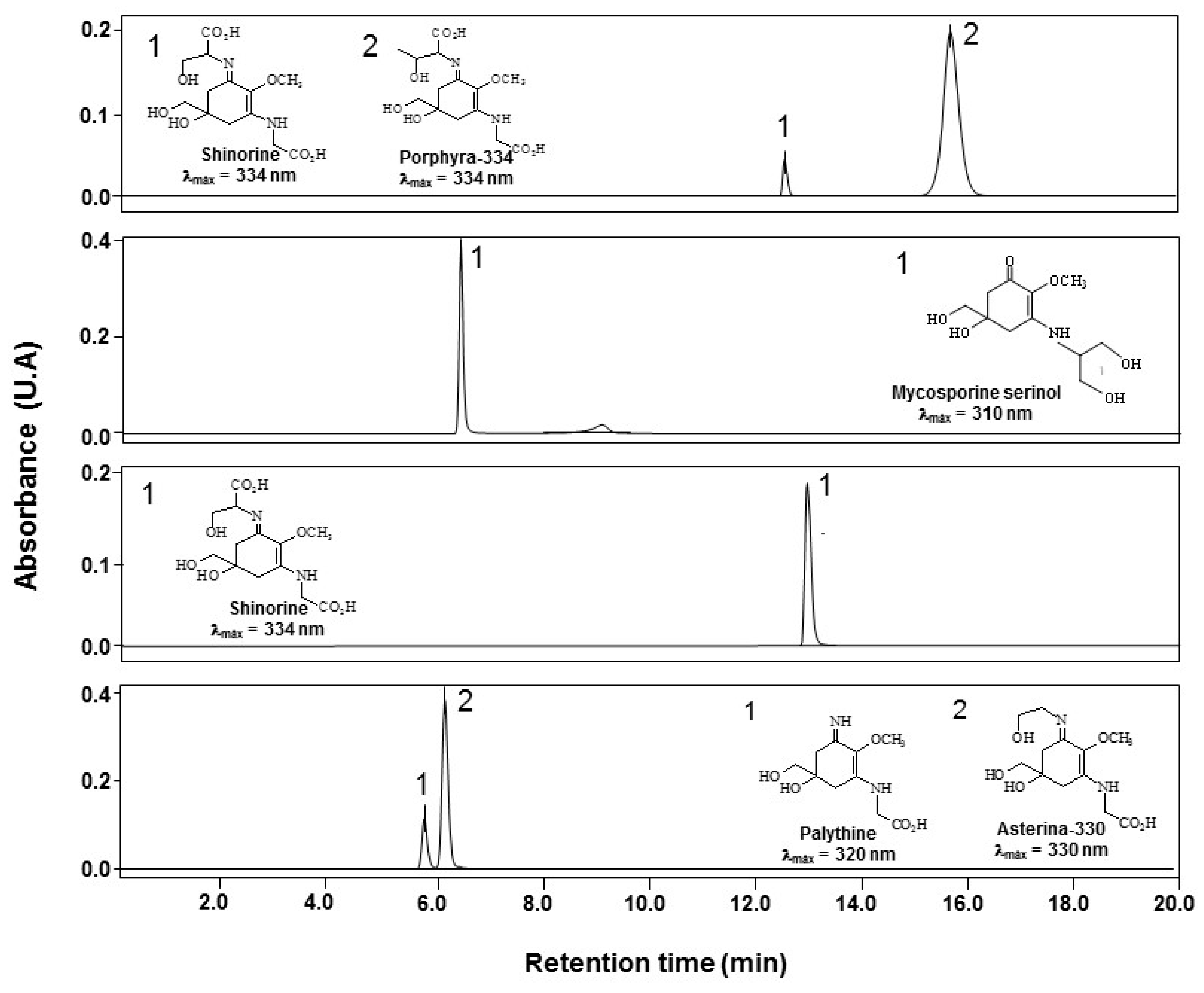
2.1. Isolation and Purification of MAAs
2.2. Influence of pH and Temperature on MAAs Stability
2.3. Galenic Formula
2.4. SPF and BEPFs Determinations
3. Discussion
4. Materials and Methods
4.1. Isolation, Identification and Characterization of MAAs
4.2. pH MAAs Stability Determination
4.3. Temperature and pH MAAs Stability Determination
4.4. Galenic Formulations
4.5. In vitro Sun Protection Factor (SPF) Determination
4.6. Biological Effective Protection Factors (BEPFs)
4.7. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Young, A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ananthaswamy, H.N. Short-term and long-term cellular and molecular events following UV irradiation of skin: Implications for molecular medicine. Expert Rev. Mol. Med. 2002, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Fernandez, B.O.; Hamilton, A.; Lang, N.N.; Gallagher, J.M.C.; Newby, D.E.; Feelisch, M.; Weller, R.B. UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase. J. Investig. Dermatol. 2014, 134, 1839–1846. [Google Scholar] [CrossRef]
- Johnson, R.S.; Titze, J.; Weller, R. Cutaneous control of blood pressure. Curr. Opin. Nephrol. Hypertens. 2016, 25, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.; Seo, I.; Liebel, F.; Southall, M.D.; Kollias, N.; Ruvolo, E. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS ONE 2015, 10, e0130949. [Google Scholar] [CrossRef] [PubMed]
- Sklar, L.R.; Almutawa, F.; Lim, H.W.; Hamzavi, I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: A review. Photochem. Photobiol. Sci. 2013, 12, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.; Lademann, J.; Darvin, M.E.; Stege, H.; Marks, C.; Bruhnke, S.; Krutmann, J. Infrared radiation-induced matrix metalloproteinase in human skin: Implications for protection. J. Investig. Dermatol. 2008, 128, 2491–2497. [Google Scholar] [CrossRef]
- Lim, H.W.; Arellano-Mendoza, M.-I.; Stengel, F. Current challenges in photoprotection. J. Am. Acad. Dermatol. 2017, 76, S91–S99. [Google Scholar] [CrossRef]
- Rundel, R.D. Action spectra and estimation of biologically effective UV radiation. Physiol. Plant. 1983, 58, 360–366. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 2443: 2012—Determination of Sunscreen UVA Photoprotection In Vitro; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Setlow, R.B. The wavelengths in sunlight effective in producing skin cancer: A theoretical analysis. Proc. Natl. Acad. Sci. USA 1974, 71, 3363–3366. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R.; Van der Leun, J.C. Estimate of the wavelength dependency of ultraviolet carcinogenesis in humans and its relevance to the risk assessment of a stratospheric ozone depletion. Health Phys. 1994, 67, 319–325. [Google Scholar] [CrossRef] [PubMed]
- De Fabo, E.; Noonan, F. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J. Exp. Med. 1983, 158, 84–98. [Google Scholar] [CrossRef] [PubMed]
- McLoone, P.; Simics, E.; Barton, A.; Norval, M.; Gibbs, N.K. An action spectrum for the production of cis-urocanic acid in human skin in vivo. J. Investig. Dermatol. 2005, 124, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.M.; Simon, J.D. Epidermal trans-urocanic acid and the UV-A-induced photoaging of the skin. Proc. Natl. Acad. Sci. USA 1998, 95, 10576–10578. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Hannon, D.P.; Orr, T.V. Wavelength dependence of histological, physical, and visible changes in chronically UV-irradiated hairless mouse skin. Photochem. Photobiol. 1989, 50, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.R.; Campos, P.M. Evaluation of the photostability of different UV filter combinations in a sunscreen. Int. J. Pharm. 2006, 307, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.R.; Camargo, F.B.; Gianeti, M.D.; Campos, P.M. Evaluation of dermatological effects of cosmetic formulations containing Saccharomyces cerevisiae extract and vitamins. Food Chem. Toxicol. 2008, 46, 3493–3500. [Google Scholar] [CrossRef]
