Influence of Preparation Methods of Chitooligosaccharides on Their Physicochemical Properties and Their Anti-Inflammatory Effects in Mice and in RAW264.7 Macrophages
Abstract
1. Introduction
2. Results
2.1. Structural and Physicochemical Characterization of COSs
2.2. Role of P1COS and P2COS in the Peritoneal Migration of Neutrophils
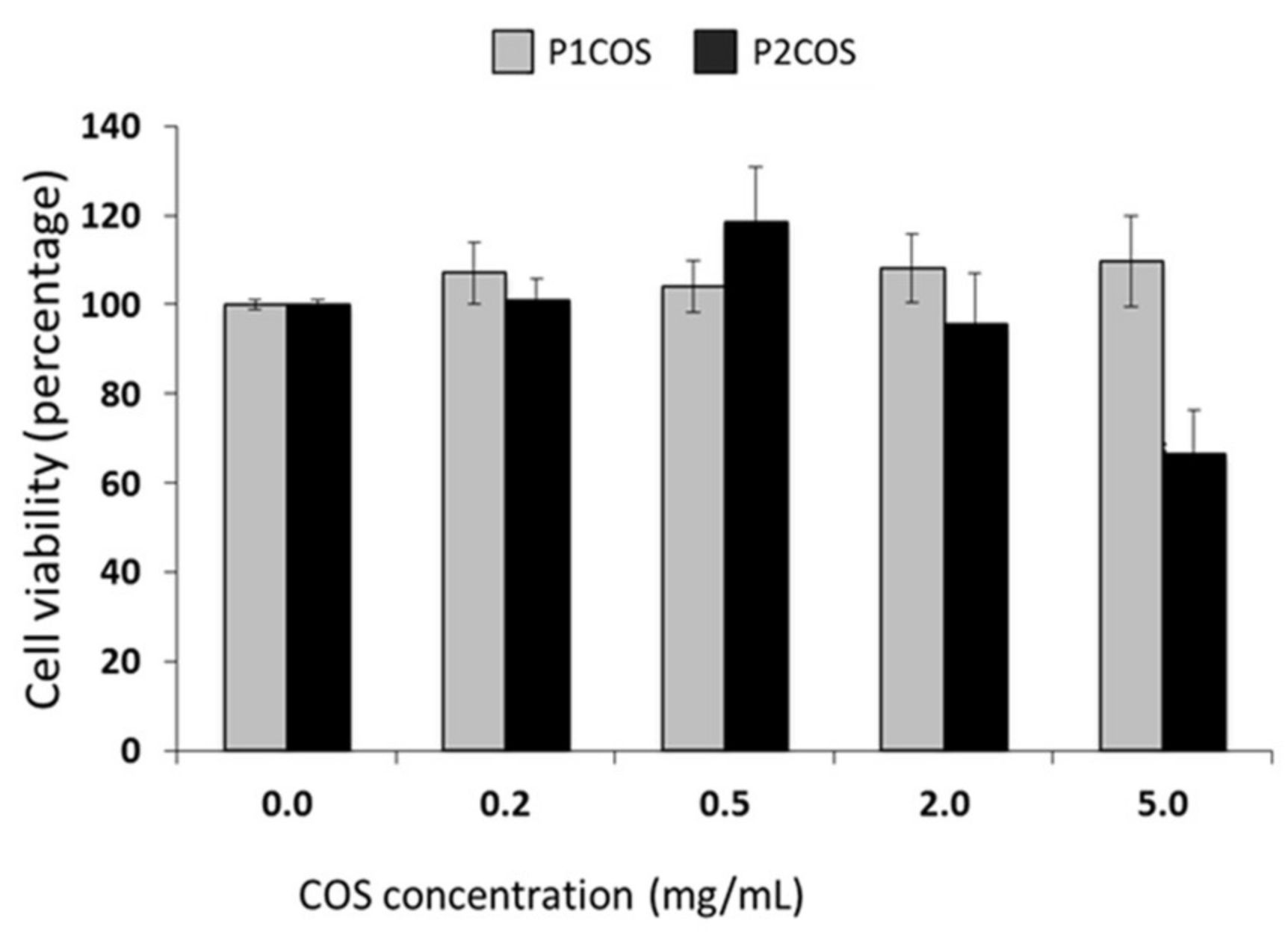
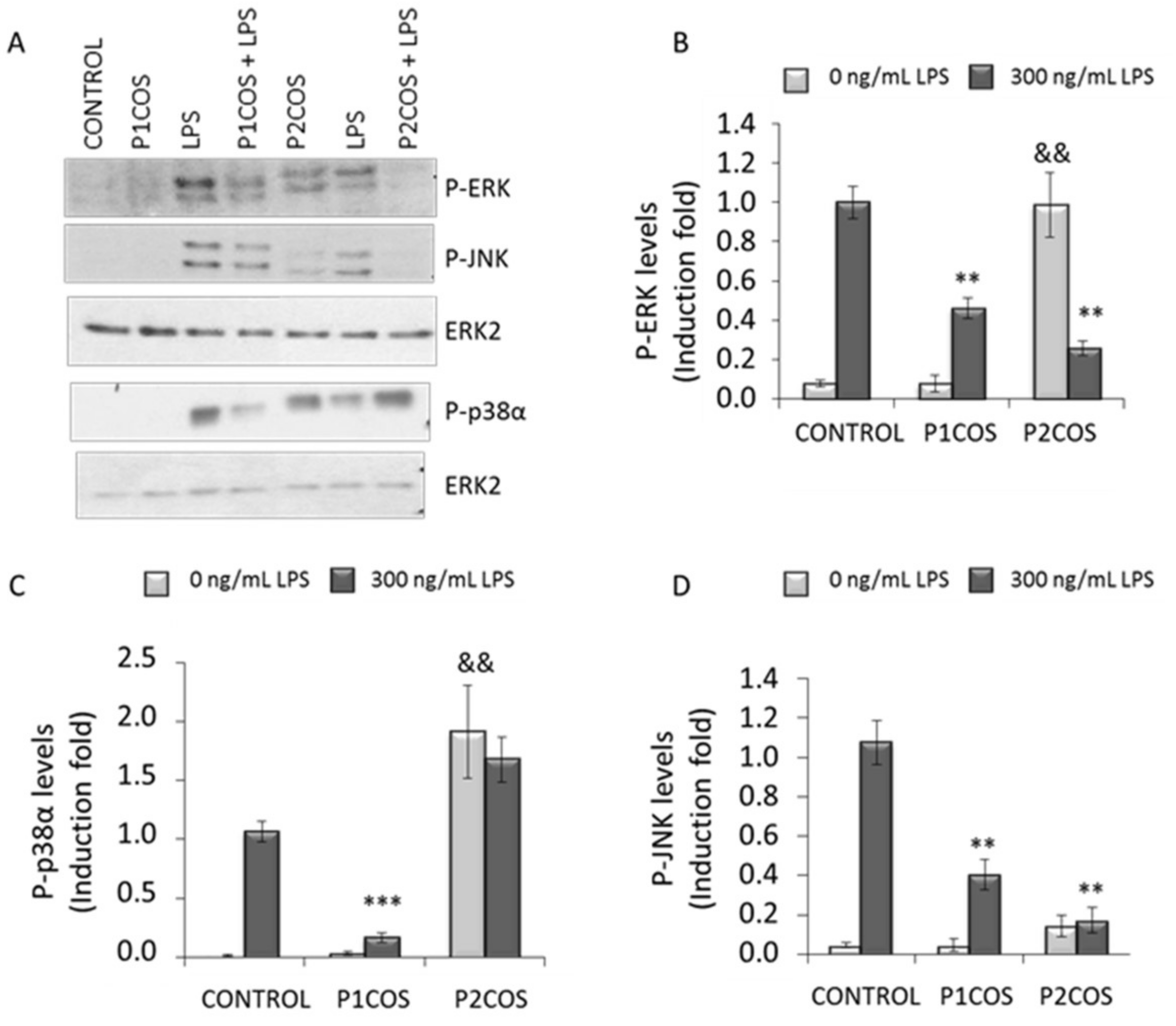
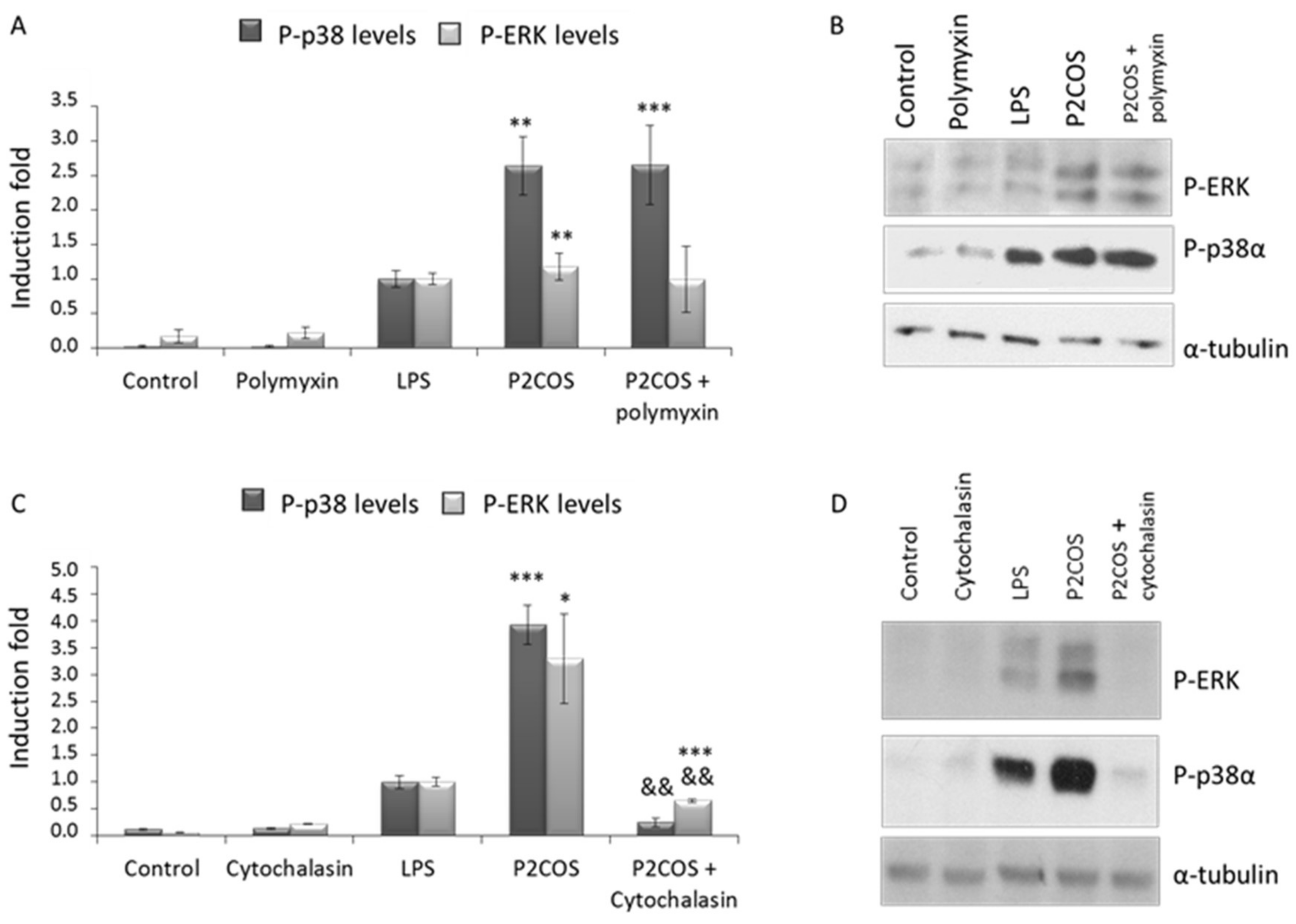
2.3. Role of P1COS and P2COS on LPS-Activated RAW264.7 Macrophage Cells
3. Discussion
3.1. The Relation between P1COS and P2COS Preparation Methods and Their Composition
3.2. Role of P1COS and P2COS in LPS-Induced Mice and LPS-Activated RAW264.7 Macrophage Cells
4. Materials and Methods
4.1. Preparation of COSs
4.2. Structural and Physicochemical Characterization of COS
4.3. Animal Model
4.4. Cell Culture, Stimuli, and Western Blotting Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Thomas, L.S.; Arnold, E.T.; CLukasek, K.; Michelsen, K.S.; Arditi, M. TLR signaling at the intestinal epithelial interface. J. Endotoxin Res. 2003, 9, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Arndt, P.G.; Suzuki, N.; Avdi, N.J.; Malcolm, K.C.; Worthen, G.S. Lipopolysaccharide-induced c-Jun NH2-terminal kinase activation in human neutrophils: Role of phosphatidylinositol 3-Kinase and Syk-mediated pathways. J. Boil. Chem. 2004, 279, 10883–10891. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Perobelli, S.M.; Galvani, R.G.; Gonçalves-Silva, T.; Xavier, C.R.; Nóbrega, A.; Bonomo, A. Plasticity of neutrophils reveals modulatory capacity. Braz. J. Med Boil. Res. 2015, 48, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.D.; Suttles, J. Functional plasticity of macrophages: Reversible adaptation to changing microenvironments. J. Leukocite Boil. 2004, 76, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T. Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Davis, R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef] [PubMed]
- English, J.M.; Cobb, M.H. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol. Sci. 2002, 23, 40–45. [Google Scholar] [CrossRef]
- Li, K.; Xing, R.; Liu, S.; Li, P. Advances in preparation, analysis and biological activities of single chitooligosaccharides. Carbohydr. Polym. 2016, 139, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Kang, K.H.; Je, J.Y.; Pham, N.D.; Byun, H.G.; Kim, S.K. Biological effects of chitosan and its derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, M.; Liu, Q.; Wang, W.; Du, Y.; Yin, H. The inhibition of LPS-induced inflammation in RAW264.7 macrophages via the PI3K/Akt pathway by highly N-acetylated chitooligosaccharide. Carbohydr. Polym. 2017, 174, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterization and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Santos-Moriano, P.; Woodley, J.M.; Plou, F.J. Continuous production of chitooligosaccharides by an immobilized enzyme in a dual-reactor system. J. Mol. Catal. B Enzym. 2016, 133, 211–217. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, X.; Li, Z.; Guo, X.; Ling, P. Application of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) in preparation of chitosan oligosaccharides (COS) with degree of polymerization (DP) 5–12 containing well-distributed acetyl groups. Int. J. Mass Spectrom. 2010, 290, 94–99. [Google Scholar] [CrossRef]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Han, J.L.; Hsieh, K.H. Kinetic study of acid depolymerization of chitosan and effects of low molecular weight chitosan on erythrocyte rouleaux formation. Carbohydr. Res. 2011, 346, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, S. Preparation of water soluble chitosan by hydrolysis with commercial a-amylase containing chitosanase activity. Food Chem. 2011, 128, 769–772. [Google Scholar] [CrossRef]
- Xia, Z.; Wu, S.; Chen, J. Preparation of water soluble chitosan by hydrolysis using hydrogen peroxide. Int. J. Boil. Macromol. 2013, 59, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, Y.; Li, K.; Yu, H.; Liu, S.; Yang, Y.; Chen, X.; Li, P. Monomer composition of chitooligosaccharides obtained by different degradation methods and their effects on immunomodulatory activities. Carbohydr. Polym. 2017, 157, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.C.P.; Cutsem, P. Preparation of chitooligosaccharides with degree of polymerization higher than 6 by acid or enzymatic degradation of chitosan. Biochem. Eng. J. 2005, 25, 165–172. [Google Scholar] [CrossRef]
- Hamer, S.N.; Cord-Landwehr, S.; Biarnés, X.; Planas, A.; Waegeman, H.; Moerschbacher, B.M.; Kolkenbrock, S. Enzymatic production of defined chitosan oligomers with a specific pattern of acetylation using a combination of chitin oligosaccharide deacetylases. Sci. Rep. 2015, 5, 8716. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.J.; Park, R.D. Bioproduction of Chitooligosaccharides: Present and Perspectives. Mar. Drugs 2014, 12, 5328–5356. [Google Scholar] [CrossRef] [PubMed]
