Eutypellenoids A–C, New Pimarane Diterpenes from the Arctic Fungus Eutypella sp. D-1
Abstract
:1. Introduction
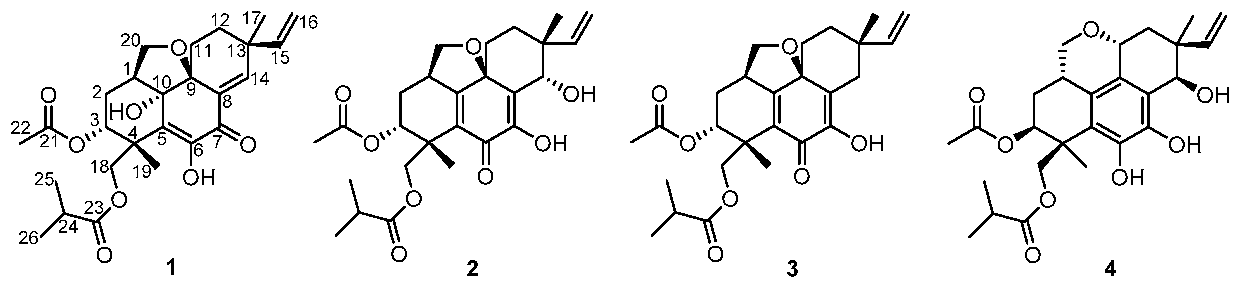
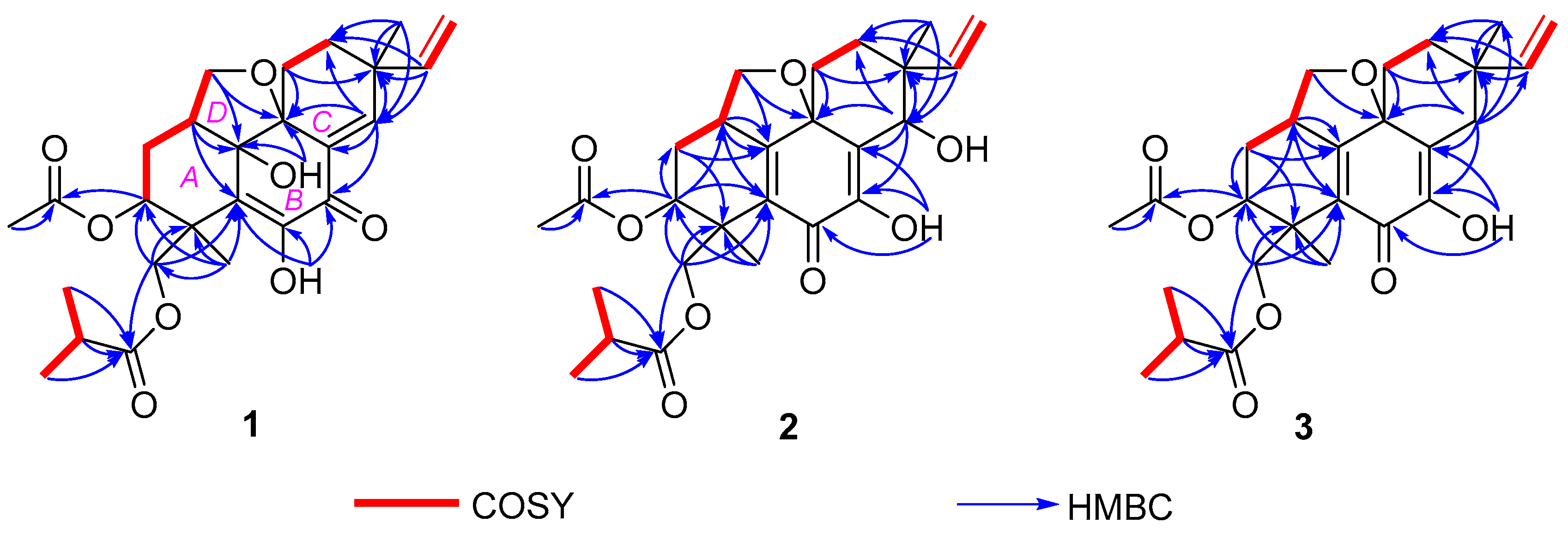
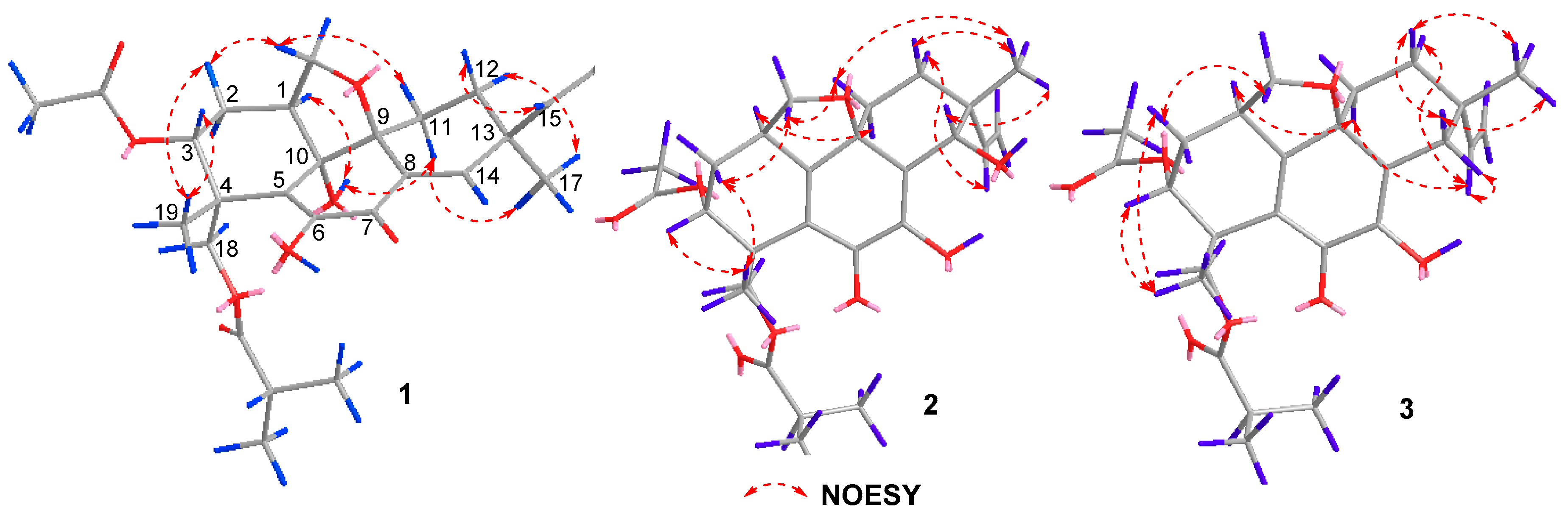
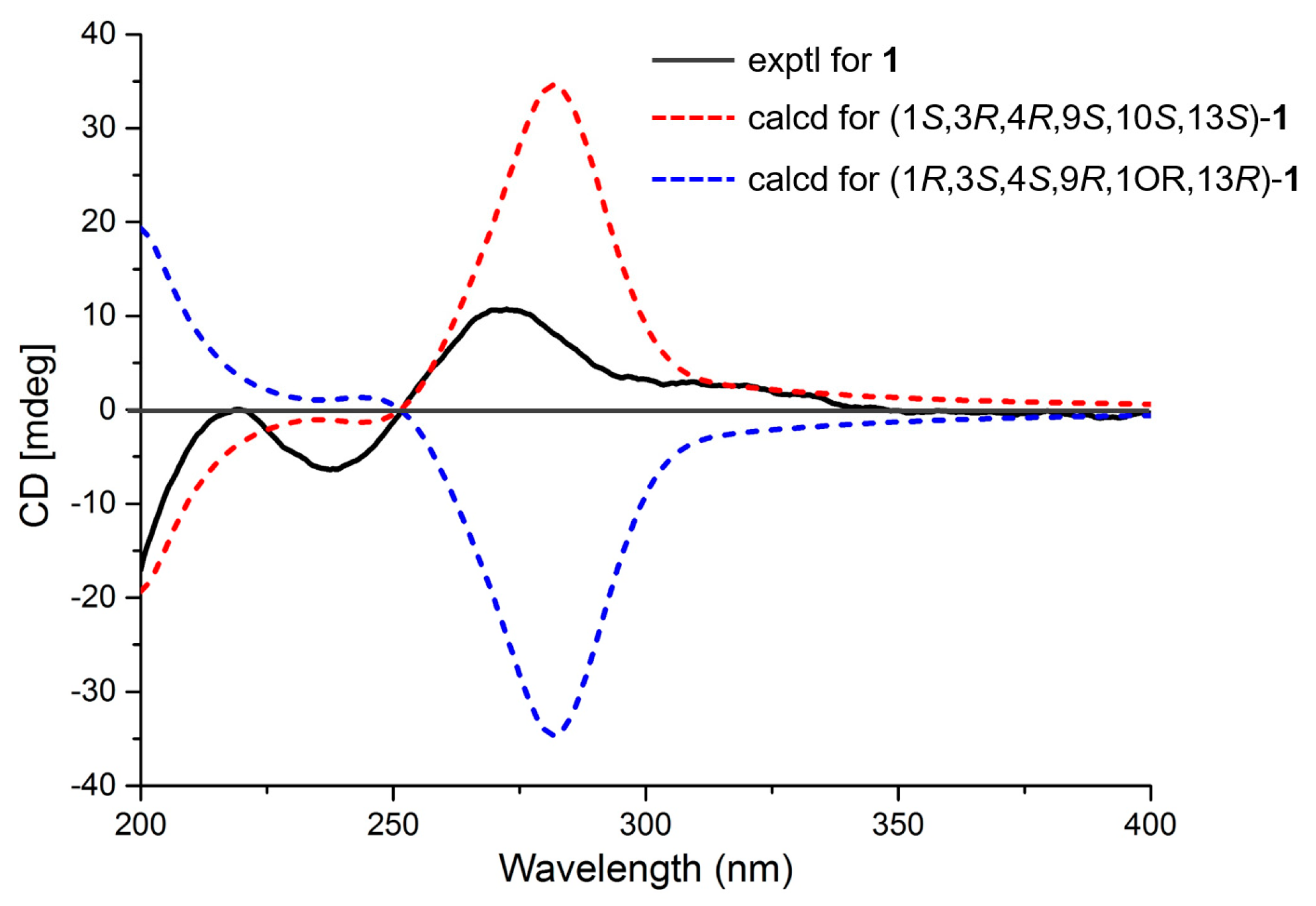
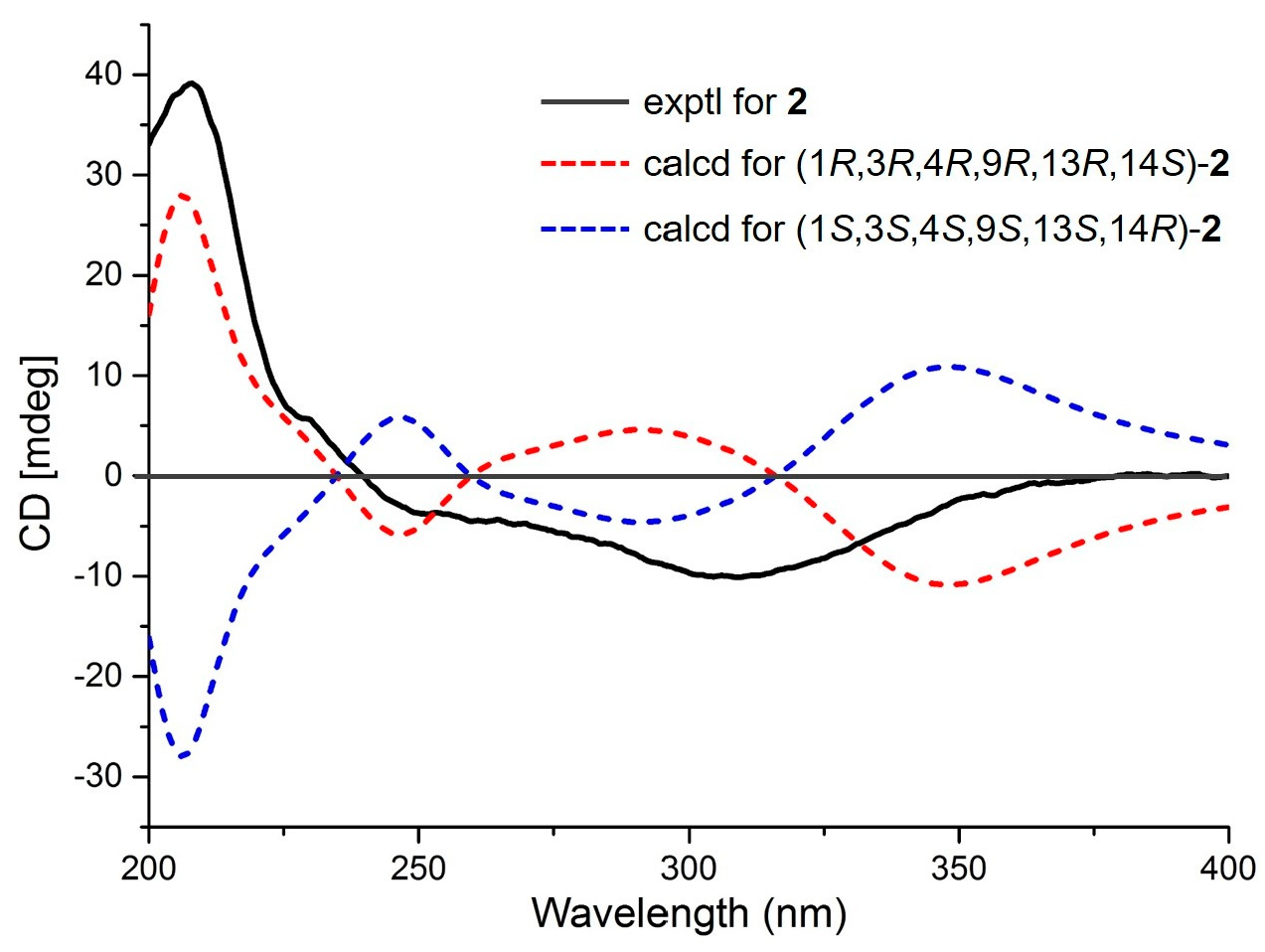
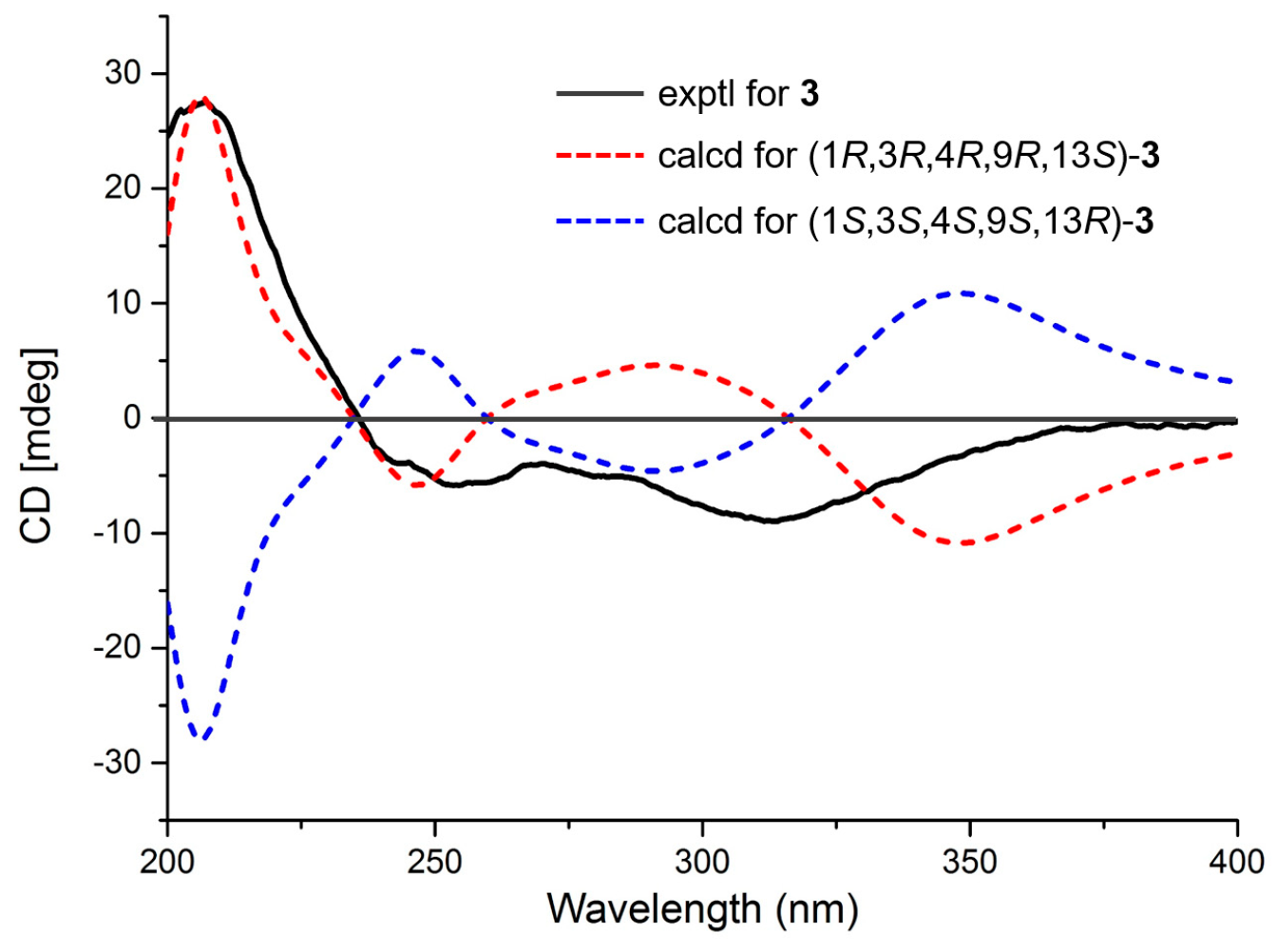
2. Results
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Strain
3.3. Fermentation, Extraction, and Isolation
3.4. ECD Calculations
3.5. Biological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ivanets, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Rasin, A.B.; Zhuravleva, O.I.; Pivkin, M.V.; Popov, R.S.; Amsberg, G.; Afiyatullov, S.S.; Dyshlovoy, S.A. Asperindoles A–D and a p-Terphenyl Derivative from the Ascidian-Derived Fungus Aspergillus sp. KMM 4676. Mar. Drugs 2018, 16, 232. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Y.-L.; Zhao, F.-C. Secondary Metabolites from Polar Organisms. Mar. Drugs 2017, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, D.; Tao, M.; Chen, Y.; Dan, F.; Zhang, W. Scopararanes C‒G: New oxygenated