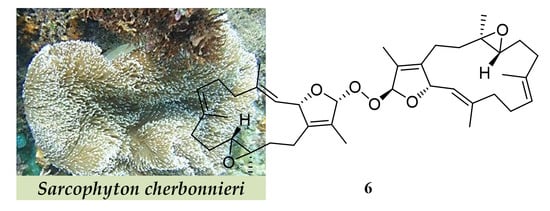
New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri
Abstract
1. Introduction
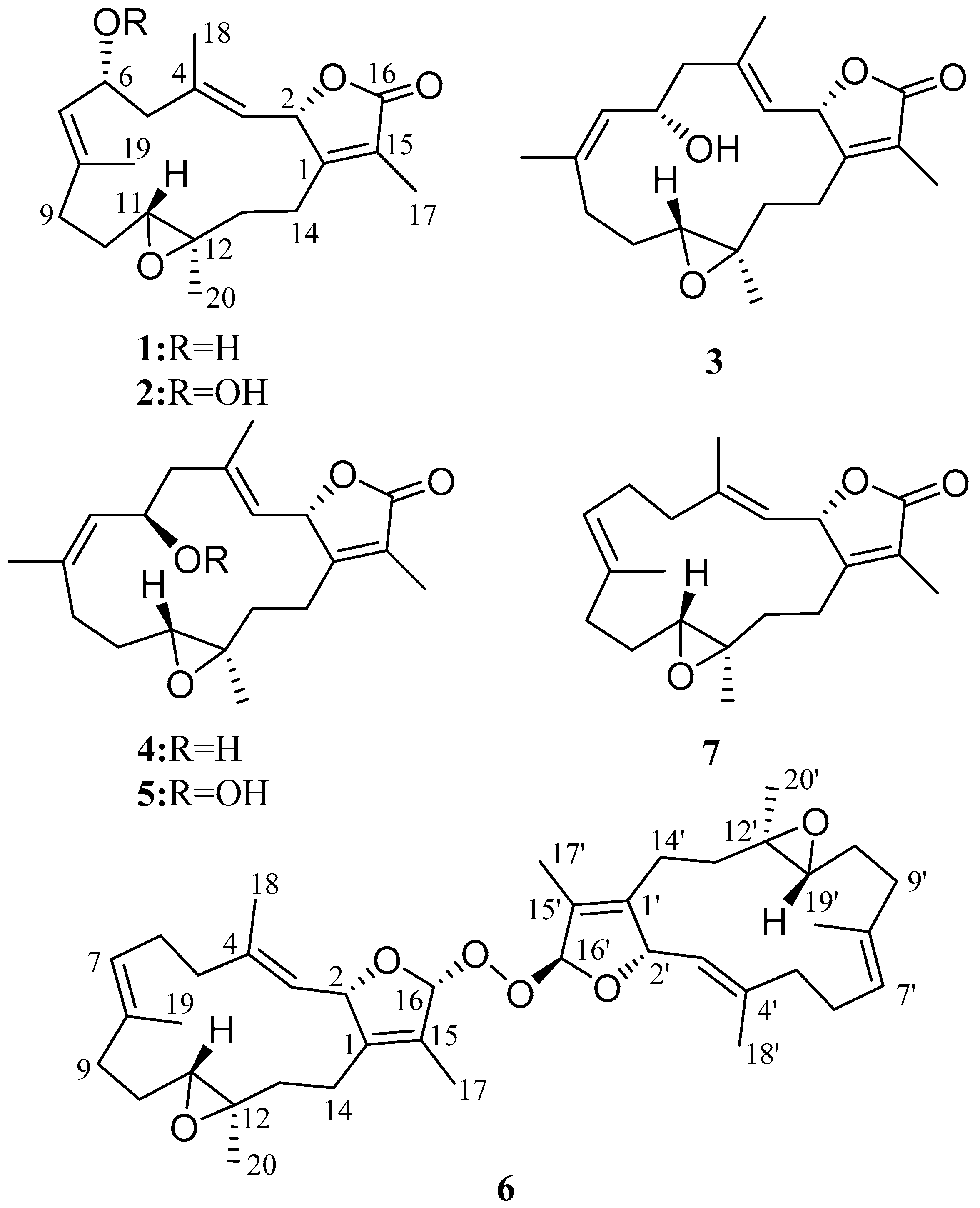
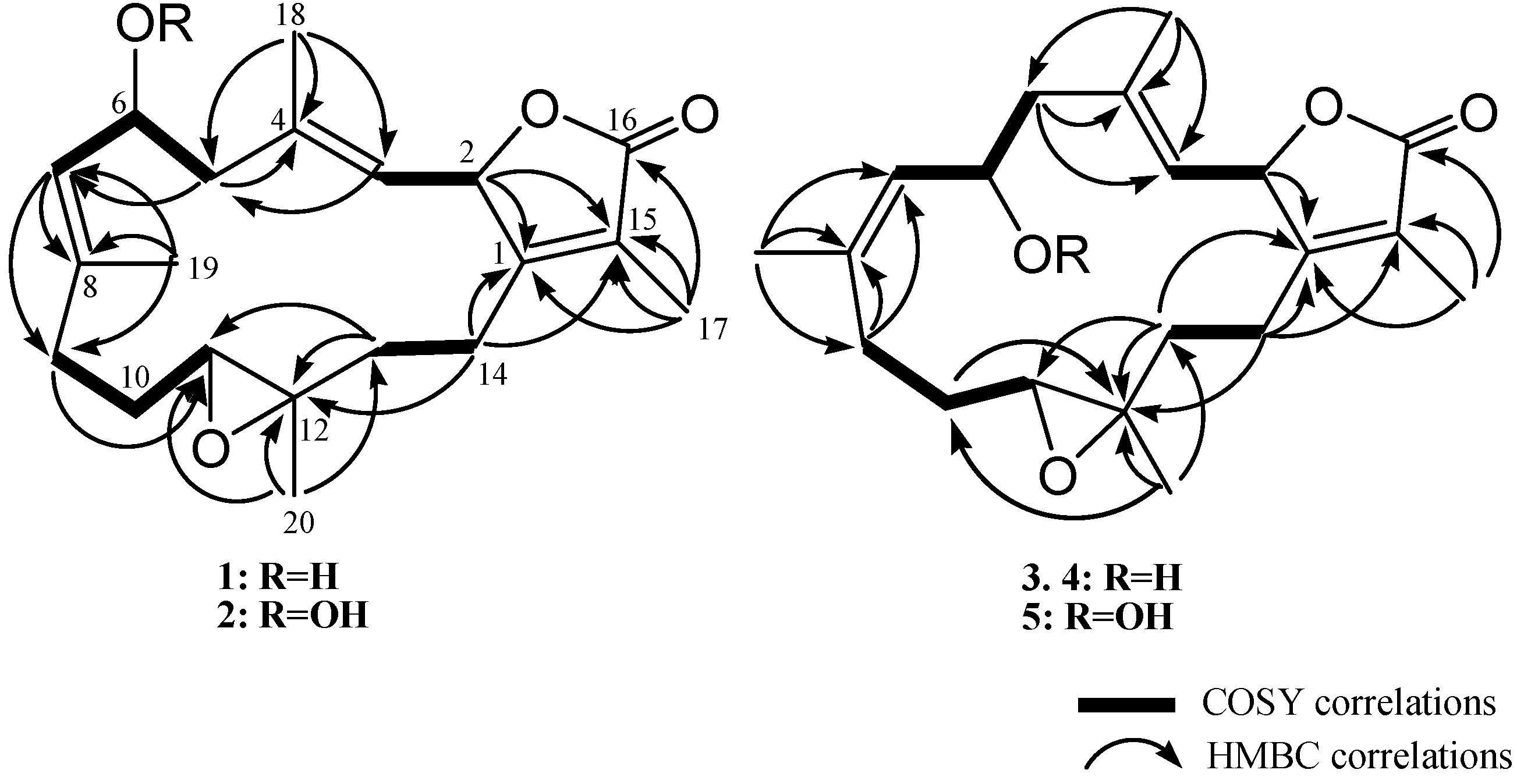
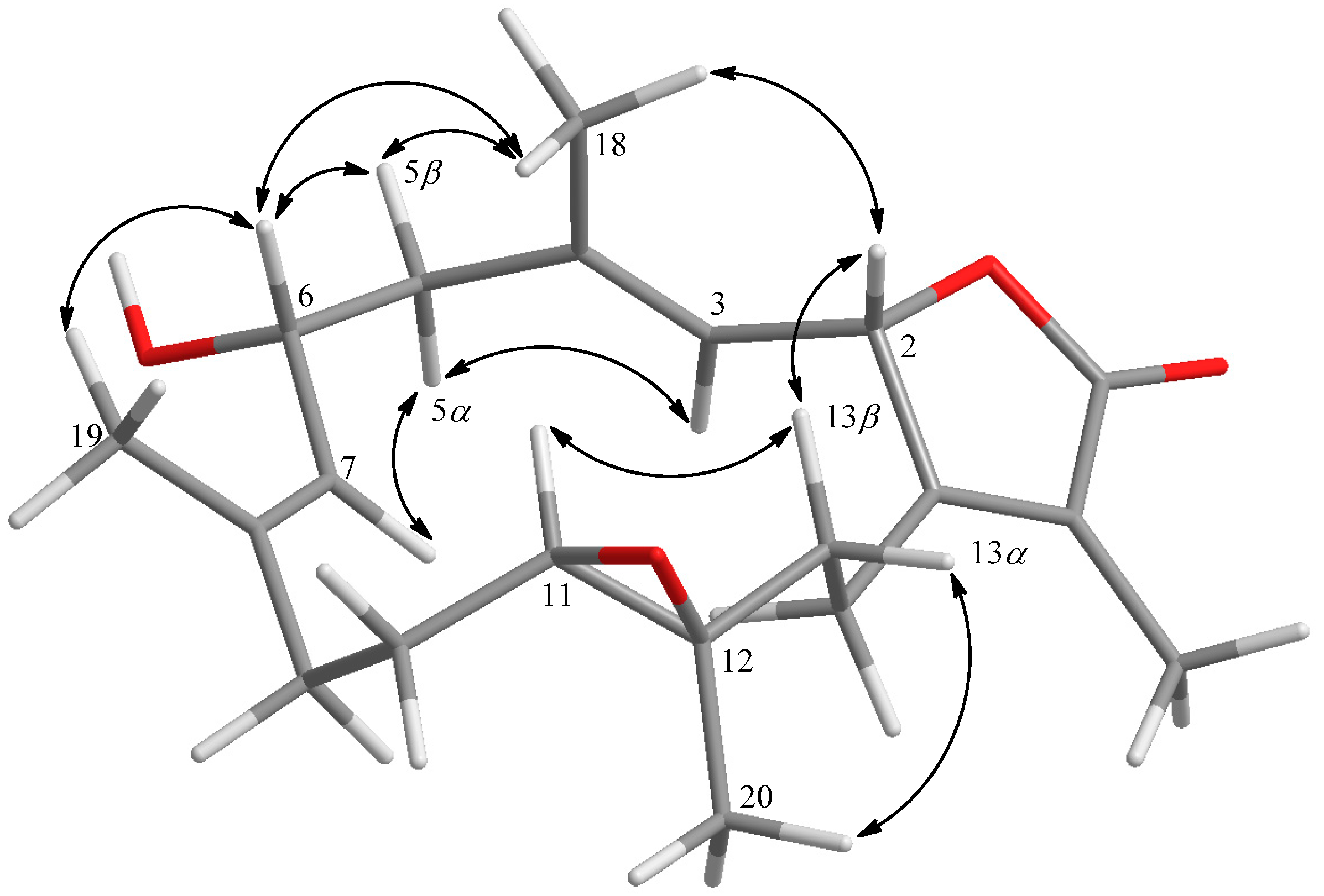
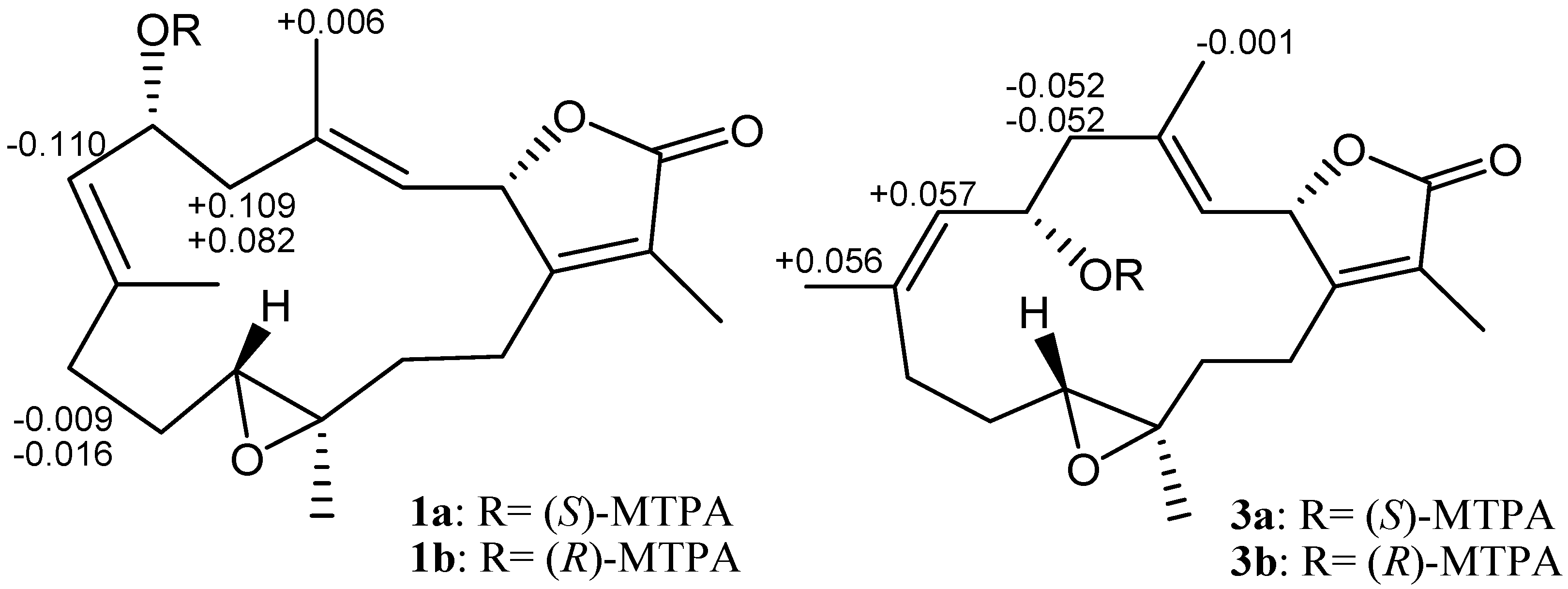
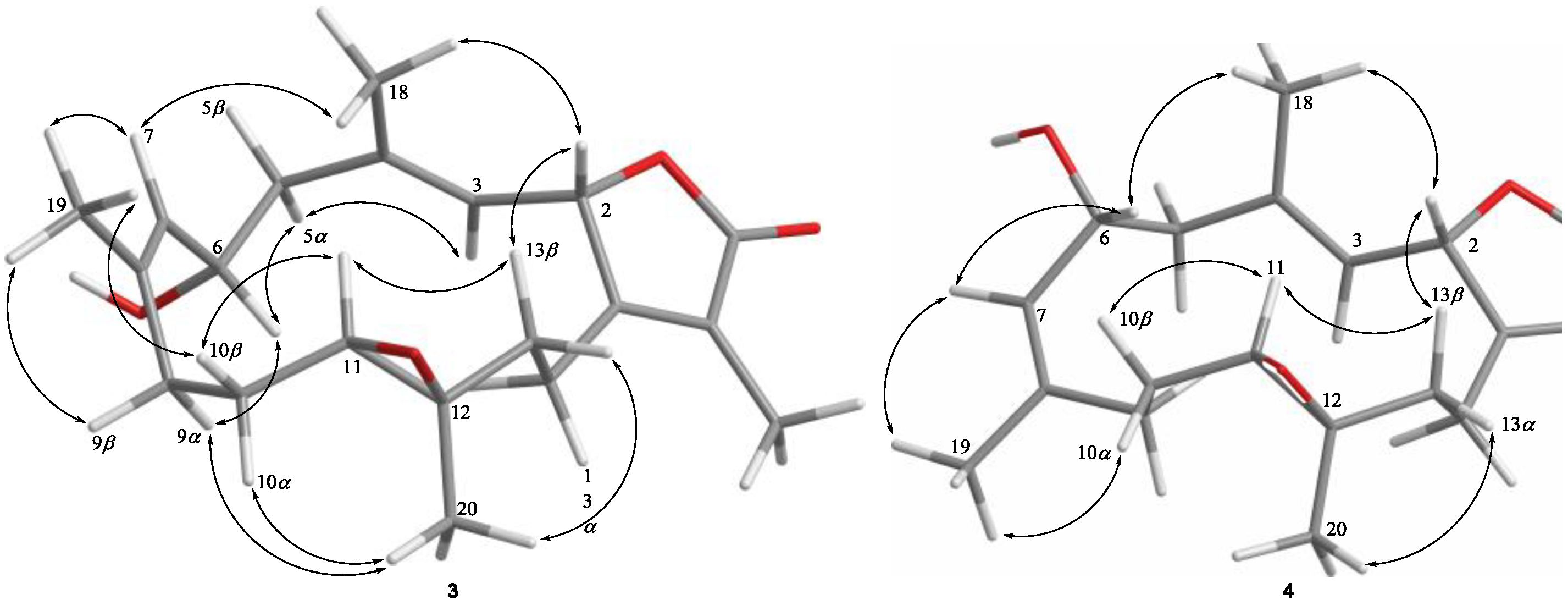
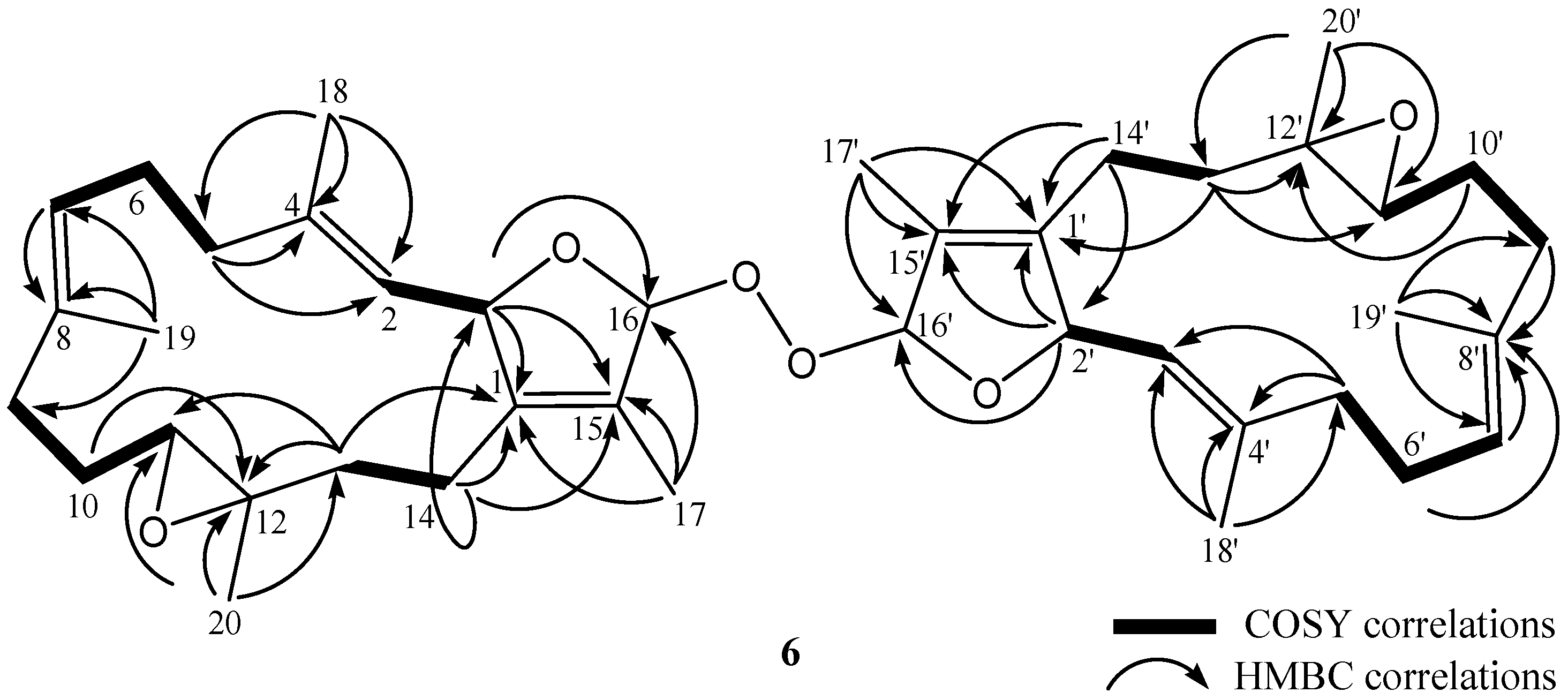
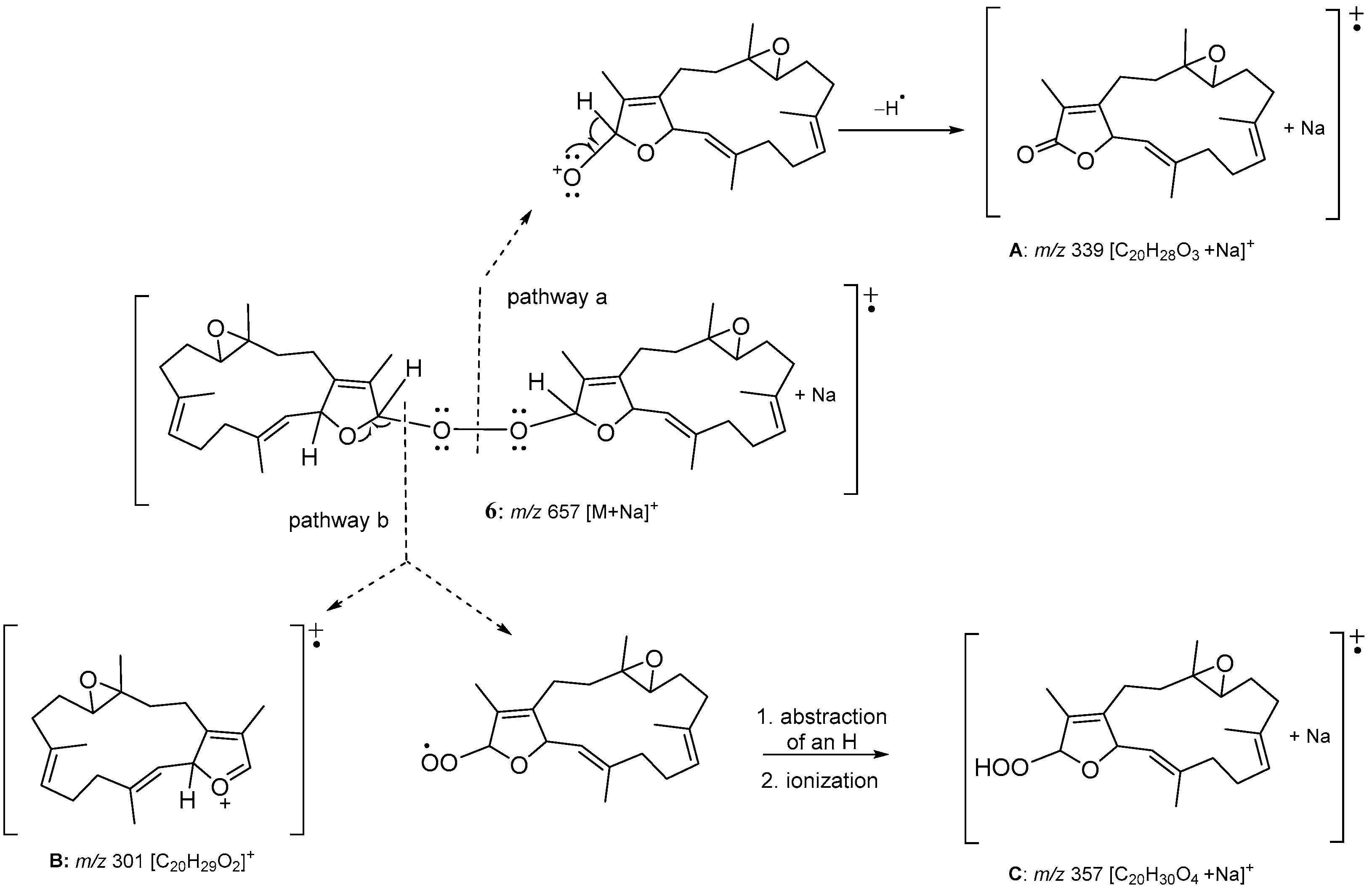
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.3.1. Cherbonnolide A (1)
3.3.2. Cherbonnolide B (2)
3.3.3. Cherbonnolide C (3)
3.3.4. Cherbonnolide D (4)
3.3.5. Cherbonnolide E (5)
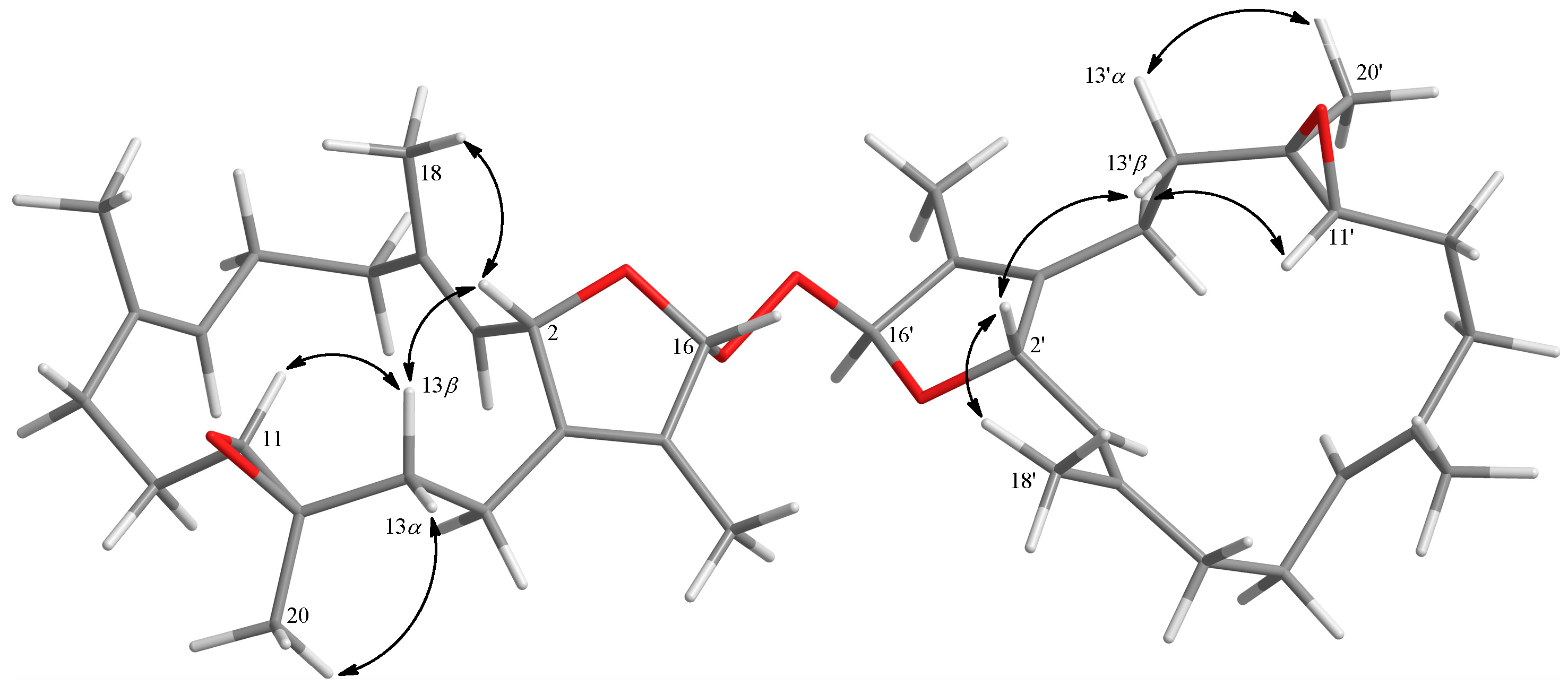
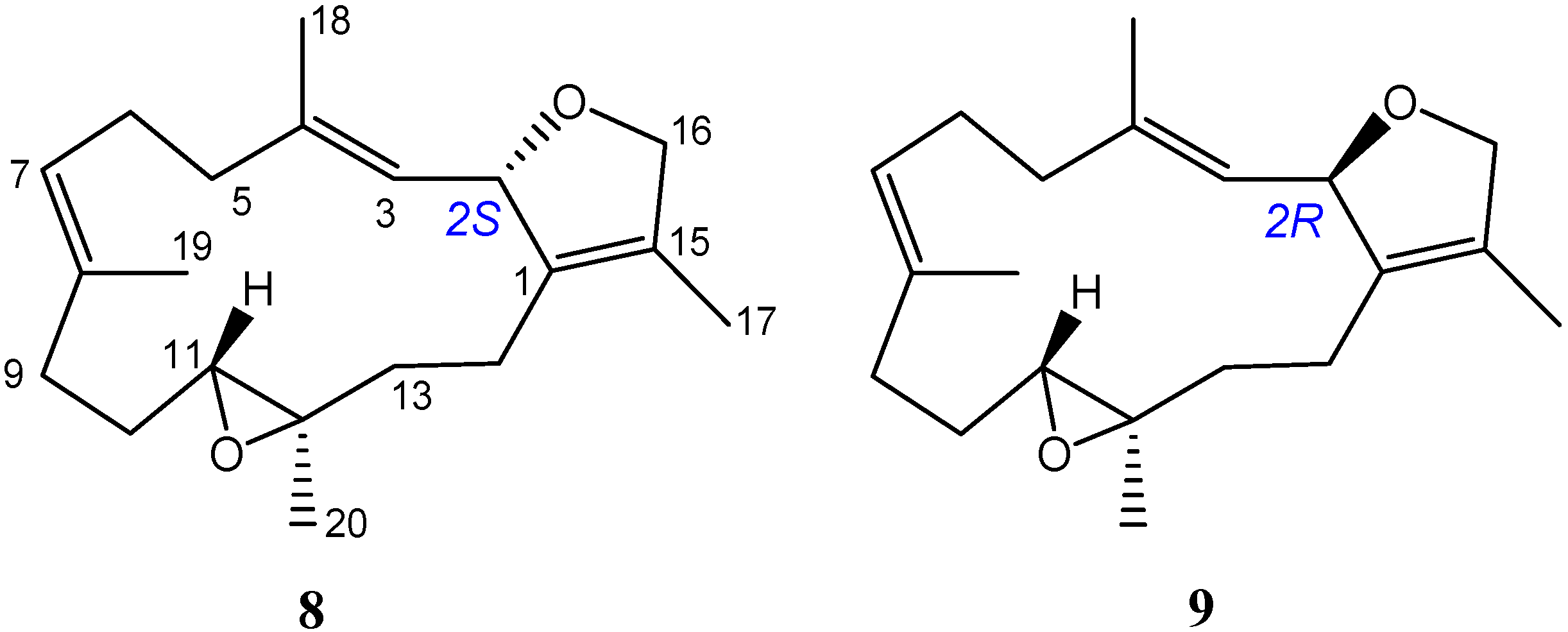
3.3.6. Bischerbolide Peroxide (6)
3.3.7. Reduction of Cherbonolides B and E (2 and 5)
3.3.8. Preparation of (S)- and (R)- MTPA Esters of 1 and 3
3.4. In Vitro Anti-Inflammatory Testing
3.4.1. Human Neutrophils
3.4.2. Superoxide Anion Generation
3.4.3. Elastase Release
3.4.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farag, M.A.; Fekry, M.I.; Al-Hammady, M.A.; Khalil, M.N.; El-Seedi, H.R.; Meyer, A.; Porzel, A.; Westphal, H.; Wessjohann, L.A. Cytotoxic effects of Sarcophyton sp. soft corals-Is there a correlation to their NMR fingerprints? Mar. Drugs 2017, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Li, W.L.; Huang, C.Y.; Ahmed, A.F.; Dai, C.F.; Wu, Y.C.; Lu, M.C.; Liaw, C.C.; Sheu, J.H. Isoprenoids from the soft coral Sarcophyton glaucum. Mar. Drugs 2017, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.F.; Elshamy, A.I.; Mohamed, T.A.; Hamed, A.R.; Ibrahim, M.A.A.; Ohta, S.; Pare, P.W. Cembrene diterpenoids with ether linkages from Sarcophyton ehrenbergi: An anti-proliferation and molecular-docking assessment. Mar. Drugs 2017, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, A.; El-Beih, A.A.; Gamal-Eldeen, A.M.; Alhammady, M.A.; Ohta, S.; Pare, P.W.; Hegazy, M.E.F. New terpenes from the Egyptian soft coral Sarcophyton ehrenbergi. Mar. Drugs 2014, 12, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Eltahawy, N.A.; Ibrahim, A.K.; Radwan, M.M.; ElSohly, M.A.; Hassanean, H.A.; Hassanean, H.A.; Ahmed, S.A. Cytotoxic cembranoids from the Red Sea soft coral, Sarcophyton auritum. Tetrahedron Lett. 2014, 55, 3984–3988. [Google Scholar] [CrossRef]