- Vårum, K.M.; Holme, H.K.; Izume, M.; Torger-Stokke, B.; Smidsrød, O. Determination of enzymatic hydrolysis specificity of partially N-acetylated chitosans. Biochim. Biophys. Acta 1996, 1291, 5–15. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G.H. Production of Chitooligosaccharides and Their Potential Applications in Medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, M.X.; Sauvageau, J.C.M.; Kumirska, J.; Thöming, J. Studies on acetylation patterns of different chitosan preparations. Carbohydr. Polym. 2009, 78, 678–684. [Google Scholar] [CrossRef]
- Tømmeraas, K.; Vårum, K.M.; Christensen, B.E.; Smidsrød, O. Preparation and characterization of oligosaccharides produced by nitrous acid depolymerisation of chitosans. Carbohydr. Res. 2001, 333, 137–144. [Google Scholar] [CrossRef]
- Soria-Castro, I.; Krzyzanowska, A.; Pelaez, M.L.; Regadera, J.; Ferrer, G.; Montoliu, L.; Rodriguez-Ramos, R.; Fernandez, M.; Alemany, S. Cot/tpl2 (MAP3K8) mediates myeloperoxidase activity and hypernociception following peripheral inflammation. J. Boil. Chem. 2010, 285, 33805–33815. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.S.; Araujo, M.I.; Góes, A.M.; Pacífico, L.G.; Oliveira, R.R.; Oliveira, S.C. Polymyxin B as inhibitor of LPS contamination of Schistosoma mansoni recombinant proteins in human cytokine analysis. Microb. Cell Fact. 2007, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- MacLean-Fletcher, S.; Pollard, T.D. Mechanism of action of cytochalasin B on actin. Cell 1980, 20, 329–341. [Google Scholar] [CrossRef]
- Ishiguro, K.; Yoshie, N.; Sakurai, M.; Inoue, Y. A 1H-NMR study of a fragment of partially N-deacetylated chitin produced by lysozyme degradation. Carbohydr. Res. 1992, 237, 333–338. [Google Scholar] [CrossRef]