- Kawakami, C.M.; Gaspar, L.R. Mangiferin and naringenin affect the photostability and phototoxicity of sunscreens containing avobenzone. J. Photochem. Photobiol. B Biol. 2015, 151, 239–247. [Google Scholar] [CrossRef]
- Krause, M.; Klit, A.; Blomberg Jensen, M.; Søeborg, T.; Frederiksen, H.; Schlumpf, M.; Lichtensteiger, W.; Skakkebaek, N.E.; Drzewiecki, K.T. Sunscreens: Are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int. J. Androl. 2012, 35, 424–436. [Google Scholar] [CrossRef]
- Sarveiya, V.; Risk, S.; Benson, H.A.E. Liquid chromatographic assay for common sunscreen agents: Application to in vivo assessment of skin penetration and systemic absorption in human volunteers. J. Chromatogr. B 2004, 803, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of sunscreens. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 91–110. [Google Scholar] [CrossRef]
- Morabito, K.; Shapley, N.C.; Steeley, K.G.; Tripathi, A. Review of sunscreen and the emergence of non-conventional absorbers and their applications in ultraviolet protection. Int. J. Cosmet. Sci. 2011, 33, 385–390. [Google Scholar] [CrossRef]
- Schauder, S.; Ippen, H. Contact and photocontact sensitivity to sunscreens: Review of a 15-year experience and of the literature. Contact Dermatitis 1997, 37, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Greenspoon, J.; Ahluwalia, R.; Juma, N.; Rosen, C.F. Allergic and photoallergic contact dermatitis: A 10-year experience. Dermatitis 2013, 24, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Navarro, N.; Figueroa, F.L.; Korbee, N.; Bonomi, J.; Álvarez-Gómez, F.; De la Coba, F. Mycosporine-Like Amino Acids from Red Algae to Develop Natural UV Sunscreens. In Sunscreens: Source, Formulations, Efficacy and Recommendations; Ragesh, P.R., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2018; pp. 99–129. ISBN 9781631172557. [Google Scholar]
- Pan, Z.; Lee, W.; Slutsky, L.; Clark, R.A.F.; Pernodet, N.; Rafailovich, M.H. Adverse effects of titanium dioxide nanoparticles on human dermal fibroblasts and how to protect cells. Small 2009, 5, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Zenker, A.; Schmutz, H.; Fent, K. Simultaneous trace determination of nine organic UV-absorbing compounds (UV filters) in environmental samples. J. Chromatogr. A 2008, 1202, 64–74. [Google Scholar] [CrossRef]
- Fent, K.; Zenker, A.; Rapp, M. Widespread occurrence of estrogenic UV-filters in aquatic ecosystems in Switzerland. Environ. Pollut. 2010, 158, 1817–1824. [Google Scholar] [CrossRef]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441. [Google Scholar] [CrossRef]
- Balmer, M.E.; Buser, H.-R.; Müller, M.D.; Poiger, T. Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from Swiss lakes. Environ. Sci. Technol. 2005, 39, 953–962. [Google Scholar] [CrossRef]
