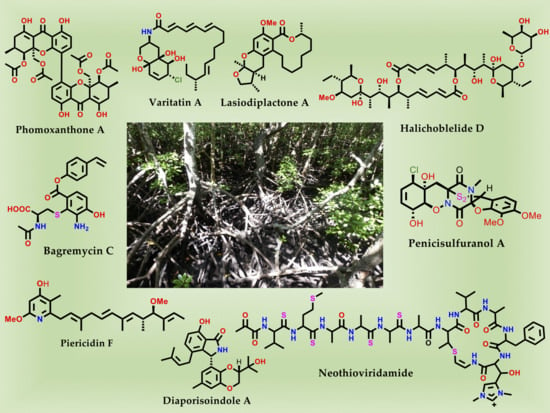
Lead Compounds from Mangrove-Associated Microorganisms
Abstract
1. Introduction
2. Bioactive Compounds from Mangrove-Associated Microorganisms
2.1. Bioactive Compounds from Endophytic Fungi
2.1.1. Cytotoxic Compounds
2.1.2. Antimicrobial Compounds
2.1.3. Compounds with Inhibitory Activity towards NO Production
2.1.4. Compounds with α-Glucosidase Inhibitory Activity
2.1.5. Compounds with Mycobacterium tuberculosis Protein Tyrosine Phosphatase B (MptpB) Inhibitory Activity
2.2. Bioactive Compounds Derived from Fungi Originating from Mangrove (Rhizosphere) Soil/Sediment Samples
2.2.1. Cytotoxic Compounds
2.2.2. Compounds with Lipid-Lowering Activity
2.3. Cytotoxic Compounds Derived from Bacteria Originating from Mangrove (Rhizosphere) Soil/Sediment Samples
3. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Tomlinson, P.B. Ecology. In The Botany of Mangroves, 2nd ed.; Cambridge University Press: Cambridge, UK, 2016; pp. 11–28. ISBN 9781107080676. [Google Scholar]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.-Y.; Pan, J.-Y.; Yang, M.-H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef] [PubMed]
- Saenger, P. Mangrove Ecology, Silviculture and Conservation; Springer: Dordrecht, The Netherlands, 2002; pp. 11–47. ISBN 9789401599627. [Google Scholar]
- Wang, L.; Mu, M.; Li, X.; Lin, P.; Wang, W. Differentiation between true mangroves and mangrove associates based on leaf traits and salt contents. J. Plant Ecol. 2011, 4, 292–301. [Google Scholar] [CrossRef]
- Yin, S.; Fan, C.-Q.; Wang, X.-N.; Lin, L.-P.; Ding, J.; Yue, J.-M. Xylogranatins A−D: Novel tetranortriterpenoids with an unusual 9,10-seco scaffold from marine mangrove Xylocarpus granatum. Org. Lett. 2006, 8, 4935–4938. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Wu, J.; Li, J.; Satyanandamurty, T.; Shen, L.; Bringmann, G. Krishnadimer A, an axially chiral non-biaryl natural product: Discovery and biomimetic synthesis. Org. Lett. 2017, 19, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.-K.; Li, P.-L.; Qiao, D.; Zhang, X.-W.; Chu, M.-J.; Qin, G.-F.; Tang, X.-L.; Li, G.-Q. Cytotoxic and antiviral triterpenoids from the mangrove plant Sonneratia paracaseolaris. Molecules 2017, 22, e1319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Satyanandamurty, T.; Shen, L.; Wu, J. Krishnolides A–D: New 2-ketokhayanolides from the Krishna mangrove, Xylocarpus moluccensis. Mar. Drugs 2017, 15, e333. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.-Y.; Bruhn, T.; Katele, F.Z.; Xiao, Q.; Pedpradab, P.; Wu, J.; Bringmann, G. Thaixylomolins A–C: Limonoids featuring two new motifs from the Thai Xylocarpus moluccensis. Org. Lett. 2013, 15, 3682–3685. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Li, X.-W.; Guo, Y.-W. Recent progress on the mangrove plants: Chemistry and bioactivity. Curr. Org. Chem. 2016, 20, 1923–1942. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, X.; Feng, S.; Jiang, G.; Luo, J.; Zhou, S.; Vrijmoed, L.L.P.; Jones, E.B.G.; Krohn, K.; Steingröver, K.; et al. Five unique compounds: Xyloketals from mangrove fungus Xylaria sp. from the South China Sea coast. J. Org. Chem. 2001, 66, 6252–6256. [Google Scholar] [CrossRef]
- Feling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew. Chem. Int. Ed. 2003, 42, 355–357. [Google Scholar] [CrossRef] [PubMed]
- MarinLit. Available online: http://pubs.rsc.org/marinlit (accessed on 25 April 2018).
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53, (and all papers of the same series prior to this). [Google Scholar] [CrossRef] [PubMed]