- Jung, W.K.; Park, P.J.; Ahn, C.B.; Je, J.Y. Preparation and antioxidant potential of maillard reaction products from (MRPs) chitooligomer. Food Chem. 2014, 145, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Mengíbar, M.; Ganan, M.; Miralles, B.; Carrascosa, A.V.; Martínez-Rodriguez, A.J.; Peter, M.G.; Heras, A. Antibacterial activity of products of depolymerization of chitosans with lysozyme and chitosanase against Campylobacter jejuni. Carbohydr. Polym. 2011, 84, 844–848. [Google Scholar] [CrossRef]
- Sánchez, Á.; Mengíbar, M.; Rivera-Rodríguez, G.; Moerchbacher, B.; Acosta, N.; Heras, A. The effect of preparation processes on the physicochemical characteristics and antibacterial activity of chitooligosaccharides. Carbohydr. Polym. 2017, 157, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Spindola, H.; de Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-Inflammatory Activity of Chitooligosaccharides in vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Bai, X.; Du, Y. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Im, S.Y.; Kim, Y.H. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 358, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Davydova, V.N.; Yermak, I.M.; Gorbach, V.I.; Krasikova, I.N.; Solov’eva, T.F. Interaction of Bacterial Endotoxins with Chitosan. Effect of Endotoxin Structure, Chitosan Molecular Mass, and Ionic Strength of the Solution on the Formation of the Complex. Biochemistry 2000, 65, 1082–1090. [Google Scholar] [PubMed]
