UPLC–MS/MS Identification of Sterol Sulfates in Marine Diatoms
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
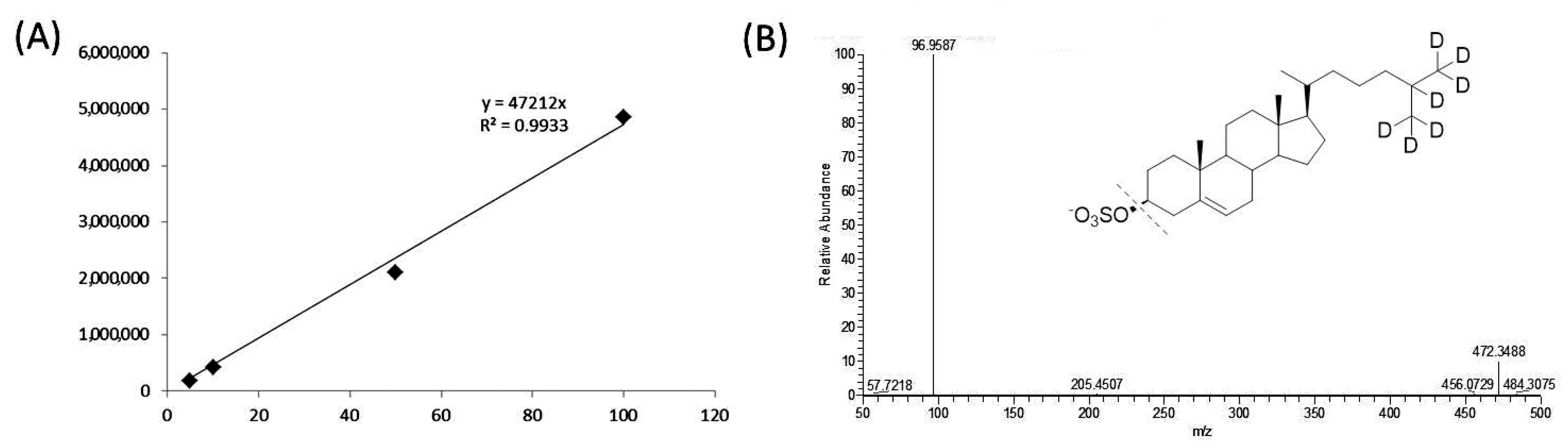
4.1. General
4.2. Chemical Derivatization of Sterols to Sterol Sulfates
4.3. Microalgal Material
4.4. Sample Preparation for LC/MS Analysis
4.5. Ultra Performance Liquid Chromatography/High Resolution Mass Spectrometry (UPLC/HRMS)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nelson, D.M.; Treguer, P.; Brzezinski, M.A.; Leynaert, A.; Queguiner, B. Production and dissolution of biogenic silica in the ocean—Revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob. Biogeochem. Cycles 1995, 9, 359–372. [Google Scholar] [CrossRef]
- Bidle, K.D.; Azam, F. Accelerated dissolution of diatom silica by marine bacterial assemblages. Nature 1999, 397, 508–512. [Google Scholar] [CrossRef]
- Benoiston, A.S.; Ibarbalz, F.M.; Bittner, L.; Guidi, L.; Jahn, O.; Dutkiewicz, S.; Bowler, C. The evolution of diatoms and their biogeochemical functions. Phil. Trans. R. Soc. B 2017, 372, 20160397. [Google Scholar] [CrossRef] [PubMed]
- Bozarth, A.; Maier, U.G.; Zauner, S. Diatoms in biotechnology: Modern tools and applications. Appl. Microbiol. Biotechnol. 2009, 82, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A. Cell signaling in marine diatoms. Commun. Integr. Biol. 2008, 2, 134–136. [Google Scholar] [CrossRef]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Ribalet, F.; Wichard, T.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. Age and nutrient limitation enhance polyunsaturated aldehyde production in marine diatoms. Phytochemistry 2007, 68, 2059–2067. [Google Scholar] [CrossRef]
- Barreiro, A.; Carotenuto, Y.; Lamari, N.; Esposito, F.; d’Ippolito, G.; Fontana, A.; Romano, G.; Ianora, A.; Miralto, A.; Guisande, C. Diatom induction of reproductive failure in copepods: The effect of PUAs versus non volatile oxylipins. J. Exp. Mar. Biol. Ecol. 2011, 401, 13–19. [Google Scholar] [CrossRef]
