Effect of Supercritical Carbon Dioxide Extraction Parameters on the Biological Activities and Metabolites Present in Extracts from Arthrospira platensis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Conditions of SFE on Extraction Yield in A. platensis Extracts
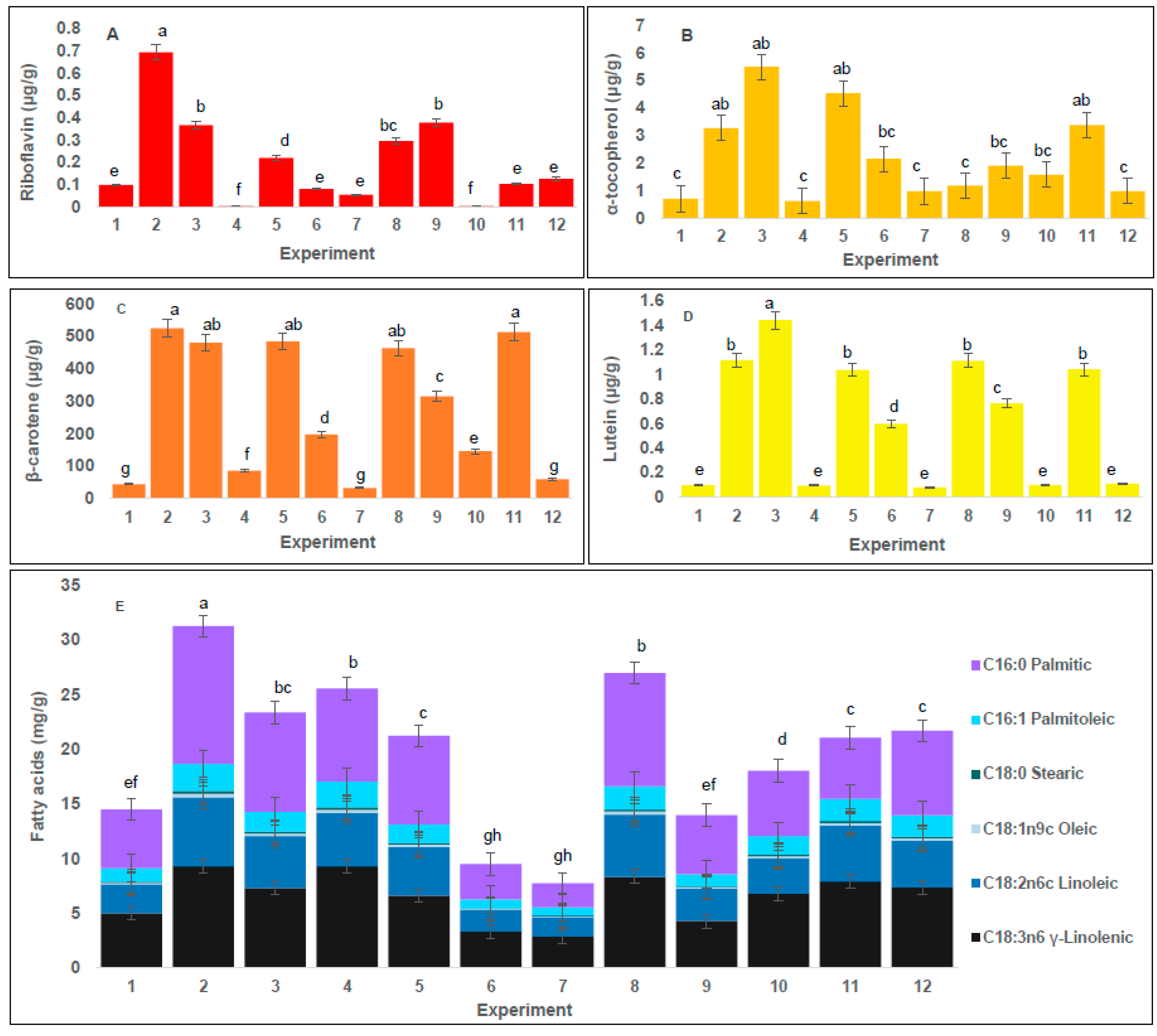
2.2. Effect of Conditions of SFE on Water-Soluble Vitamins Content in A. platensis Extracts
2.3. Effect of Conditions of SFE on Tocopherol Content in A. platensis Extracts
2.4. Effect of Conditions of SFE on Carotenoids Content in A. platensis Extracts
2.5. Effect of Conditions of SFE on Fatty Acid Content in A. platensis Extracts
2.6. Effect of Conditions of SFE on Antimicrobial Activity in A. platensis Extracts
2.7. Effect of Conditions of SFE on Antioxidant Activity in A. platensis Extracts
3. Materials and Methods
3.1. Chemicals
3.2. Samples
3.3. Extraction Method
3.4. Experimental Design
3.5. Determination of Bioactive Metabolites in Samples
3.5.1. Water-Soluble Vitamins Analysis (HPLC-PDA-FLD)
3.5.2. Carotenoid Analysis (HPLC-PDA)
3.5.3. Tocopherol Analysis (GC-MS)
3.5.4. Fatty Acid Analysis (GC-FID)
3.6. Determination of Biological Activities in Samples
3.6.1. Antimicrobial Activity
Test Microorganisms
Antimicrobial Testing
3.6.2. Antioxidant Activity
Determination of Total Phenolics Folin–Ciocalteu Assay
DPPH Free Radical Scavenging Assay
FRAP Assay
TEAC Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RH | Relative humidity |
| ps | Particle size |
| P | Pressure |
| CX | Co-solvent (ethanol) |
| SX | Static extraction |
| DX | Dynamic extraction |
| T | Temperature |
| Di | Dispersant |
| dw | Dry weight |
| PE | Polar extract |
| NPE | Non-polar extract |
| PDA | Photodiode array detector |
| FLD | Fluorescence detector |
| MS | Mass detector |
| FID | Flame ionization detector |
| SFE | Supercritical fluid extraction |
| LOQ | Limit of quantification |
| GAE | Gallic acid equivalents |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| FRAP | Ferric reducing ability of plasma |
| TEAC | Trolox equivalent antioxidant capacity |
| TE | Trolox equivalents |
| ABTS | 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) |
References
- Weststrate, J.; Van Poppel, G.; Verschuren, P. Functional foods, trends and future. Br. J. Nutr. 2002, 88, S233–S235. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Subhashini, J.; Mahipal, S.V.; Reddy, M.C.; Reddy, M.M.; Rachamallu, A.; Reddanna, P. Molecular mechanisms in C-Phycocyanin induced apoptosis in human chronic myeloid leukemia cell line-K562. Biochem. Pharmacol. 2004, 68, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Kumar, A.; Rao, A. Modulatory influence of Spirulina fusiformis on 7, 12-dimethylbenz (a) anthracene induced papillomagenesis in the skin of mice. Pharm. Biol. 1998, 36, 341–346. [Google Scholar] [CrossRef]
- Hirahashi, T.; Matsumoto, M.; Hazeki, K.; Saeki, Y.; Ui, M.; Seya, T. Activation of the human innate immune system by Spirulina: Augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int. Immunopharmacol. 2002, 2, 423–434. [Google Scholar] [CrossRef]