- Lin, W.Y.; Lu, Y.; Su, J.H.; Wen, Z.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Bioactive cembranoids from the dongsha atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2011, 9, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Su, J.H.; Lu, Y.; Wen, Z.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Cytotoxic and anti-inflammatory cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Bioorg. Med. Chem. 2010, 18, 1936–1941. [Google Scholar] [CrossRef] [PubMed]
- Iwagawa, T.; Hashimoto, K.; Yokogawa, Y.; Okamura, H.; Nakatani, M.; Doe, M.; Morimoto, Y.; Takemura, K. Cytotoxic biscembranes from the soft coral Sarcophyton glaucum. J. Nat. Prod. 2009, 72, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Tseng, Y.J.; Chokkalingam, U.; Hwang, T.L.; Hsu, C.H.; Dai, C.F.; Sung, P.J.; Sheu, J.H. Bioactive isoprenoid-derived natural products from a Dongsha Atoll soft coral Sinularia erecta. J. Nat. Prod. 2016, 79, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.J.; Yang, Y.C.; Wang, S.K.; Duh, C.Y. Numerosol A-D, new cembranoid diterpenes from the soft coral Sinularia numerosa. Mar. Drugs 2014, 12, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Lillsunde, K.E.; Festa, C.; Adel, H.; De Marino, S.; Lombardi, V.; Tilvi, S.; Nawrot, D.A.; Zampella, A.; D’Souza, L.; D’Auria, M.V.; et al. Bioactive cembrane derivatives from the Indian Ocean soft coral, Sinularia kavarattiensis. Mar. Drugs 2014, 12, 4045–4068. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Cytotoxic cembranoid diterpenes from a soft coral Sinularia gibberosa. J. Nat. Prod. 2005, 68, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Wen, Z.H.; Wang, S.K.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Chiang, M.Y.; Duh, C.Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral Lobophytum durum. J. Nat. Prod. 2009, 72, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Wen, Z.H.; Wu, Y.C.; Yeh, H.C.; Sheu, J.H. Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.H.; You, W.J.; Lin, C.C.; El-Shazly, M.; Liao, Z.J.; Su, J.H. Anti-Inflammatory cembranoids from the soft coral Lobophytum crassum. Mar. Drugs 2017, 15, 327. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Tseng, Y.J.; Chen, B.W.; Hwang, T.L.; Chen, H.Y.; Dai, C.F.; Sheu, J.H. Tortuosenes A and B, new diterpenoid metabolites from the Formosan soft coral Sarcophyton tortuosum. Org. Lett. 2014, 16, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Wu, C.Y.; Huang, C.Y.; Wang, H.C.; Dai, C.F.; Wu, Y.C.; Sheu, J.H. Cubitanoids and cembranoids from the soft coral Sinularia nanolobata. Mar. Drugs 2016, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.W.; Chao, C.H.; Su, J.H.; Huang, C.Y.; Dai, C.F.; Wen, Z.H.; Sheu, J.H. A novel symmetric sulfur-containing biscembranoid from the formosan soft coral Sinularia flexibilis. Tetrahedron Lett 2010, 51, 764–766. [Google Scholar] [CrossRef]
- Huang, C.Y.; Sung, P.J.; Uvarani, C.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Dai, C.F.; Wu, S.L.; Sheu, J.H. Glaucumolides A and B, biscembranoids with new structural type from a cultured soft coral Sarcophyton glaucum. Sci. Rep. 2015, 5, 15624. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Kurtan, T.; Mandi, A.; Yan, X.H.; Zhang, W.; Guo, Y.W. Biscembranoids formed from an alpha, beta-unsaturated gamma-lactone ring as a dienophile: Structure revision and establishment of their absolute configurations using theoretical calculations of electronic circular dichroism spectra. J. Org. Chem. 2013, 78, 3113–3119. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, T.; Igari, M.; Ishitsuka, M.O.; Ichikawa, A.; Itezono, Y.; Nakayama, N.; Kakisawa, H. A Novel chlorinated biscembranoid from the marine soft coral Sarcophyton glaucum. J. Org. Chem. 1990, 55, 6286–6289. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Ahmed, A.F.; Dai, C.F.; Chiang, M.Y.; Sheu, J.H. Sinulochmodins A-C, three novel terpenoids from the soft coral Sinularia lochmodes. Org. Lett. 2005, 7, 3813–3816. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pattenden, G. Biomimetic syntheses of ineleganolide and sinulochmodin C from 5-episinuleptolide via sequences of transannular Michael reactions. Tetrahedron 2011, 67, 10045–10052. [Google Scholar] [CrossRef]
