Enhanced Adhesion of Fish Ovarian Germline Stem Cells on Solid Surfaces by Mussel-Inspired Polymer Coating
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Animals
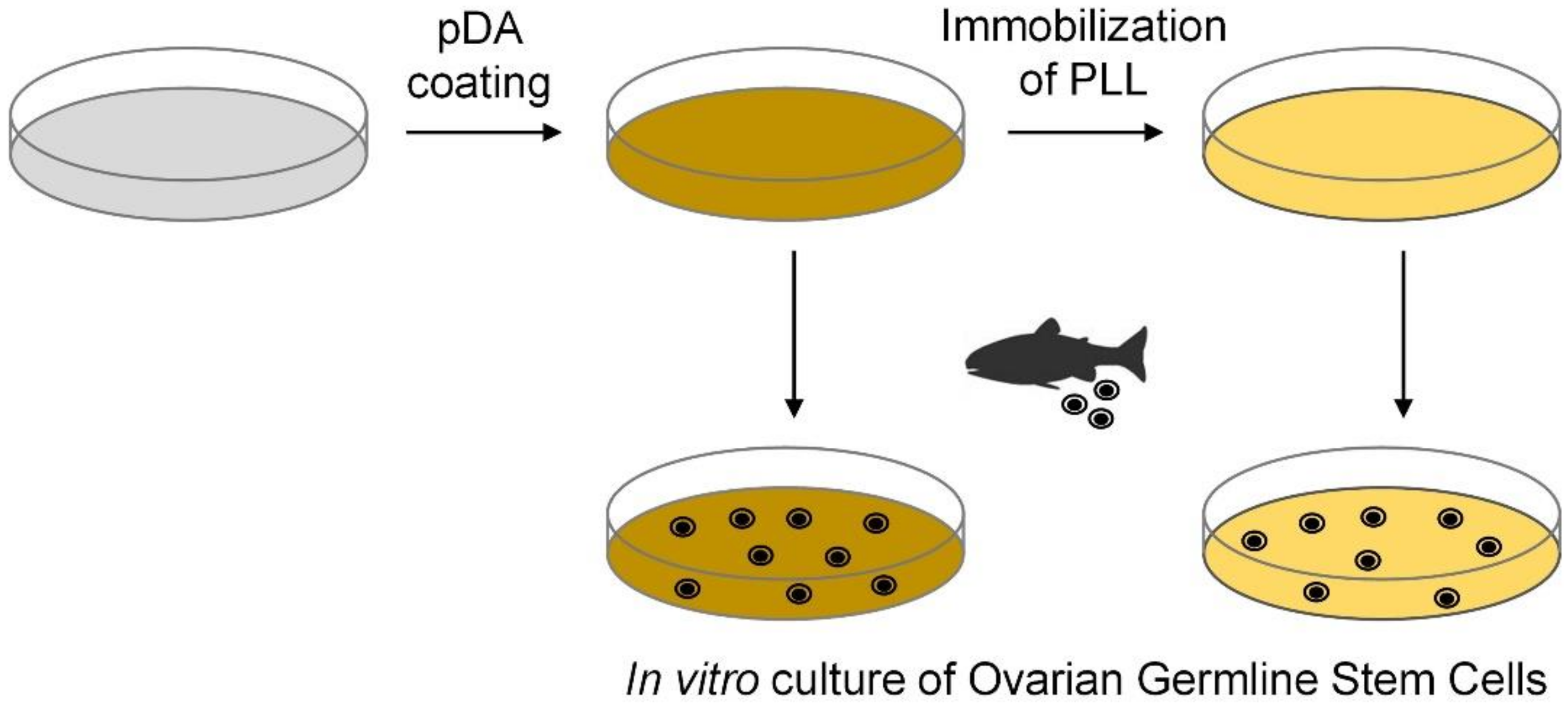
2.3. Polydopamine (pDA) Coating
2.4. Immobilization of PLL
2.5. Enrichment of Ovarian Germline Stem Cells (OGSCs)
2.6. Cell Culture
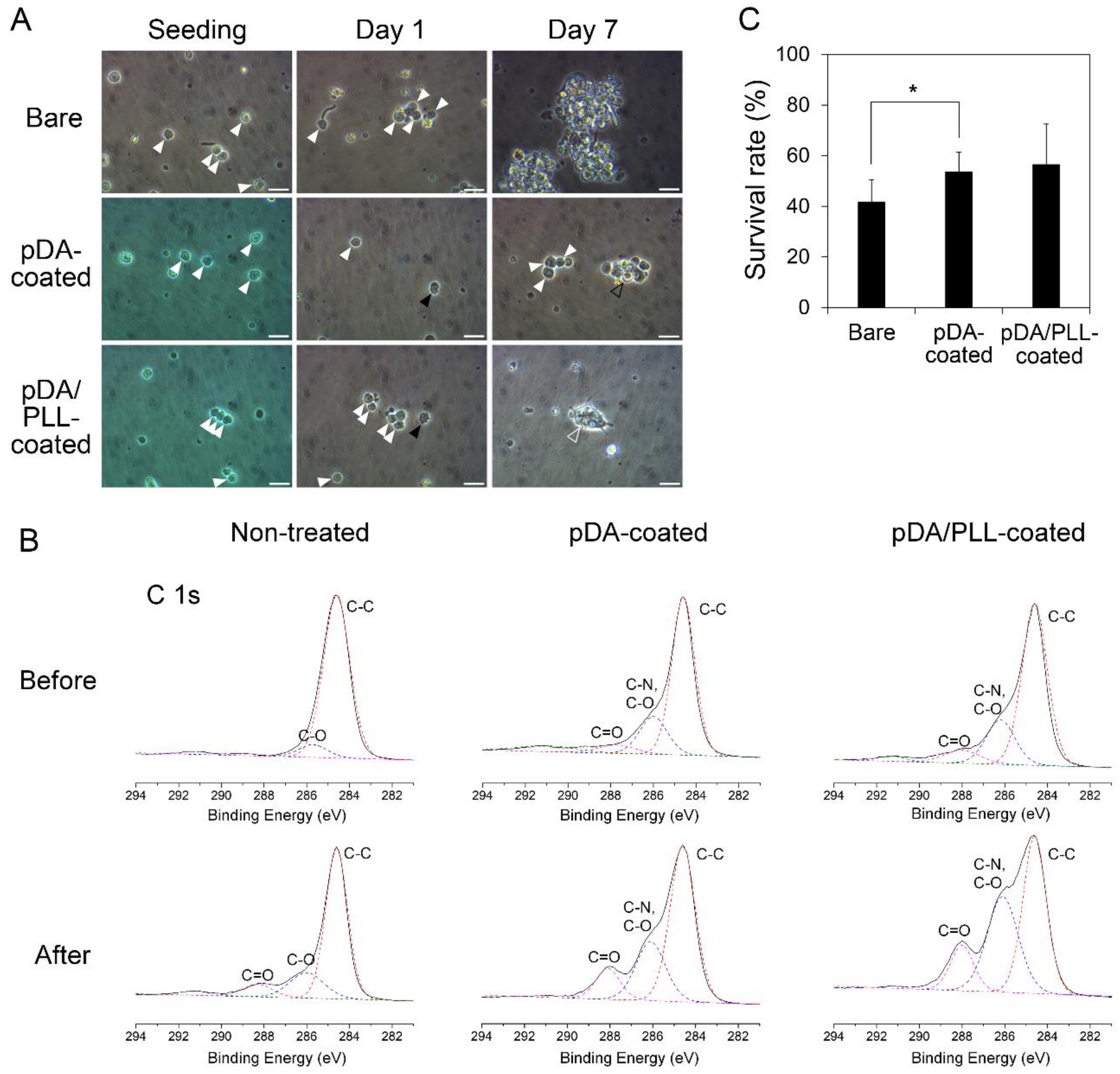
2.7. Cell Viability
2.8. Cell Transplantation
2.9. Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Quantitative RT-PCR (qRT-PCR)
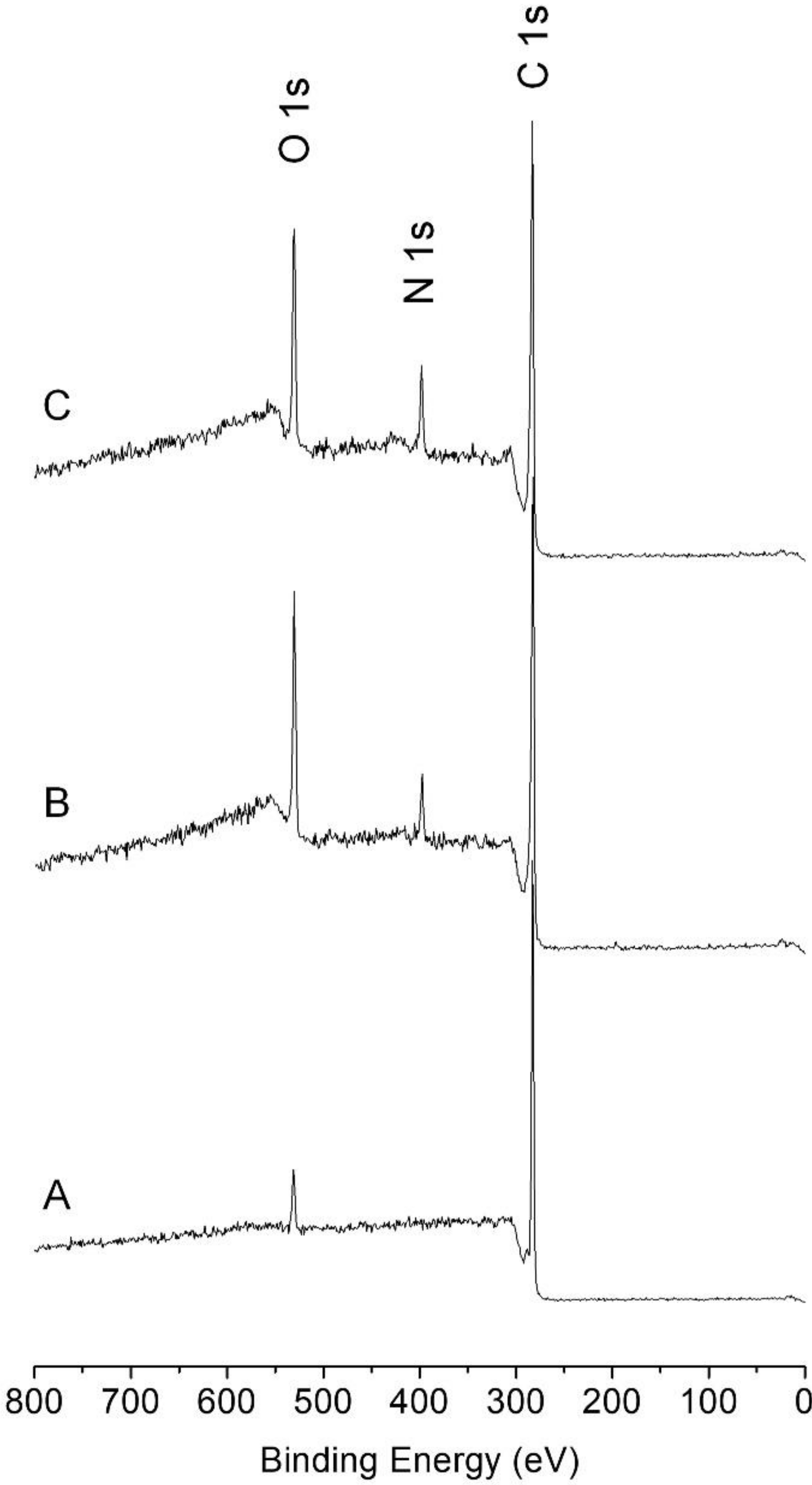
2.10. Characterizations
2.11. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shikina, S.; Yoshizaki, G. Improved in vitro culture conditions to enhance the survival, mitotic activity, and transplantability of rainbow trout type a spermatogonia. Biol. Reprod. 2010, 83, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Liu, T.; Zhao, H.; Xu, H.; Wang, W.; Liu, R.; Chen, T.; Deng, J.; Gui, J. Establishment of a normal medakafish spermatogonial cell line capable of sperm production in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 8011–8016. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kobayashi, K.; Nishimura, T.; Higashijima, S.; Tanaka, M. Identification of germline stem cells in the ovary of the teleost medaka. Science 2010, 328, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Li, Z.; Hong, Y. Fish stem cell cultures. Int. J. Biol. Sci. 2011, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kobayashi, K.; Nishimura, T.; Tanaka, M. Ovarian germline stem cells in the teleost fish, medaka (Oryzias latipes). Int. J. Biol. Sci. 2011, 7, 403. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-T.; Saito, T.; Crodian, J.; Collodi, P. Zebrafish germline chimeras produced by transplantation of ovarian germ cells into sterile host larvae. Biol. Reprod. 