- Qureshi, M.; Garlich, J.; Kidd, M. Dietary Spirulina platensis enhances humoral and cell-mediated immune functions in chickens. Immunopharmacol. Immunotoxicol. 1996, 18, 465–476. [Google Scholar] [CrossRef] [PubMed]
- KG, M.G.; Sarada, R.; Ravishankar, G. Supercritical CO2 extraction of functional compounds from Spirulina and their biological activity. J. Food Sci. Technol. 2014, 52, 3627–3633. [Google Scholar]
- Careri, M.; Furlattini, L.; Mangia, A.; Musci, M.; Anklam, E.; Theobald, A.; Von Holst, C. Supercritical fluid extraction for liquid chromatographic determination of carotenoids in Spirulina Pacifica algae: A chemometric approach. J. Chromatogr. A 2001, 912, 61–71. [Google Scholar] [CrossRef]
- Mendiola, J.; Jaime, L.; Santoyo, S.; Reglero, G.; Cifuentes, A.; Ibanez, E.; Senorans, F. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007, 102, 1357–1367. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, N.; Tixier, A.S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefin. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; Ibarra-Garza, I.P.; Rodríguez-Rodríguez, J.; Cuéllar-Bermúdez, S.P.; Rostro-Alanis, M.; Alemán-Nava, G.; García-Pérez, J.S.; Parra-Saldivar, R. Green extraction technologies for high-value metabolites from algae: A review. Biofuels Bioprod. Biorefin. 2017, 11, 215–231. [Google Scholar] [CrossRef]
- Pereda, S.; Bottini, S.; Brignole, E.A. Fundamentals of supercritical fluid technology. In Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds; Martinez, J.L., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–21. [Google Scholar]
- Aresta, M. Carbon dioxide: Utilization options to reduce its accumulation in the atmosphere. In Carbon Dioxide as Chemical Feedstock; Aresta, M., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 6–13. [Google Scholar]
- Sajilata, M.; Singhal, R.S.; Kamat, M.Y. Supercritical CO2 extraction of γ-linolenic acid (GLA) from Spirulina platensis ARM 740 using response surface methodology. J. Food Eng. 2008, 84, 321–326. [Google Scholar] [CrossRef]
- Mendiola, J.A.; García-Martínez, D.; Rupérez, F.J.; Martín-Álvarez, P.J.; Reglero, G.; Cifuentes, A.; Barbas, C.; Ibanez, E.; Señoráns, F.J. Enrichment of vitamin E from Spirulina platensis microalga by SFE. J. Supercrit. Fluids 2008, 43, 484–489. [Google Scholar] [CrossRef]
- Sharif, K.; Rahman, M.; Azmir, J.; Mohamed, A.; Jahurul, M.; Sahena, F.; Zaidul, I. Experimental design of supercritical fluid extraction—A review. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Kamali, H.; Ghaziaskar, H.S.; Dadkhah, A.A. Optimization of Supercritical Extraction of Linalyl Acetate from Lavender via Box Behnken Design. Chem. Eng. Technol. 2012, 35, 1641–1648. [Google Scholar] [CrossRef]