- Ianora, A.; Bastianini, M.; Carotenuto, Y.; Casotti, R.; Roncalli, V.; Miralto, A.; Romano, G.; Gerecht, A.; Fontana, A.; Turner, J.T. Non-volatile oxylipins render some diatom blooms more toxic for copepod reproduction. Harmful Alga 2015, 44, 1–7. [Google Scholar] [CrossRef]
- Fontana, A.; D’Ippolito, G.; Cutignano, A.; Romano, G.; Lamari, N.; Gallucci, A.M.; Ianora, A. LOX-induced lipid peroxidation mechanism responsible for the detrimental effect of marine diatoms on zooplankton grazers. Chembiochem 2007, 8, 1810–1818. [Google Scholar] [CrossRef]
- Fontana, A.; d’Ippolito, G.; Cutignano, A.; Miralto, A.; Ianora, A.; Romano, G.; Cimino, G. Chemistry of oxylipin pathways in marine diatoms. Pure Appl. Chem. 2007, 79, 481–490. [Google Scholar] [CrossRef]
- d’Ippolito, G.; Nuzzo, G.; Sardo, A.; Manzo, E.; Gallo, C.; Fontana, A. Lipoxygenases and lipoxygenase products in marine diatoms. Methods Enzymol. 2018, 605, 69–100. [Google Scholar] [PubMed]
- Cimino, G.; Ciavatta, M.L.; Fontana, A.; Gavagnin, M. Metabolites of marine opisthobranchs: Chemistry and biological activity. In Bioactive Compounds from Natural Sources; Tringali, C., Ed.; Taylor & Francis: London, UK, 2001; pp. 579–637. [Google Scholar]
- Marthi, R.; Fontana, A.; Uriz, M.J.; Cimino, G. Quantitative assessment of natural toxicity in sponge: Toxicity bioassay versus compound quantification. J. Chem. Ecol. 2003, 29, 1307–1318. [Google Scholar] [CrossRef]
