Dietary Supplementation with a Magnesium-Rich Marine Mineral Blend Enhances the Diversity of Gastrointestinal Microbiota
Abstract
1. Introduction
2. Results
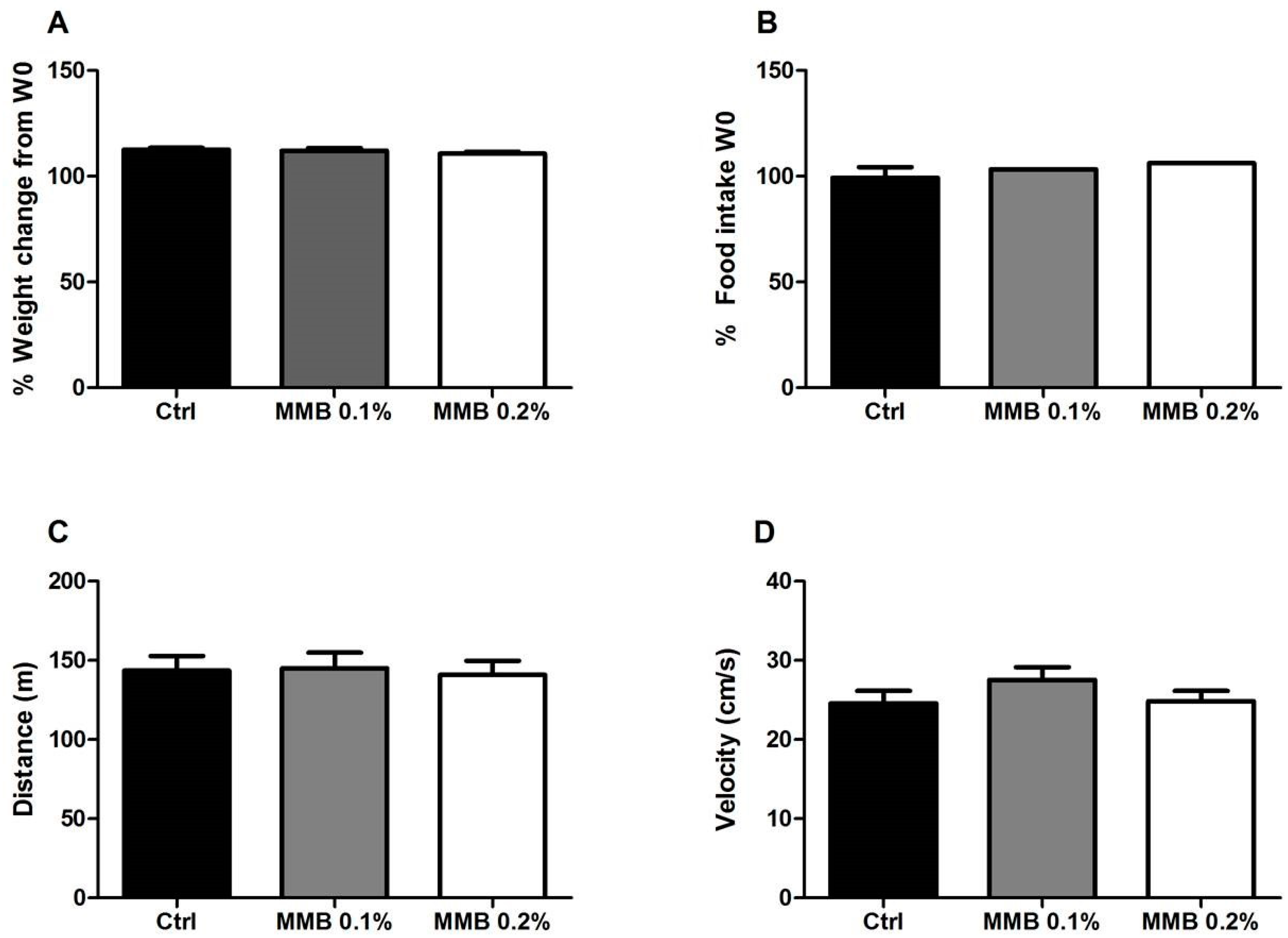
2.1. MMB Supplementation Had No Effect on Weight Gain, Food Intake or Locomotor Activity
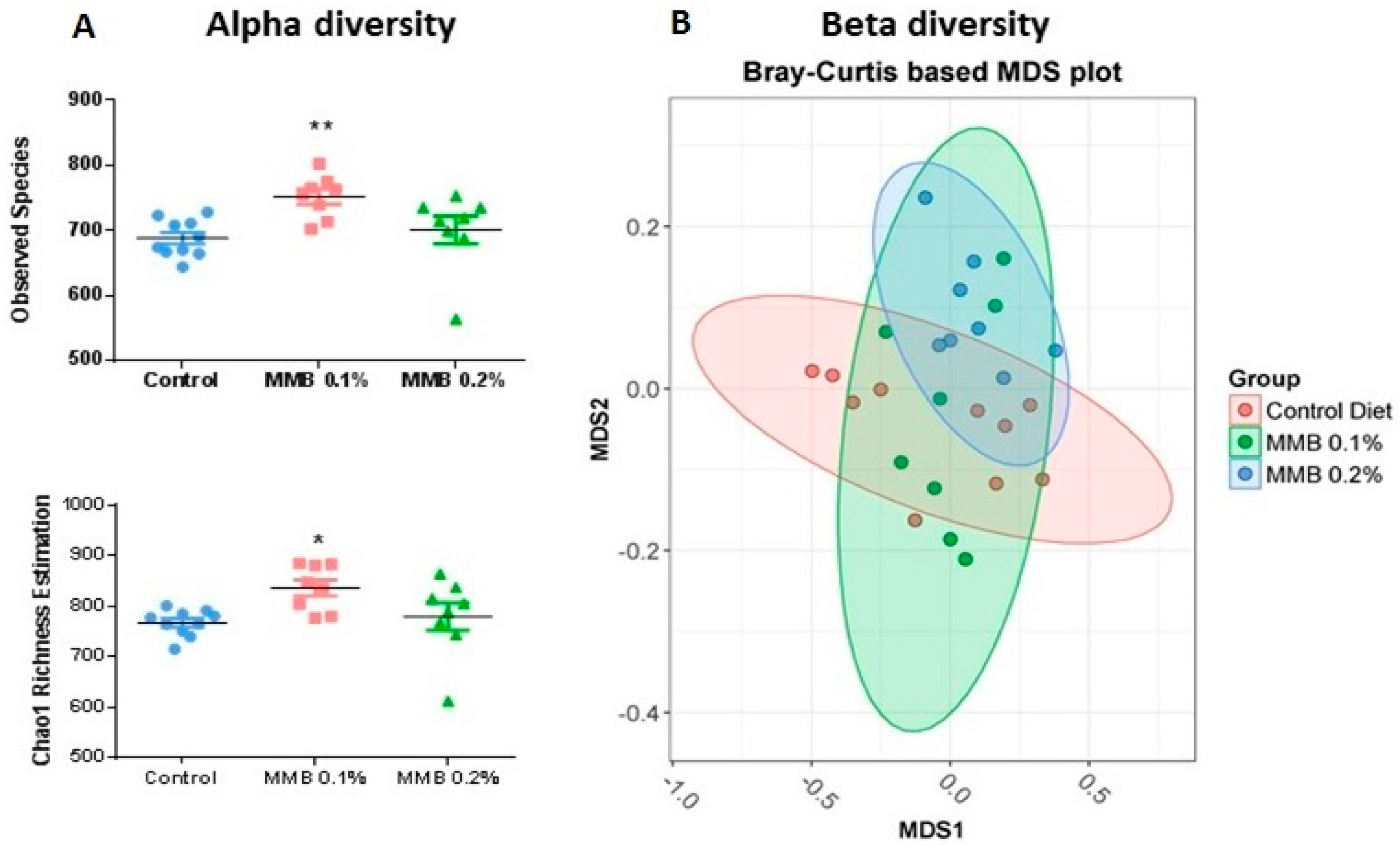
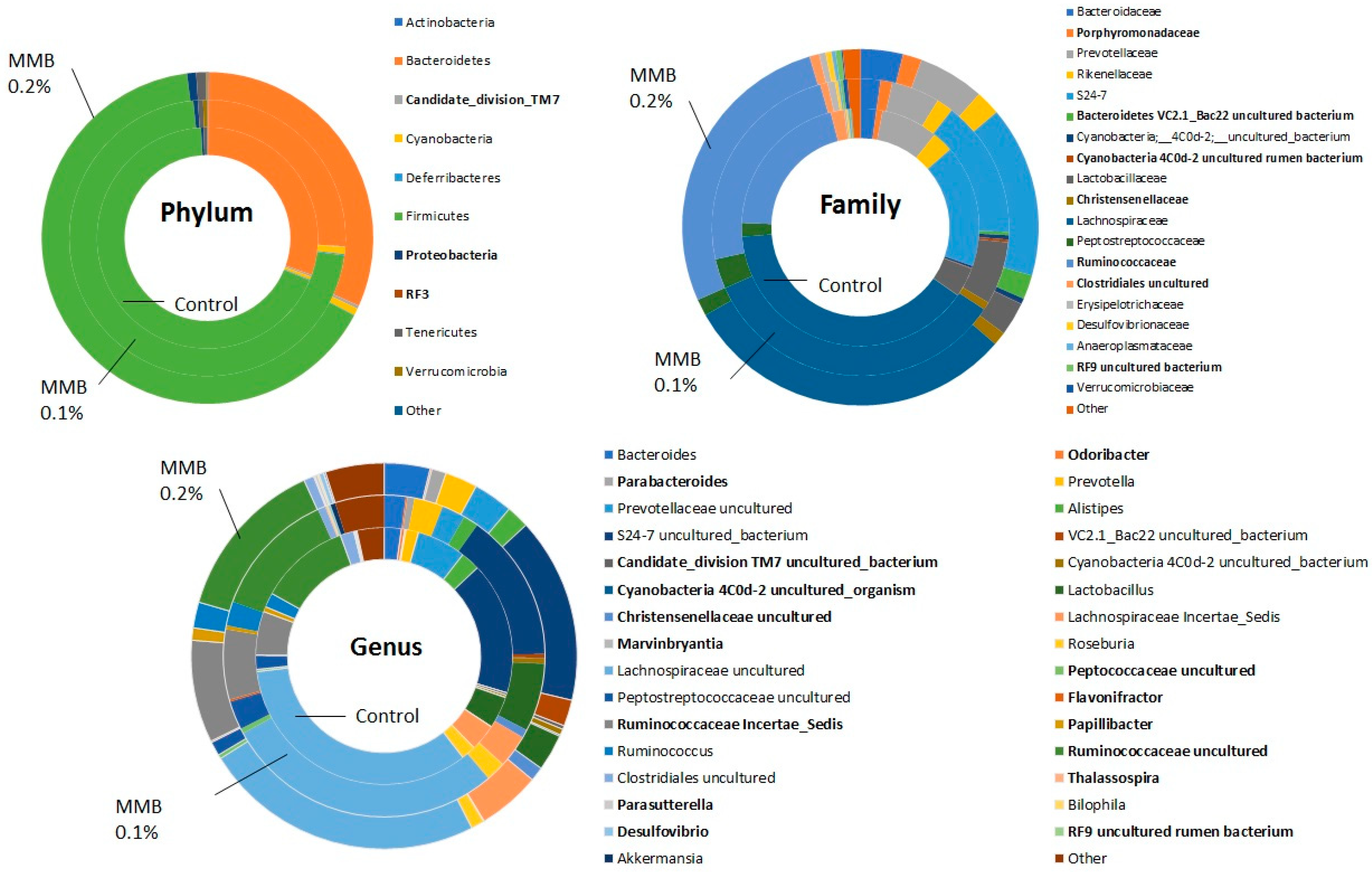
2.2. MMB Supplementation Increased the Diversity of the Gut Microbiota
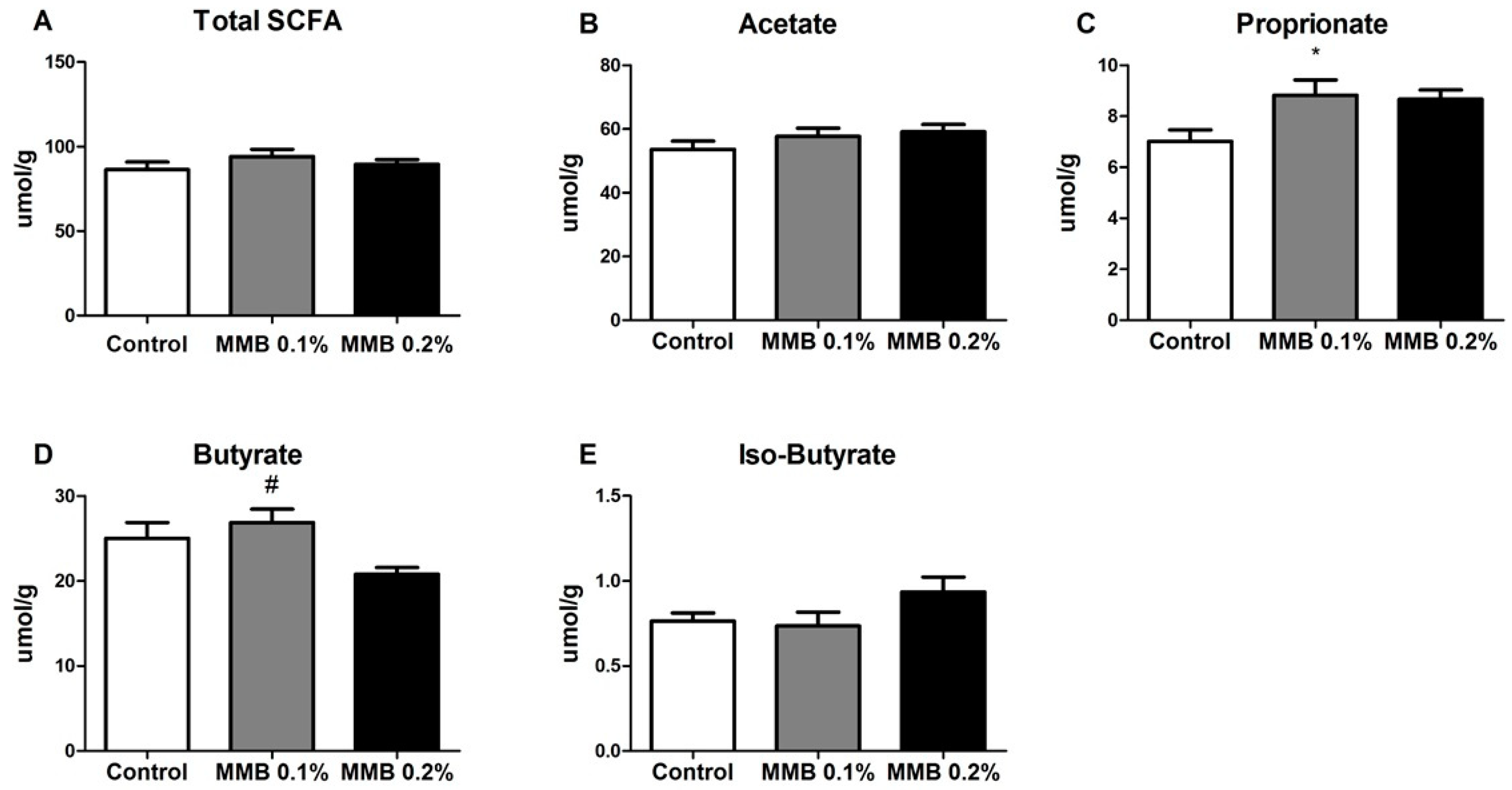
2.3. MMB Supplementation Altered the Short Chain Fatty Acid (SCFA) Profile in the Gut
3. Discussion
4. Materials and Methods
4.1. Dietary Supplementation and Experimental Design
4.2. Open Field
4.3. DNA Extraction
4.5. Short-Chain Fatty Acid (SCFA) Analysis
4.6. Bioinformatic Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.; Howard, J.M.L.; Henein, M.Y. A review of the effect of diet on cardiovascular calcification. Int. J. Mol. Sci. 2015, 16, 8861–8883. [Google Scholar] [CrossRef] [PubMed]
- Peterlik, M.; Cross, H.S. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur. J. Clin. Investig. 2005, 3, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Vaskonen, T. Dietary minerals and modification of cardiovascular risk factors. J. Nutr. Biochem. 2003, 14, 492–506. [Google Scholar] [CrossRef]
- Moore-Schiltz, L.; Albert, J.M.; Singer, M.E.; Swain, J.; Nock, N.L. Dietary intake of calcium and magnesium and the metabolic syndrome in the National Health and Nutrition Examination (NHANES) 2001–2010 data. Br. J. Nutr. 2015, 114, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Rayssiguier, Y.; Libako, P.; Nowacki, W.; Rock, E. Magnesium deficiency and metabolic syndrome: Stress and inflammation may reflect calcium activation. Magnes. Res. 2010, 23, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.F.; Stamler, J.; Dennis, B.; Moag-Stahlberg, A.; Okuda, N.; Robertson, C.; Zhao, L.; Chan, Q.; Elliott, P. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: TheINTERMAP Study. J. Hum. Hypertens. 