- Yamini, Y.; Fat’hi, M.R.; Alizadeh, N.; Shamsipur, M. Solubility of dihydroxybenzene isomers in supercritical carbon dioxide. Fluid Phase Equilib. 1998, 152, 299–305. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.; López, V.; Rodríguez-Rodríguez, J.; Alemán-Nava, G.; Cuéllar-Bermúdez, S.; Rostro-Alanis, M.; Parra-Saldívar, R. Supercritical Carbon Dioxide and Microwave-Assisted Extraction of Functional Lipophilic Compounds from Arthrospira platensis. Int. J. Mol. Sci. 2016, 17, 658. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.A.; Marín, F.R.; Hernandez, S.; Arredondo, B.O.; Señoráns, F.J.; Ibañez, E.; Reglero, G. Characterization via liquid chromatography coupled to diode array detector and tandem mass spectrometry of supercritical fluid antioxidant extracts of Spirulina platensis microalga. J. Sep. Sci. 2005, 28, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.L.; Reis, A.D.; Palavra, A.F. Supercritical CO2 extraction of γ-linolenic acid and other lipids from Arthrospira (Spirulina) maxima: Comparison with organic solvent extraction. Food Chem. 2006, 99, 57–63. [Google Scholar] [CrossRef]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Crampon, C.; Mouahid, A.; Toudji, S.-A.A.; Lépine, O.; Badens, E. Influence of pretreatment on supercritical CO2 extraction from Nannochloropsis oculata. J. Supercrit. Fluids 2013, 79, 337–344. [Google Scholar] [CrossRef]
- Blake, C.J. Analytical procedures for water-soluble vitamins in foods and dietary supplements: A review. Anal. Bioanal. Chem. 2007, 389, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Mendiola, J.; Oliveira, M.; Ibáñez, E.; Herrero, M. Sequential determination of fat-and water-soluble vitamins in green leafy vegetables during storage. J. Chromatogr. A 2012, 1261, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Cocero, M.; González, S.; Pérez, S.; Alonso, E. Supercritical extraction of unsaturated products. Degradation of β-carotene in supercritical extraction processes. J. Supercrit. Fluids 2000, 19, 39–44. [Google Scholar] [CrossRef]
- Schneider, C. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 2005, 49, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Oyarzún, B.; Quezada, N.; del Valle, J.M. Solubility of carotenoid pigments (lycopene and astaxanthin) in supercritical carbon dioxide. Fluid Phase Equilib. 2006, 247, 90–95. [Google Scholar]
- Canela, A.P.R.; Rosa, P.T.; Marques, M.O.; Meireles, M.A.A. Supercritical fluid extraction of fatty acids and carotenoids from the microalgae Spirulina maxima. Ind. Eng. Chem. Res. 2002, 41, 3012–3018. [Google Scholar] [CrossRef]
- Gómez-Prieto, M.S.; del Castillo, M.L.R.; Flores, G.; Santa-María, G.; Blanch, G.P. Application of Chrastil’s model to the extraction in SC-CO2 of β-carotene and lutein in Mentha spicata L. J. Supercrit. Fluids 2007, 43, 32–36. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.; Fernandez-Sevilla, J.; Fernández, F.A.; García, M.C.; Grima, E.M. Supercritical fluid extraction of carotenoids from Scenedesmus almeriensis. Food Chem. 2010, 123, 928–935. [Google Scholar] [CrossRef]
- Carvalho, R.N.; Moura, L.S.; Rosa, P.T.; Meireles, M.A.A. Supercritical fluid extraction from rosemary (Rosmarinus officinalis): Kinetic data, extract’s global yield, composition, and antioxidant activity. J. Supercrit. Fluids 2005, 35, 197–204. [Google Scholar] [CrossRef]