- Buser, H.-R.; Balmer, M.E.; Schmid, P.; Kohler, M. Occurrence of UV filters 4-methylbenzylidene camphor and octocrylene in fish from various Swiss rivers with inputs from wastewater treatment plants. Environ. Sci. Technol. 2006, 40, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Alonso, M.B.; Bertozzi, C.P.; Marigo, J.; Barbosa, L.; Cremer, M.; Secchi, E.R.; Azevedo, A.; Lailson-Brito, J., Jr.; Torres, J.P.M. First determination of UV filters in marine mammals. Octocrylene levels in Franciscana dolphins. Environ. Sci. Technol. 2013, 47, 5619–5625. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Sieratowicz, A.; Zielke, H.; Oetken, M.; Hollert, H.; Oehlmann, J. Ecotoxicological effect characterisation of widely used organic UV filters. Environ. Pollut. 2012, 163, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Sunscreens as a source of hydrogen peroxide production in coastal waters. Environ. Sci. Technol. 2014, 48, 9037–9042. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Cho, J.-K.; Mok, J.Y.; Jeon, I.H.; Kim, H.S.; Kang, H.J.; Jang, S. Il Protective effect of astragalin and quercetin on ultraviolet (UV)-irradiated damage in HaCaT cells and Balb/c mice. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 443–446. [Google Scholar] [CrossRef]
- Wei, H.; Saladi, R.; Lu, Y.; Wang, Y.; Palep, S.R.; Moore, J.; Phelps, R.; Shyong, E.; Lebwohl, M.G. Isoflavone genistein: Photoprotection and clinical implications in dermatology. J. Nutr. 2003, 133, 3811S–3819S. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2000, 56, 139–144. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M.; Sandra, C.M.; Carlos, M.P. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem. Photobiol. Sci. 2004, 3, 960–967. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Alonso-Varona, A.; Palomares, T.; Zubillaga, V.; Labidi, J.; Bulone, V. Exploiting mycosporines as natural molecular sunscreens for the fabrication of UV-absorbing green materials. ACS Appl. Mater. Interfaces 2015, 7, 16558–16564. [Google Scholar] [CrossRef]
- De La Coba, F.; Aguilera, J.; Figueroa, F.L.; De Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 105–114. [Google Scholar] [CrossRef]
- De la Coba, F. Evaluación de la capacidad fotoprotectora y antioxidante de aminoácidos tipo micosporina. Aplicaciones biotecnológicas; Universidad de Málaga. Servicio de publicaciones: Málaga, Spain, 2007. [Google Scholar]
- Torres, A.; Enk, C.D.; Hochberg, M.; Srebnik, M. Porphyra-334, a potential natural source for UVA protective sunscreens. Photochem. Photobiol. Sci. 2006, 5, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- De la Coba-Luque, F.; Aguilera-Arjona, J.; López-Figueroa, F. Uso de Aminoácido Tipo Micosporina (porfira 334) en Productos Para Prevención y Tratamiento de Eritema Actínico, Fotocarcinogénesis y Fotoenvejecimiento. ES Patent 2,301,435 B1, 4 April 2009. [Google Scholar]
- De la Coba-Luque, F.; Aguilera-Arjona, J.; López-Figueroa, F. Uso de una Mezcla Purificada de Aminoácidos Tipo Micosporina (Asterina 330 + Palitina) en Productos para Prevención y Tratamiento de Eritema actínico, Fotocarcinogénesis y Fotoenvejecimiento. ES Patent 2,303,487 B1, 7 May 2009. [Google Scholar]
- De la Coba-Luque, F.; Aguilera-Arjona, J.; López-Figueroa, F. Uso de una Mezcla Purificada de Aminoácidos Tipos Micosporina (Asterina 330 + Palitina) en la Prevención de la Oxidación de Productos Cosméticos y Farmacéuticos 2009. ES Patent 2,307,438 B1, 18 August 2009. [Google Scholar]
- De la Coba-Luque, F.; Aguilera-Arjona, J.; López-Figueroa, F. Uso de Aminoácido Tipos Micosporina (Porfira 334) en la Prevención de la Oxidación de Productos Cosméticos y Farmacéuticos 2009. ES Patent 2,301,437 B1, 15 April 2009. [Google Scholar]
- De la Coba-Luque, F.; Aguilera-Arjona, J.; López-Figueroa, F. Uso de Aminoácido Tipo Micosporina (Shinorine) en la Prevención de la Oxidación de Productos Cosméticos y Farmacéuticos 2009. ES Patent 2,301,428 B1, 15 April 2009. [Google Scholar]