- Simões, M.F.; Antunes, A.; Ottoni, C.A.; Amini, M.S.; Alam, I.; Alzubaidy, H.; Mokhtar, N.-A.; Archer, J.A.C.; Bajic, V.B. Soil and rhizosphere associated fungi in gray mangroves (Avicennia marina) from the Red Sea—A metagenomic approach. Genom. Proteom. Bioinform. 2015, 13, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Sanka Loganathachetti, D.; Poosakkannu, A.; Muthuraman, S. Fungal community assemblage of different soil compartments in mangrove ecosystem. Sci. Rep. 2017, 7, e8560. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.; Tsai, S. Variations of bacterial community structure and composition in mangrove sediment at different depths in Southeastern Brazil. Diversity 2014, 6, e827. [Google Scholar] [CrossRef]
- Basak, P.; Pramanik, A.; Sengupta, S.; Nag, S.; Bhattacharyya, A.; Roy, D.; Pattanayak, R.; Ghosh, A.; Chattopadhyay, D.; Bhattacharyya, M. Bacterial diversity assessment of pristine mangrove microbial community from Dhulibhashani, Sundarbans using 16S rRNA gene tag sequencing. Genom. Data 2016, 7, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-B.; Chen, Y.-Q.; Lan, C.-Y.; Tam, N.F.Y.; Zan, Q.-J.; Huang, L.-N. Recovery of novel bacterial diversity from mangrove sediment. Mar. Biol. 2007, 150, 739–747. [Google Scholar] [CrossRef]
- De Souza Sebastianes, F.L.; Romão-Dumaresq, A.S.; Lacava, P.T.; Harakava, R.; Azevedo, J.L.; de Melo, I.S.; Pizzirani-Kleiner, A.A. Species diversity of culturable endophytic fungi from Brazilian mangrove forests. Curr. Genet. 2013, 59, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-B.; Ye, W.-W.; Han, Y.; Deng, Z.-X.; Hong, K. Natural products from mangrove actinomycetes. Mar. Drugs 2014, 12, 2590–2613. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Meng, L.-H.; Li, X.-M.; Lv, C.-T.; Huang, C.-G.; Wang, B.-G. Brocazines A–F, cytotoxic bisthiodiketopiperazine derivatives from Penicillium brocae MA-231, an endophytic fungus derived from the marine mangrove plant Avicennia marina. J. Nat. Prod. 2014, 77, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Wang, C.-Y.; Mándi, A.; Li, X.-M.; Hu, X.-Y.; Kassack, M.U.; Kurtán, T.; Wang, B.-G. Three diketopiperazine alkaloids with spirocyclic skeletons and one bisthiodiketopiperazine derivative from the mangrove-derived endophytic fungus Penicillium brocae MA-231. Org. Lett. 2016, 18, 5304–5307. [Google Scholar] [CrossRef] [PubMed]
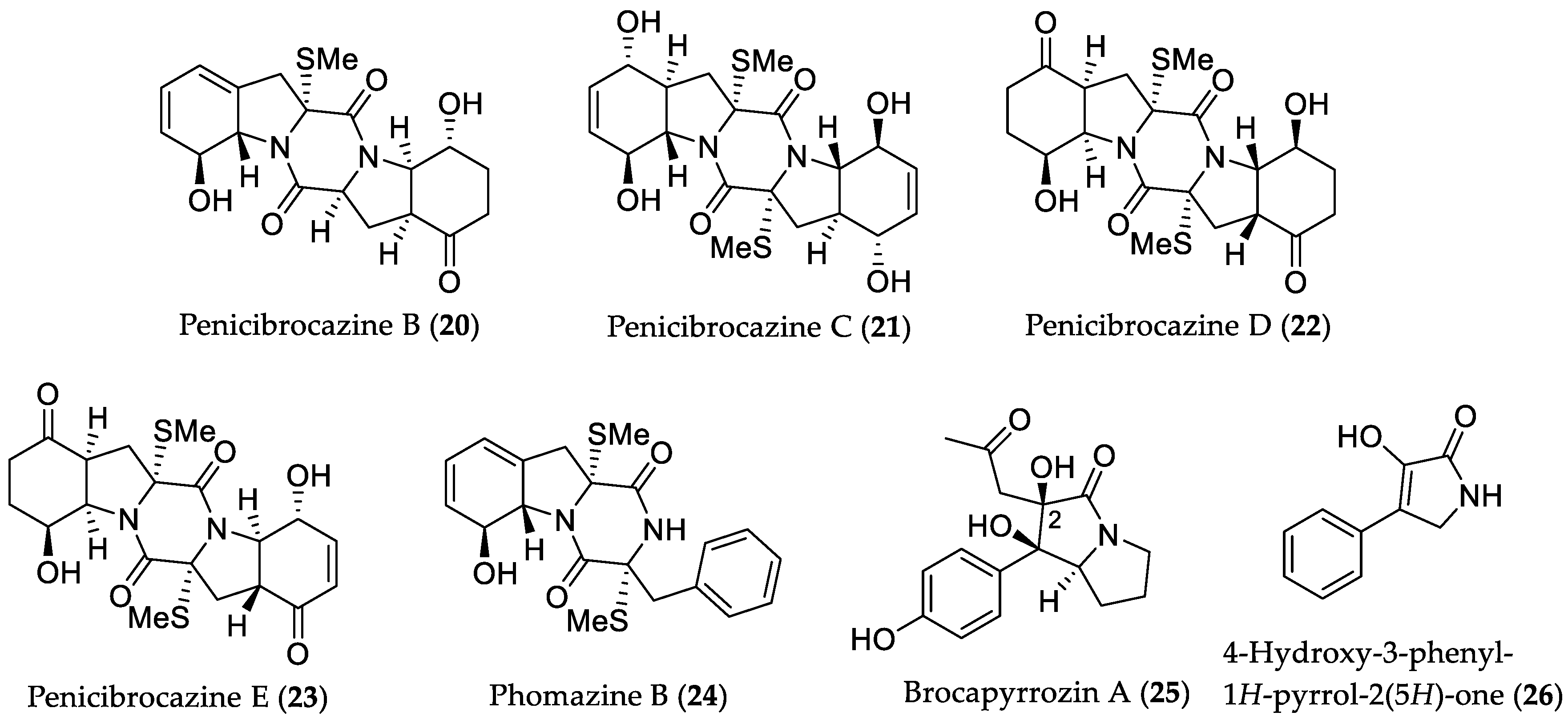
- Meng, L.-H.; Zhang, P.; Li, X.-M.; Wang, B.-G. Penicibrocazines A–E, five new sulfide diketopiperazines from the marine-derived endophytic fungus Penicillium brocae. Mar. Drugs 2015, 13, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Li, X.-M.; Liu, Y.; Xu, G.-M.; Wang, B.-G. Antimicrobial alkaloids produced by the mangrove endophyte Penicillium brocae MA-231 using the OSMAC approach. RSC Adv. 2017, 7, 55026–55033. [Google Scholar] [CrossRef]
- Chen, S.; Chen, D.; Cai, R.; Cui, H.; Long, Y.; Lu, Y.; Li, C.; She, Z. Cytotoxic and antibacterial preussomerins from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. J. Nat. Prod. 2016, 79, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
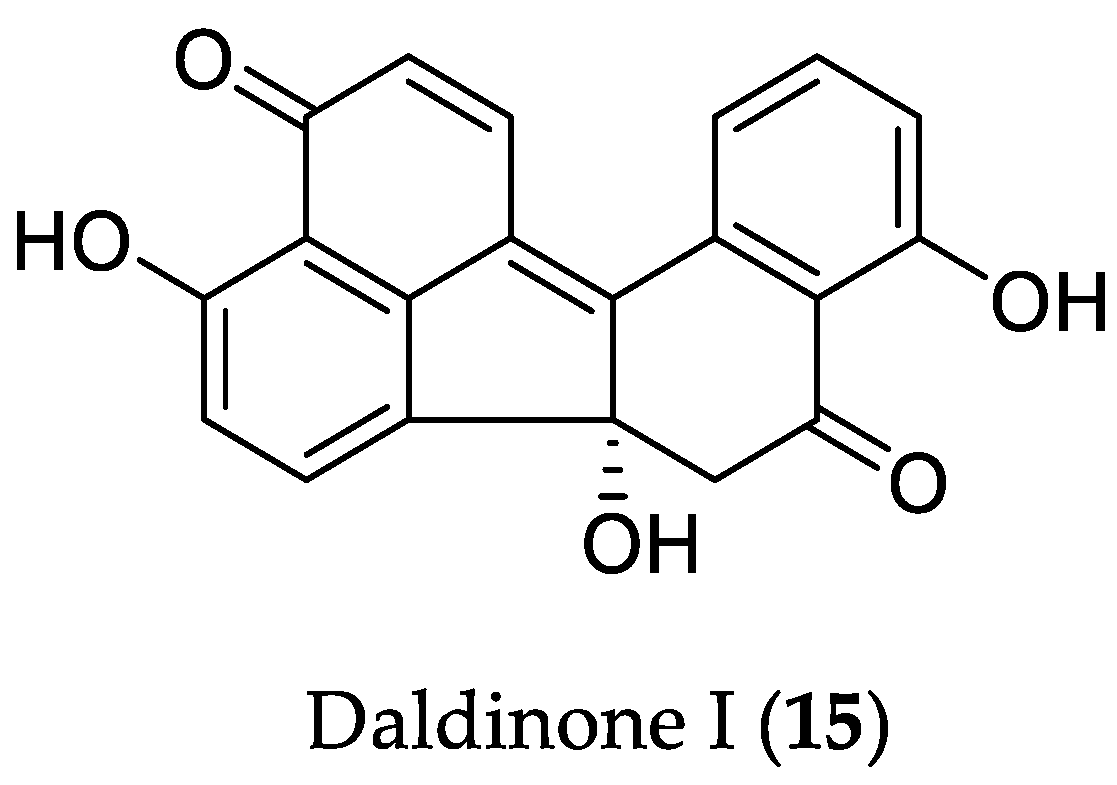
- Liu, Y.; Stuhldreier, F.; Kurtan, T.; Mandi, A.; Arumugam, S.; Lin, W.; Stork, B.; Wesselborg, S.; Weber, H.; Henrich, B.; et al. Daldinone derivatives from the mangrove-derived endophytic fungus Annulohypoxylon sp. RSC Adv. 2017, 7, 5381–5393. [Google Scholar] [CrossRef]
- Siridechakorn, I.; Yue, Z.; Mittraphab, Y.; Lei, X.; Pudhom, K. Identification of spirobisnaphthalene derivatives with anti-tumor activities from the endophytic fungus Rhytidhysteron rufulum AS21B. Bioorg. Med. Chem. 2017, 25, 2878–2882. [Google Scholar] [CrossRef] [PubMed]
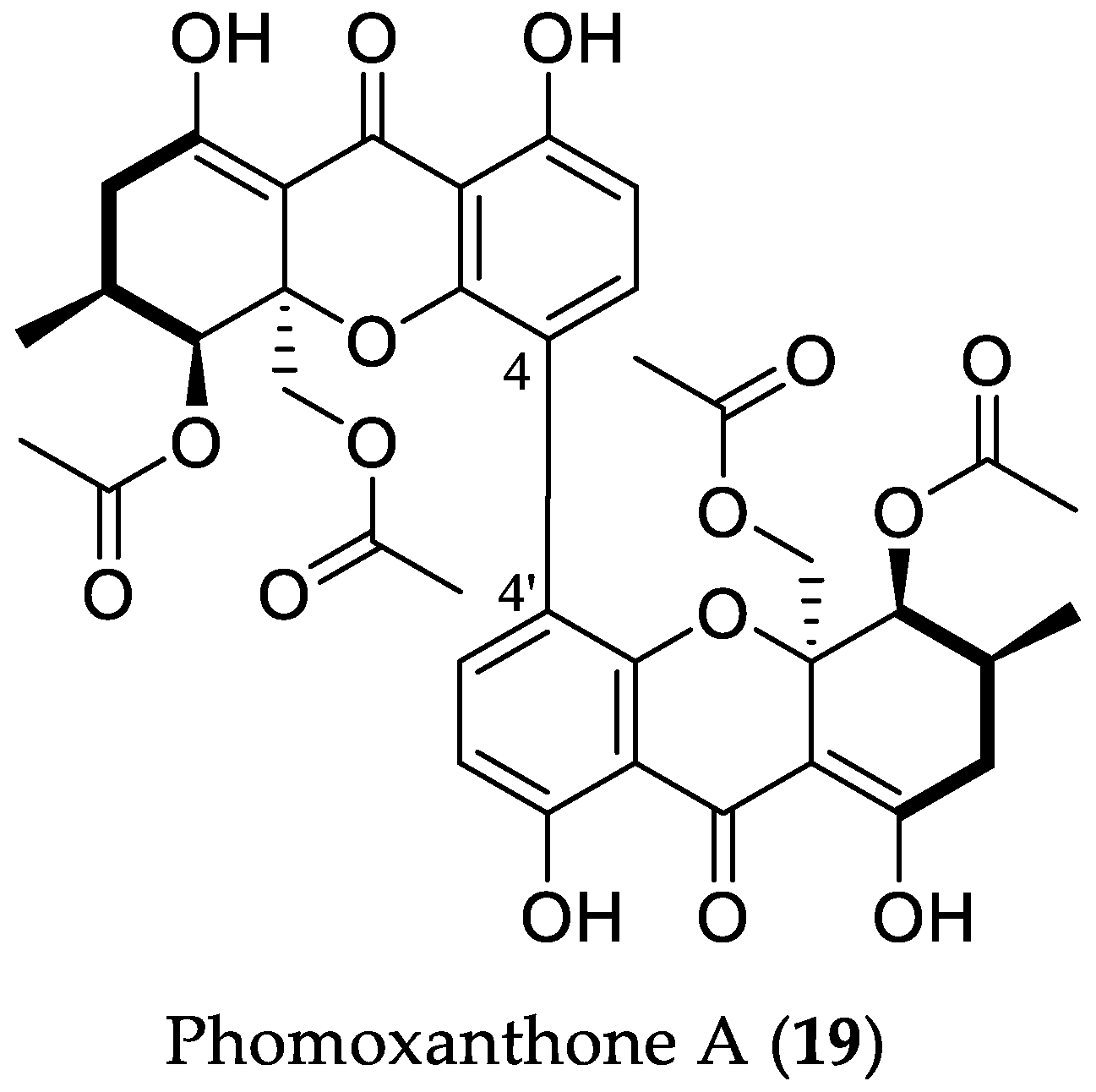
- Marian, F.; Hendrik, N.; Philip, B.; Björn, S.; Sebastian, W.; Wenhan, L.; Peter, P. Phomoxanthone A—from mangrove forests to anticancer therapy. Curr. Med. Chem. 2015, 22, 3523–3532. [Google Scholar] [CrossRef]
- Rönsberg, D.; Debbab, A.; Mándi, A.; Vasylyeva, V.; Böhler, P.; Stork, B.; Engelke, L.; Hamacher, A.; Sawadogo, R.; Diederich, M.; et al. Pro-apoptotic and immunostimulatory tetrahydroxanthone dimers from the endophytic fungus Phomopsis longicolla. J. Org. Chem. 2013, 78, 12409–12425. [Google Scholar] [CrossRef] [PubMed]