pimarane diterpenes from the marine sediment-derived fungus Eutypella scoparia FS26. Mar. Drugs 2012, 10, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhang, J.; Li, Y. Secondary Metabolites from Eutypella species and their Bioactivities. Mycosystema 2017, 36, 1181–1191. [Google Scholar]
- Niu, S.; Liu, D.; Shao, Z.; Proksch, P.; Lin, W. Eutypellazines A‒M, Thiodiketopiperazine-type Alkaloids from Deep Ssea Derived Fungus Eutypella sp. MCCC 3A00281. RSC Adv. 2017, 7, 33580–33590. [Google Scholar] [CrossRef]
- Pongcharoen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Rungjindamai, N.; Sakayaroj, J. Pimarane Diterpene and Cytochalasin Derivatives from the Endophytic Fungus Eutypella scoparia PSU-D44. J. Nat. Prod. 2006, 69, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Ciavatta, M.L.; Lopez-Gresa, M.P.; Gavagnin, M.; Nicoletti, R.; Manzo, E.; Mollo, E.; Guo, Y.-W.; Cimino, G. Cytosporin-related Compounds from the Marine-derived Fungus Eutypella scoparia. Tetrahedron 2008, 64, 5365–5369. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Lapanun, S.; Chanthaket, R.; Boonyuen, N.; Lumyong, S. γ-Lactones and ent-Eudesmane Sesquiterpenes from the Endophytic Fungus Eutypella sp. BCC 13199. J. Nat. Prod. 2009, 72, 1720–1722. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-L.; Liu, J.-T.; Liu, X.-Y.; Gao, Y.; Zhang, J.; Jiao, B.-H.; Zheng, H. Pimarane Diterpenes from the Arctic Fungus Eutypella sp. D-1. J. Antibiot. 2014, 67, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Q.; Chen, X.-C.; Zhu, S.-G.; Yang, Y.-F.; Chen, K.-X.; Li, Y.-M.; Chen, Z.-Q.; Wang, G.-M.; Chen, K.-X.; Liu, X.-Y. Eutypenoids A‒C: Novel Pimarane Diterpenoids from the Arctic Fungus Eutypella sp. D-1. Mar. Drugs 2016, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-B.; Wang, X.-L.; Zhang, Y.-X.; Xu, W.-H.; Zhang, J.-P.; Zhou, X.-Y.; Lu, X.-L.; Liu, X.-Y.; Jiao, B.-H. Libertellenones O−S and Eutypellenones A and B, Pimarane Diterpene Derivatives from the Arctic Fungus Eutypella sp. D-1. J. Nat. Prod. 2018, 81, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, B.B.; Sun, F.; Xu, J.R.; Jiao, W.H.; Yu, H.B.; Han, B.N.; Yang, F.; Zhang, X.C.; Lin, H.W. Sesquiterpene Quinones/Hydroquinones from the Marine Sponge Spongia pertusa Esper. J. Nat. Prod. 2017, 80, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-B.; Jiao, H.; Zhu, Y.