- López-Figueroa, F.; Aguilera Arjona, J.; de la Coba-Luque, F.; Korbee Peinado, N. Composición para Protección solar a Base de Extractos de Algas y Líquenes. ES Patent 2,317,741 A1, 16 April 2009. [Google Scholar]
- Diffey, B.L.; Tanner, P.R.; Matts, P.J.; Nash, J.F. In vitro assessment of the broad-spectrum ultraviolet protection of sunscreen products. J. Am. Acad. Dermatol. 2000, 43, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- COLIPA. Guideline for the Colorimetric Determination of Skin Colour Typing and Prediction of the Minimal Erythemal Dose (MED) without UV Exposure; The European Cosmetic and Perfumery Association: Brussels, Belgium, 2007. [Google Scholar]
- COLIPA. In Vitro Method for the Determination of the UVA Protection Factor and “Critical Wavelength” Values of Sunscreen Products; The European Cosmetic and Perfumery Association: Brussels, Belgium, 2011. [Google Scholar]
- Barceló-Villalobos, M.; Figueroa, F.L.; Korbee, N.; Álvarez-Gómez, F.; Abreu, M.H. Production of Mycosporine-Like Amino Acids from Gracilaria vermiculophylla (Rhodophyta) Cultured Through One Year in an Integrated Multi-trophic Aquaculture (IMTA) System. Mar. Biotechnol. 2017, 19, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–172. [Google Scholar] [CrossRef]
- Wolf, R.; Tüzün, B.; Tüzün, Y. Sunscreens. In Dermatologic Therapy; Wiley Online Library: Hoboken, NJ, USA, 2001; pp. 208–2014. [Google Scholar]
- Schmid, D.; Schürch, C.; Zülli, F. Mycosporine-like amino acids from red algae protect against premature skin-aging. Eur. Cosmet. 2006, 9, 1–4. [Google Scholar]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-like Amino Acids for Skin Photoprotection. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kullavanijaya, P.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2005, 52, 937–958. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Greci, L.; Parsons, R.; Knowland, J. Nitroxide radicals protect DNA from damage when illuminated in vitro in the presence of dibenzoylmethane and a common sunscreen ingredient. Free Radic. Biol. Med. 1999, 26, 809–816. [Google Scholar] [CrossRef]
- Armeni, T.; Damiani, E.; Battino, M.; Greci, L.; Principato, G. Lack of in vitro protection by a common sunscreen ingredient on UVA-induced cytotoxicity in keratinocytes. Toxicology 2004, 203, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Dondi, D.; Albini, A.; Serpone, N. Interactions between different solar UVB/UVA filters contained in commercial suncreams and consequent loss of UV protection. Photochem. Photobiol. Sci. 2006, 5, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.A. Sunscreens: Progress and perspectives on photoprotection of human skin against UVB and UVA radiation. J. Dermatol. 1996, 23, 783–800. [Google Scholar] [CrossRef]
- Chatelain, E.; Gabard, B. Photostabilization of Butyl methoxydibenzoylmethane (Avobenzone) and Ethylhexyl methoxycinnamate by Bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a New UV Broadband Filter. Photochem. Photobiol. 2001, 74, 401–406. [Google Scholar] [CrossRef]
- Zhang, Z.; Tashiro, Y.; Matsukawa, S.; Ogawa, H. Influence of pH and temperature on the ultraviolet-absorbing properties of porphyra-334. Fish. Sci. 2005, 71, 1382–1384. [Google Scholar] [CrossRef]
- Gröniger, A.; Häder, D.-P. Stability of mycosporine-like amino acids. Recent Res. Dev. Photochem. Photobiol. 2000, 247–252. [Google Scholar]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 2014, 87, 244–256. [Google Scholar] [CrossRef]