- Kusumi, T.; Yamada, K.; Ishitsuka, M.O.; Fujita, Y.; Kakisawa, H. New cembranoids from the Okinawan soft coral Sinularia mayi. Chem. Lett. 1990, 19, 1315–1318. [Google Scholar] [CrossRef]
- Uchio, Y.; Eguchi, S.; Kuramoto, J.; Nakayama, M.; Hase, T. Denticulatolide, an ichthyotoxic peroxide-containing cembranolide from the soft coral Lobophytum denticulatum. Tetrahedron Lett. 1985, 26, 4487–4490. [Google Scholar] [CrossRef]
- Hegazy, M.E.; Gamal Eldeen, A.M.; Shahat, A.A.; Abdel-Latif, F.F.; Mohamed, T.A.; Whittlesey, B.R.; Pare, P.W. Bioactive hydroperoxyl cembranoids from the Red Sea soft coral Sarcophyton glaucum. Mar. Drugs 2012, 10, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Casteel, D.A. Peroxy natural products. Nat. Prod. Rep. 1992, 9, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.F.; Chen, W.T.; Li, X.W.; Wang, H.Y.; Guo, Y.W. New bicyclic cembranoids from the South China Sea soft coral Sarcophyton trocheliophorum. Sci. Rep. 2017, 7, 46584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yin, J.; Jiang, W.; Ma, M.; Lei, X.; Xiang, Z.; Dong, J.; Huang, K.; Yan, P. Cytotoxic and antibacterial cembranoids from a South China Sea soft coral, Lobophytum sp. Mar. Drugs 2013, 11, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, H.O.; Berger, S.; Braun, S. Carbon-13 NMR Spectroscopy; John Wiley & Sons: Chichester, UK, 1988. [Google Scholar]
- Dale, J.A.; Mosher, H.S. Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and alpha-methoxy-alpha-trifluoromethylphenylacetate (MTPA) esters. J. Am. Chem. Soc. 1973, 95, 512–519. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-Field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Duh, C.Y.; Chia, M.C.; Wang, S.K.; Chen, H.J.; El-Gamal, A.A.; Dai, C.F. Cytotoxic dolabellane diterpenes from the Formosan soft coral Clavularia inflata. J. Nat. Prod. 2001, 64, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Corminboeuf, O.; Overman, L.E.; Pennington, L.D. Total synthesis of the reputed structure of alcyonin and reassignment of its structure. Org. Lett. 2003, 5, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.P.; Chen, B.W.; Dai, C.F.; Sung, P.J.; Wu, Y.C.; Sheu, J.H. Sarcophyton F and G new dihydrofuranocembranoids from a Donsha atoll soft coral Sarcophyton sp. Bull. Chem. Soc. Jpn. 2012, 85, 920–922. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Heaton, A.; Konig, G.; Bruck, M.A.; Cramer, R.E.; Klein, D.M.; Scheuer, P.J. The structure of four isometric dihydrofuran-containing cembranoid diterpenes from several species of soft corals. J. Nat. Prod. 1987, 50, 650–659. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C. Studies of Australian soft corals. XLV. Epoxidation reaction of cembranoid diterpenes: Stereochemical outcomes. Heterocycles 1989, 28, 669–672. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hirase, T. Marine terpenes and terpenoids. XI. Structures of new dihydrofuranocembranoids isolated from a Sarcophyton sp. soft coral of Okinawa. Chem. Pharm. Bull. 1990, 38, 2442–2445. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Li, G.L.; Lan, Y.H.; Chia, Y.C.; Hsieh, P.W.; Wu, Y.H.; Wu, Y.C. Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free. Radic. Biol. Med. 2009, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Su, Y.C.; Chang, H.L.; Leu, Y.L.; Chung, P.J.; Kuo, L.M.; Chang, Y.J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res. 2009, 50, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Minami, H.; Hayashi, E.; Kodama, M.; Kawazu, K.; Fukuyama, Y. Neovibsanin C, a macrocyclic peroxide-containing neovibsane-type diterpene from Viburnum awabuki. Tetrahedron Lett. 1999, 40, 6261–6265. [Google Scholar] [CrossRef]
- Kamchonwongpaisan, S.; Nilanonta, C.; Tarnchompoo, B.; Thebtaranonth, C.; Thebtaranonth, Y.; Yuthavong, Y.; Kongsaeree, P.; Clardy, J. An antimalarial peroxide from Amomum krervanh Pierre. Tetrahedron Lett. 1995, 36, 1821–1824. [Google Scholar] [CrossRef]
- Higgs, M.D.; Faulkner, D.J. Plakortin, an antibiotic from Plakortis halichondrioides. J. Org. Chem. 1978, 34, 3454–3457. [Google Scholar] [CrossRef]
- Wells, R.J. A novel peroxyketal from a sponge. Tetrahedron Lett. 1976, 17, 2637–2638. [Google Scholar] [CrossRef]
