New Eudesmane-Type Sesquiterpenoids from the Mangrove-Derived Endophytic Fungus Penicillium sp. J-54
Abstract
1. Introduction
2. Results
2.1. Structural Elucidation
2.2. The Bioactivities of Compounds 1–4 from Penicillium sp. J-54
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Purification and Identification
3.5. Preparation of S-MTPA and R-MTPA Esters 1a, 1b, 2a, 2b, 3a, and 3b of Compounds 1, 2, and 3
3.6. Absolute Configuration of the 1, 2-Diol Moiety in 1
3.7. Bioassays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gunatilaka, A.A.L. Natural products from plant-associatedmicroorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Wang, Y.; Sun, X.; Tang, K. Bioactive natural products from endophytes: A review. Appl. Biochem. Microbiol. 2008, 44, 136–142. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munroa, M.H.G.; Prinsepd, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munroa, M.H.G.; Prinsepd, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munroa, M.H.G.; Prinsepd, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munroa, M.H.G.; Prinsepd, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munroa, M.H.G.; Prinsepd, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munroa, M.H.G.; Prinsepd, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Jiang, J.Y.; Liu, Z.M.; Lin, S.; Xia, G.P.; Xia, X.K.; Dingm, B.; He, L.; Lu, Y.J.; She, Z.G. Peniphenones A–D from the mangrove fungus Penicillium dipodomyicola HN4-3A as inhibitors of mycobacterium tuberculosis phosphatase (MptpB). J. Nat. Prod. 2014, 77, 800–806. [Google Scholar] [CrossRef] [PubMed]
- An, C.Y.; Li, X.M.; Li, C.S.; Wang, M.H.; Xu, G.M.; Wang, B.G. 4-Phenyl-3,4-dihydroquinolone derivatives from Aspergillus nidulans MA-143 an endophytic fungus isolated from the mangrove plant Rhizophora stylosa. J. Nat. Prod. 2013, 76, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.D.; Wang, Y.; Liu, P.P.; Tian, H.D.; Zhu, W.M. Thiodiketopiperazines from the marine-derived Fungus Phoma sp. OUCMDZ-1847. J. Nat. Prod. 2014, 77, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.F.; Zuo, W.J.; Guo, Z.K.; Mei, W.L.; Dai, H.F. Metabolites from the endophytic fungus Penicillium sp. FJ-1 of Ceriops tagal. Acta Pharm. Sin. 2013, 48, 1688–1691. [Google Scholar]