- Matsuyama, K.; Matsumoto, J.; Yamamoto, S.; Nagasaki, K.; Inoue, Y.; Nishijima, M.; Mori, T. pH-independent charge resonance mechanism for UV protective functions of shinorine and related mycosporine-like amino acids. J. Phys. Chem. A 2015, 119, 12722–12729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, X.; Yuri, T.; Shingo, M.; Hiroo, O. Researches on the stability of porphyra-334 solution and its influence factors. J. Ocean Univ. China 2004, 3, 166–170. [Google Scholar] [CrossRef]
- Yoshiki, M.; Tsuge, K.; Tsuruta, Y.; Yoshimura, T.; Koganemaru, K.; Sumi, T.; Matsui, T.; Matsumoto, K. Production of new antioxidant compound from mycosporine-like amino acid, porphyra-334 by heat treatment. Food Chem. 2009, 113, 1127–1132. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. UV radiation-induced accumulation of photoprotective compounds in the green alga Tetraspora sp. CU2551. Plant Physiol. Biochem. 2013, 70, 7–13. [Google Scholar] [CrossRef]
- Korbee-Peinado, N. Fotorregulación y Efecto del Nitrógeno Inorgánico en la Acumulación de Aminoácidos Tipo Micosporina en Algas Rojas; Universidad de Málaga, Servicio de Publicaciones: Málaga, Spain, 2003. [Google Scholar]
- McKinlay, A.F.; Diffey, B.L. A reference action spectrum for ultraviolet induced erythema in human skin. CIE J. 1987, 6, 17–22. [Google Scholar]
- de la Coba, F.; Aguilera, J.; De Galvez, M.V.; Alvarez, M.; Gallego, E.; Figueroa, F.L.; Herrera, E. Prevention of the ultraviolet effects on clinical and histopathological changes, as well as the heat shock protein-70 expression in mouse skin by topical application of algal UV-absorbing compounds. J. Dermatol. Sci. 2009, 55, 161–169. [Google Scholar] [CrossRef]
- Misonou, T.; Saitoh, J.; Oshiba, S.; Tokitomo, Y.; Maegawa, M.; Inoue, Y.; Hori, H.; Sakurai, T. UV-absorbing substance in the red alga Porphyra yezoensis (Bangiales, Rhodophyta) block thymine photodimer production. Mar. Biotechnol. 2003, 5, 194–200. [Google Scholar] [CrossRef]
- Schmid, D.; Schürch, C.; Zülli, F.; Nissen, H.-P.; Prieur, H. Mycosporine-like amino acids: Natural UV-screening compounds from red algae to protect the skin against photoaging. SÖFW-J. 2003, 129, 38–42. [Google Scholar]
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi, Y.; Nam, T.-J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 796–803. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Morliere, P.; Annie, M.; Isabelle, T. Action spectrum for UV-inducen lipid peroxidation in cultured human skin fibroblast. Free Radic. Biol. Med. 1995, 19, 365–371. [Google Scholar] [CrossRef]
- Kaye, E.T.; Levin, J.A.; Blank, I.H.; Arndt, K.A.; Anderson, R.R. Efficiency of opaque photoprotective agents in the visible light range. Arch. Dermatol. 1991, 127, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Douki, T.; Brash, D.E. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The role of carotenoids in human skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Gonzalez, S.; Pathak, M.A.; Cuevas, J.; Villarrubia, V.G.; Fitzpatrick, T.B. Topical or oral administration with an extract of Polypodium leucotomos prevents acute sunburn and psoralen-induced phototoxic reactions as well as depletion of Langerhans cells in human skin. Photodermatol. Photoimmunol. Photomed. 1997, 13, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Santos, J.P.; Chow, F.; Ferreira, M.J.P.; dos Santos, D.Y.A.C. Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Tsujino, I.; Yabe, K.; Sekekawa, I. Isolation and structure of a new amino acid, shinorine, from the red alga Chondrus yendoi. Bot. Mar. 1980, 23, 65–68. [Google Scholar]
- Korbee, N.; Abdala Díaz, R.T.; Figueroa, F.L.; Helbling, E.W.; Peinado, N.K.; Abdala Díaz, R.T.; Figueroa, F.L.; Helbling, E.W. Ammonium and UV radiation stimulate the accumulation of mycosporine-like amino acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina. J. Phycol. 2004, 40, 248–259. [Google Scholar]