- Bu, M.; Yang, B.B.; Hu, L. Natural endoperoxides as drug lead compounds. Curr. Med. Chem. 2016, 23, 383–405. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Hsieh, P.W.; Chang, Y.J.; Chung, P.J.; Kuo, L.M.; Hwang, T.L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free. Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]

| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH, m (J in Hz) a | δC b, type | δH, m (J in Hz) a | δC b, type | δH, m (J in Hz) c | δC d, type | δH, m (J in Hz) c | δC d, type | |
| 1 | 160.7, C | 160.6, C | 160.6, C | 159.8, C | ||||
| 2 | 5.44, dd (10.0, 1.6) | 77.8, CH | 5.43, dd (10.0, 1.6) | 77.8, CH | 4.91, dd (10.0, 1.6) | 78.4, CH | 4.98, d (10.4) | 77.8, CH |
| 3 | 4.90, d (10.0) | 122.2, CH | 4.91, d (10.0) | 122.7, CH | 4.55, d (10.0) | 123.9, CH | 4.45, d (10.4) | 123.5, CH |
| 4 | 141.6, C | 140.8, C | 140.2, C | 139.4, C | ||||
| 5α | 2.20, m | 47.9, CH2 | 2.18, dd (12.4, 10.8) | 42.6, CH2 | 2.42, dd (12.0, 3.2) | 49.1, CH2 | 1.98, m | 49.3, CH2 |
| 5β | 2.76, dd (12.8, 5.2) | 2.87, dd (12.4, 4.4) | 189, m | 2.25, dd (12.8, 3.6) | ||||
| 6 | 4.70, ddd (10.4, 10.4, 5.2) | 65.2, CH | 4.97, ddd (10.8, 9.2, 4.4) | 78.3, CH | 3.84, dd (9.2, 9.2) | 69.6, CH | 4.21, ddd (11.2, 9.2, 3.6) | 64.8, CH |
| 7 | 5.20, d (10.4) | 128.1, CH | 5.05, d (9.2) | 123.1, CH | 5.09, d (9.2) | 131.6, CH | 4.84, d (9.2) | 131.2, CH |
| 8 | 139.8, C | 144.2, C | 138.4, C | 139.4, C | ||||
| 9α | 2.03, m | 36.8, CH2 | 2.07, m | 36.9, CH2 | 2.21, ddd (13.6, 13.6, 2.4) | 28.2, CH2 | 1.58, m | 28.5, CH2 |
| 9β | 2.38, m | 2.42, m | 1.63, m | 2.30, dd (13.2, 4.8) | ||||
| 10α | 1.29, m | 23.5, CH2 | 1.35, m | 23.6, CH2 | 1.16, m | 23.9, CH2 | 1.60, m | 22.7, CH2 |
| 10β | 2.51, m | 2.17, m | 1.84, m | 1.28, m | ||||
| 11 | 2.42, dd (10.8, 2.8) | 61.4, CH | 2.43, m | 61.4, CH | 2.24, dd (10.4, 2.4) | 58.9, CH | 1.98, m | 62.6, CH |
| 12 | 60.8, C | 60.8, C | 59.7, CH | 60.9, C | ||||
| 13α | 2.03, m | 36.9, CH2 | 2.02, m | 37.0, CH2 | 1.49, m | 35.5, CH2 | 1.61, m | 37.1, CH2 |
| 13β | 1.06, m | 1.07, m | 0.98, m | 0.65, m | ||||
| 14α | 2.49, m | 23.7, CH2 | 2.52, m | 23.7, CH2 | 1.58, m | 22.2, CH2 | 2.08, m | 23.1, CH2 |
| 14β | 2.01, m | 2.03, m | 1.58, m | 1.65, m | ||||
| 15 | 123.8, C | 123.8, C | 123.8, C | 123.7, C | ||||
| 16 | 174.4, C | 174.4, C | 173.9, C | 174.3, C | ||||