-P.; Zhang, J.-P.; Lu, X.-L.; Liu, X.-Y. Bioactive metabolites from the Arctic fungus Nectria sp. B-13. J. Asian Nat. Prod. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Klink, J.W.; Larsen, L.; Perry, N.B.; Weavers, R.T.; Cook, G.M.; Bremer, P.J.; MacKenzie, A.D.; Kirikae, T. Triketones Active Against Antibiotic-resistant Bacteria: Synthesis, Structure-activity Relationships, and Mode of Action. Bioorg. Med. Chem. 2006, 13, 6651–6662. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-B.; Yang, F.; Sun, F.; Li, J.; Jiao, W.-H.; Gan, J.-H.; Hu, W.-Z.; Lin, H.-W. Aaptamine Derivatives with Antifungal and Anti-HIV-1 Activities from the South China Sea Sponge Aaptos aaptos. Mar. Drugs 2014, 12, 6003–6013. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast; Approved Standard, 3rd ed.; Document M27 A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, 2nd ed.; Document M38 A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
| Position | 1 a | 2 b | 3 b | |||
|---|---|---|---|---|---|---|
| δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | |
| 1 | 40.6, CH | 2.93, m | 32.3, CH | 3.27, m | 33.0, CH | 3.32, m |
| 2α | 26.0, CH2 | 2.32, m | 26.5, CH2 | 2.35, m | 26.8, CH2 | 2.33, m |
| 2β | 1.70, m | 1.46, m | 1.46, m | |||
| 3 | 74.1, CH | 5.11, t (7.0, 3.5) | 72.7, CH | 5.12, d (1.2) | 72.9, CH | 5.11, d (0.8) |
| 4 | 42.8, C | 40.5, C | 40.5, C | |||
| 5 | 124.7, C | 127.3, C | 126.9, C | |||
| 6 | 145.9, C | 180.3, C | 179.9, C | |||
| 6-OH | 7.17, s | |||||
| 7 | 181.3, C | 142.3, C | 142.4, C | |||
| 7-OH | 6.66, s | 6.52, s | ||||
| 8 | 129.5, C | 122.7, C | 125.8, C | |||
| 9 | 80.9, C | 79.2, C | 77.7, C | |||
| 10 | 76.2, C | 164.8, C | 164.8, C | |||
| 10-OH | 4.41, s | |||||
| 11α | 24.4, CH2 | 1.72, m | 35.8, CH2 | 2.04, m | 35.4, CH2 | 2.04, m |
| 11β | 2.09, m | 1.54, m | 1.41, m | |||
| 12α | 29.7, CH2 | 1.82, m | 26.9, CH2 | 1.57, m | 32.6, CH2 | 1.60, m |
| 12β | 1.64, m | 2.08, m | 1.93, m | |||
| 13 | 39.8, C | 44.9, C | 41.1, C | |||
| 14α | 152.7, CH | 7.11, s | 72.2, CH | 4.68, s | 35.0, CH2 | 2.96, dd (12.8, 2.0) |
| 14β | 2.15, d (12.8) | |||||