2011, 84, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, S.; Costa, G.; Campos-Junior, P.; Segatelli, T.; Yazawa, R.; Takeuchi, Y.; Morita, T.; Yoshizaki, G.; França, L. Germ cell transplantation as a potential biotechnological approach to fish reproduction. Fish Physiol. Biochem. 2013, 39, 3–11. [Google Scholar] [CrossRef]
- Lacerda, S.M.; Costa, G.M.; de França, L.R. Biology and identity of fish spermatogonial stem cell. Gen. Comp. Endocrinol. 2014, 207, 56–65. [Google Scholar] [CrossRef]
- Lee, S.; Iwasaki, Y.; Shikina, S.; Yoshizaki, G. Generation of functional eggs and sperm from cryopreserved whole testes. Proc. Natl. Acad. Sci. USA 2013, 110, 1640–1645. [Google Scholar] [CrossRef]
- Lee, S.; Seki, S.; Katayama, N.; Yoshizaki, G. Production of viable trout offspring derived from frozen whole fish. Sci. Rep. 2015, 5, 16045. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Fujinuma, K.; Iwasaki, Y.; Okutsu, T.; Shikina, S.; Yazawa, R.; Takeuchi, Y. Spermatogonial transplantation in fish: a novel method for the preservation of genetic resources. Comp. Biochem. Physiol. Part D Genom. Proteom. 2011, 6, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Gutzeit, H.O. Effect of 17-α-ethynylestradiol on germ cell proliferation in organ and primary culture of medaka (Oryzias latipes) testis. Dev. Growth Differ. 2003, 45, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Shikina, S.; Ihara, S.; Yoshizaki, G. Culture conditions for maintaining the survival and mitotic activity of rainbow trout transplantable type A spermatogonia. Mol. Reprod. Dev. 2008, 75, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Shikina, S.; Nagasawa, K.; Hayashi, M.; Furuya, M.; Iwasaki, Y.; Yoshizaki, G. Short-term in vitro culturing improves transplantability of type A spermatogonia in rainbow trout (Oncorhynchus mykiss). Mol. Reprod. Dev. 2013, 80, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, L.; Song, W.; Wu, Z.; Li, D. Biocompatible polymer materials: role of protein–surface interactions. Prog. Polym. Sci. 2008, 33, 1059–1087. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Lee, J.S.; Park, C.B. Spatial control of cell adhesion and patterning through mussel-inspired surface modification by polydopamine. Langmuir 2010, 26, 15104–15108. [Google Scholar] [CrossRef]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef]
- Kang, K.; Choi, I.S.; Nam, Y. A biofunctionalization scheme for neural interfaces using polydopamine polymer. Biomaterials 2011, 32, 6374–6380. [Google Scholar] [CrossRef]
- Yang, K.; Lee, J.S.; Kim, J.; Lee, Y.B.; Shin, H.; Um, S.H.; Kim, J.B.; Park, K.I.; Lee, H.; Cho, S.-W. Polydopamine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials 2012, 33, 6952–6964. [Google Scholar] [CrossRef]
- Kang, S.M.; Choi, I.S. Control of Cell Adhesion on a Superhydrophobic Surface by Polydopamine Coating. Bull. Korean Chem. Soc. 2013, 34, 2525–2527. [Google Scholar] [CrossRef]
- Park, H.-J.; Yang, K.; Kim, M.-J.; Jang, J.; Lee, M.; Kim, D.-W.; Lee, H.; Cho, S.-W. Bio-inspired oligovitronectin-grafted surface for enhanced self-renewal and long-term maintenance of human pluripotent stem cells under feeder-free conditions. Biomaterials 2015, 50, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cortez-Jugo, C.; Choi, G.H.; Björnmalm, M.; Dai, Y.; Yoo, P.J.; Caruso, F. Patterned poly (dopamine) films for enhanced cell adhesion. Bioconjugate Chem. 2016, 28, 75–80. [Google Scholar] [CrossRef]