| 17 | 1.86, s | 8.8, CH3 | 1.86, s | 8.7, CH3 | 1.64, s | 8.8, CH3 | 1.63, s | 8.8, CH3 |
| 18 | 1.70, s | 15.9, CH3 | 1.72, s | 15.9, CH3 | 1.31, s | 18.1, CH3 | 1.28, s | 16.9, CH3 |
| 19 | 1.86, s | 14.9, CH3 | 1.89, s | 15.3, CH3 | 1.45, s | 22.2, CH3 | 1.32, s | 21.8, CH3 |
| 20 | 1.33, s | 15.8, CH3 | 1.33, s | 15.8, CH3 | 1.00, s | 17.1, CH3 | 0.99, s | 16.4, CH3 |
| 6-OOH | 7.99, br s | |||||||
| Position | 5 | 6 | |||||
|---|---|---|---|---|---|---|---|
| δH, m (J in Hz) a | δC b, type | δH, m (J in Hz) c | δC d, type | δH, m (J in Hz) c | δC d, type | ||
| 1 | 160.4, C | 141.4, C | 1′ | 141.5, C | |||
| 2 | 4.95, d (10.0) | 78.4, CH | 5.28, d (10.0) | 82.7, CH | 2′ | 5.50, d (10.0) | 81.9, CH |
| 3 | 4.42, d (10.0) | 124.6, CH | 5.06, d (10.0) | 126.4, CH | 3′ | 4.92, d (10.0) | 125.1, CH |
| 4 | 139.2, C | 140.2, C | 4′ | 141.0, C | |||
| 5α | 1.95, m | 44.6, CH2 | 2.21, m | 38.5, CH2 | 5′α | 2.21, m | 38.8, CH2 |
| 5β | 2.47, br d (11.0) | 2.32, m | 5′β | 2.31, m | |||
| 6 | 4.58, ddd (11.0, 9.5, 2.5) | 78.9, CH | 24.2, CH2 | 6′ | 24.2, CH2 | ||
| 6α | 2.07, m | 6′α | 2.07, m | ||||
| 6β | 2.42, m | 6′β | 2.42, m | ||||
| 7 | 4.78, d (9.5) | 126.6, CH | 4.98, dd (9.2, 9.2) | 125.6, CH | 7′ | 4.95, dd (9.2, 9.2) | 125.5, CH |
| 8 | 143.8, C | 133.1, C | 8′ | 133.3, C | |||
| 9α | 1.65, m | 29.8, CH2 | 1.96, m | 36.6, CH2 | 9′α | 1.96, m | 36.6, CH2 |
| 9β | 2.52, dd (14.0, 4.5) | 2.27, m | 9′β | 2.27, m | |||
| 10α | 1.28, m | 23.4, CH2 | 1.22, m | 23.6, CH2 | 10′α | 1.22, m | 23.7, CH2 |
| 10β | 1.62, m | 2.04, m | 10′β | 2.04, m | |||
| 11 | 1.97, m | 63.3, CH | 2.51, m | 62.1, CH | 11′ | 2.51, m | 62.2, CH |
| 12 | 61.5, C | 61.2, CH | 12′ | 61.3, C | |||
| 13α | 1.59, m | 37.6, CH2 | 1.83, m | 37.3, CH2 | 13′α | 1.83, m | 37.4, CH2 |
| 13β | 0.64, m | 0.95, m | 13′β | 0.95, m | |||
| 14α | 2.07, m | 23.7, CH2 | 2.33, m | 22.6, CH2 | 14′α | 2.33, m | 22.7, CH2 |
| 14β | 1.61, m | 1.81, m | 14′β | 1.81, m | |||
| 15 | 124.3, C | 124.9, C | 15′ | 124.9, C | |||
| 16 | 174.3, C | 6.13, br s | 114.3, C | 16′ | 6.17, d (3.6) | 114.4, CH | |
| 17 | 1.63, s | 9.4, CH3 | 1.72, s | 10.2, CH3 | 17′ | 1.73, s | 10.2, CH3 |