- Nazifi, E.; Wada, N.; Asano, T.; Nishiuchi, T.; Iwamuro, Y.; Chinaka, S.; Matsugo, S.; Sakamoto, T. Characterization of the chemical diversity of glycosylated mycosporine-like amino acids in the terrestrial cyanobacterium Nostoc commune. J. Photochem. Photobiol. B Biol. 2015, 142, 154–168. [Google Scholar] [CrossRef]
- Takano, S.; Uemura, D.; Hirata, Y. Isolation and structure of two new amino acids, palythinol and palythene, from the zoanthid Palythoa tuberculosa. Tetrahedron Lett. 1978, 26, 4909–4912. [Google Scholar] [CrossRef]
- Takano, S.; Daisuke, U.; Yoshimasa, H. Isolation and structure of a new amino acid, palythine, from the zoanthid Palythoa tuberculosa. Tetrahedron Lett. 1978, 19, 2229–2300. [Google Scholar]
- Dunlap, W.C.; Chalker, B.E.; Oliver, J.K. Bathymetric adaptations of reef-building corals at Davies Reef, Great Barrier Reef, Australia. III. UV-B absorbing compounds. J. Exp. Mar. Bio. Ecol. 1986, 104, 239–248. [Google Scholar] [CrossRef]
- Gleason, D.F. Differential effects of ultraviolet radiation on green and brown morphs of the Caribbean coral Porites astreoides. Limnol. Oceanogr. 1993, 38, 1452–1463. [Google Scholar] [CrossRef]
- Diffey, B.L.; Robson, J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J. Soc. Cosmet. Chem. 1989, 40, 127–133. [Google Scholar]
- Diffey, B.L. Indices of protection from in vitro assay of sunscreens. Sunscreens Dev. Eval. Regul. Asp. 1997, 589–600. [Google Scholar]
- Diffey, Bl. A method for broad spectrum classification of sunscreens. Int. J. Cosmet. Sci. 1994, 16, 47–52. [Google Scholar] [CrossRef]
- Garoli, D.; Pelizzo, M.G.; Nicolosi, P.; Peserico, A.; Tonin, E.; Alaibac, M. Effectiveness of different substrate materials for in vitro sunscreen tests. J. Dermatol. Sci. 2009, 56, 89–98. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Introducción a la Bioestadística; Reverté: Barcelona, Spain, 1986; Volume 5, ISBN 8429118624. [Google Scholar]
 ), 7.5 (
), 7.5 ( ), 8.5 (
), 8.5 ( ) or 10.5 (
) or 10.5 ( ) at room temperature (25 °C). Initial absorbance (
) at room temperature (25 °C). Initial absorbance ( ). (A) P-334 (+SH), (B) M-Ser (OH), (C) AS-330 (+PNE) and (D) SH.
). (A) P-334 (+SH), (B) M-Ser (OH), (C) AS-330 (+PNE) and (D) SH.
 ), 7.5 (
), 7.5 ( ), 8.5 (
), 8.5 ( ) or 10.5 (
) or 10.5 ( ) at room temperature (25 °C). Initial absorbance (
) at room temperature (25 °C). Initial absorbance ( ). (A) P-334 (+SH), (B) M-Ser (OH), (C) AS-330 (+PNE) and (D) SH.
). (A) P-334 (+SH), (B) M-Ser (OH), (C) AS-330 (+PNE) and (D) SH.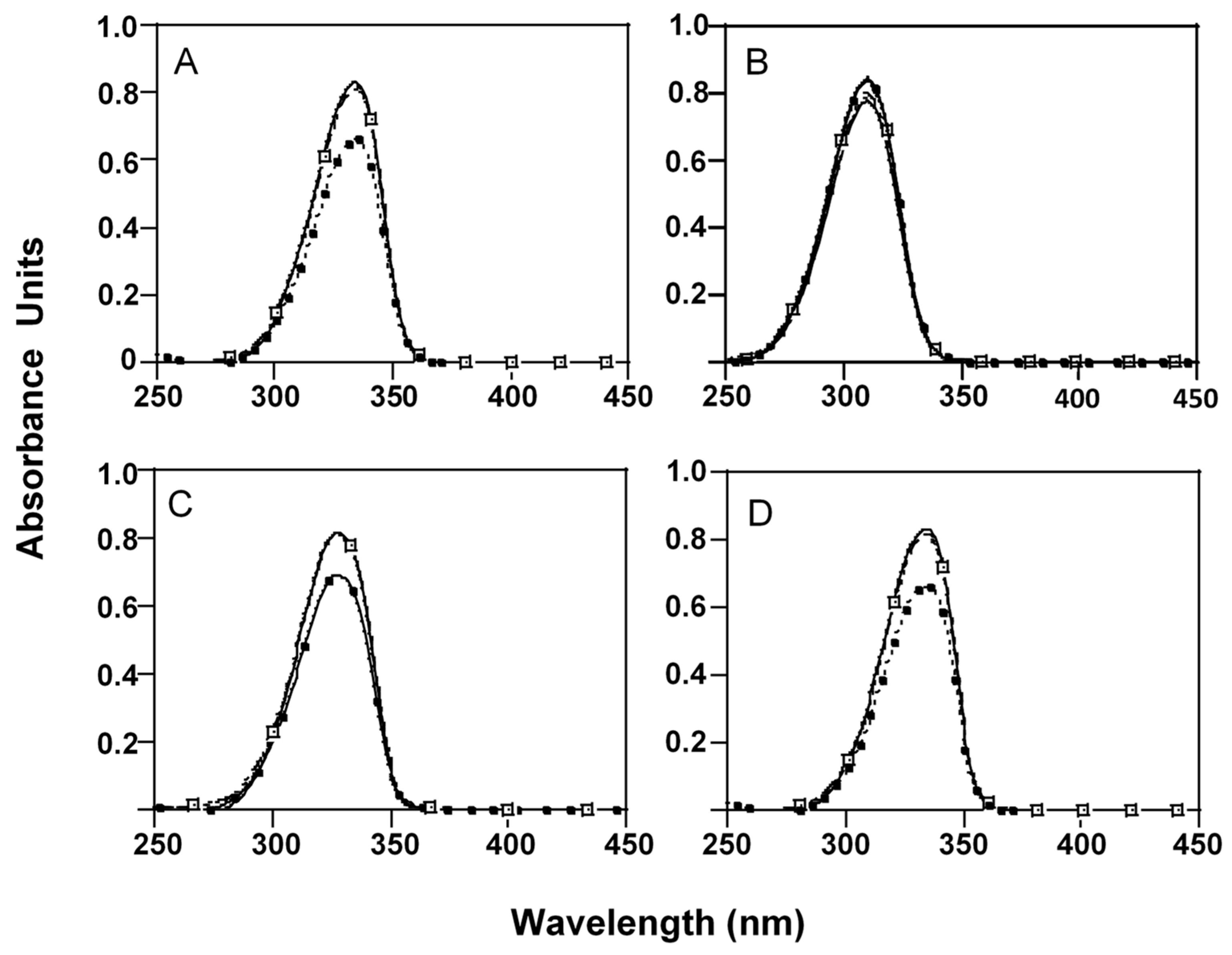
 ), 3 (
), 3 ( ), 4.5 (
), 4.5 ( ) and 6 (
) and 6 ( ) hours of incubation in 50 mM phosphate buffer at pH 10.5 and 50 °C. Initial absorbance (
) hours of incubation in 50 mM phosphate buffer at pH 10.5 and 50 °C. Initial absorbance ( ). (A) P-334 (+SH), (B) M-Ser (OH), (C) AS-330 (+PNE) and (D) SH.
). (A) P-334 (+SH), (B) M-Ser (OH), (C) AS-330 (+PNE) and (D) SH.
 ), 3 (
), 3 ( ), 4.5 (
), 4.5 ( ) and 6 (
) and 6 ( ) hours of incubation in 50 mM phosphate buffer at pH 10.5 and 50 °C. Initial absorbance (
) hours of incubation in 50 mM phosphate buffer at pH 10.5 and 50 °C. Initial absorbance ( ). (A) P-334 (+SH), (B) M-Ser (OH), (C) AS-330 (+PNE) and (D) SH.
). (A) P-334 (+SH), (B) M-Ser (OH), (C) AS-330 (+PNE) and (D) SH.
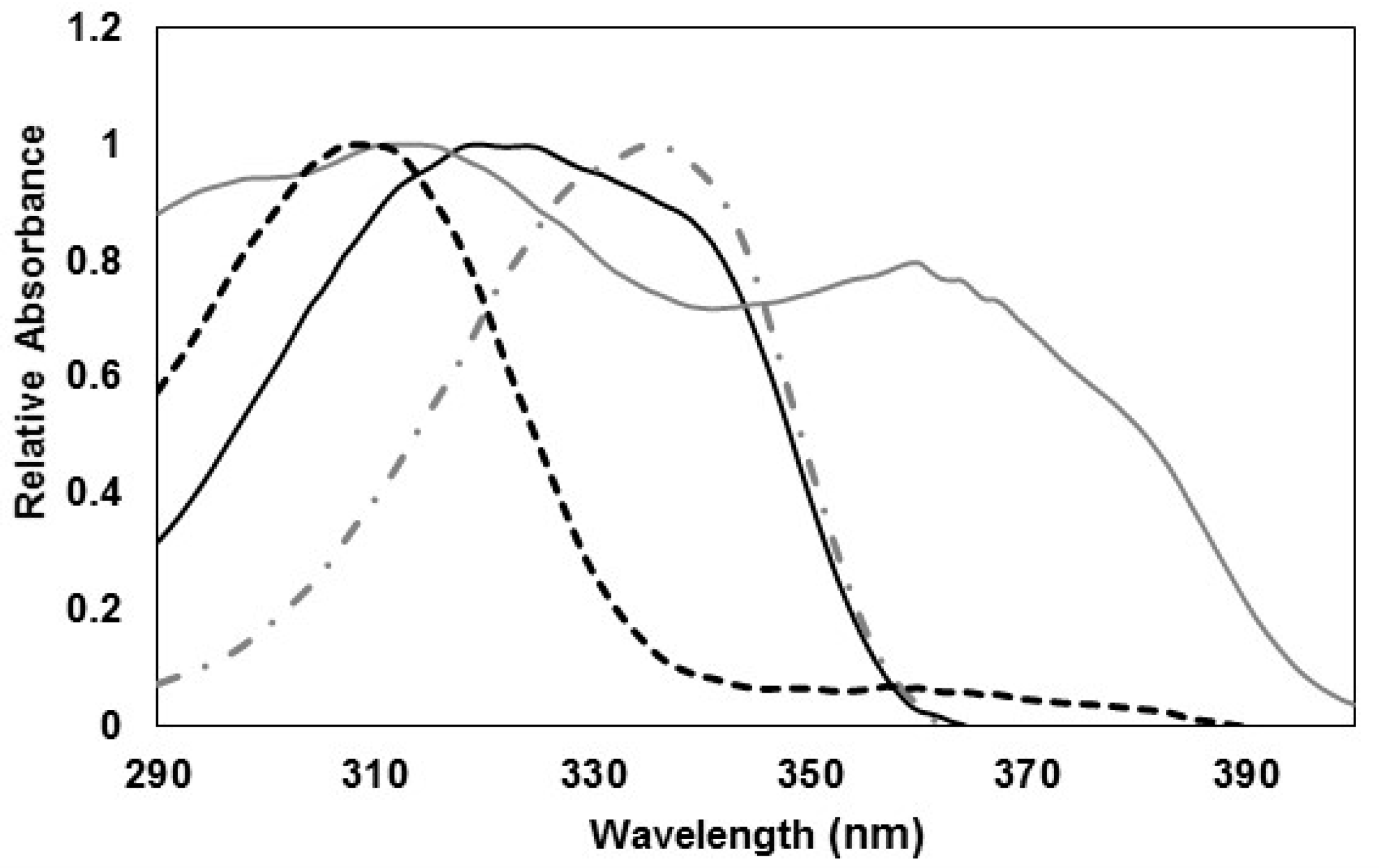
 ) reference (OMC Y BMDM) (
) reference (OMC Y BMDM) ( ), P-334 (+SH) (
), P-334 (+SH) ( ) and M-Ser (OH) (
) and M-Ser (OH) ( ).