- Yermak, I.M.; Davidova, V.N.; Gorbach, V.I.; Luk’yanov, P.A.; Solov’eva, T.F.; Ulmer, A.J.; Buwitt-Beckmann, U.; Rietschel, E.T.; Ovodov, Y.S. Forming and immunological properties of some lipopolysaccharide–chitosan complexes. Biochimie 2006, 88, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Davydova, V.N.; Naberezhnykh, G.A.; Yermak, I.M.; Gorbach, I.N.; Solov’eva, T.F. Determination of Binding Constants of Lipopolysaccharides of Different Structure with Chitosan. Biochemistry 2006, 71, 332–339. [Google Scholar] [CrossRef]
- Naberezhnykh, G.A.; Gorbach, V.I.; Likhatskaya, G.N.; Davidova, V.N.; Solov’eva, T.F. Interaction of Chitosans and Their N-Acylated Derivatives with Lipopolysaccharide of Gram-Negative Bacteria. Biochemistry 2008, 73, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Naberezhnykh, G.A.; Gorbach, V.I.; Kalmykova, E.N.; Solov’eva, T.F. Determination of the parameters of binding between lipopolysaccharide and chitosan and its N-acetylated derivative using a gravimetric piezoquartz biosensor. Biophys. Chem. 2015, 198, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Khavari, T.A.; Rinn, J. Ras/Erk MAPK signaling in epidermal homeostasis and neoplasia. Cell Cycle 2007, 6, 2928–2931. [Google Scholar] [CrossRef] [PubMed]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Minakami, M.; Kitagawa, N.; Iida, H.; Anan, H.; Inai, T. p38 mitogen-activated protein kinase and c-jun NH2-terminal protein kinase regulate the accumulation of a tight junction protein, ZO-1, in cell–cell contacts in HaCaT cells. Tissue Cell 2015, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT–Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- NTP. NTP toxicology and carcinogenesis studies of 5-(Hydroxymethyl)-2-furfural (CAS No. 67-47-0) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2010, 554, 7–13, 15–19, 21–31. [Google Scholar]