| 18 | 1.29, s | 17.3, CH3 | 1.58, s | 14.6, CH3 | 18′ | 1.59, s | 14.6, CH3 |
| 19 | 1.34, s | 22.5, CH3 | 1.65, s | 14.7, CH3 | 19′ | 1.65, s | 14.7, CH3 |
| 20 | 0.98, s | 16.9, CH3 | 1.27, s | 15.7, CH3 | 20′ | 1.27, s | 15.7, CH3 |
| 6-OOH | 7.25, br s | ||||||
| Position | 6 | 8 a | 9 a |
|---|---|---|---|
| H-11 | δH 2.51 (H-11, H-11′) | δH 2.50 | δH 2.75 |
| C-11 | δC 62.1 (C-11) | δC 62.3 | δC 61.2 |
| δC 62.2 (C-11′) | |||
| C-12 | δC 61.2 (C-12) | δC 61.4 | δC 60.7 |
| δC 61.3 (C-12′) | |||
| C-13 | δC 37.3 (C-13) | δC 37.4 | δC 35.4 |
| δC 37.4 (C-13′) | |||
| C-14 | δC 22.6 (C-14) | δC 22.5 | δC 20.4 |
| δC 22.7 (C-14′) | |||
| H3-18 | δH 1.58 (H3-18) | δH 1.58 | δH 1.70 |
| δH 1.59 (H3-18′) | |||
| H3-20 | δH 1.27 (H3-20, H3-20′) | δH 1.28 | δH 1.18 |
| C-20 | δC 15.7 (C-20, C-20′) | δC 15.7 | δC 17.7 |
| Compounds | Superoxide Anion | Elastase Release | |
|---|---|---|---|
| IC50 (μM) a | Inh b % | Inh b % | |
| 1 | >30 | 32.1 ± 4.3 ** | 37.6 ± 5.0 ** |
| 2 | >30 | 4.0 ± 6.7 | 23.5 ± 6.6 * |
| 3 | >30 | 44.5 ± 4.6 *** | 35.6 ± 6.2 ** |
| 4 | >30 | 6.4 ± 4.2 | 27.6 ± 6.4 ** |
| 5 | >30 | 2.6 ± 6.2 | 30.5 ± 4.6 ** |
| 6 | 26.2 ± 1.0 | 64.6 ± 0.8 *** | 42.4 ± 5.1 ** |
| 7 | >30 | 3.5 ± 5.3 | 20.7 ± 4.1 ** |
| Idelalisib | 0.07 ± 0.01 | 102.8 ± 2.2 *** | 99.6 ± 4.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.-C.; Huang, C.-Y.; Ahmed, A.F.; Hwang, T.-L.; Dai, C.-F.; Sheu, J.-H. New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri. Mar. Drugs 2018, 16, 276. https://doi.org/10.3390/md16080276
Peng C-C, Huang C-Y, Ahmed AF, Hwang T-L, Dai C-F, Sheu J-H. New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri. Marine Drugs. 2018; 16(8):276. https://doi.org/10.3390/md16080276
Chicago/Turabian StylePeng, Chia-Chi, Chiung-Yao Huang, Atallah F. Ahmed, Tsong-Long Hwang, Chang-Feng Dai, and Jyh-Horng Sheu. 2018. "New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri" Marine Drugs 16, no. 8: 276. https://doi.org/10.3390/md16080276
APA StylePeng, C.-C., Huang, C.-Y., Ahmed, A. F., Hwang, T.-L., Dai, C.-F., & Sheu, J.-H. (2018). New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri. Marine Drugs, 16(8), 276. https://doi.org/10.3390/md16080276