- Liu, M.L.; Duan, Y.H.; Zhang, J.B.; Yu, Y.; Dai, Y.; Yao, X.S. Novel sesquiterpenes from Nardostachys chinensis Batal. Tetrahedron 2013, 69, 6574–6578. [Google Scholar] [CrossRef]
- Di, B.L.; Pescitelli, G.; Pratelli, C.; Pini, D.; Salvadori, P. Determination of absolute configuration of acyclic 1,2-diols with Mo2(OAc)4.1.Snatzke’s method revisited. J. Org. Chem. 2001, 66, 4819–4825. [Google Scholar]
- Gorecki, M.; Jablonska, E.; Kruszewska, A.; Suszczynska, A.; Urbanczyk-Lipkowska, Z.; Gerards, M.; Morzycki, J.W.; Szczepek, W.J.; Frelek, J. Practical method for the absolute configuration assignment of tert/tert 1,2-diols using their complexes with Mo2(OAc)4. J. Org. Chem. 2007, 72, 2906–2916. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fu, P.; Kong, F.D.; Wang, Y.F.; Wang, Y.; Liu, P.P.; Zuo, G.Y.; Zhu, W.M. Antibiotic metabolites from the coral-associated actinomycete Streptomyces sp. OUCMDZ-1703. Chin. J. Chem. 2013, 31, 100–104. [Google Scholar] [CrossRef]
- Chen, S.X.; Ren, F.X.; Niu, S.B.; Liu, X.Z.; Che, Y.S. Dioxatricyclic and oxabicyclic polyketides from Trichocladium opacum. J. Nat. Prod. 2014, 77, 9–14. [Google Scholar] [CrossRef] [PubMed]
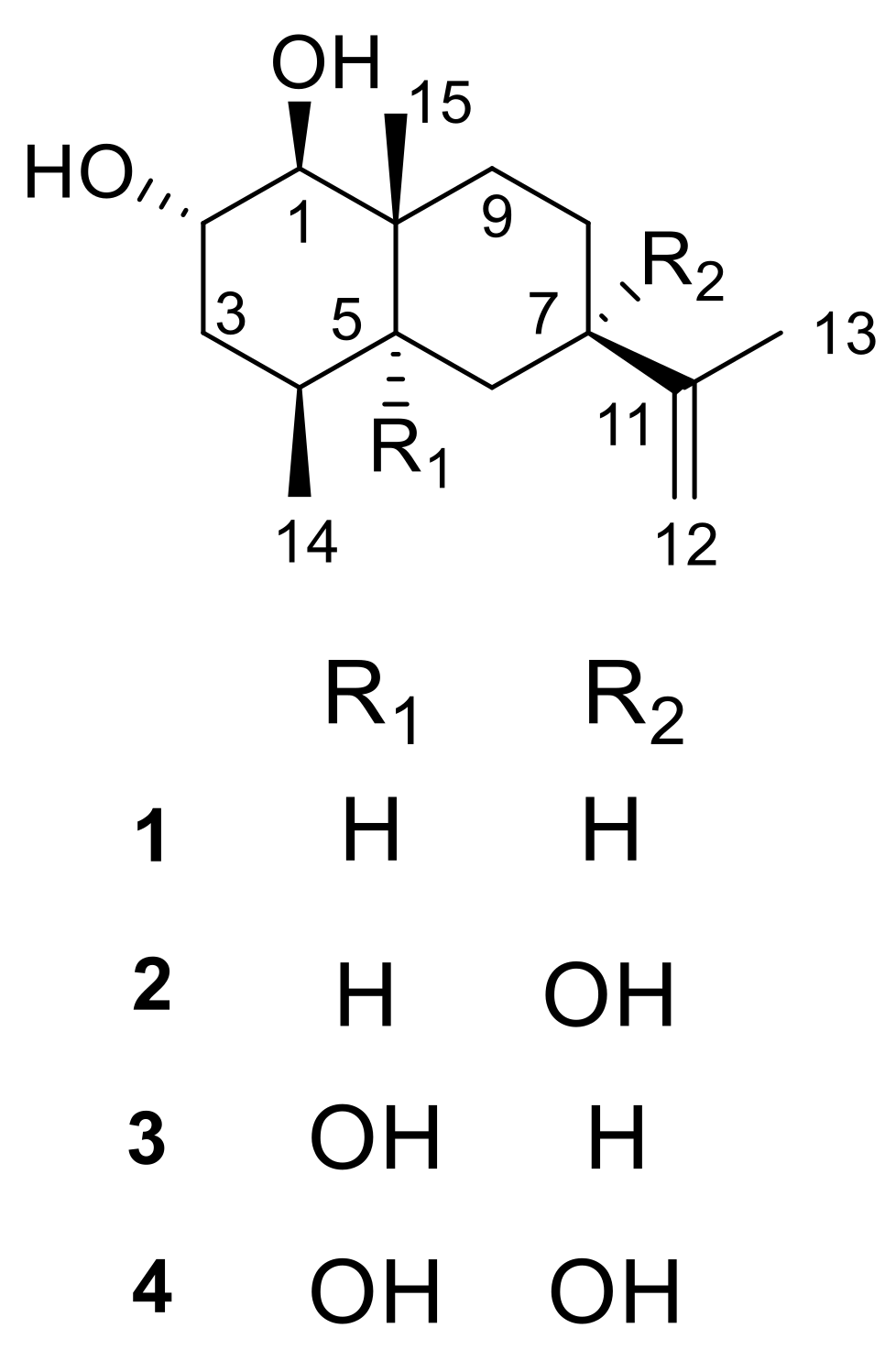
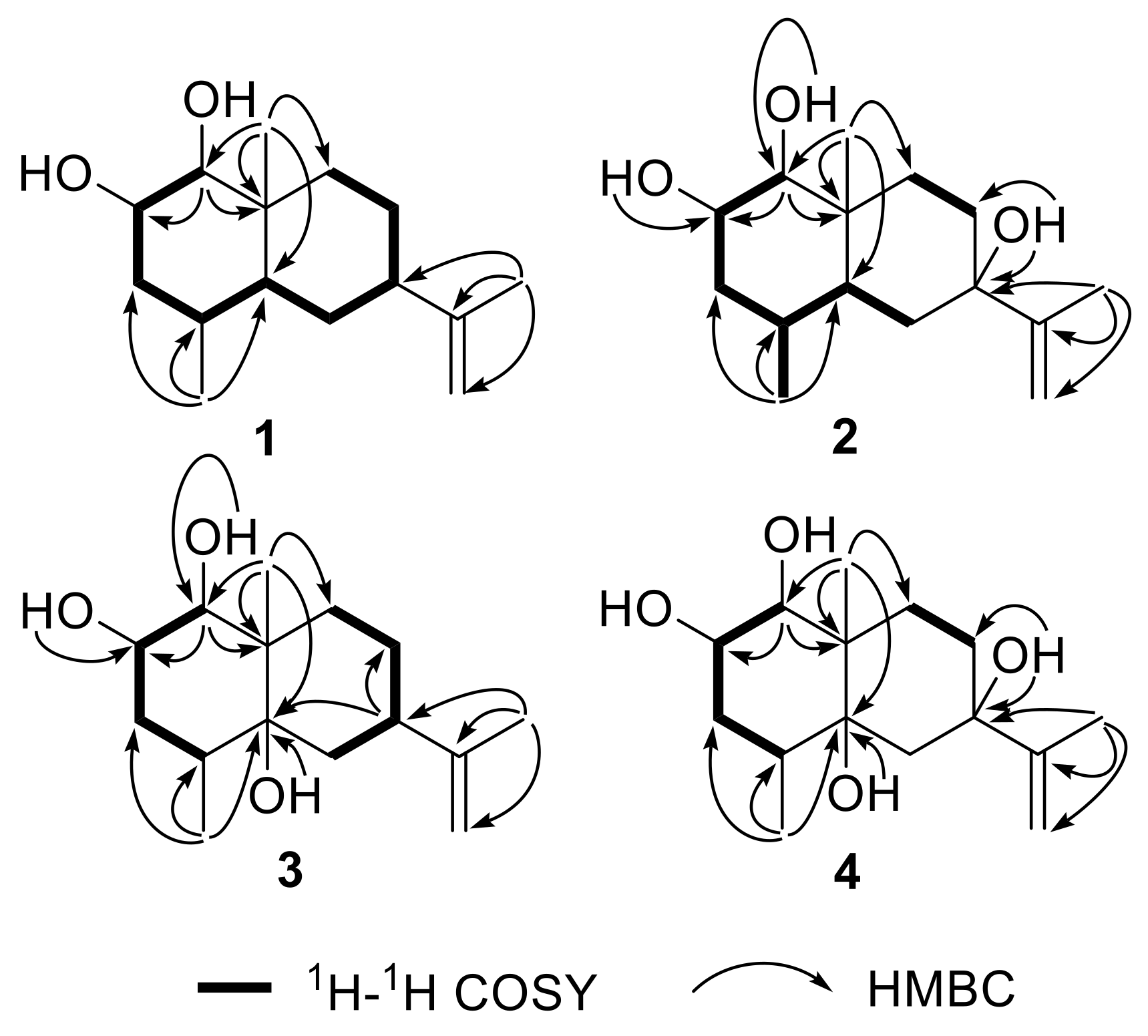