- Tomas, D.; Karin, K.; Stephanie, D.; Imre, B. Analysis of Amadori compounds by high-performance cation exchange chromatography coupled to tandem mass spectrometry. Anal. Chem. 2005, 77, 140–147. [Google Scholar]
- Zeng, L.; Qin, C.; Chi, W.; Wang, L.; Ku, Z.; Li, W. Browning of chitooligomers and their optimum preservation. Carbohydr. Polym. 2007, 67, 551–558. [Google Scholar] [CrossRef]
- Basta, G. Receptor for advanced glycation end products and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis 2008, 196, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Boil. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.G.; Peyron, M. Molecular weight manipulation of chitosan I: Kinetics of depolymerization by nitrous acid. Carbohydr. Res. 1995, 277, 257–272. [Google Scholar] [CrossRef]
- Mengíbar, M.; Mateos-Aparicio, I.; Miralles, B.; Heras, A. Influence of the physico-chemical characteristics of chito-oligosaccharides (COS) on antioxidant activity. Carbohydr. Polym. 2013, 97, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H- NMR spectroscopy. Polym. Bullettin 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Madhuprakash, J.; El Gueddari, N.E.; Moerchbacher, B.M.; Podile, A.R. Production of bioactive chitosan oligosaccharides using the hypertransglycosylating chitinase-D from serratia proteamaculans. Bioresour. Technol. 2015, 198, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Rodríguez, C.; López, P.; Pozo, M.; Duce, A.M.; López-Pelaéz, M.; Fernández, M.; Alemany, S. COX2 expression and Erk1/Erk2 activity mediate Cot-induced cell migration. Cell. Signal 2008, 20, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
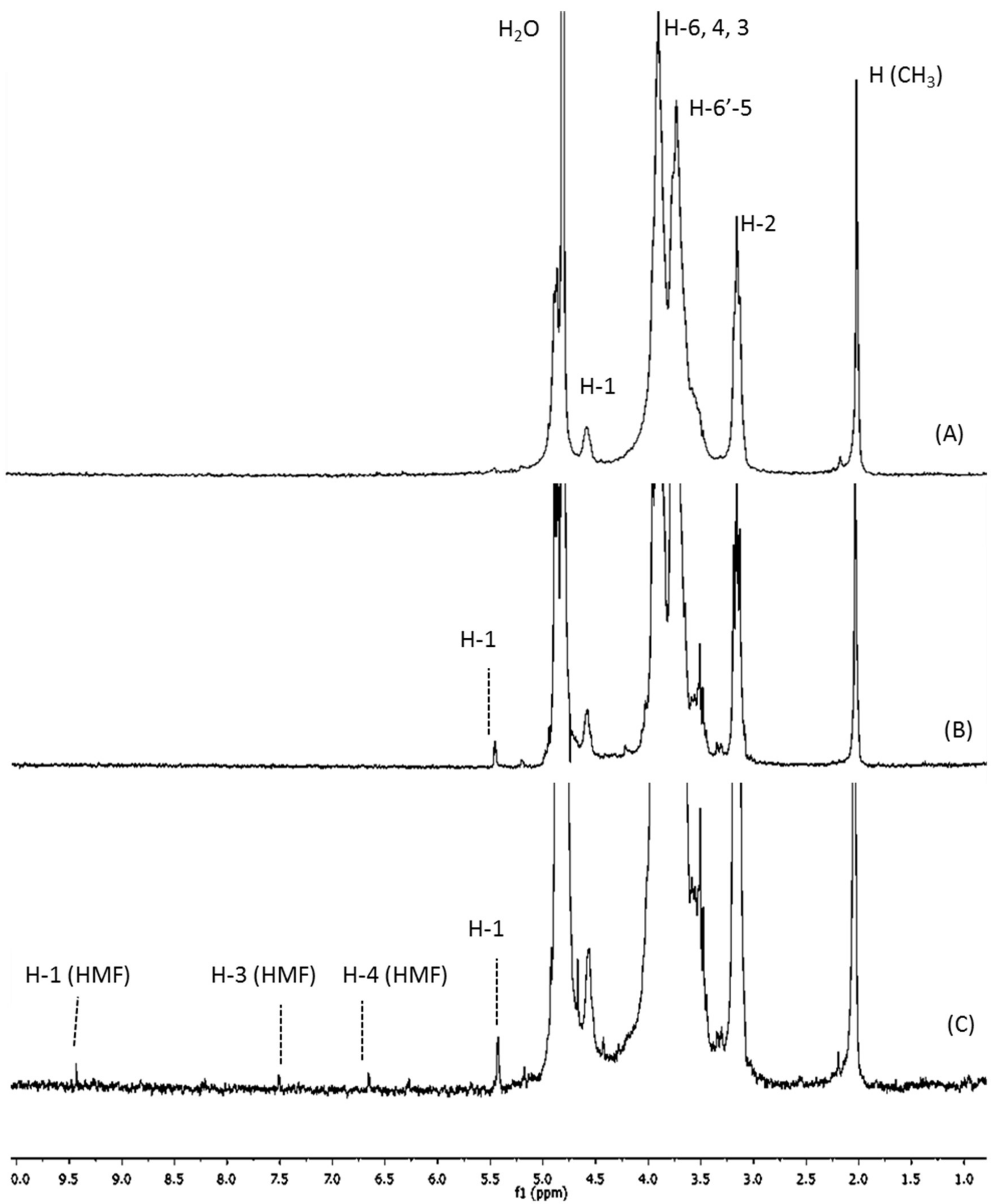