- Mollo, E.; Fontana, A.; Roussis, V.; Polese, G.; Amodeo, P.; Ghiselin, M.T. Sensing marine biomolecules: Smell, taste, and the evolutionary transition from aquatic to terrestrial life. Front. Chem. 2014, 2, 92. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Ciavatta, M.L.; D’Souza, L.; Mollo, E.; Naik, C.G.; Parameswaran, P.S.; Cimino, G. Selected chemo-ecological studies of marine opisthobranchs from Indian coasts. J. Indian Inst. Sci. 2001, 81, 403–415. [Google Scholar]
- Gallo, C.; d’Ippolito, G.; Nuzzo, G.; Sardo, A.; Fontana, A. Autoinhibitory sterol sulfates mediate programmed cell death in a bloom-forming marine diatom. Nat. Commun. 2018, 8, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Gallo, C.; Nuzzo, G.; d’Ippolito, G.; Manzo, E.; Sardo, A.; Fontana, A. Sterol sulfates and sulfotransferases in marine diatoms. Methods Enzymol. 2018, 605, 101–138. [Google Scholar]
- Rampen, S.W.; Abbas, B.A.; Schouten, S.; Sinninghe Dams, J.S. A comprehensive study of sterols in marine diatoms (Bacillariophyta): Implications for their use as tracers for diatom productivity. Limnol. Oceanogr. 2010, 55, 91–105. [Google Scholar] [CrossRef]
- McKee, T.C.; Cardellina, J.H.; Riccio, R.; D’Auria, M.V.; Iorizzi, M.; Minale, L.; Buckheit, R.W. HIV-inhibitory natural products. 11. Comparative studies of sulfated sterols from marine invertebrates. J. Med. Chem. 1994, 37, 793–797. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Breen, A.; Carte, B.; Johnson, R.K. Halistanol disulfate B, a novel sulfated sterol from the sponge Pachastrella sp.: Inhibitor of endothelin converting enzyme. J. Nat. Prod. 1996, 59, 606–608. [Google Scholar] [CrossRef]
- Rudi, A.; Yosief, T.; Loya, S.; Hizi, A.; Schleyer, M.; Kashman, Y. Clathsterol, a novel anti-HIV-1 RT sulfated sterol from the sponge clathria species. J. Nat. Prod. 2001, 64, 1451–1453. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Shen, X.R.; Kong, J.L. Arenicolsterol A, a novel cytotoxic enolic sulfated sterol from a marine annelid Arenicola cristata. Chin. J. Chem. 2005, 23, 599–602. [Google Scholar] [CrossRef]