| 15 | 142.7, CH | 5.71, dd (17.5, 10.5) | 142.4, CH | 5.72, dd (17.6, 10.8) | 144.0, CH | 5.68, dd (17.6, 10.8) |
| 16a | 114.2, CH2 | 4.86, d (17.5) | 113.7, CH2 | 5.03, d (10.8) | 112.9, CH2 | 4.96, d (10.8) |
| 16b | 5.04, d (10.5) | 5.09, d (17.6) | 5.12, d (17.6) | |||
| 17 | 27.4, CH3 | 1.23, s | 25.2, CH3 | 1.20, s | 30.1, CH3 | 1.15, s |
| 18α | 64.9, CH2 | 4.42, d (14.0) | 65.2, CH2 | 4.35, d (10.8) | 65.3, CH2 | 4.35, d, (10.4) |
| 18β | 4.57, d (14.0) | 4.79, d (10.8) | 4.81, d (10.4) | |||
| 19 | 20.6, CH3 | 1.61, s | 20.9, CH3 | 1.31, s | 20.9, CH3 | 1.31, s |
| 20α | 72.5, CH2 | 4.22, t (18.5, 9.5) | 72.9, CH2 | 4.42, t (8.8) | 71.9, CH2 | 4.36, t (8.0) |
| 20β | 3.47, t (18.0, 9.0) | 3.77, t (8.8) | 3.70, t (8.0) | |||
| 21 | 168.6, C | 169.9, C | 170.0, C | |||
| 22 | 21.1, CH3 | 2.08, s | 21.0, CH3 | 1.99, s | 21.0, CH3 | 1.99, s |
| 23 | 176.6, C | 176.4, C | 176.4, C | |||
| 24 | 34.1, CH | 2.51, m | 34.1, CH | 2.52, m | 34.1, CH | 2.52, m |
| 25 | 18.8, CH3 | 1.13, d (7.0) | 18.8, CH3 | 1.13, d (6.8) | 18.8, CH3 | 1.13, d (6.8) |
| 26 | 18.9, CH3 | 1.14, d (7.0) | 18.9, CH3 | 1.14, d (6.8) | 18.9, CH3 | 1.13, d (6.8) |