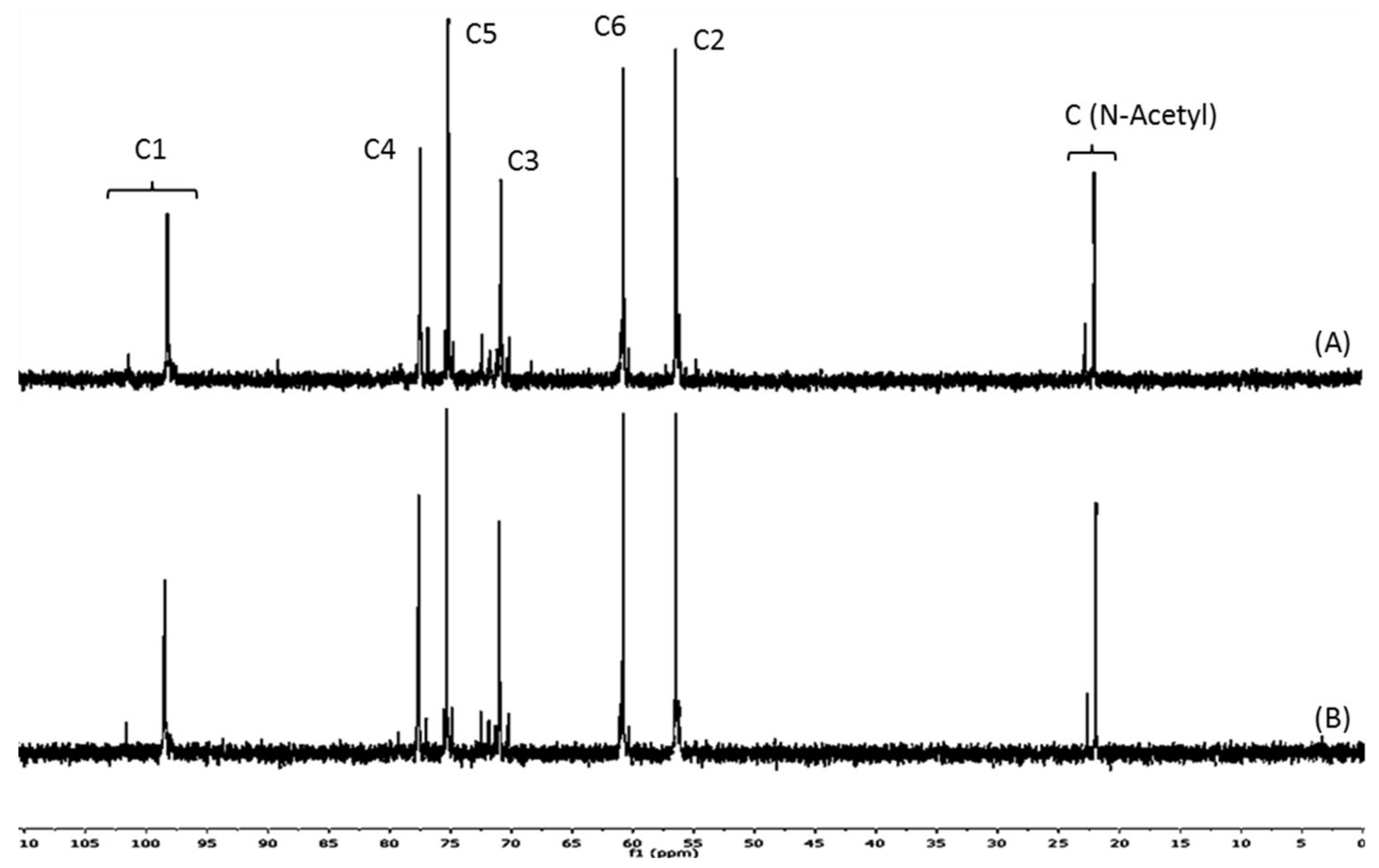
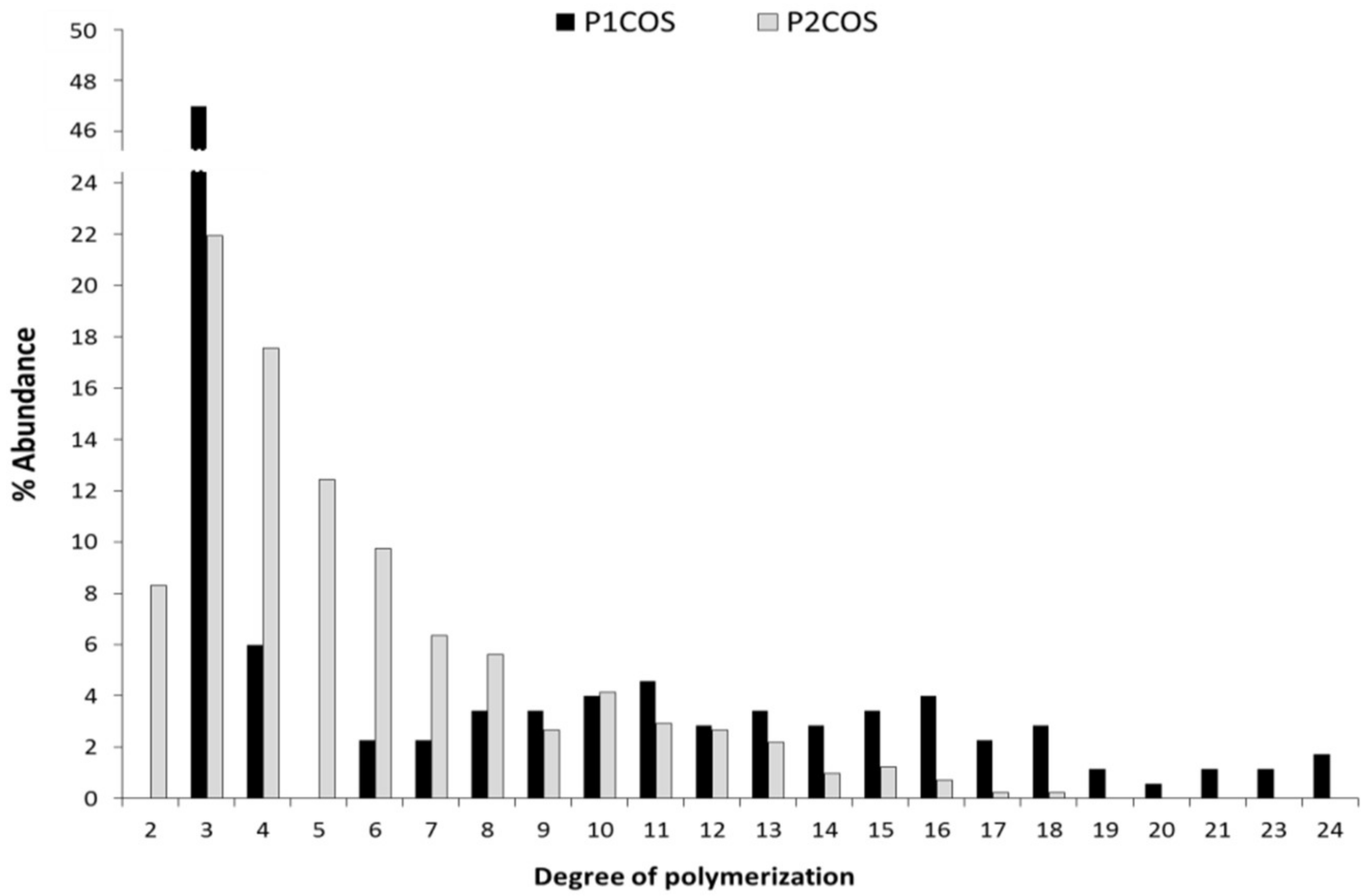

| Sample | Mw (kDa) | DA (%) | ζ (mV) | PA | A0 (%) | A1 (%) | A2 (%) | A3 (%) | A4–A24 (%) |
|---|---|---|---|---|---|---|---|---|---|
| P1COS | 8 | 13 | 56.4 ± 4.9 | 0.8 | 42 | 54 | 3 | 1 | 0 |
| P2COS | 5 | 11 | 33.1 ± 1.9 | 0.9 | 50 | 27 | 14 | 9 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, Á.; Mengibar, M.; Fernández, M.; Alemany, S.; Heras, A.; Acosta, N. Influence of Preparation Methods of Chitooligosaccharides on Their Physicochemical Properties and Their Anti-Inflammatory Effects in Mice and in RAW264.7 Macrophages. Mar. Drugs 2018, 16, 430. https://doi.org/10.3390/md16110430
Sánchez Á, Mengibar M, Fernández M, Alemany S, Heras A, Acosta N. Influence of Preparation Methods of Chitooligosaccharides on Their Physicochemical Properties and Their Anti-Inflammatory Effects in Mice and in RAW264.7 Macrophages. Marine Drugs. 2018; 16(11):430. https://doi.org/10.3390/md16110430
Chicago/Turabian StyleSánchez, Ángela, María Mengibar, Margarita Fernández, Susana Alemany, Angeles Heras, and Niuris Acosta. 2018. "Influence of Preparation Methods of Chitooligosaccharides on Their Physicochemical Properties and Their Anti-Inflammatory Effects in Mice and in RAW264.7 Macrophages" Marine Drugs 16, no. 11: 430. https://doi.org/10.3390/md16110430
APA StyleSánchez, Á., Mengibar, M., Fernández, M., Alemany, S., Heras, A., & Acosta, N. (2018). Influence of Preparation Methods of Chitooligosaccharides on Their Physicochemical Properties and Their Anti-Inflammatory Effects in Mice and in RAW264.7 Macrophages. Marine Drugs, 16(11), 430. https://doi.org/10.3390/md16110430