- Yang, S.W.; Buivich, A.; Chan, T.M.; Smith, M.; Lachowicz, J.; Pomponi, S.A.; Chu, M. A new sterol sulfate, Sch 572423, from a marine sponge, Topsentia sp. Bioorg. Med. Chem. Lett. 2003, 13, 1791–1794. [Google Scholar] [CrossRef]
- DiGirolamo, J.A.; Li, X.-C.; Jacob, M.R.; Clark, A.M.; Ferreira, D. Reversal of fluconazole resistance by sulfated sterols from the marine sponge Topsentia sp. J. Nat. Prod. 2009, 72, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Morinaka, B.I.; Pawlik, J.R.; Molinski, T.F. Amaroxocanes A and B: Sulfated dimeric sterols defend the caribbean coral reef sponge Phorbas amaranthus from fish predators. J. Nat. Prod. 2009, 72, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Festa, C.; De Marino, S.; Dauria, M.V.; Bifulco, G.; Renga, B.; Fiorucci, S.; Zampella, A. Solomonsterols A and B from Theonella swinhoei. the first example of C-24 and C-23 sulfated sterols from a marine source endowed with a PXR agonistic activity. J. Med. Chem. 2011, 54, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Sepe, V.; Bifulco, G.; Renga, B.; D’Amore, C.; Fiorucci, S.; Zampella, A. Discovery of sulfated sterols from marine invertebrates as a new class of marine natural antagonists of farnesoid-x-receptor. J. Med. Chem. 2011, 54, 1314–1320. [Google Scholar] [CrossRef]
- Casanovas, A.; Hannibal-Bach, H.K.; Jensen, O.N.; Ejsing, C.S. Shotgun lipidomic analysis of chemically sulfated sterols compromises analytical sensitivity: Recommendation for large-scale global lipidome analysis. Eur. J. Lipid Sci. Technol. 2014, 116, 1618–1620. [Google Scholar] [CrossRef]
- Gouveia, M.J.; Brindley, P.-J.; Santos, L.L.; Correia da Costa, J.M.; Gomes, P.; Vale, N. Mass spectrometry techniques in the survey of steroid metabolites as potential disease biomarkers: A review. Metabolism 2013, 62, 1206–1217. [Google Scholar] [CrossRef]