- Ding, Y.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurf. Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- Kim, S.; Moon, J.M.; Choi, J.S.; Cho, W.K.; Kang, S.M. Mussel-Inspired Approach to Constructing Robust Multilayered Alginate Films for Antibacterial Applications. Adv. Funct. Mater. 2016, 26, 4099–4105. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.; Ryu, J.H.; Kim, K.; Han, Y.-M.; Lee, H. Long-term, feeder-free maintenance of human embryonic stem cells by mussel-inspired adhesive heparin and collagen type I. Acta Biomater. 2016, 32, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, G.; Wang, K.; Wan, Q.; Tao, L.; Zhang, X.; Wei, Y. Recent developments in polydopamine: an emerging soft matter for surface modification and biomedical applications. Nanoscale 2016, 8, 16819–16840. [Google Scholar] [CrossRef]
- Kim, S.; Gim, T.; Jeong, Y.; Ryu, J.H.; Kang, S.M. Facile Construction of Robust Multilayered PEG Films on Polydopamine-Coated Solid Substrates for Marine Antifouling Applications. ACS Appl. Mater. Interfaces 2017, 10, 7626–7631. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Lynge, M.E.; van der Westen, R.; Postma, A.; Städler, B. Polydopamine—A nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Rho, J.; Choi, I.S.; Messersmith, P.B.; Lee, H. Norepinephrine: Material-Independent, Multifunctional Surface Modification Reagent. J. Am. Chem. Soc. 2009, 131, 13224–13225. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kinoshita, M.; Kobayashi, D.; Nagahama, Y. Establishment of medaka (Oryzias latipes) transgenic lines with the expression of green fluorescent protein fluorescence exclusively in germ cells: a useful model to monitor germ cells in a live vertebrate. Proc. Natl Acad. Sci. USA 2001, 98, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Nakamura, S.; Ishikawa, Y.; Tanaka, M. Expression and syntenic analyses of four nanos genes in medaka. Zool. Sci. 2009, 26, 112–118. [Google Scholar] [CrossRef] [PubMed]


| Genes | Primer Sequences (5′ > 3′) | Product Size (bp) | Accession Number |
|---|---|---|---|