- Böhler, P.; Stuhldreier, F.; Anand, R.; Kondadi, A.K.; Schlutermann, D.; Berleth, N.; Deitersen, J.; Wallot-Hieke, N.; Wu, W.; Frank, M.; et al. The mycotoxin phomoxanthone A disturbs the form and function of the inner mitochondrial membrane. Cell Death Dis. 2018, 9, e286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-M.; Zheng, C.-J.; Chen, G.-Y.; Song, X.-P.; Han, C.-R.; Li, G.-N.; Fu, Y.-H.; Chen, W.-H.; Niu, Z.-G. Bioactive anthraquinone derivatives from the mangrove-derived fungus Stemphylium sp. 33231. J. Nat. Prod. 2014, 77, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
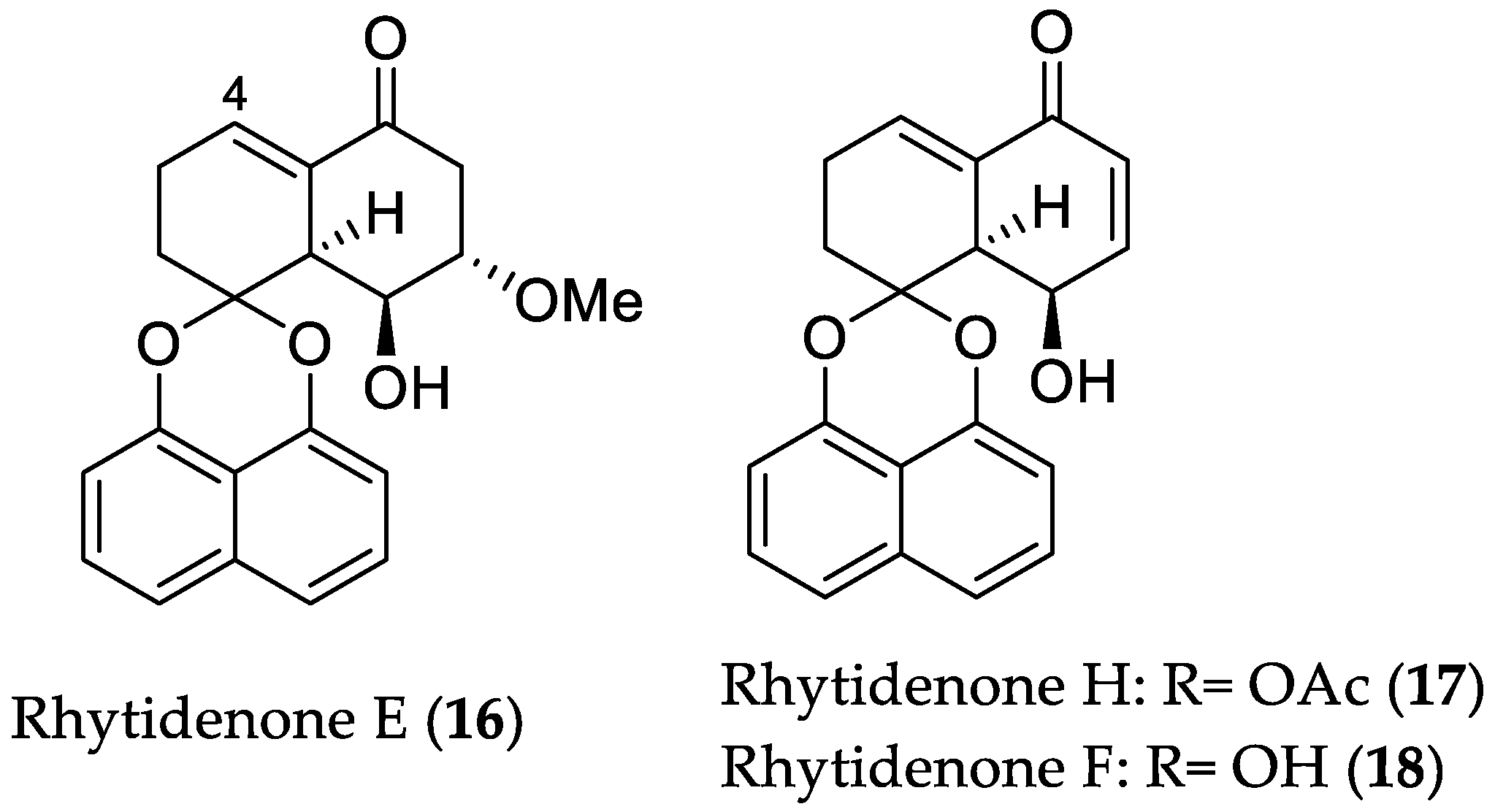
- Pudhom, K.; Teerawatananond, T. Rhytidenones A–F, spirobisnaphthalenes from Rhytidhysteron sp. AS21B, an endophytic fungus. J. Nat. Prod. 2014, 77, 1962–1966. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Z.; Liu, H.; Long, Y.; Chen, D.; Lu, Y.; She, Z. Lasiodiplactone A, a novel lactone from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. Org. Biomol. Chem. 2017, 15, 6338–6341. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Liu, Z.; Lu, Y.; Xia, G.; Liu, H.; He, L.; She, Z. Bioactive metabolites from mangrove endophytic fungus Aspergillus sp. 16–5B. Mar. Drugs 2015, 13, 3091–3102. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Liu, Z.; Cai, R.; Lu, Y.; Huang, X.; She, Z. Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromyces amestolkiae possess α-glucosidase inhibitory and antibacterial activities. RSC Adv. 2016, 6, 26412–26420. [Google Scholar] [CrossRef]
- Cui, H.; Lin, Y.; Luo, M.; Lu, Y.; Huang, X.; She, Z. Diaporisoindoles A–C: Three isoprenylisoindole alkaloid derivatives from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. Org. Lett. 2017, 19, 5621–5624. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, J.; Liu, Z.; Lin, S.; Xia, G.; Xia, X.; Ding, B.; He, L.; Lu, Y.; She, Z. Peniphenones A–D from the mangrove fungus Penicillium dipodomyicola HN4-3A as inhibitors of Mycobacterium tuberculosis phosphatase MptpB. J. Nat. Prod. 2014, 77, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, L.; Chen, D.; Cai, R.; Long, Y.; Lu, Y.; She, Z. Talaramide A, an unusual alkaloid from the mangrove endophytic fungus Talaromyces sp. (HZ-YX1) as an inhibitor of mycobacterial PknG. New J. Chem. 2017, 41, 4273–4276. [Google Scholar] [CrossRef]
- Yu, G.; Zhou, G.; Zhu, M.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Neosartoryadins A and B, fumiquinazoline alkaloids from a mangrove-derived fungus Neosartorya udagawae HDN13-313. Org. Lett. 2016, 18, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Liu, Y.; Li, X.-M.; Xu, G.-M.; Ji, N.-Y.; Wang, B.-G. Citrifelins A and B, citrinin adducts with a tetracyclic framework from cocultures of marine-derived isolates of Penicillium citrinum and Beauveria felina. J. Nat. Prod. 2015, 78, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Q.; Xia, G.; Huang, H.; Li, H.; Ma, L.; Lu, Y.; He, L.; Xia, X.; She, Z. Polyketides with α-glucosidase inhibitory activity from a mangrove endophytic fungus, Penicillium sp. HN29-3B1. J. Nat. Prod. 2015, 78, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Niaz, S.; Khan, D.; Wang, Z.; Zhu, Y.; Zhou, H.; Lin, Y.; Li, J.; Liu, L. Induction of diverse bioactive secondary metabolites from the mangrove endophytic fungus Trichoderma sp. (strain 307) by co-cultivation with Acinetobacter johnsonii (strain B2). Mar. Drugs 2017, 15, e35. [Google Scholar] [CrossRef] [PubMed]