- Sánchez-Guijo, A.; Oji, V.; Hartmann, M.F.; Schuppe, H.C.; Traupe, H.; Wudy, S.A. High levels of oxysterol sulfates in serum of patients with steroid sulfatase deficiency. J. Lipid Res. 2015, 56, 403–412. [Google Scholar] [CrossRef]
- Sánchez-Guijo, A.; Oji, V.; Hartmann, M.F.; Traupe, H.; Wudy, S.A. Simultaneous quantification of cholesterol sulfate, androgen sulfates, and progestagen sulfates in human serum by LC–MS/MS. J. Lipid Res. 2015, 56, 1843–1851. [Google Scholar] [CrossRef]
- Strott, C.A.; Higashi, Y. Cholesterol sulfate in human physiology: What’s it all about? J. Lipid Res. 2003, 44, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Raniello, R.; Iannicelli, M.M.; Nappo, M.; Avila, C.; Zupo, V. Production of Cocconeis neothumensis (Bacillariophyceae) biomass in batch cultures and bioreactors for biotechnological applications: Light and nutrient requirements. J. Appl. Phycol. 2007, 19, 383–391. [Google Scholar] [CrossRef]
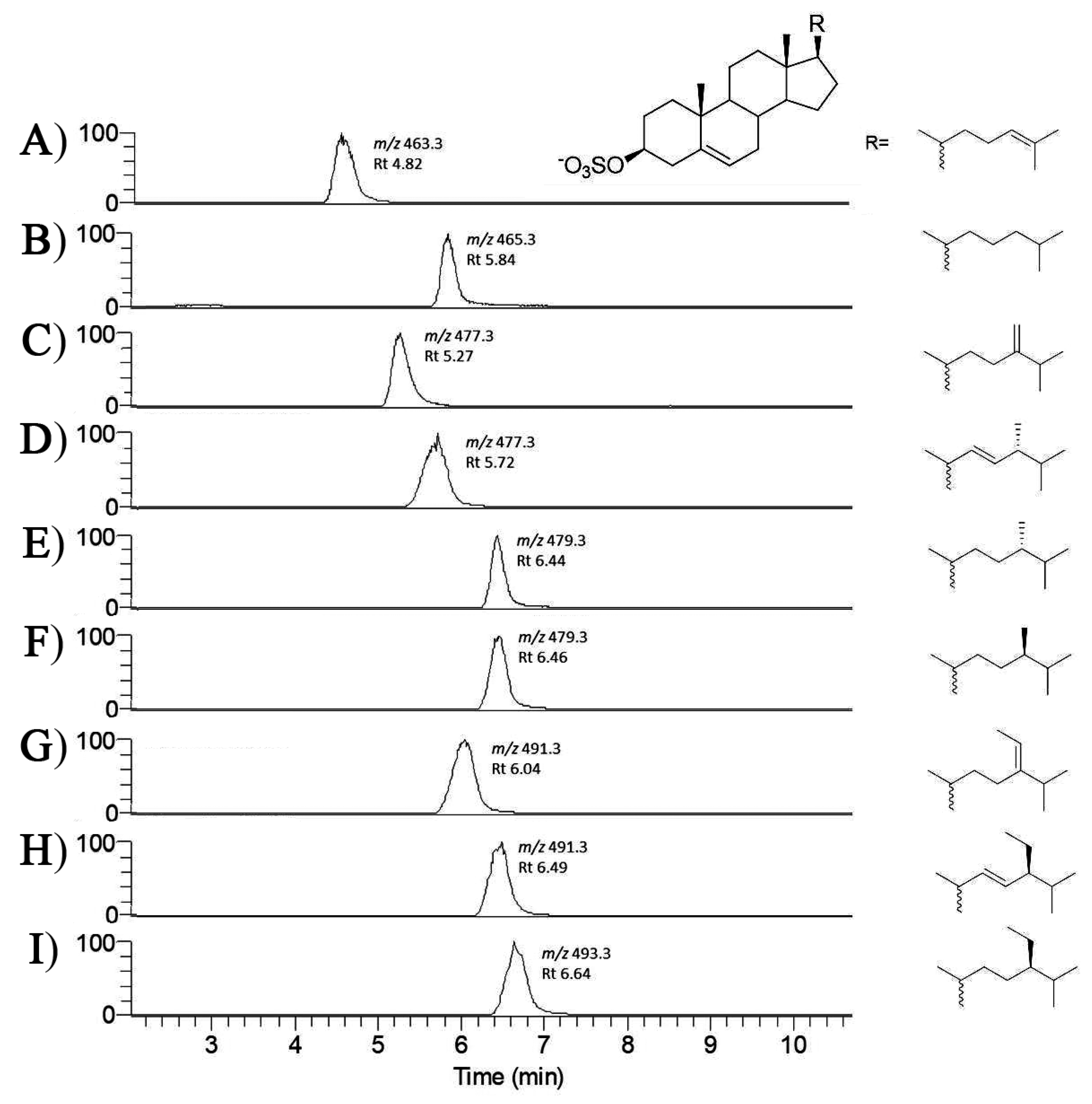
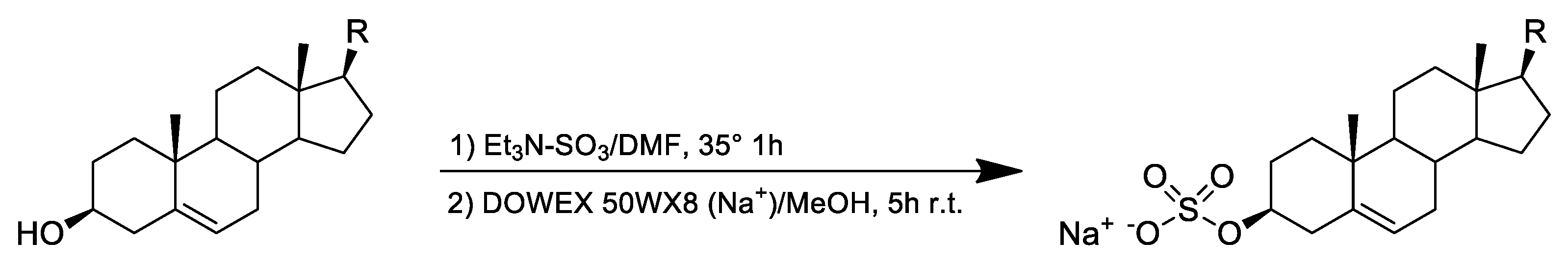
| Compounds | Molecular Formula | Calculated MS | Measured m/z | Species Distribution |
|---|---|---|---|---|
| Desmosterol sulfate | C27H43O4S− | 463.2887 | 463.2896 | C. closterium P. tricornutum |
| Cholesterol sulfate | C27H45O4S− | 465.3044 | 465.3047 | S. marinoi |
| 24-methylene cholesterol sulfate | C28H45O4S− | 477.3044 | 477.3043 | S. marinoi C. cryptica C. scutellum P. tricornutum T. pseudonana T. rotula |
| Brassicasterol sulfate (24β-methyl-22-dehydrocholesterol) | C28H45O4S− | 477.3044 | 477.3052 | N. shiloi C. scutellum Diploneis sp. |
| 24-methyl cholesterol sulfate | C28H47O4S− | 479.3200 | 479.3205 | S. marinoi 1 C. Cryptica 1 T. pseudonana T. rotula |
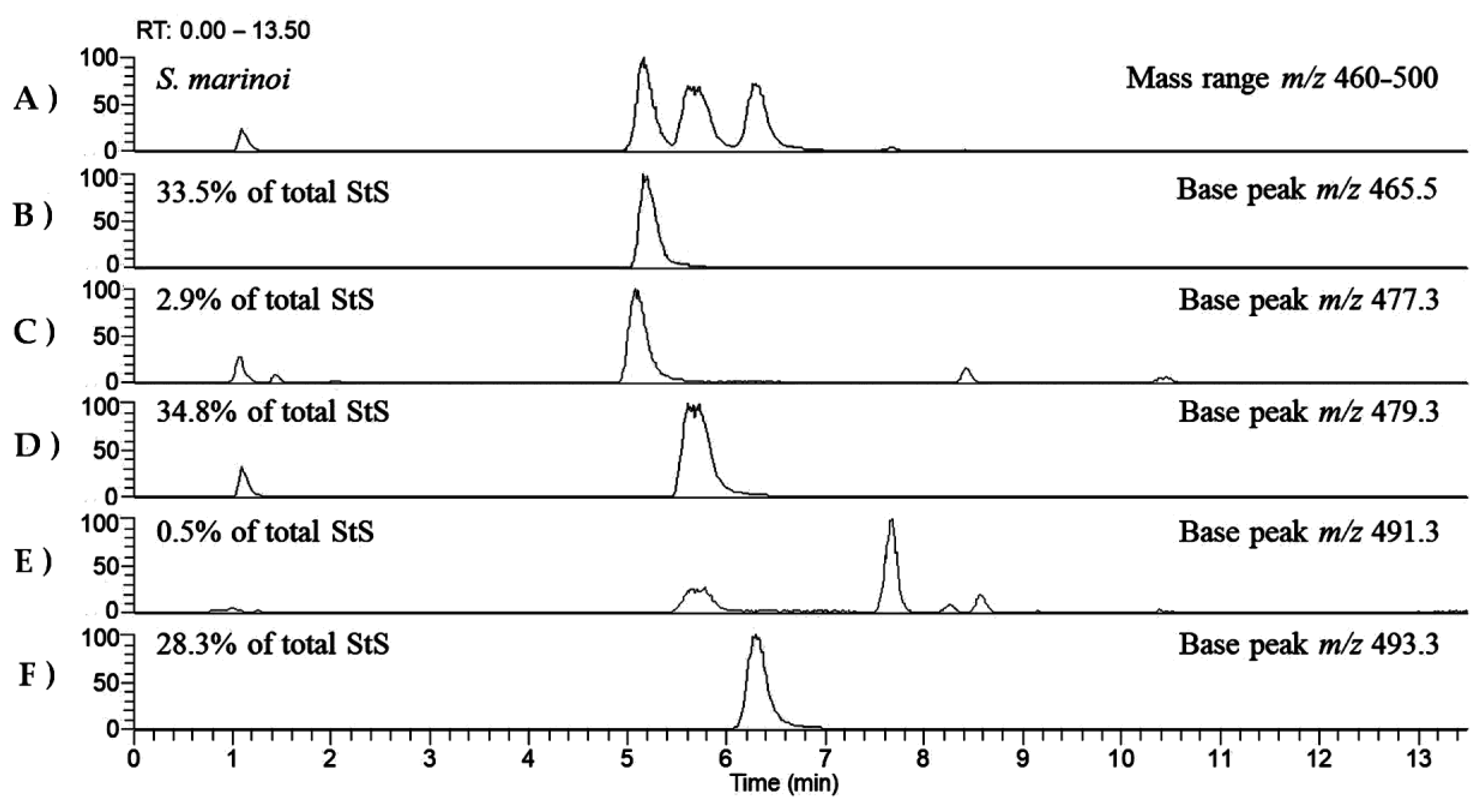