).
 ) reference (OMC Y BMDM) (
) reference (OMC Y BMDM) ( ), P-334 (+SH) (
), P-334 (+SH) ( ) and M-Ser (OH) (
) and M-Ser (OH) ( ).
).
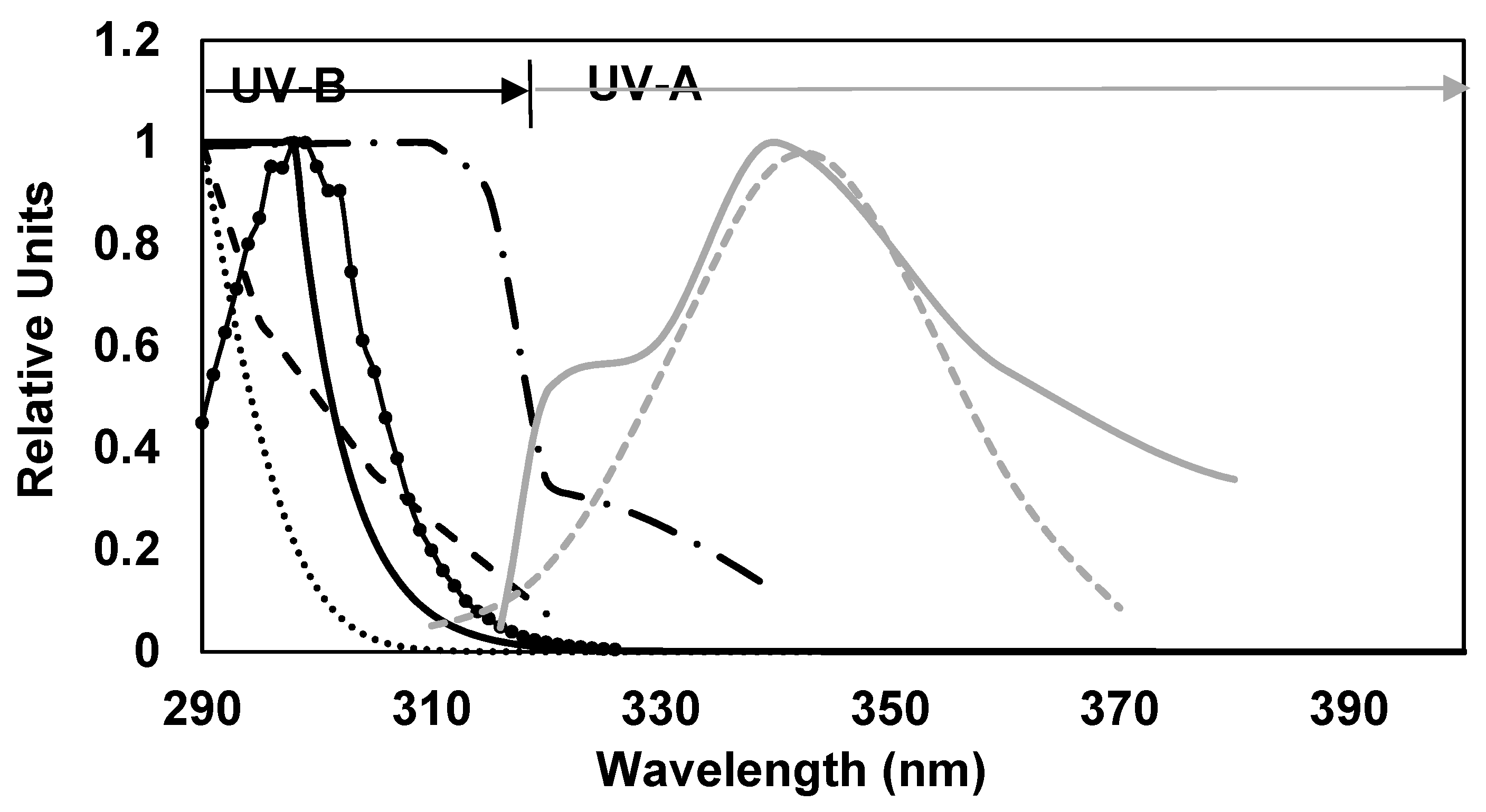
 ), erythema (
), erythema ( ), photocarcinogenesis (NMSC) (
), photocarcinogenesis (NMSC) ( ), induction of systemic suppression of CHS (
), induction of systemic suppression of CHS ( ), photoisomerization of urocanic acid (
), photoisomerization of urocanic acid ( ), formation of singlet oxygen (
), formation of singlet oxygen ( ) and photoaging (
) and photoaging ( ).
).
 ), erythema (
), erythema ( ), photocarcinogenesis (NMSC) (
), photocarcinogenesis (NMSC) ( ), induction of systemic suppression of CHS (
), induction of systemic suppression of CHS ( ), photoisomerization of urocanic acid (
), photoisomerization of urocanic acid ( ), formation of singlet oxygen (
), formation of singlet oxygen ( ) and photoaging (
) and photoaging ( ).