2003, 17, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S.; Derbyshire, E.; Toribio-Mateas, M. Role of fatty acids and micronutrients in healthy ageing: A systematic review of randomised controlled trials set in the context of European dietary surveys of older adults. J. Hum. Nutr. Diet. 2016, 29, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Burnett-Hartman, A.; Fitzpatrick, A.; Kun Gao, M.; Jackson, S.; Schreiner, P. Supplement use contributes to meeting recommended dietary intakes for calcium, magnesium and vitamin C in four ethnicities of middle-aged and older Americans: The Multi-Ethnic Study of Atherosclerosis. J. Am. Diet. Assoc. 2009, 109, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Mokdad, A.H. Dietary Magnesium Intake in a National Sample of U.S. Adults. J. Nutr. 2003, 133, 2879–2882. [Google Scholar] [CrossRef] [PubMed]
- Power, S.E.; Jeffery, I.B.; Ross, R.P.; Stanton, C.; O’Toole, P.W.; O’Connor, E.M.; Fitzgerald, G.F. Food and nutrient intake of Irish community-dwelling elderly subjects: Who is at nutritional risk? J. Nutr. Health Aging 2014, 18, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Soderstrom, L.; Rosenblad, A.; Adolfsson, E.T.; Wolk, A.; Hakansson, N.; Bergkvist, L. A high energy intake from dietary fat among middle-aged and older adults is associated with increased risk of malnutrition 10 years later. Br. J. Nutr. 2015, 114, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Han, H.; Li, M.; Liang, C.; Fan, Z.; Aaseth, J.; He, J.; Montgomery, S.; Cao, Y. Dose-response relationship between dietary magnesium intake and risk of type 2 diabetes mellitus: A systematic review and meta-regression analysis of prospective cohort studies. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Ryder, K.M.; Shorr, R.I.; Bush, A.J.; Kritchevsky, S.B.; Harris, T.; Stone, K.; Cauley, J.; Tylavsky, F.A. Magnesium intake from food and supplements is associated with bone mineral density in healthy older white subjects. J. Am. Geriatr. Soc. 2005, 53, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.J.; Youn, C.H.; Kim, H.M.; Cho, Y.J.; Lee, G.H.; Lee, W.K. Dietary magnesium intake and risk of cancer: A meta-analysis of epidemiologic studies. Nutr. Cancer 2014, 66, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Pang, Z.; Liu, Q.F. Magnesium intake and risk of colorectal cancer: A meta-analysis of prospective studies. Eur. J. Clin. Nutr. 2012, 66, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Martinez, K.B.; Leone, V.; Chang, E.B. Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut Microbes 2017, 8, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut Microbiota: The Conductor in the Orchestra of Immune-Neuroendocrine Communication. Clin. Ther. 2015, 37, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Mackay, C.; Kaye, D. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018, 15, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2017, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.T.K.; Chan, G.C.F.; Li, J.C.B. Systemic effects of gut microbiota and its relationship with disease and modulation. BMC Immunol. 2015, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Neff, C.; Krueger, O.; Xiong, K.; Arif, S.; Nusbacher, N.; Schneider, J.M.; Cunningham, A.W.; Armstrong, A.; Li, S.; McCarter, M.D.; et al. Faecal Microbiota Composition Drives Immune Activation in HIV-infected Individuals. EBioMedicine 2018, 20, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Katsimichas, T.; Ohtani, T.; Motooka, D.; Tsukamoto, Y.; Kioka, H.; Nakamoto, K.; Konishi, S.; Chimura, M.; Sengoku, K.; Miyawaki, H.; et al. Non-Ischemic Heart Failure With Reduced Ejection Fraction Is Associated With Altered Intestinal Microbiota. Circ. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tang, W. Contributory Role of Gut Microbiota and Their Metabolites Toward Cardiovascular Complications in Chronic Kidney Disease. Semin. Nephrol. 2018, 38, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, J.; Guo, J.; Geng, B.; Ji, W.; Zhao, Q.; Li, J.; Liu, X.; Liu, J.; Guo, Z.; et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Chen, W.D.; Wang, Y.D. Gut microbiota: An integral moderator in health and disease. Front. Microbiol. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goubet, A.G.; Daillère, R.; Routy, B.; Derosa, L.; Roberti, P.M.; Zitvogel, L. The impact of the intestinal microbiota in therapeutic responses to cancer. Comptes Rendus-Biol. 2018, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Perales-Puchalt, A.; Perez-Sanz, J.; Payne, K.K.; Svoronos, N.; Allegrezza, M.J.; Chaurio, R.A.; Anadon, C.; Calmette, J.; Biswas, S.; Mine, J.A.; et al. Microbiota reconstitution restores intestinal integrity after cisplatin therapy. J. Leukoc. Biol. 2018, 1–7. [Google Scholar] [CrossRef]
- Opazo, M.C.; Ortega-Rocha, E.M.; Coronado-Arrázola, I.; Bonifaz, L.C.; Boudin, H.; Neunlist, M.; Bueno, S.M.; Kalergis, A.M.; Riedel, C.A. Intestinal microbiota influences non-intestinal related autoimmune diseases. Front. Microbiol. 2018, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Antza, C.; Stabouli, S.; Kotsis, V. Gut microbiota in kidney disease and hypertension. Pharmacol. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cosola, C.; Rocchetti, M.T.; Cupisti, A.; Gesualdo, L. Microbiota metabolites: Pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol. Res. 2018, 0–1. [Google Scholar] [CrossRef] [PubMed]
- Zoetendal, E.; de Vos, W. Effect of diet on the intestinal microbiota and its activity. Curr. Opin. Gastroenterol. 2014, 30, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M. Diet, gut microbiome, and bone health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.G.; Costa, J.A.; Alfenas, R.C. Could the beneficial effects of dietary calcium on obesity and diabetes control be mediated by changes in intestinal microbiota and integrity? Br. J. Nutr. 2015, 114, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Coudray, C.; Demigne, C.; Rayssiguier, Y. Effects of Dietary Fibers on Magnesium Absorption in Animals and Humans. J. Nutr. 2003, 133, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Ahrens, K.E.; Schaafsma, G.; Van den Heuvel, E.G.H.M.; Schrezenmeir, J. Effects of prebiotics on mineral metabolism. Am. J. Clin. Nutr. 2001, 73 (Suppl. 2), 459S–464S. [Google Scholar] [CrossRef] [PubMed]