| Fucosterol sulfate | C29H47O4S− | 491.3200 | 491.3201 | S. marinoi N. shiloi |
| β-Sitosterol sulfate (24β-ethyl cholesterol) | C29H49O4S− | 493.3357 | 493.3361 | S. marinoi |
| Diatom Species | Compounds | µg StS/mg C |
|---|---|---|
| C. closterium | Desmosterol sulfate | 26.21 ± 2.6 |
| Total StS | 26.21 ± 2.6 | |
| N. shiloi | Brassicasterol sulfate | 5.94 ± 1.0 |
| Fucosterol sulfate | 60.69 ± 7.3 | |
| Total StS | 66.63 ± 5.2 | |
| C. scutellum | 24-methylene cholesterol sulfate | 10.09 ± 1.3 |
| Brassicasterol sulfate | 3.91 ± 0.1 | |
| Total StS | 14.00 ± 1.45 | |
| Diploneis sp. | Brassicasterol sulfate | 11.03 ± 2.9 |
| Total StS | 11.03 ± 2.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzzo, G.; Gallo, C.; D’Ippolito, G.; Manzo, E.; Ruocco, N.; Russo, E.; Carotenuto, Y.; Costantini, M.; Zupo, V.; Sardo, A.; et al. UPLC–MS/MS Identification of Sterol Sulfates in Marine Diatoms. Mar. Drugs 2019, 17, 10. https://doi.org/10.3390/md17010010
Nuzzo G, Gallo C, D’Ippolito G, Manzo E, Ruocco N, Russo E, Carotenuto Y, Costantini M, Zupo V, Sardo A, et al. UPLC–MS/MS Identification of Sterol Sulfates in Marine Diatoms. Marine Drugs. 2019; 17(1):10. https://doi.org/10.3390/md17010010
Chicago/Turabian StyleNuzzo, Genoveffa, Carmela Gallo, Giuliana D’Ippolito, Emiliano Manzo, Nadia Ruocco, Ennio Russo, Ylenia Carotenuto, Maria Costantini, Valerio Zupo, Angela Sardo, and et al. 2019. "UPLC–MS/MS Identification of Sterol Sulfates in Marine Diatoms" Marine Drugs 17, no. 1: 10. https://doi.org/10.3390/md17010010
APA StyleNuzzo, G., Gallo, C., D’Ippolito, G., Manzo, E., Ruocco, N., Russo, E., Carotenuto, Y., Costantini, M., Zupo, V., Sardo, A., & Fontana, A. (2019). UPLC–MS/MS Identification of Sterol Sulfates in Marine Diatoms. Marine Drugs, 17(1), 10. https://doi.org/10.3390/md17010010