| Compound | MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | B. subtilis | V. alginolyticus | V. vulnificus | S. agalactiae | A. hydrophila | |
| 1 | 32 | 32 | 64 | 64 | 32 | 64 | 64 |
| 2 | 8 | 8 | 32 | 32 | 32 | 32 | 32 |
| 3 | 32 | 32 | 32 | 64 | 64 | 64 | 64 |
| 4 | 32 | 64 | 64 | 64 | 64 | 64 | 64 |
| Chloromycetin | 4 | 4 | 2 | 1 | 1 | 1 | 1 |
| Compound | MIC (μg/mL) | |||||
|---|---|---|---|---|---|---|
| C. parapsilosis | C. albicans | C. glabrata | C. neoformans | M. gypseum | C. tropicalis | |
| 1 | >64 | >64 | >64 | >64 | >64 | >64 |
| 2 | 8 | 8 | 16 | >64 | >64 | 32 |
| 3 | >64 | >64 | 64 | >64 | >64 | >64 |
| 4 | >64 | >64 | 64 | >64 | >64 | >64 |
| Fluconazole | 0.50 | 2 | 0.50 | 1 | 2 | 0.25 |
| Posaconazole | 0.50 | 0.12 | 0.50 | 0.02 | 1 | 0.02 |
| Voriconazole | 0.02 | 0.03 | 0.02 | 0.02 | 0.06 | 0.02 |
| Compound | IC50 (μM) | ||||
|---|---|---|---|---|---|
| HeLa | MCF-7 | HCT-116 | K562 | SW1990 | |
| 1 | 24.4 | 26.2 | 20.7 | 30.9 | 23.6 |
| 2 | 15.1 | 20.3 | 3.7 | 23.3 | 33.6 |
| 3 | 41.5 | 36.5 | 31.6 | >50 | >50 |
| 4 | 46.5 | 40.1 | 27.1 | 32.1 | >50 |