- Brennan, O.; Sweeney, J.; O’Meara, B.; Widaa, A.; Bonnier, F.; Byrne, H.J.; O’Gorman, D.M.; O’Brien, F.J. A Natural, Calcium-Rich Marine Multi-mineral Complex Preserves Bone Structure, Composition and Strength in an Ovariectomised Rat Model of Osteoporosis. Calcif. Tissue Int. 2017, 101, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Brennan, O.; Stenson, B.; Widaa, A.; O’Gorman, D.M.; O’Brien, F.J. Incorporation of the natural marine multi-mineral dietary supplement Aquamin enhances osteogenesis and improves the mechanical properties of a collagen-based bone graft substitute. J. Mech. Behav. Biomed. Mater. 2015, 47, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Paruchuri, T.; Bhagavathula, N.; Varani, J. A Mineral-Rich Red Algae Extract Inhibits Polyp Formation and Inflammation in the Gastrointestinal Tract of Mice on a High-Fat Diet. Integr. Cancer Ther. 2010, 9, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Amu, S.; Saunders, S.P.; Fallon, P.G. A mineral extract from red algae ameliorates chronic spontaneous colitis in IL-10 deficient mice in a mouse strain dependent manner. Phyther. Res. 2014, 28, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Cronin, B.E.; Allsopp, P.J.; Slevin, M.M.; Magee, P.J.; Livingstone, M.B.E.; Strain, J.J.; McSorley, E.M. Effects of supplementation with a calcium-rich marine-derived multi-mineral supplement and short-chain fructo-oligosaccharides on serum lipids in postmenopausal women. Br. J. Nutr. 2016, 115, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.N.; Bassis, C.M.; Zhang, L.; Zaidi, S.; Varani, J.; Bergin, I.L. Calcium reduces liver injury in mice on a high-fat diet: Alterations in microbial and bile acid profiles. PLoS ONE 2016, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Parra, P.; Laraichi, S.; Serra, F.; Palou, A. Calcium