| nanos2 | Forward, GGTGCAAACAACTGTGGATG | 262 | NM_001160447.1 |
| Reverse, CTTGCAGAAGCGGCAGTAAT | |||
| vasa | Forward, GAGAAGGTTCCGACCACCAG | 177 | NM_001104676.1 |
| Reverse, AATGGTGTTGGGCAGGTCAA | |||
| β−actin | Forward, CCACCATGTACCCTGGAATC | 153 | NM_001104808.1 |
| Reverse, GCTGGAAGGTGGACAGAGAG |
| C 1s | N 1s | O 1s | N 1s/O 1s | |
|---|---|---|---|---|
| Non-treated | 95.8 | 0 | 4.2 | 0 |
| pDA-coated | 77.4 | 5.5 | 17.1 | 0.32 |
| pDA/PLL-coated | 74.8 | 8.8 | 16.4 | 0.54 |
| Substrate conditions of culture dishes | Number of recipients transplanted | Number (%) a of recipients survived to 20 dpf | Number (%) a of recipients harboring transplanted OGSCs in their gonads |
|---|---|---|---|
| Non-treated | 28 | 20 (71) bc | 3 (11) bc |
| pDA-coated | 30 | 24 (80) b | 6 (20) b |
| pDA/PLL-coated | 30 | 15 (50) c | 0 (0) c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.; Ryu, J.H.; Nam, Y.K.; Gong, S.P.; Kang, S.M. Enhanced Adhesion of Fish Ovarian Germline Stem Cells on Solid Surfaces by Mussel-Inspired Polymer Coating. Mar. Drugs 2019, 17, 11. https://doi.org/10.3390/md17010011
Jeong Y, Ryu JH, Nam YK, Gong SP, Kang SM. Enhanced Adhesion of Fish Ovarian Germline Stem Cells on Solid Surfaces by Mussel-Inspired Polymer Coating. Marine Drugs. 2019; 17(1):11. https://doi.org/10.3390/md17010011
Chicago/Turabian StyleJeong, Yeonwoo, Jun Hyung Ryu, Yoon Kwon Nam, Seung Pyo Gong, and Sung Min Kang. 2019. "Enhanced Adhesion of Fish Ovarian Germline Stem Cells on Solid Surfaces by Mussel-Inspired Polymer Coating" Marine Drugs 17, no. 1: 11. https://doi.org/10.3390/md17010011
APA StyleJeong, Y., Ryu, J. H., Nam, Y. K., Gong, S. P., & Kang, S. M. (2019). Enhanced Adhesion of Fish Ovarian Germline Stem Cells on Solid Surfaces by Mussel-Inspired Polymer Coating. Marine Drugs, 17(1), 11. https://doi.org/10.3390/md17010011