| Cisplatin | 0.5 | 4.5 | 2.7 | 2.7 | 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-B.; Wang, X.-L.; Xu, W.-H.; Zhang, Y.-X.; Qian, Y.-S.; Zhang, J.-P.; Lu, X.-L.; Liu, X.-Y. Eutypellenoids A–C, New Pimarane Diterpenes from the Arctic Fungus Eutypella sp. D-1. Mar. Drugs 2018, 16, 284. https://doi.org/10.3390/md16080284
Yu H-B, Wang X-L, Xu W-H, Zhang Y-X, Qian Y-S, Zhang J-P, Lu X-L, Liu X-Y. Eutypellenoids A–C, New Pimarane Diterpenes from the Arctic Fungus Eutypella sp. D-1. Marine Drugs. 2018; 16(8):284. https://doi.org/10.3390/md16080284
Chicago/Turabian StyleYu, Hao-Bing, Xiao-Li Wang, Wei-Heng Xu, Yi-Xin Zhang, Yi-Sen Qian, Jian-Peng Zhang, Xiao-Ling Lu, and Xiao-Yu Liu. 2018. "Eutypellenoids A–C, New Pimarane Diterpenes from the Arctic Fungus Eutypella sp. D-1" Marine Drugs 16, no. 8: 284. https://doi.org/10.3390/md16080284
APA StyleYu, H.-B., Wang, X.-L., Xu, W.-H., Zhang, Y.-X., Qian, Y.-S., Zhang, J.-P., Lu, X.-L., & Liu, X.-Y. (2018). Eutypellenoids A–C, New Pimarane Diterpenes from the Arctic Fungus Eutypella sp. D-1. Marine Drugs, 16(8), 284. https://doi.org/10.3390/md16080284