- El-Elimat, T.; Figueroa, M.; Raja, H.A.; Graf, T.N.; Swanson, S.M.; Falkinham, J.O.; Wani, M.C.; Pearce, C.J.; Oberlies, N.H. Biosynthetically distinct cytotoxic polyketides from Setophoma terrestris. Eur. J. Org. Chem. 2015, 2015, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-S.; Li, X.-M.; Williams, K.; Proksch, P.; Ji, N.-Y.; Wang, B.-G. Rhizovarins A–F, indole-diterpenes from the mangrove-derived endophytic fungus Mucor irregularis QEN-189. J. Nat. Prod. 2016, 79, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dai, H.; Makhloufi, G.; Heering, C.; Janiak, C.; Hartmann, R.; Mándi, A.; Kurtán, T.; Müller, W.E.G.; Kassack, M.U.; et al. Cytotoxic 14-membered macrolides from a mangrove-derived endophytic fungus, Pestalotiopsis microspora. J. Nat. Prod. 2016, 79, 2332–2340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, X.; Feng, H.; Dai, J.; Li, J.; Che, Q.; Gu, Q.; Zhu, T.; Li, D. Penicisulfuranols A–F, alkaloids from the mangrove endophytic fungus Penicillium janthinellum HDN13-309. J. Nat. Prod. 2017, 80, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-J.; Bai, M.; Zhou, X.-M.; Huang, G.-L.; Shao, T.-M.; Luo, Y.-P.; Niu, Z.-G.; Niu, Y.-Y.; Chen, G.-Y.; Han, C.-R. Cytotoxic indole diterpenes from the mangrove-derived fungus Eupenicillium sp. HJ002. J. Nat. Prod. 2018, 81, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Lin, X.; Tao, H.; Wang, J.; Li, J.; Yang, B.; Zhou, X.; Liu, Y. Isochromophilones A–F, cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yu, G.; Kurtán, T.; Mándi, A.; Peng, J.; Mo, X.; Liu, M.; Li, H.; Sun, X.; Li, J.; et al. Cytotoxic xanthone–chromanone dimers from the marine-derived fungus Aspergillus versicolor HDN1009. J. Nat. Prod. 2015, 78, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, Z.; Chen, Y.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Varitatin A, a highly modified fatty acid amide from Penicillium variabile cultured with a DNA methyltransferase inhibitor. J. Nat. Prod. 2015, 78, 2841–2845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, X.; Zhang, G.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Inducing secondary metabolite production by combined culture of Talaromyces aculeatus and Penicillium variabile. J. Nat. Prod. 2017, 80, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, Y.; Miao, C.; Liu, P.; Hong, K.; Zhu, W. Sesquiterpenoids and benzofuranoids from the marine-derived fungus Aspergillus ustus 094102. J. Nat. Prod. 2009, 72, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Lu, Z.; Fan, J.; Wang, L.; Zhu, G.; Wang, Y.; Li, X.; Hong, K.; Piyachaturawat, P.; Chairoungdua, A.; et al. Ophiobolins from the mangrove fungus Aspergillus ustus. J. Nat. Prod. 2018, 81, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lu, Z.; Meng, L.; Wei, S.; Hong, K.; Zhu, W.; Huang, C. The novel agent ophiobolin O induces apoptosis and cell cycle arrest of MCF-7 cells through activation of MAPK signaling pathways. Bioorg. Med. Chem. Lett. 2012, 22, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Qin, W.; Zhu, T.; Wei, S.; Hong, K.; Zhu, W.; Chen, R.; Huang, C. Ophiobolin O isolated from Aspergillus ustus induces G1 arrest of MCF-7 cells through interaction with AKT/GSK3β/Cyclin D1 signaling. Mar. Drugs 2015, 13, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lv, C.; Zhu, T.; Yang, X.; Wei, S.; Sun, J.; Hong, K.; Zhu, W.; Huang, C. Ophiobolin O reverses adriamycin resistance via cell cycle arrest and apoptosis sensitization in adriamycin-resistant human breast carcinoma (MCF-7/ADR) cells. Mar. Drugs 2013, 11, 4570–4584. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, Y.; Chen, R.; Liu, D.; Liu, M.; Proksch, P.; Guo, P.; Lin, W. Phenolic metabolites from mangrove-associated Penicillium pinophilum fungus with lipid-lowering effects. RSC Adv. 2016, 6, 21969–21978. [Google Scholar] [CrossRef]