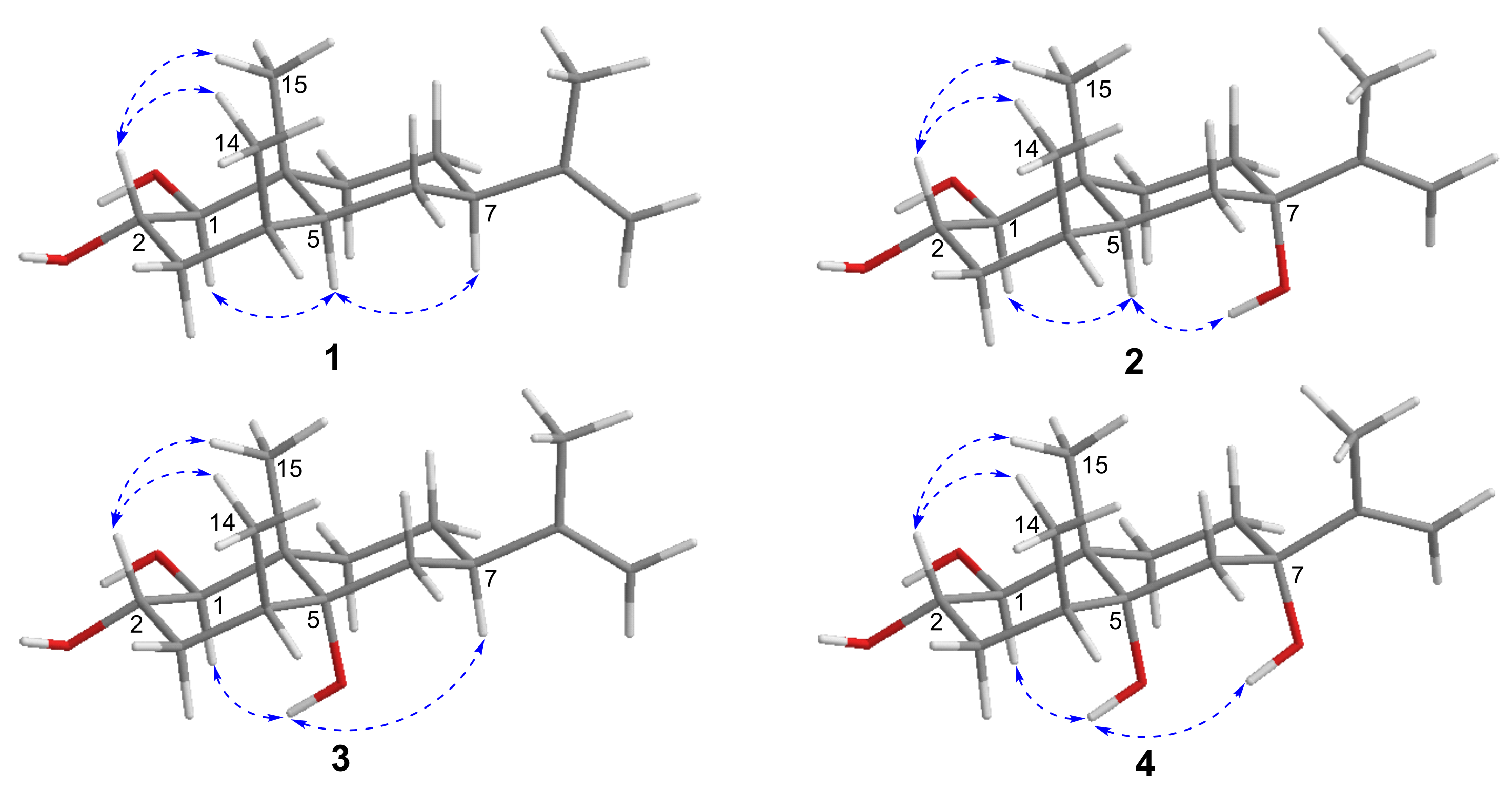
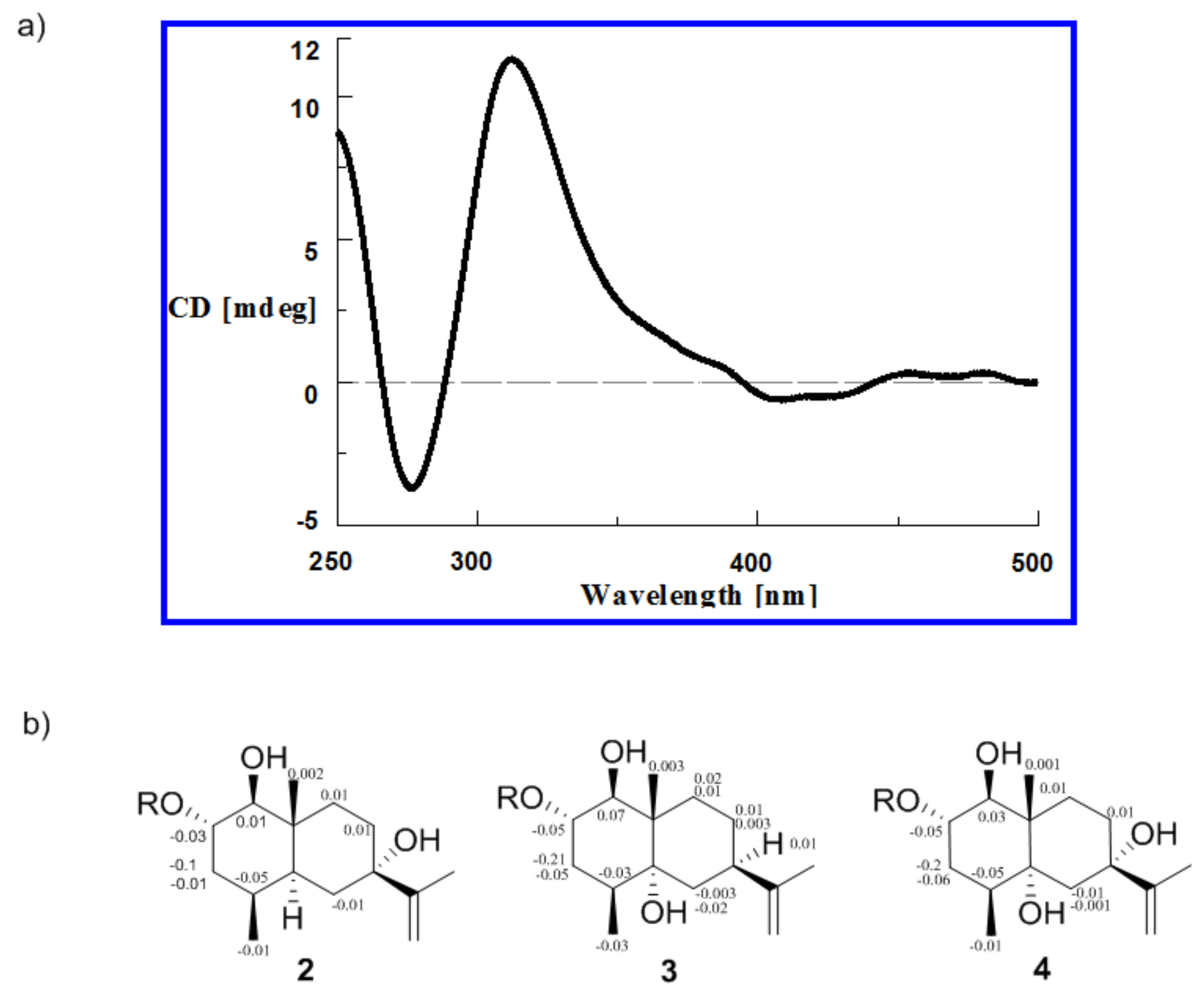
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH mult. (J in Hz) | δC, Type | δH, mult. (J in Hz) | |
| 1 | 83.9, CH | 2.74, dd, (9.2, 4.0) | 83.8, CH | 2.77, dd, (9.2, 3.9) |
| 2 | 66.7, CH | 3.54, m | 66.6, CH | 3.64, m |
| 3 | 40.5, CH2 | 1.79, m | 40.5, CH2 | 1.68, m |
| 1.43, m | 1.42, m | |||
| 4 | 33.7, CH | 1.71, m | 33.3, CH | 1.59, m |
| 5 | 45.7, CH | 1.31, m | 39.1, CH | 1.76, m |
| 6 | 31.3, CH2 | 1.41, m | 35.8, CH2 | 1.51, m |
| 1.25, m | 1.11, d, (13.0, 2.5) | |||
| 7 | 45.6, CH | 1.90, m | 72.7, qC | |
| 8 | 26.2, CH2 | 1.44, m | 30.4, CH2 | 1.54, m |
| 1.27, m | 1.33, m | |||
| 9 | 40.3, CH2 | 1.67, m | 35.9, CH2 | 1.66, m |
| 0.98, m | 1.35, m | |||
| 10 | 39.2, qC | 38.9, qC | ||
| 11 | 150.3, qC | 153.2, qC | ||
| 12 | 108.7, CH2 | 4.67, s | 108.5, CH2 | 4.96, d, (1.8) |
| 4.64, s | 4.68 d, (1.8) | |||
| 13 | 21.1, CH3 | 1.68, s | 19.2, CH3 | 1.74, s |
| 14 | 15.6, CH3 | 0.81, s | 14.6, CH3 | 0.79, s |
| 15 | 16.0, CH3 | 0.85, d, (7.6) | 15.9, CH3 | 0.86, d, (7.6) |
| 1-OH | 4.38, d, (3.7) | |||
| 2-OH | 4.41, d, overlap | 4.37, d, (3.7) | ||