supplementation modulates gut microbiota in a prebiotic manner in dietary obese mice. Mol. Nutr. Food Res. 2016, 60, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Pyndt Jørgensen, B.; Winther, G.; Kihl, P.; Nielsen, D.S.; Wegener, G.; Hansen, A.K.; Sørensen, D.B. Dietary magnesium deficiency affects gut microbiota and anxiety-like behaviour in C57BL/6N mice. Acta Neuropsychiatr. 2015, 27, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Winther, G.; Pyndt Jørgensen, B.M.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sorensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Dinis, J.M.; Barton, D.E.; Ghadiri, J.; Surendar, D.; Reddy, K.; Velasquez, F.; Chaffee, C.L.; Lee, M.C.W.; Gavrilova, H.; Ozuna, H.; et al. In search of an uncultured human-associated TM7 bacterium in the environment. PLoS ONE 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kuehbacher, T.; Rehman, A.; Lepage, P.; Hellmig, S.; Fölsch, U.R.; Schreiber, S.; Ott, S.J. Intestinal TM7 bacterial phylogenies in active inflammatory bowel disease. J. Med. Microbiol. 2008, 57, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Goodrich, J.; Waters, J.; Poole, A.; Sutter, J.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.; et al. Human genetics shape the gut microbiome. PubMed Commons Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Baxter, N.T.; Iverson, K.D.; Sadler, W.D.; Petrosino, J.F.; Chen, G.Y.; Schloss, P.D. The Gut Microbiome Modulates Colon Tumorigenesis. mBio 2013, 4, e00692-13. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.C.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D.; et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Fermentation in the human large intestine: Its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 2011, 45, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front. Behav. Neurosci. 2017, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Gorman, D.M.O.; Nolan, Y.M. Evidence that the Marine-derived Multi-mineral Aquamin has Anti-inflammatory Effects on Cortical Glial-enriched Cultures. Phyther. Res. 2011, 767, 765–767. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, D.M.; O’Carroll, C.; Carmody, R.J. Evidence that marine-derived, multi-mineral, aquamin inhibits the NF-κB signaling pathway in vitro. Phyther. Res. 2012, 26, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chassany, O.; Michaux, A.; Bergmann, J. Drug-induced diarrhoea. Drug Saf. 2000, 22, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, I.; Abumaria, N.; Wu, L.J.; Huang, C.; Zhang, L.; Li, B.; Zhao, X.; Govindarajan, A.; Zhao, M.G.; Zhuo, M.; et al. Enhancement of Learning and Memory by Elevating Brain Magnesium. Neuron 2010, 65, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Hahn, A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium—An Update. Curr. Nutr. Food Sci. 2017, 13, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Strause, L.; Saltman, P.; Smith, K.; Bracker, M.; Andon, M. Spinal Bone Loss in Postmenopausal Women Supplemented with Calcium and Trace Minerals. J. Nutr. 1994, 124, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 108, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Ida, M.; Peterson, V.L.; Prenderville, J.A.; Moloney, G.M.; Izumo, T.; Murphy, K.; Murphy, A.; Ross, R.P.; Stanton, C.; et al. Revisiting Metchnikoff: Age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav. Immun. 2017, 65, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Marques, T.M.; O’Sullivan, O.; Ross, R.P.; Shanahan, F.; Quigley, E.M.; Dinan, T.G.; Kiely, B.; Fitzgerald, G.F.; Cotter, P.D.; et al. Contrasting effects of Bifidobacterium breve NCIMB 702258 and Bifidobacterium breve DPC 6330 on the composition of murine brain fatty acids and gut microbiota. Am. J. Clin. Nutr. 2012, 95, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; Desantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- R-3.3.1 for Windows (32/64 bit). Available online: https://cran.r-project.org/bin/windows/base/old/3.3.1/ (accessed on 19 June 2018).