- Han, Y.; Tian, E.; Xu, D.; Ma, M.; Deng, Z.; Hong, K. Halichoblelide D, a new elaiophylin derivative with potent cytotoxic activity from mangrove-derived Streptomyces sp. 219807. Molecules 2016, 21, e970. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chai, W.; Wang, W.; Song, T.; Lian, X.-Y.; Zhang, Z. Cytotoxic bagremycins from mangrove-derived Streptomyces sp. Q22. J. Nat. Prod. 2017, 80, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Che, Q.; Zhu, T.; Keyzers, R.A.; Liu, X.; Li, J.; Gu, Q.; Li, D. Polycyclic hybrid isoprenoids from a reed rhizosphere soil derived Streptomyces sp. CHQ-64. J. Nat. Prod. 2013, 76, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Che, Q.; Li, T.; Liu, X.; Yao, T.; Li, J.; Gu, Q.; Li, D.; Li, W.; Zhu, T. Genome scanning inspired isolation of reedsmycins A–F, polyene-polyol macrolides from Streptomyces sp. CHQ-64. RSC Adv. 2015, 5, 22777–22782. [Google Scholar] [CrossRef]
- Han, X.; Liu, Z.; Zhang, Z.; Zhang, X.; Zhu, T.; Gu, Q.; Li, W.; Che, Q.; Li, D. Geranylpyrrol A and piericidin F from Streptomyces sp. CHQ-64 ΔrdmF. J. Nat. Prod. 2017, 80, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Sasaki, K.; Nagai, K.; Shin-ya, K.; Furihata, K. Structure of thioviridamide, a novel apoptosis inducer from Streptomyces olivoviridis. J. Antibiot. 2006, 59, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Izawa, M.; Kawasaki, T.; Hayakawa, Y. Cloning and heterologous expression of the thioviridamide biosynthesis gene cluster from Streptomyces olivoviridis. Appl. Environ. Microbiol. 2013, 79, 7110–7113. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Izumikawa, M.; Kozone, I.; Hashimoto, J.; Kagaya, N.; Koiwai, H.; Komatsu, M.; Fujie, M.; Sato, N.; Ikeda, H.; et al. Neothioviridamide, a polythioamide compound produced by heterologous expression of a Streptomyces sp. Cryptic RiPP biosynthetic gene cluster. J. Nat. Prod. 2018, 81, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shao, J.; Sun, C.; Song, Y.; Li, Q.; Lu, L.; Hu, Y.; Gui, C.; Zhang, H.; Ju, J.; et al. Cytotoxic and cell migration inhibiting agents from mangrove-derived Streptomyces rochei SCSIO ZJ89. Bioorg. Med. Chem. 2018, 26, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Ruan, B.; Bovee, M.L.; Sacher, M.; Stathopoulos, C.; Poralla, K.; Francklyn, C.S.; Söll, D. A unique hydrophobic cluster near the active site contributes to differences in borrelidin inhibition among threonyl-tRNA synthetases. J. Biol. Chem. 2005, 280, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jiang, Y.; Han, L.; Chen, X.; Hu, C.; Su, H.; Mu, Y.; Guan, P.; Huang, X. Effect of borrelidin on hepatocellular carcinoma cells in vitro and in vivo. RSC Adv. 2017, 7, 44401–44409. [Google Scholar] [CrossRef]
- Sidhu, A.; Miller, J.R.; Tripathi, A.; Garshott, D.M.; Brownell, A.L.; Chiego, D.J.; Arevang, C.; Zeng, Q.; Jackson, L.C.; Bechler, S.A.; et al. Borrelidin induces the unfolded protein response in oral cancer cells and chop-dependent apoptosis. ACS Med. Chem. Lett. 2015, 6, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Li, X.-M.; Liu, Y.; Wang, B.-G. Penicibilaenes A and B, sesquiterpenes with a tricyclo[6.3.1.01,5]dodecane skeleton from the marine isolate of Penicillium bilaiae MA-267. Org. Lett. 2014, 16, 6052–6055. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, X.-M.; Wang, B.-G. Penicisimpins A–C, three new dihydroisocoumarins from Penicillium simplicissimum MA-332, a marine fungus derived from the rhizosphere of the mangrove plant Bruguiera sexangula var. rhynchopetala. Phytochem. Lett. 2016, 17, 114–118. [Google Scholar] [CrossRef]
- Li, H.-L.; Xu, R.; Li, X.-M.; Yang, S.-Q.; Meng, L.-H.; Wang, B.-G. Simpterpenoid A, a meroterpenoid with a highly functionalized cyclohexadiene moiety featuring gem-propane-1,2-dione and methylformate groups, from the mangrove-derived Penicillium simplicissimum MA-332. Org. Lett. 2018, 20, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shen, N.-X.; Chen, Z.-Q.; Zhang, F.-M.; Chen, Y. Penicilones A–D, anti-MRSA azaphilones from the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2017, 80, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Du, F.-Y.; Li, X.-M.; Pedpradab, P.; Xu, G.-M.; Wang, B.