- Temelli, F. Perspectives on supercritical fluid processing of fats and oils. J. Supercrit. Fluids 2009, 47, 583–590. [Google Scholar] [CrossRef]
- Güçlü-Üstündağ, Ö.; Temelli, F. Solubility behavior of ternary systems of lipids, cosolvents and supercritical carbon dioxide and processing aspects. J. Supercrit. Fluids 2005, 36, 1–15. [Google Scholar] [CrossRef]
- Mendes, R.L.; Reis, A.D.; Pereira, A.P.; Cardoso, M.T.; Palavra, A.F.; Coelho, J.P. Supercritical CO2 extraction of γ-linolenic acid (GLA) from the cyanobacterium Arthrospira (Spirulina) maxima: Experiments and modeling. Chem. Eng. J. 2005, 105, 147–151. [Google Scholar] [CrossRef]
- Andrich, G.; Zinnai, A.; Nesti, U.; Venturi, F. Supercritical fluid extraction of oil from microalga Spirulina (Arthrospira) platensis. Acta Aliment. Hung. 2006, 35, 195–203. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Romero-Ogawa, M.A.; Vannela, R.; Lai, Y.S.; Rittmann, B.E.; Parra-Saldivar, R. Effects of light intensity and carbon dioxide on lipids and fatty acids produced by Synechocystis sp. PCC6803 during continuous flow. Algal Res. 2015, 12, 10–16. [Google Scholar] [CrossRef]
- Kumar, V.; Bhatnagar, A.; Srivastava, J. Antibacterial activity of crude extracts of Spirulina platensis and its structural elucidation of bioactive compound. J. Med. Plants Res. 2011, 5, 7043–7048. [Google Scholar]
- Benkendorff, K.; Davis, A.R.; Rogers, C.N.; Bremner, J.B. Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Biol. Ecol. 2005, 316, 29–44. [Google Scholar] [CrossRef]
- Hameed, M.A.; Hassan, S.; Mohammed, R.; Gamal, R. Isolation and characterization of antimicrobial active compounds from the cyanobacterium Nostoc commune Vauch. J. Pure Appl. Microbiol. 2013, 7, 109–116. [Google Scholar]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- El-Baky, H.H.A.; El-Baroty, G.S. Characterization and bioactivity of phycocyanin isolated from Spirulina maxima grown under salt stress. Food Funct. 2012, 3, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Kawaguchipeptin B, an antibacterial cyclic undecapeptide from the cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 1997, 60, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Kopecký, J.; Benešová, L.; Vacek, J. Solid-phase/supercritical-fluid extraction for liquid chromatography of phenolic compounds in freshwater microalgae and selected cyanobacterial species. J. Chromatogr. A 2009, 1216, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.M.; Luong-Van, J.T. Seasonal Variation in the Chemical Composition of Tropical Australian Marine Macroalgae. In Proceedings of the Eighteenth International Seaweed Symposium, Bergen, Norway, 20–25 June 2004; Springer: Dordrecht, The Netherlands, 2007; pp. 155–161. [Google Scholar]