| 5-OH | 4.41, d, overlap | |||
| 7-OH | 4.22, s | |||
| Position | 3 | 4 | ||
|---|---|---|---|---|
| δC, Type | δH, mult. (J in Hz) | δC, Type | δH mult. (J in Hz) | |
| 1 | 77.8, CH | 3.45, dd, (9.1, 2.5) | 77.5, CH | 3.40, d, (9.2) |
| 2 | 67.7, CH | 3.66, m | 67.3, CH | 3.60, m |
| 3 | 35.9, CH2 | 2.06, m | 35.0, CH2 | 1.93, m |
| 1.48, m | 1.40, m | |||
| 4 | 41.2, CH | 1.71, m | 40.5, CH | 1.67, m |
| 5 | 75.3, qC | 76.7, qC | ||
| 6 | 37.1, CH2 | 1.79, m | 38.7, CH2 | 1.99, d, (14.0) |
| 1.25, dd, (13.3, 3.0) | 1.15, d, (14.0) | |||
| 7 | 39.2, CH | 2.53, m | 75.1, qC | |
| 8 | 25.4, CH2 | 1.49, m | 30.3, CH2 | 1.46, m |
| 1.34, m | ||||
| 9 | 33.3, CH2 | 1.76, m | 29.8, CH2 | 1.76, m |
| 1.43, m | 1.40, m | |||
| 10 | 41.9, qC | 42.1, qC | ||
| 11 | 150.7, qC | 151.6, qC | ||
| 12 | 108.7, CH2 | 4.73, d, (1.6) | 109.3, CH2 | 4.96, s |
| 4.75, d, (1.6) | 4.74, s | |||
| 13 | 21.3, CH3 | 1.67, s | 19.0, CH3 | 1.74, s |
| 14 | 17.8, CH3 | 0.96, d, (7.8) | 17.4, CH3 | 0.95, d, (7.8) |
| 15 | 16.9, CH3 | 0.86, s | 16.7, CH3 | 0.87, s |
| 1-OH | 4.29, d, (3.7) | 4.26, br s | ||
| 2-OH | 4.37, d, (2.8) | 4.20, br s | ||
| 5-OH | 3.74, s | 5.66, s | ||