).
| Fraction | MAAs | Mol.formula | UV λmax (nm) | Exact (ppm) | m/z [M + H]+ | |
|---|---|---|---|---|---|---|
| Parent Ion | Theoretical | |||||
| P-334 (+SH) | Porphyra-334 | C14H22N2O8 | 334 | 3.1 | 347.1460 | 347.1449 |
| Shinorine | C13H20N2O8 | 334 | 2.5 | 333.1301 | 333.1292 | |
| M-Ser (OH) | Mycosporine-serinol | C11H19NO6 | 310 | 3.4 | 262.1294 | 262.1285 |
| SH | Shinorine | C13H20N2O8 | 334 | 3.0 | 333.1302 | 333.1292 |
| AS-330 (+PNE) | Asterina-330 | C12H20N2O6 | 331 | 1.3 | 289.1398 | 289.1394 |
| Palythine | C10H16N2O5 | 320 | 1.3 | 245.1135 | 245.1132 | |
| MAAs Extract | pH | Time (Hours) | ||
|---|---|---|---|---|
| 1.5 | 3 | 4.5 | ||
| P-334 (+SH) | 4 | 6.9 ± 0.04 a | 11.2 ± 0.04 b | 18.3 ± 0.05 c |
| 7.5 | 41.8 ± 1.19 a | 67.6 ± 1.62 b | 80.4 ±1.12 c | |
| 8.5 | 93.5 ± 0.07 a | 100b | ||
| 10.5 | 100 | |||
| M-Ser (OH) | 4 | 7.4 ±1.03 a | 14.7± 1.92 b | 17.4 ± 2.03 b |
| 7.5 | - | 6.4 ± 0.08 a | 10.0 ± 0.08 b | |
| 8.5 | 17.2 ± 0.03 a | 20.7 ±0.04 b | 25.1 ± 0.04 c | |
| 10.5 | 69.5± 0.06 a | 100b | ||
| AS-330 (+PNE) | 4 | - | - | 12.6 ± 0.05 |
| 7.5 | 54.9 ± 1.23 a | 77.6 ± 1.56 b | 86.7 ± 0.08 c | |
| 8.5 | 100 | |||
| 10.5 | 100 | |||
| SH | 4 | - | 12.8 ± 0.03 a | 17.6 ± 0.03 b |
| 7.5 | 50.6 ± 2.03 a | 74.6 ± 1.87 b | 85.4 ± 1.23 c | |
| 8.5 | 89.8 ± 0.08 a | 100b | - | |
| 10.5 | 100 | |||
| UV-mediated Effects | P-334 (+SH) | M-Ser (OH) | MAA Combination | Reference |
|---|---|---|---|---|
| Erythema (SPF) | 4.53 ± 1.58 a | 6.47 ± 1 ab | 8.37 ± 2.12 bc | 9.54 ± 1.53 c |
| DNA Damage | 4.17 ± 1.55 a | 9.27 ± 1.98 b | 10.18 ± 2.99 b | 9.71 ± 1.58 b |
| Photocarcinogenesis (NMSC) | 4.60 ± 1.63 a | 7.55 ± 1.31 b | 8.74 ± 2.24 b | 9.50 ± 1.49 b |
| Systemic Immunosuppression (CHS) | 5.63 ± 2.23 a | 9.73 ± 2.17 b | 10.72 ± 2.99 b | 10.41 ± 1.68 b |
| Urocanic acid photoisomerization | 7.22 ± 3.21 a | 9.21 ± 2.37 a | 10.90 ± 2.95 a | 11.00 ± 2.38 a |
| Formation of singlet oxygen radicals | 6.51 ± 2.25 b | 2.62 ± 0.18 a | 6.45 ± 1.69 b | 9.74 ± 1.89 c |
| Photoaging | 4.81 ± 1.72 b | 2.16 ± 0.10 a | 4.37 ± 0.94 b | 10.63 ± 2.63 c |
| Biological Effects | λmax (nm) | UVB/UVA | Experimental Model |
|---|---|---|---|
| Erythema | 250–298 | 99/1 | Human skin [77] |
| DNA damage | 270 | 100/0 | Function [12] |
| Photocarcinogenesis (NMSC) | 298 | 99/1 | SCUP-h (human) [13] |
| Inmunosuppression CHS | 270 | 100/0 | Balb/c mouse [14] |
| Photoisomerization of urocanic acid | 303–309 | 86/14 | Human skin [15] |
| Formation of Singlet oxygen | 342–343 | 4/96 | In vitro [16] |
| Photo-aging | 340 | 4/96 | SKHR-1 H mouse [17] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Coba, F.; Aguilera, J.; Korbee, N.; de Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs). Mar. Drugs 2019, 17, 55. https://doi.org/10.3390/md17010055
de la Coba F, Aguilera J, Korbee N, de Gálvez MV, Herrera-Ceballos E, Álvarez-Gómez F, Figueroa FL. UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs). Marine Drugs. 2019; 17(1):55. https://doi.org/10.3390/md17010055
Chicago/Turabian Stylede la Coba, Francisca, José Aguilera, Nathalie Korbee, María Victoria de Gálvez, Enrique Herrera-Ceballos, Félix Álvarez-Gómez, and Félix L. Figueroa. 2019. "UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs)" Marine Drugs 17, no. 1: 55. https://doi.org/10.3390/md17010055
APA Stylede la Coba, F., Aguilera, J., Korbee, N., de Gálvez, M. V., Herrera-Ceballos, E., Álvarez-Gómez, F., & Figueroa, F. L. (2019). UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs). Marine Drugs, 17(1), 55. https://doi.org/10.3390/md17010055