-G. Rubrumazines A–C, indolediketopiperazines of the isoechinulin class from Eurotium rubrum MA-150, a fungus obtained from marine mangrove-derived rhizospheric soil. J. Nat. Prod. 2015, 78, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, D.; Peng, J.; Zhu, T.; Gu, Q.; Li, D. Penicitols A–C and penixanacid A from the mangrove-derived Penicillium chrysogenum HDN11-24. J. Nat. Prod. 2015, 78, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; MacMillan, J.B. Thiasporines A–C, thiazine and thiazole derivatives from a marine-derived Actinomycetospora chlora. J. Nat. Prod. 2015, 78, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Misiek, M.; Hoffmeister, D. In vivo and in vitro production options for fungal secondary metabolites. Mol. Pharm. 2008, 5, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J. Plants and endophytes: Equal partners in secondary metabolite production? Biotechnol. Lett. 2015, 37, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Deepika, V.B.; Murali, T.S.; Satyamoorthy, K. Modulation of genetic clusters for synthesis of bioactive molecules in fungal endophytes: A review. Microbiol. Res. 2016, 182, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Daletos, G.; Ebrahim, W.; Ancheeva, E.; El-Neketi, M.; Lin, WH.; Proksch, P. Microbial co-culture and OSMAC approach as strategies to induce cryptic fungal biogenetic gene clusters. In Chemical Biology of Natural Products; Grothaus, P., Cragg, G.M., Newman, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 233–284. ISBN 9781439841945. [Google Scholar]
- Nützmann, H.-W.; Reyes-Dominguez, Y.; Scherlach, K.; Schroeckh, V.; Horn, F.; Gacek, A.; Schümann, J.; Hertweck, C.; Strauss, J.; Brakhage, A.A. Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc. Natl. Acad. Sci. USA 2011, 108, 14282–14287. [Google Scholar] [CrossRef] [PubMed]
- Vasundhara, M.; Kumar, A.; Reddy, M.S. Molecular approaches to screen bioactive compounds from endophytic fungi. Front. Microbiol. 2016, 7, 1774. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Sharma, T.; Dhar, M.K. “Omics” tools for better understanding the plant–endophyte interactions. Front. Plant Sci. 2016, 7, e955. [Google Scholar] [CrossRef] [PubMed]
- Ziemert, N.; Alanjary, M.; Weber, T. The evolution of genome mining in microbes—A review. Nat. Prod. Rep. 2016, 33, 988–1005. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, T.A.J.; Medema, M.H. Computational strategies for genome-based natural product discovery and engineering in fungi. Fungal Genet. Biol. 2016, 89, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Pickens, L.B.; Tang, Y.; Chooi, Y.-H. Metabolic engineering for the production of natural products. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 211–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Wang, Y.; Ang, E.L.; Zhao, H. Engineering microbial hosts for production of bacterial natural products. Nat. Prod. Rep. 2016, 33, 963–987. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Harrison, S.; Zonder, J.; Badros, A.; Laubach, J.; Bergin, K.; Khot, A.; Zimmerman, T.; Chauhan, D.; Levin, N.; et al. A phase 1 clinical trial evaluating marizomib, pomalidomide and low-dose dexamethasone in relapsed and refractory multiple myeloma (NPI-0052-107): Final study results. Br. J. Haematol. 2018, 180, 41–51. [Google Scholar] [CrossRef] [PubMed]
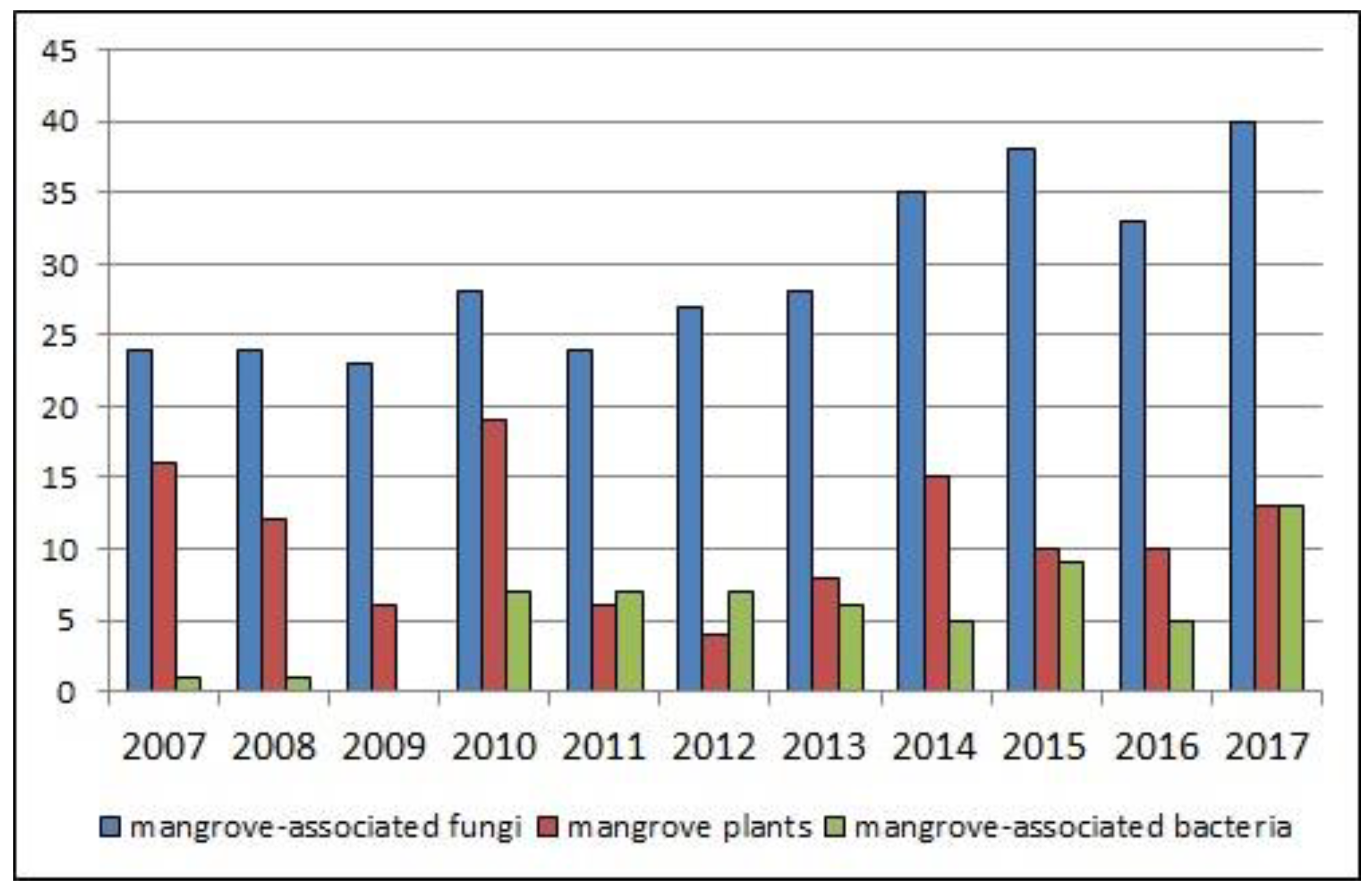
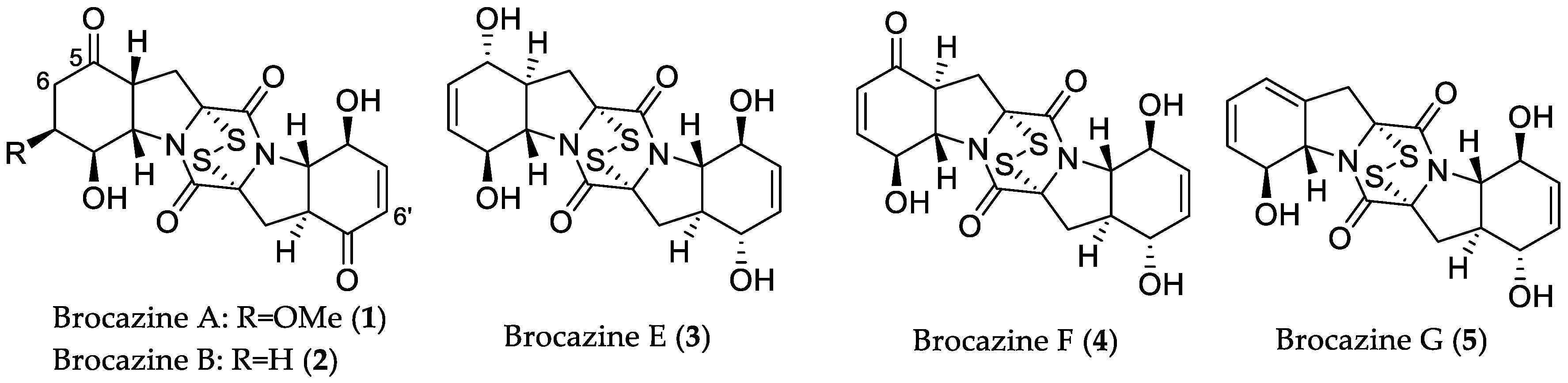
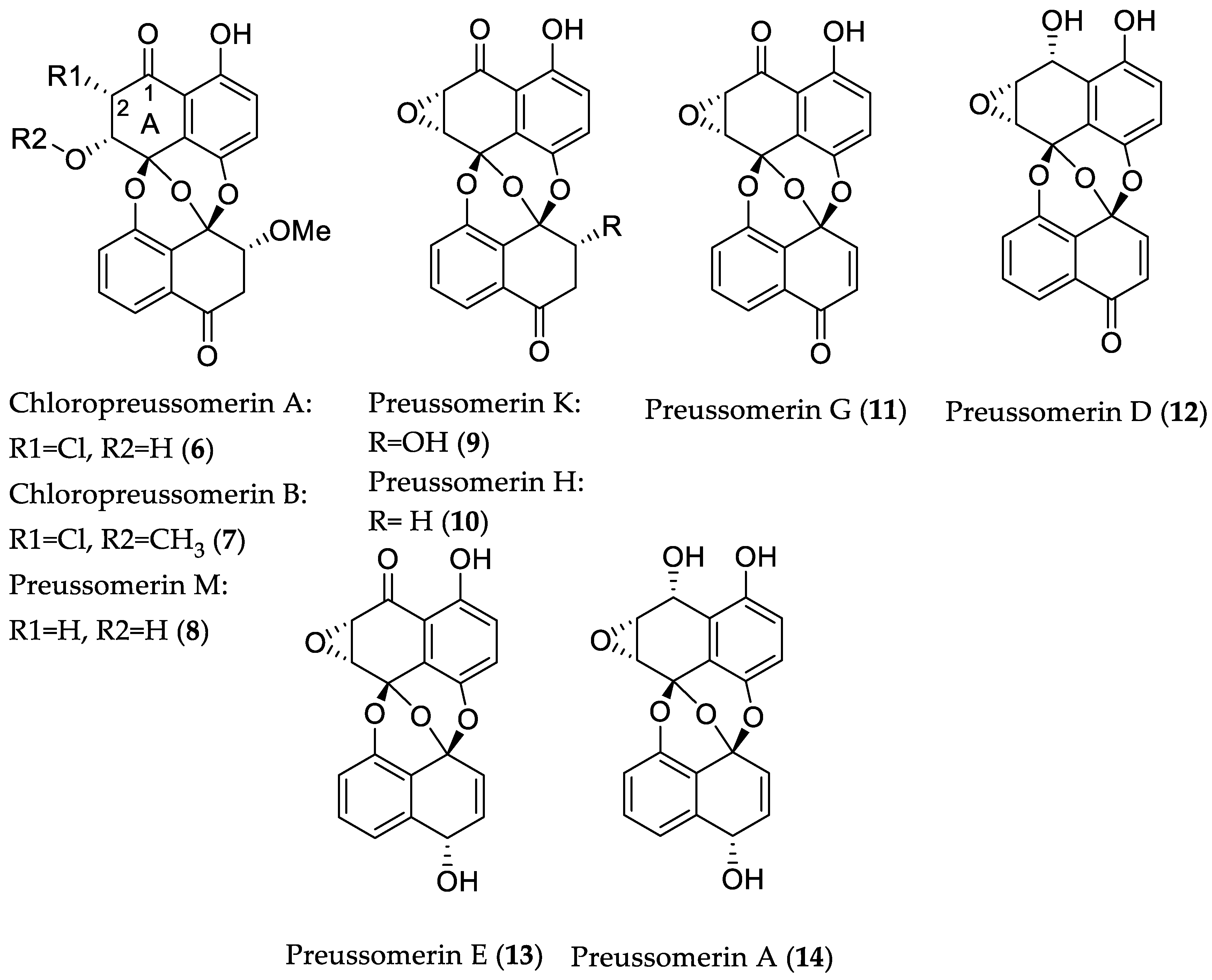
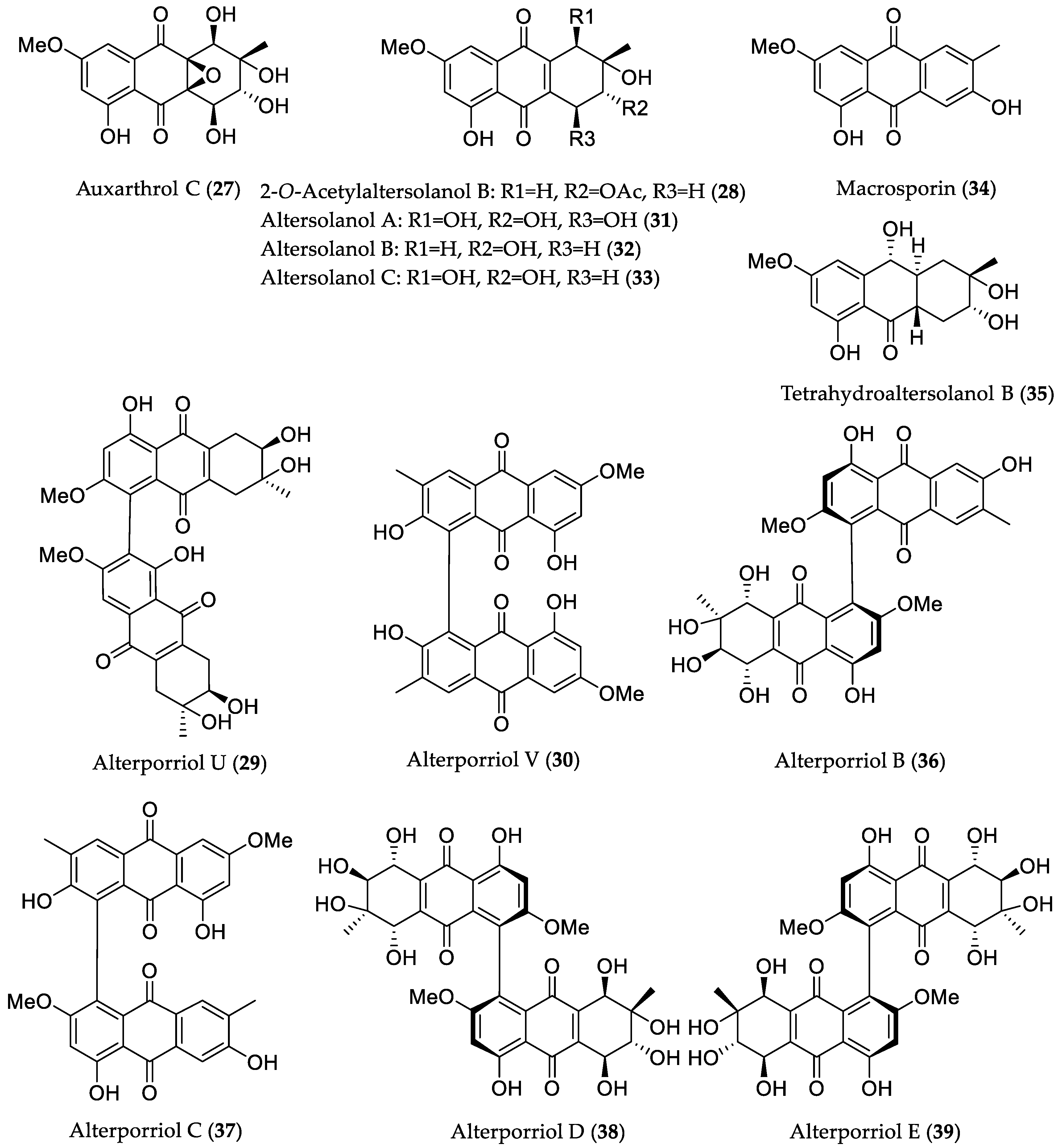
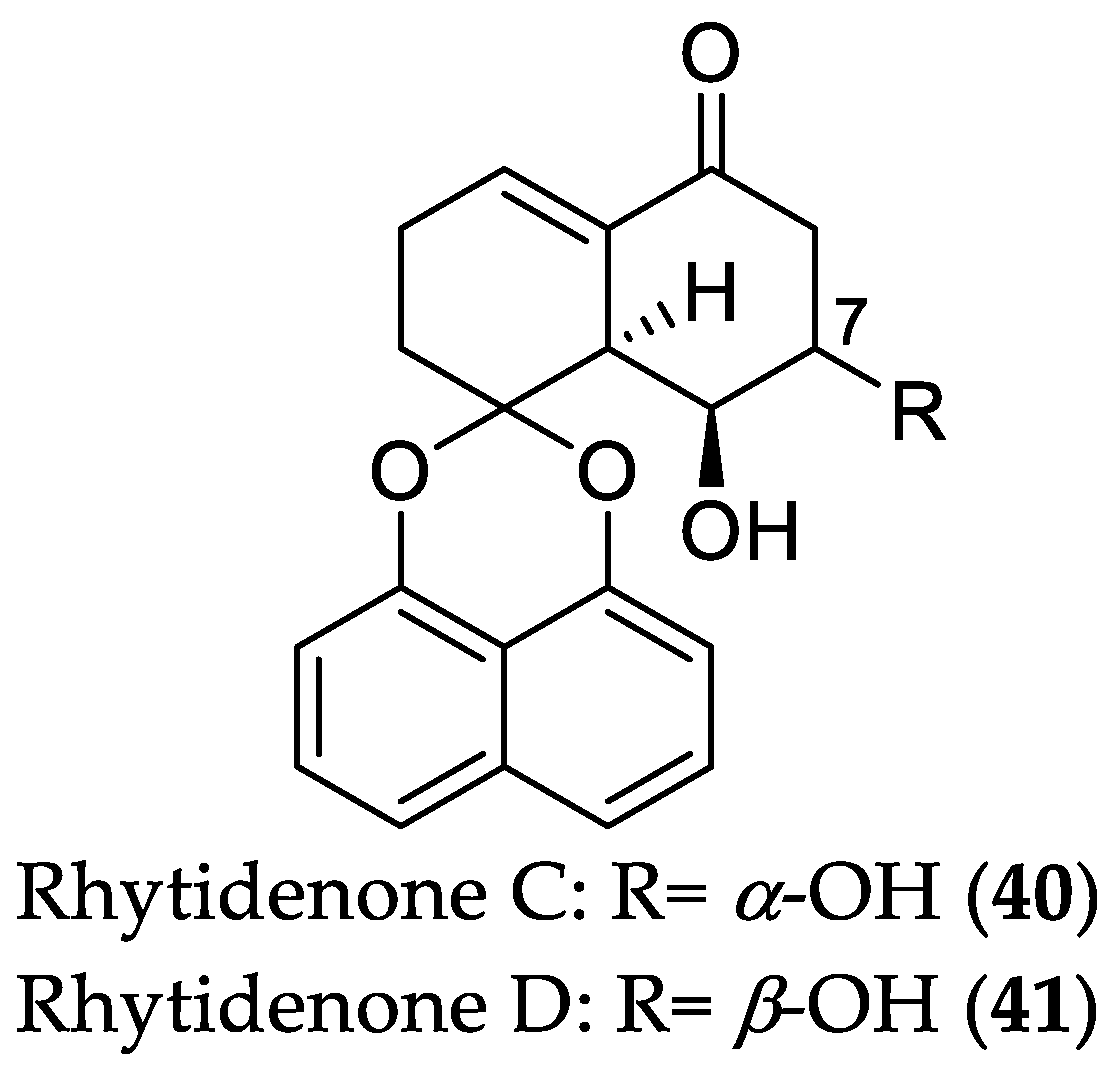
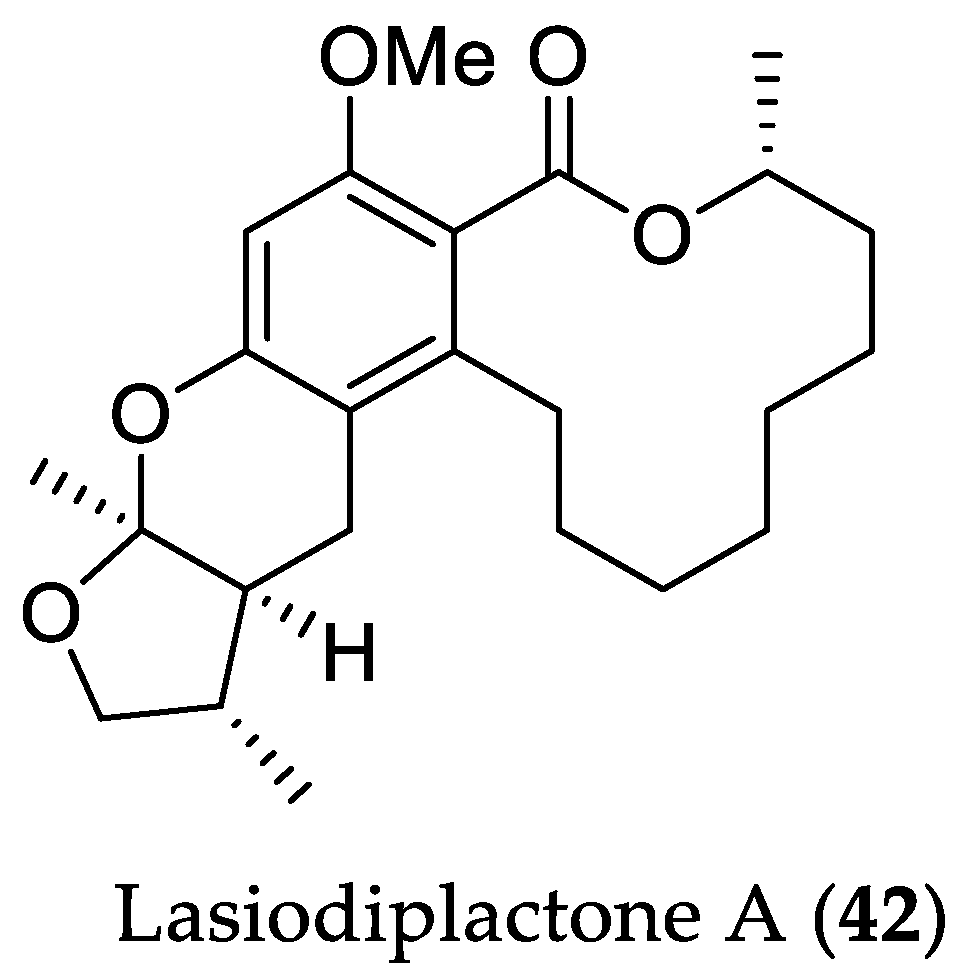

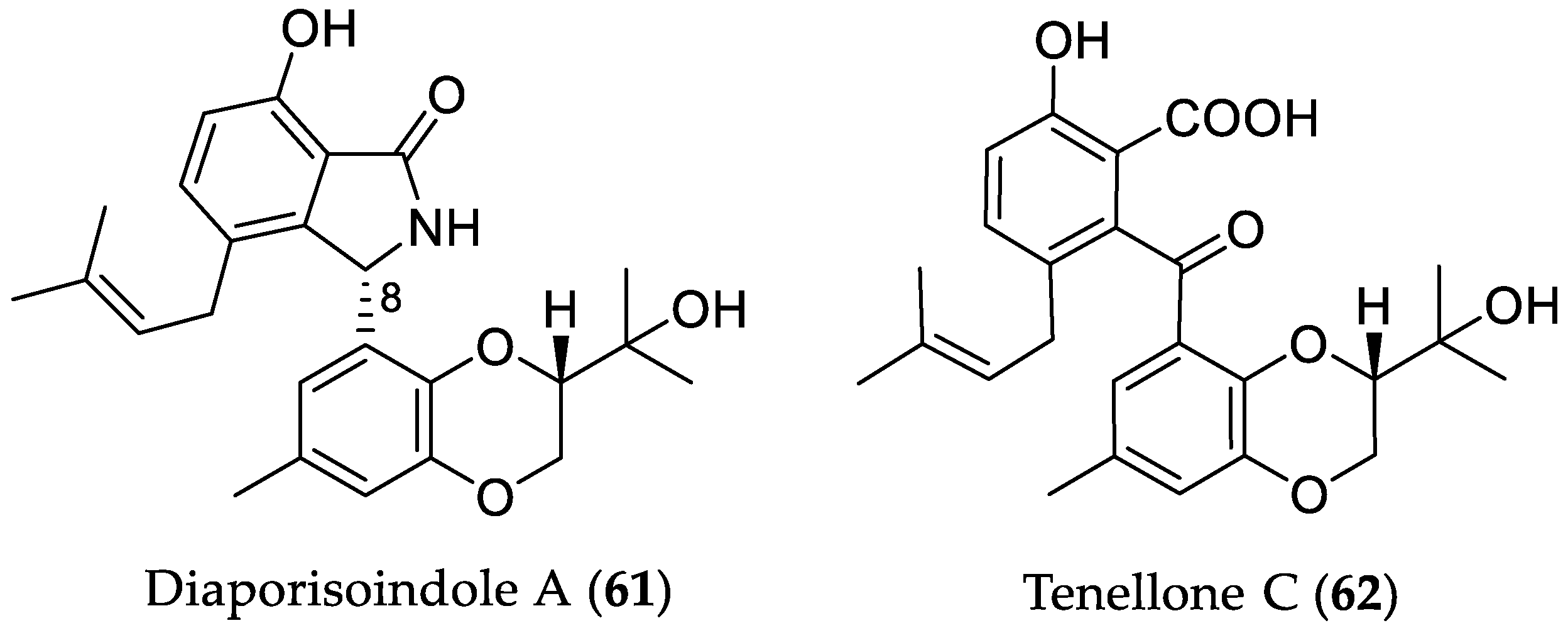
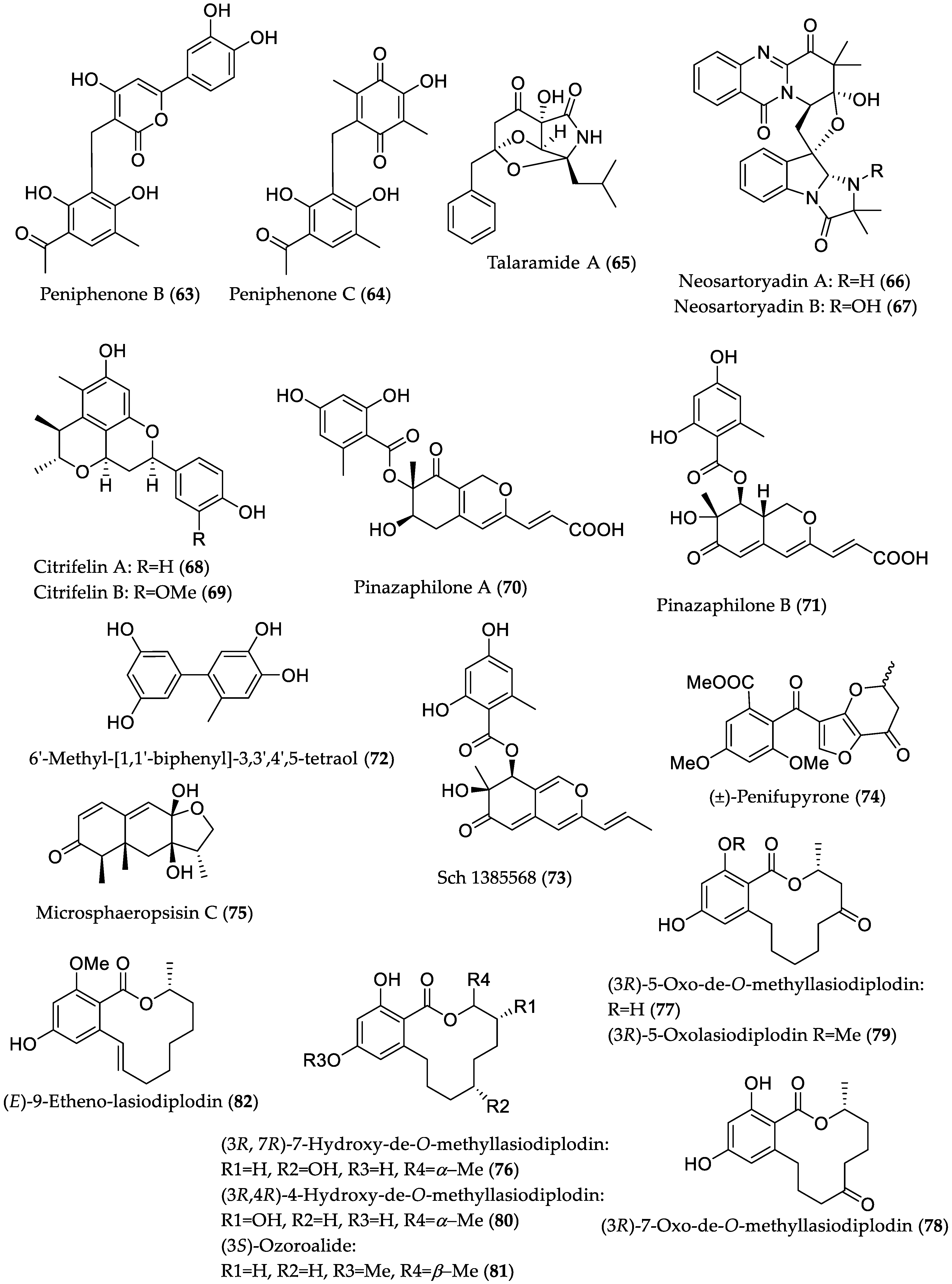
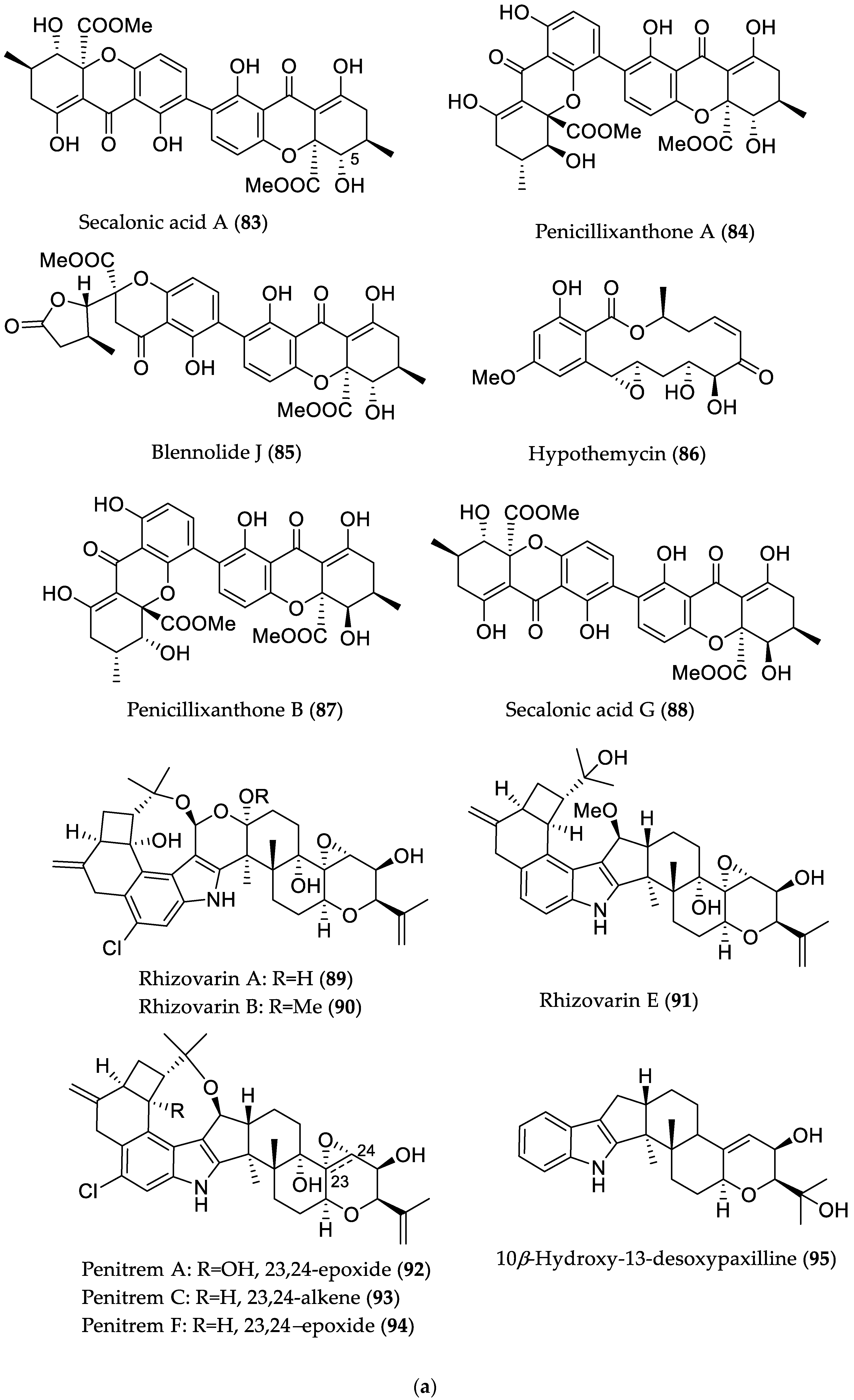
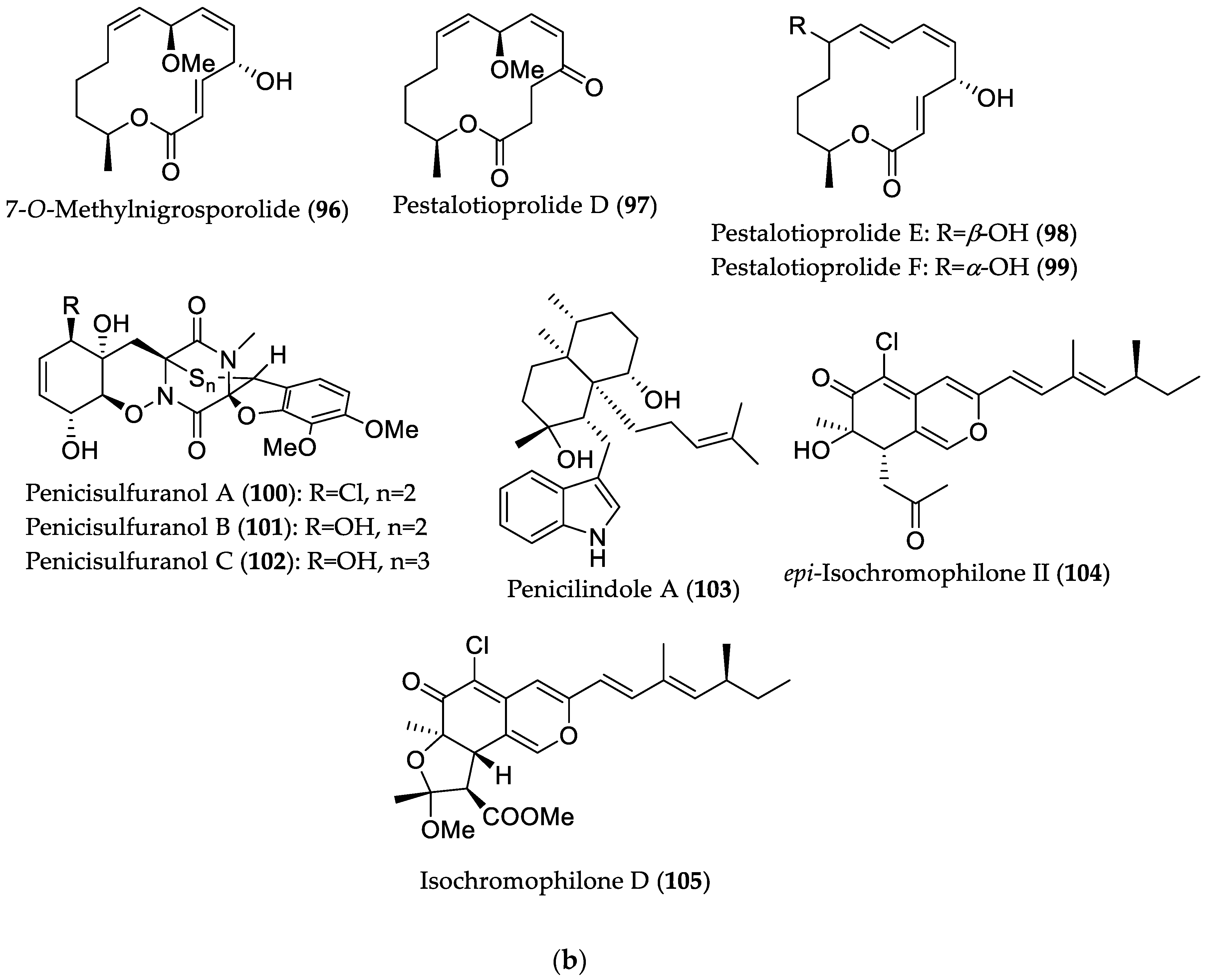
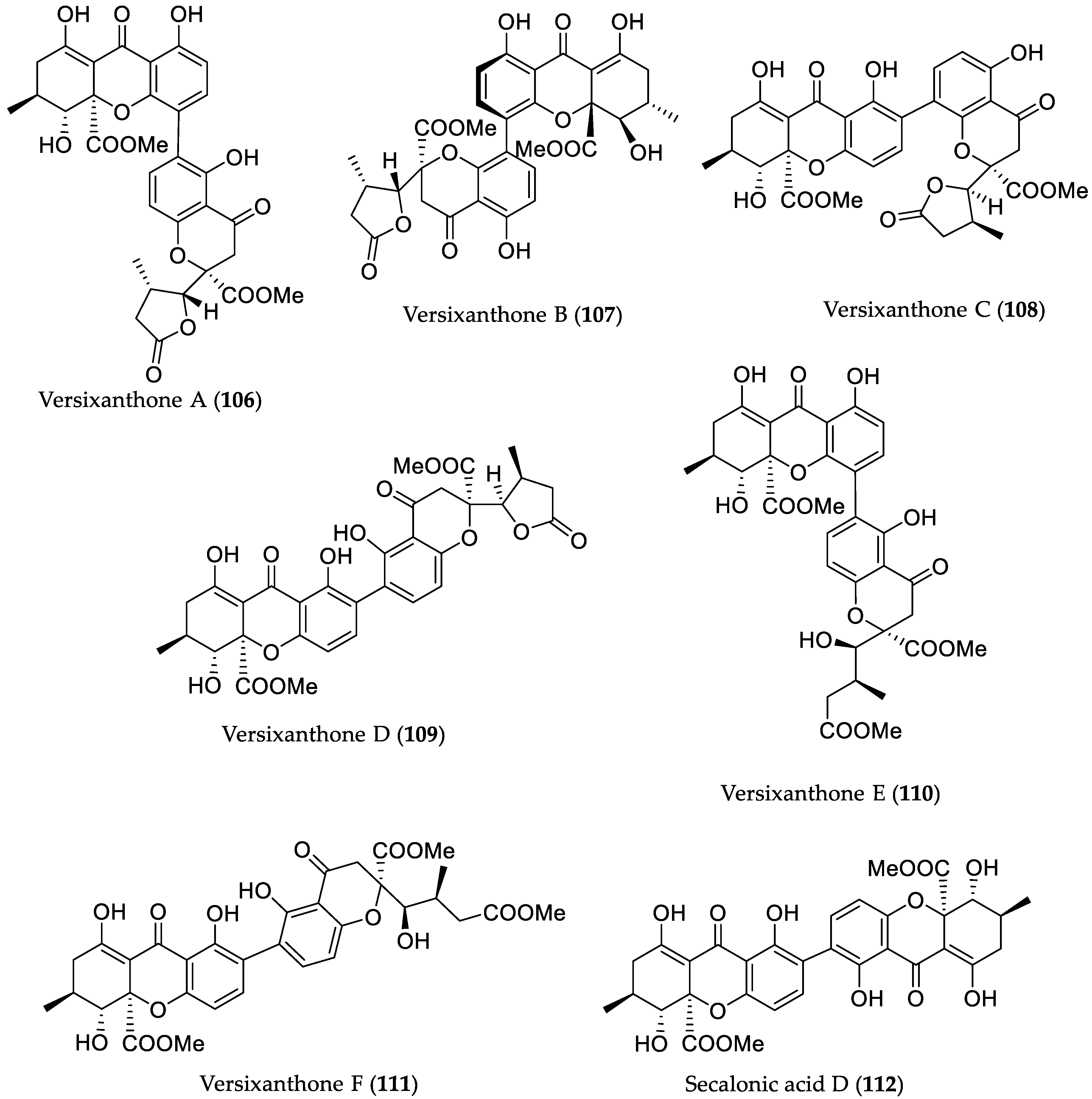
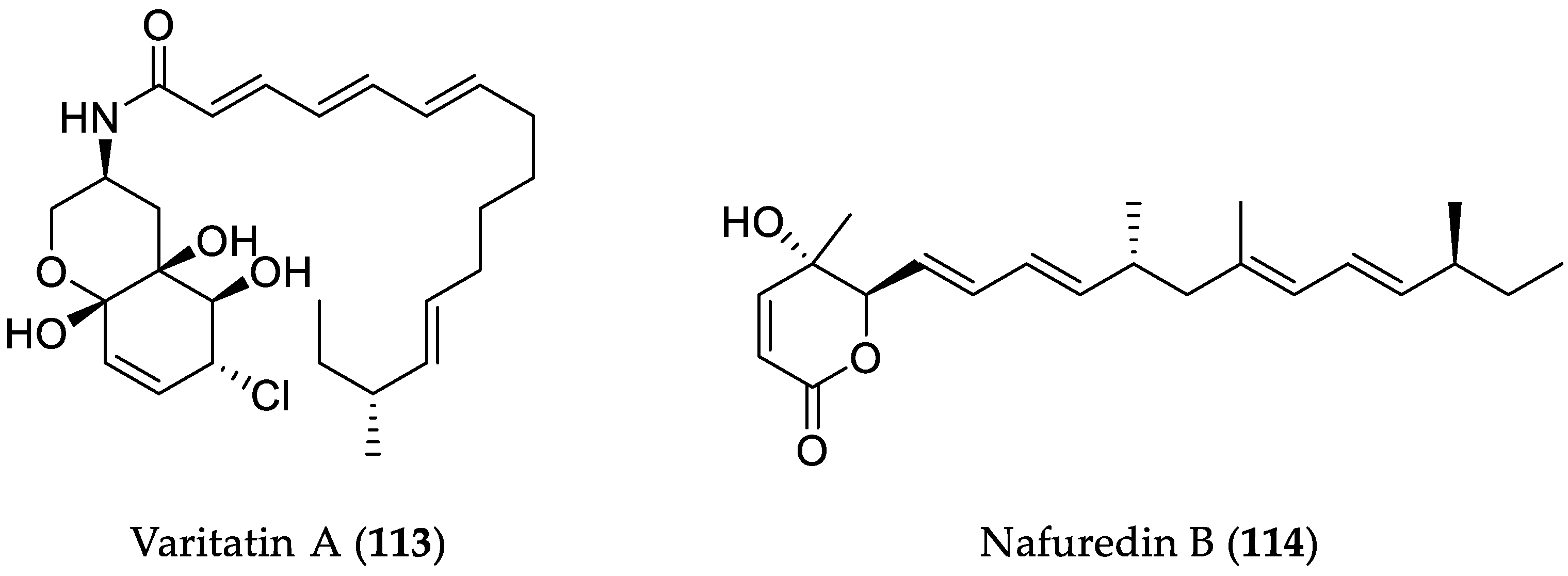
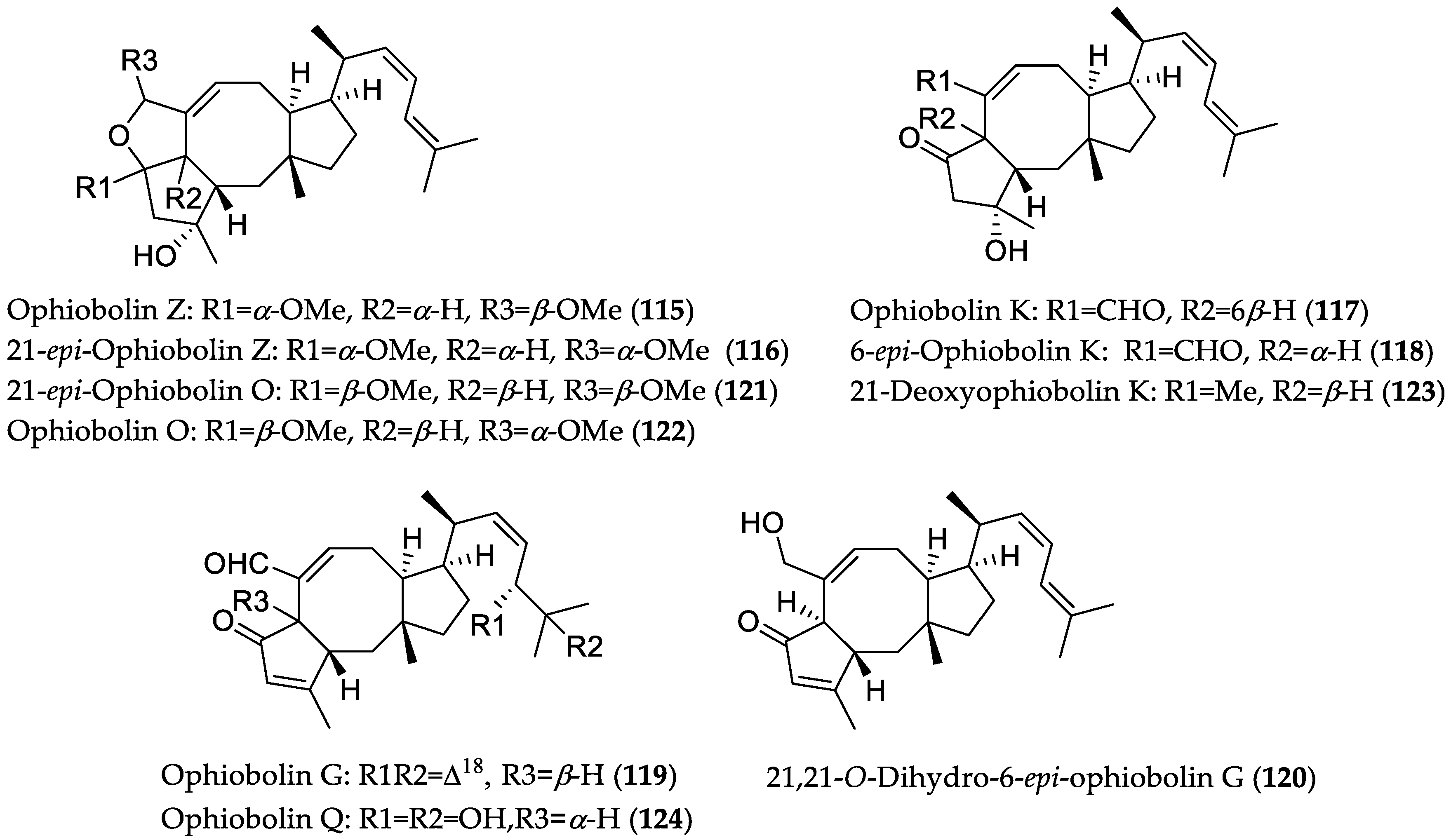

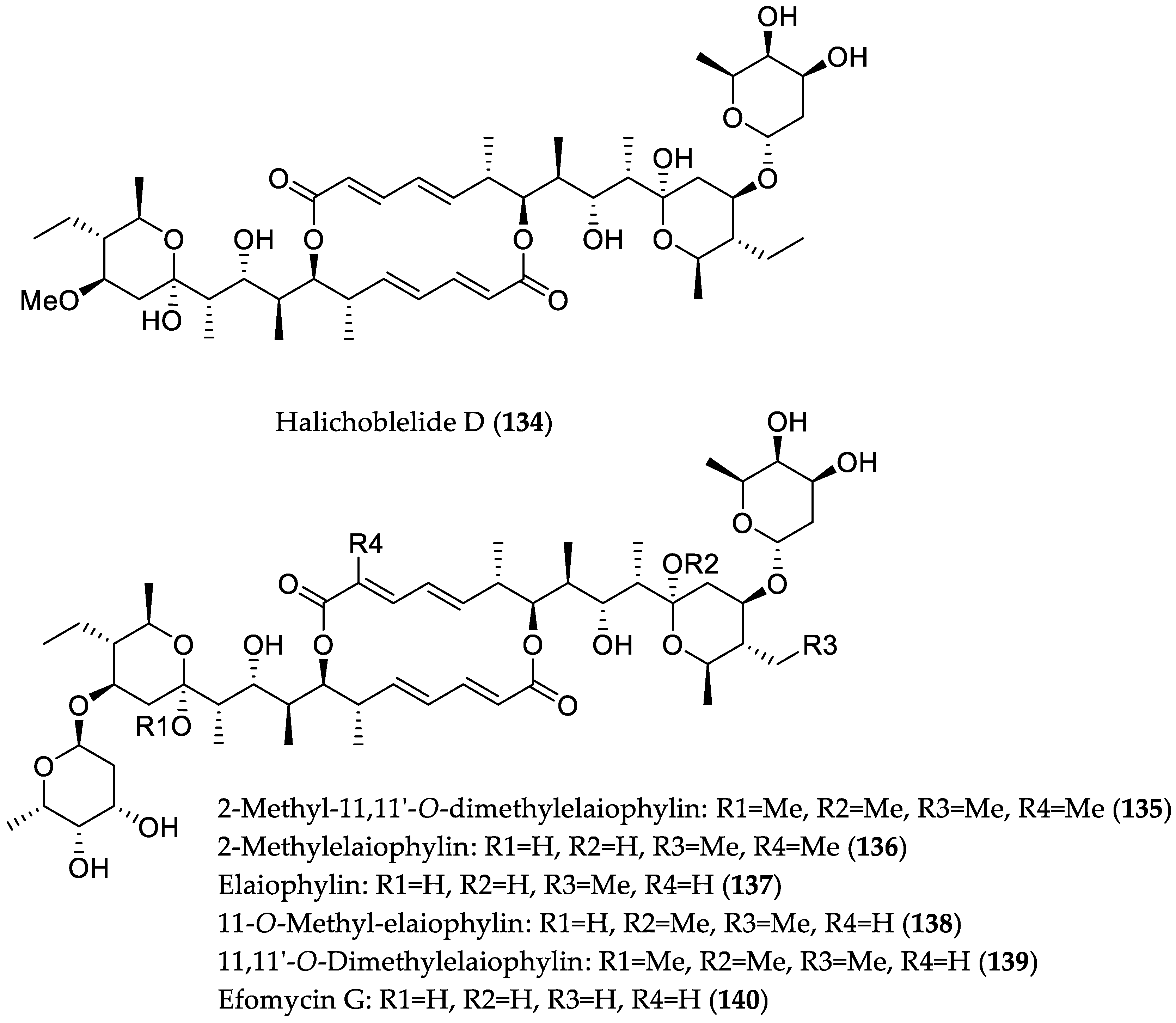
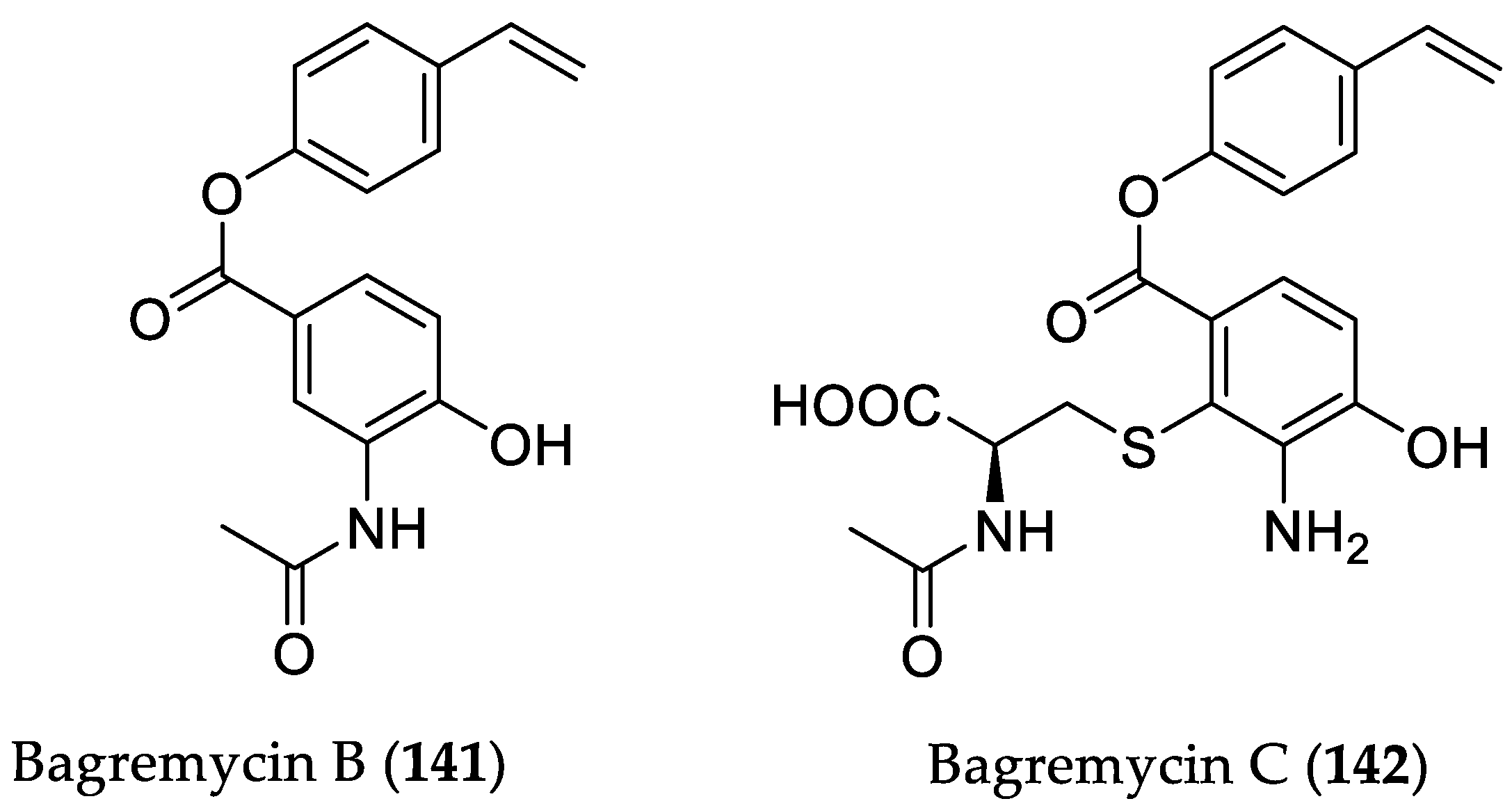



| Compound Name | Source | Type of Activity [Ref.] | IC50 or MIC Values |