| 7-OH | 5.63 s | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Wang, P.; Liao, G.; Zeng, Y.; Cai, C.; Kong, F.; Guo, Z.; Proksch, P.; Dai, H.; Mei, W. New Eudesmane-Type Sesquiterpenoids from the Mangrove-Derived Endophytic Fungus Penicillium sp. J-54. Mar. Drugs 2018, 16, 108. https://doi.org/10.3390/md16040108
Qiu L, Wang P, Liao G, Zeng Y, Cai C, Kong F, Guo Z, Proksch P, Dai H, Mei W. New Eudesmane-Type Sesquiterpenoids from the Mangrove-Derived Endophytic Fungus Penicillium sp. J-54. Marine Drugs. 2018; 16(4):108. https://doi.org/10.3390/md16040108
Chicago/Turabian StyleQiu, Liuming, Pei Wang, Ge Liao, Yanbo Zeng, Caihong Cai, Fandong Kong, Zhikai Guo, Peter Proksch, Haofu Dai, and Wenli Mei. 2018. "New Eudesmane-Type Sesquiterpenoids from the Mangrove-Derived Endophytic Fungus Penicillium sp. J-54" Marine Drugs 16, no. 4: 108. https://doi.org/10.3390/md16040108
APA StyleQiu, L., Wang, P., Liao, G., Zeng, Y., Cai, C., Kong, F., Guo, Z., Proksch, P., Dai, H., & Mei, W. (2018). New Eudesmane-Type Sesquiterpenoids from the Mangrove-Derived Endophytic Fungus Penicillium sp. J-54. Marine Drugs, 16(4), 108. https://doi.org/10.3390/md16040108