| (>5 ppm) | ppm | (<5 ppm) | ppm | (<1 ppm) | (<0.2 ppm) |
|---|---|---|---|---|---|
| Calcium | 285,000 | Iodine | 4.75 | Hafnium | Rhodium |
| Magnesium | 214,650 | Barium | 4.62 | Cadmium | Tin |
| Carbon | 106,500 | Chromium | 4.33 | Antimony | Gallium |
| Sulfur | 3174 | Copper | 3.73 | Bismuth | Europium |
| Sodium | 2835 | Fluoride | 2.97 | Gold | Holmium |
| Chloride | 1451 | Zinc | 2.77 | Lithium | Terbium |
| Strontium | 1262 | Cerium | 2.13 | Selenium | Lutetium |
| Iron | 975.50 | Silver | 2.07 | Tellurium | Thulium |
| Silicon | 380.00 | Neodymium | 1.92 | Thallium | Rubidium |
| Aluminum | 270.00 | Lanthanum | 1.66 | Dysprosium | Tantalum |
| Manganese | 265.35 | Molybdenum | 1.62 | Praseodymium | Germanium |
| Potassium | 142.60 | Arsenic | 1.47 | Gadolinium | Cesium |
| Boron | 110.80 | Scandium | 1.37 | Erbium | Mercury |
| Phosphorus | 96.65 | Cobalt | 1.24 | Palladium | Platinum |
| Titanium | 23.65 | Nickel | 1.19 | Samarium | Iridium |
| Zirconium | 10.45 | Beryllium | 1.10 | Lead | Osmium |
| Vanadium | 9.64 | Ruthenium | 1.10 | Ytterbium | Rhenium |
| Thorium | 9.08 | Indium | |||
| Niobium | 6.25 | ||||
| Tungsten | 5.57 | ||||
| Yttrium | 5.47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crowley, E.K.; Long-Smith, C.M.; Murphy, A.; Patterson, E.; Murphy, K.; O’Gorman, D.M.; Stanton, C.; Nolan, Y.M. Dietary Supplementation with a Magnesium-Rich Marine Mineral Blend Enhances the Diversity of Gastrointestinal Microbiota. Mar. Drugs 2018, 16, 216. https://doi.org/10.3390/md16060216
Crowley EK, Long-Smith CM, Murphy A, Patterson E, Murphy K, O’Gorman DM, Stanton C, Nolan YM. Dietary Supplementation with a Magnesium-Rich Marine Mineral Blend Enhances the Diversity of Gastrointestinal Microbiota. Marine Drugs. 2018; 16(6):216. https://doi.org/10.3390/md16060216
Chicago/Turabian StyleCrowley, Erin K., Caitriona M. Long-Smith, Amy Murphy, Elaine Patterson, Kiera Murphy, Denise M. O’Gorman, Catherine Stanton, and Yvonne M. Nolan. 2018. "Dietary Supplementation with a Magnesium-Rich Marine Mineral Blend Enhances the Diversity of Gastrointestinal Microbiota" Marine Drugs 16, no. 6: 216. https://doi.org/10.3390/md16060216
APA StyleCrowley, E. K., Long-Smith, C. M., Murphy, A., Patterson, E., Murphy, K., O’Gorman, D. M., Stanton, C., & Nolan, Y. M. (2018). Dietary Supplementation with a Magnesium-Rich Marine Mineral Blend Enhances the Diversity of Gastrointestinal Microbiota. Marine Drugs, 16(6), 216. https://doi.org/10.3390/md16060216