|---|---|---|---|
| Peniphenone B (63) | Penicillium dipodomyicola HN4-3A from Acanthus ilicifolius (Hainan Province, China) | MptpB inhibitory [39] | IC50 0.16 μM |
| Peniphenone C (64) | IC50 1.37 μM | ||
| Talaramide A (65) | Talaromyces sp. HZ-YX1 from Kandelia obovata (Guangdong Province, China) | Mycobacterial serine/threonine protein kinase G (PknG) inhibitory [40] | IC50 55 μM, positive control AX20017 (IC50 = 98 μM) 1 |
| Neosartoryadin A (66) | Neosartorya udagawae HDN13-313 from Avicennia marina (Hainan Province, China) | Anti-influenza A virus (H1N1) [41] | IC50 66 μM; positive control ribavirin (IC50 94 μM); no cytotoxicity against the human leukemia (HL-60) cell line |
| Neosartoryadin B (67) | IC50 58 μM; -//- | ||
| Citrifelin A (68) | Co-culture of Penicillium citrinum MA-197 (from Lumnitzera racemosa) and Beauveria felina EN-135 | Antibacterial against E. coli and S. aureus [42] | MIC 24.5 μM/8.0 μg/mL (both E. coli and S. aureus) |
| Citrifelin B (69) | MICs 5.6 μM/2.0 μg/mL (E. coli) and 11.2 μM/4.0 μg/mL (S. aureus) | ||
| Pinazaphilone A (70) | Penicillium sp. HN29-3B1 from Cerbera manghas (Hainan Island, China) | α-Glucosidase inhibitory [43] | IC50 81.7 μM; positive control acarbose (IC50 = 446.7 μM) |
| Pinazaphilone B (71) | IC50 28.0 μM; -//- | ||
| 6′-Methyl-[1,1′-biphenyl]-3,3′,4′,5-tetraol (72) | IC50 2.2 μM; -//- | ||
| Sch 1385568 (73) | IC50 16.6 μM; -//- | ||
| (±)-Penifupyrone (74) | IC50 14.4 μM; -//- | ||
| Microsphaeropsisin C (75) | Co-culture of Trichoderma sp. 307 (from Clerodendrum inerme; Guangdong Province, China) with Acinetobacter johnsonii B2 | α-Glucosidase inhibitory [44] | IC50 188.7 μM; positive control acarbose (IC50 = 703.8 μM) |
| (3R,7R)-7-Hydroxy-de-O-methyllasiodiplodin (76) | IC50 25.8 μM; -//- | ||
| (3R)-5-Oxo-de-O-methyllasiodiplodin (77) | IC50 54.6 μM; -//- | ||
| (3R)-7-Oxo-de-O-methyllasiodiplodin (78) | IC50 178.5 μM; -//- | ||
| (3R)-5-Oxolasiodiplodin (79) | IC50 176.8 μM; -//- | ||
| (3R,4R)-4-Hydroxy-de-O-methyllasiodiplodin (80) | IC50 60.3 μM; -//- | ||
| (3S)-Ozoroalide (81) | IC50 198.1 μM; -//- | ||
| (E)-9-Etheno-lasiodiplodin (82) | IC50 101.3 μM; -//- | ||
| Secalonic acid A (83) | Plant endophyte Setophoma terrestris from a leaf litter collected in a mangrove habitat | Cytotoxic against MDA-MB-435 (melanoma) and SW-620 (colon cancer) cell lines [45] | IC50 0.16 (MDA-MB-435) and 0.41 μM (SW-620) |
| Penicillixanthone A (84) | IC50 0.18 (MDA-MB-435) and 0.21 μM (SW-620) | ||
| Blennolide J (85) | IC50 4.06 (MDA-MB-435) and 6.14 μM (SW-620) | ||
| Hypothemycin (86) | IC50 0.58 (MDA-MB-435) and 2.14 μM (SW-620) | ||
| Penicillixanthone B (87) | IC50 5.20 (MDA-MB-435) and 5.55 μM (SW-620) | ||
| Secalonic acid G (88) | Cytotoxic against MDA-MB-435 and SW-620 cell lines/antibacterial against M. luteus [45] | IC50 3.27 (MDA-MB-435) and 3.67 μM (SW-620)/MIC 7.83 μM (/5 μg/mL) | |
| Rhizovarin A (89) | Mucor irregularis QEN-189 from Rhizophora stylosa (Hainan Island, China) | Cytotoxic against A549 and/or HL-60 (promyelocytic leukemia) cancer cell lines [46] | 9.6 μM (HL-60) |
| Rhizovarin B (90) | 6.3 (A549) and 5.0 μM (HL-60) | ||
| Rhizovarin E (91) | 9.2 μM (A549) | ||
| Penitrem A (92) | 8.4 (A549) and 7.0 μM (HL-60) | ||
| Penitrem C (93) | 8.0 (A549) and 4.7 μM (HL-60) | ||
| Penitrem F (94) | 8.2 (A549) and 3.3 μM (HL-60) | ||
| 10β-Hydroxy-13-desoxypaxilline (95) | 4.6 (A549) and 2.6 μM (HL-60) | ||
| 7-O-Methylnigrosporolide (96) | Pestalotiopsis microspora from Drepanocarpus lunatus (Cameroon) | Cytotoxic against L5178Y (murine lymphoma) cell line or human ovarian (A2780) cancer cell line [47] | IC50 0.7 μM (L5178Y) |
| Pestalotioprolide D (97) | IC50 5.6 μM (L5178Y) | ||
| Pestalotioprolide E (98) | IC50 3.4 (L5178Y) and 1.2 μM; (A2780) | ||
| Pestalotioprolide F (99) | IC50 3.9 μM (L5178Y) | ||
| Penicisulfuranol A (100) | Penicillium janthinellum HDN13-309 from Sonneratia caseolaris (Hainan Province, China) | Cytotoxic against HeLa and HL-60 cell lines [48] | IC50 0.5 (HeLa) and 0.1 (HL-60) μM; |
| Penicisulfuranol B (101) | IC50 3.9 (HeLa) and 1.6 μM (HL-60) | ||
| Penicisulfuranol C (102) | IC50 0.3 (HeLa) and 1.2 μM (HL-60) | ||
| Penicilindole A (103) | Eupenicillium sp. HJ002 from Xylocarpus granatum (South China Sea) | Cytotoxic against A549 and HepG2 cell lines [49] | IC50 5.5 (A549) and 1.5 (HepG2) μM |
| epi-Isochromophilone II (104) | Diaporthe sp. SCSIO 41011 from Rhizophora stylosa (Hainan Province, China) | Cytotoxic against renal carcinoma cell lines: ACHN, OS-RC-2, and 786-O [50] | IC50 4.4 (ACHN), 3.0 (786-O) and 3.9 μM (OS-RC-2) |
| Isochromophilone D (105) | IC50 14 (ACHN), 8.9 (786-O) and 13 μM (OS-RC-2); induced apoptosis (in 786-O cells) in a dose- and time-dependent manner, whereas it did not induce cell cycle arrest at a concentration level up to 10 μM. |
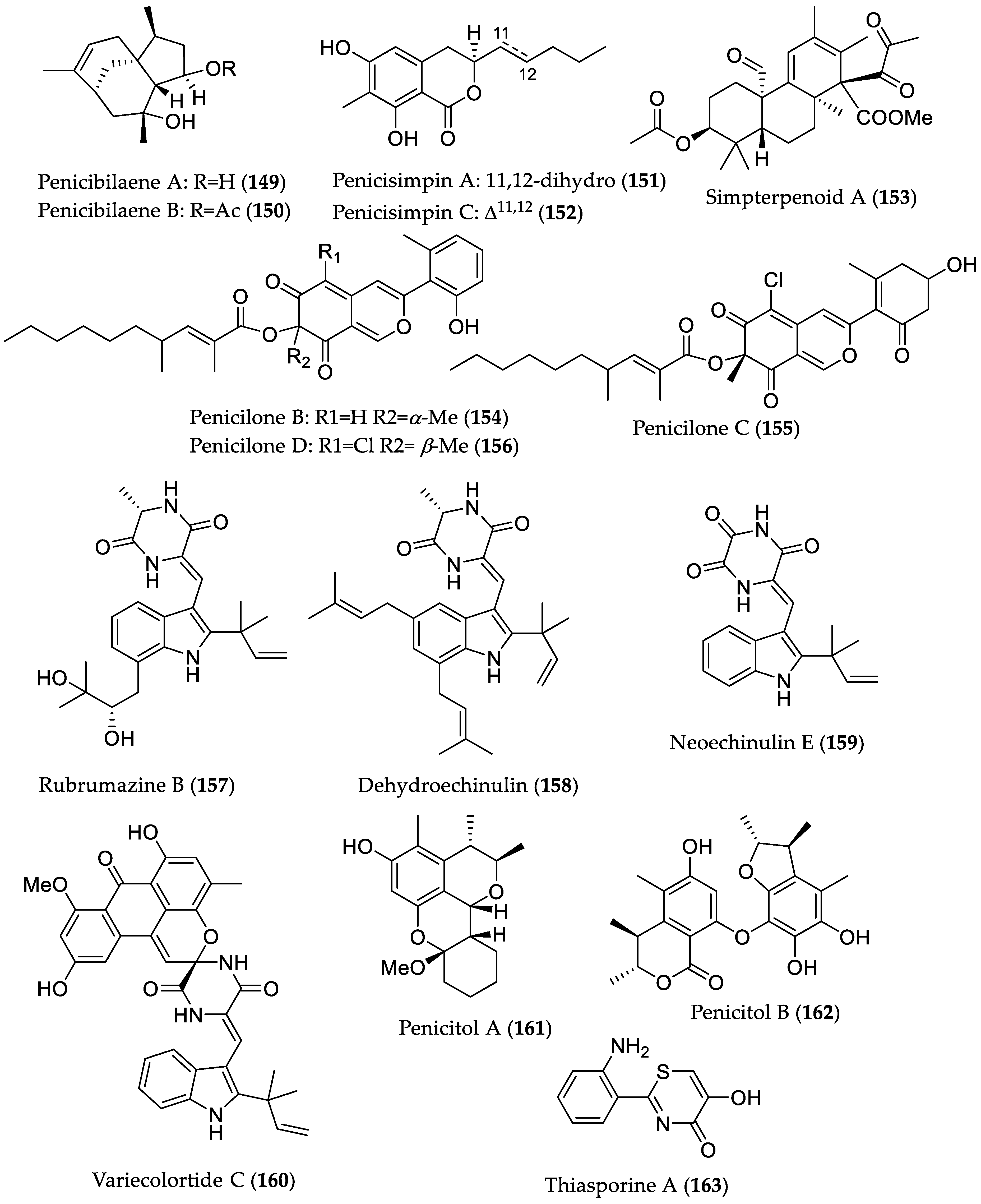
| Penicibilaene A (149) | Penicillium bilaiae MA-267 from the rhizospheric soil of Lumnitzera racemosa (Hainan Island, China) | Antifungal against Colletotrichum gloeosporioides [72] | MIC 4.23 μM/1.0 μg/mL |
| Penicibilaene B (150) | MIC 0.45 μM/0.125 μg/mL | ||
| Penicisimpin A (151) | Penicillium simplicissimum MA-332 from the rhizospheric soil of B. sexangula var. rhynchopetala (Hainan Island, China) | Antibacterial and antifungal [73] | MIC 15.1 μM/4.0 μg/mL (E. coli, P. aeruginosa, Vibrio harveyi, Vibrio parahaemolyticus C. gloeosporioides) and 30.3 μM (8 μg/mL) (M. luteus, Vibrio alginolyticus) MIC 15.1 μM/4.0 μg/mL (C. gloeosporioides) |
| Penicisimpin C (152) | MIC 30.5 μM/8.0 μg/mL (E. coli, P. aeruginosa, V. harveyi, V. parahaemolyticus) MIC 30.5 μM (8.0 μg/mL) (C. gloeosporioides) | ||
| Simpterpenoid A (153) | Influenza neuraminidase inhibitory activity [74] | IC50 8.1 nM | |
| Penicilone B (154) | Penicillium janthinellum HK1-6, isolated from mangrove rhizosphere soil (Dongzhaigang, Hainan Island) | Antibacterial against methicillin-resistant and -susceptible S. aureus, vancomycin-resistant Enterococcus faecalis, and -susceptible Enterococcus faecium strains [75] | MIC 6.54 μM/3.13 μg/mL |
| Penicilone C (155) | MIC 11.8–23.5 μM (6.25–12.5 μg/mL) | ||
| Penicilone D (156) | MIC 6.1–24.4 μM (3.13–12.5 μg/mL) | ||
| Rubrumazine B (157) | Eurotium rubrum MA-150 from mangrove-derived rhizospheric soil (Andaman Sea coastline, Thailand) | Cytotoxic in brine shrimp assay [76] | LD50 2.4 μM |
| Dehydroechinulin (158) | LD50 3.5 μM | ||
| Neoechinulin E (159) | LD50 3.9 μM | ||
| Variecolortide C (160) | LD50 9.8 μM | ||
| Penicitol A (161) | Penicillium chrysogenum HND 11–24 from the rhizosphere soil of Acanthus ilicifolius | Cytotoxic against several cancer cell lines and HEK 293 [77] | IC50 4.6–7.6 μM; HeLa, HEK 293, HCT-116, and A549 cell lines |
| Penicitol B (162) | IC50 3.4–9.6 μM; HeLa, BEL-7402 (hepatocellular carcinoma), HEK 293, HCT-116, and A549 cell lines | ||
| Thiasporine A (163) | Actinomycetospora chlora SNC-032 from mangrove swamp sediment sample (Vava’u, Tonga) | Cytotoxic toward non-small-cell lung cancer H2122 cell line [78] | IC50 5.4 μM |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ancheeva, E.; Daletos, G.; Proksch, P. Lead Compounds from Mangrove-Associated Microorganisms. Mar. Drugs 2018, 16, 319. https://doi.org/10.3390/md16090319
Ancheeva E, Daletos G, Proksch P. Lead Compounds from Mangrove-Associated Microorganisms. Marine Drugs. 2018; 16(9):319. https://doi.org/10.3390/md16090319
Chicago/Turabian StyleAncheeva, Elena, Georgios Daletos, and Peter Proksch. 2018. "Lead Compounds from Mangrove-Associated Microorganisms" Marine Drugs 16, no. 9: 319. https://doi.org/10.3390/md16090319
APA StyleAncheeva, E., Daletos, G., & Proksch, P. (2018). Lead Compounds from Mangrove-Associated Microorganisms. Marine Drugs, 16(9), 319. https://doi.org/10.3390/md16090319