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.; Mäki-Arvela, P.; Mikkola, J.-P.; Lienqueo, M. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.L.; Fernandes, H.L.; Coelho, J.; Reis, E.C.; Cabral, J.M.; Novais, J.M.; Palavra, A.F. Supercritical CO2 extraction of carotenoids and other lipids from Chlorella vulgaris. Food Chem. 1995, 53, 99–103. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Nuhu, A.A. Spirulina (Arthrospira): An important source of nutritional and medicinal compounds. J. Mar. Biol. 2013. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Esquivel-Hernandez, D.A.; Rodriguez-Rodriguez, J.; Rostro-Alanis, M.; Cuellar-Bermudez, S.P.; Mancera-Andrade, E.; Nuñez-Echevarria, J.E.; García-Pérez, J.S.; Parra-Saldivar, R.; Chandra, R. Advancement of Green Process Through Microwave-Assisted Extraction of Bioactive Metabolites from Arthrospira platensis and Bioactivity Evaluation. Bioresour. Technol. 2017, 224, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Sandate-Flores, L.; Rostro-Alanis, M.D.J.; Mancera-Andrade, E.I.; Esquivel-Hernandez, D.A.; Brambila-Paz, C.; Parra-Saldívar, R.; Welti-Chanes, J.; Escobedo-Avellaneda, Z.; Rodríguez-Rodríguez, J. Using high hydrostatic pressures to retain the antioxidant compounds and to reduce the enzymatic activity of a pitaya–pineapple (Stenocereus sp.–Fragaria ananassa) beverage. J. Food Sci. Technol. 2017, 54, 611–619. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, J.S.; Cuéllar-Bermúdez, S.P.; Arévalo-Gallegos, A.; Rodríguez-Rodríguez, J.; Iqbal, H.; Parra-Saldivar, R. Identification of Bioactivity, Volatile and Fatty Acid Profile in Supercritical Fluid Extracts of Mexican arnica. Int. J. Mol. Sci. 2016, 17, 1528. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]

| Treatment | CX (g/min) | P (Bar) | SX (min) | DX (min) | T (°C) | Di (g) | Yield 2 (%) |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 450 | 5 | 25 | 40 | 35 | 1.90 ± 0.13 h |
| 2 | 11 | 450 | 15 | 25 | 60 | 35 | 5.59 ± 0.09 c |
| 3 | 11 | 450 | 5 | 55 | 60 | 0 | 6.85 ± 0.16 b |
| 4 | 4 | 150 | 5 | 55 | 60 | 35 | 2.02 ± 0.07 h |
| 5 | 11 | 150 | 15 | 55 | 40 | 35 | 5.62 ± 0.11 c |
| 6 | 4 | 150 | 15 | 55 | 60 | 0 | 2.34 ± 0.15 g |
| 7 | 4 | 150 | 5 | 25 | 40 | 0 | 1.52 ± 0.08 l |
| 8 | 11 | 450 | 5 | 55 | 40 | 0 | 7.48 ± 0.15 a |
| 9 | 11 | 150 | 15 | 25 | 40 | 0 | 3.71 ± 0.09 e |
| 10 | 4 | 450 | 15 | 55 | 40 | 35 | 2.48 ± 0.10 g |
| 11 | 11 | 150 | 5 | 25 | 60 | 35 | 4.78 ± 0.14 d |
| 12 | 4 | 450 | 15 | 25 | 60 | 0 | 3.01 ± 0.11 f |
| Diameter of Effective Zone of Inhibition (cm) 1 | ||||
|---|---|---|---|---|
| Treatment | S. aureus | P. aeruginosa | C. albicans | E. coli |
| 1 | 0.75 ± 0.21 b | 0.65 ± 0.07 c | 0.80 ± 0.01 b | 0.65 ± 0.07 d |
| 2 | 0.75 ± 0.07 b | 0.65 ± 0.07 c | 0.85 ± 0.06 a | 0.70 ± 0.02 d |
| 3 | 0.65 ± 0.05 d | 0.75 ± 0.04 a | 0.65 ± 0.01 d | 0.65 ± 0.05 d |
| 4 | 0.85 ± 0.19 a | 0.70 ± 0.03 b | 0.65 ± 0.08 d,e | 0.75 ± 0.04 c,d |
| 5 | 0.60 ± 0.01 e | 0.60 ± 0.09 c | 0.70 ± 0.02 c | 0.80 ± 0.01 b |
| 6 | 0.70 ± 0.02 c | -- | 0.70 ± 0.01 c | 0.75 ± 0.12 b,c |
| 7 | 0.70 ± 0.01 c | -- | 0.90 ± 0.17 a | 0.65 ± 0.08 d |
| 8 | -- | -- | 0.60 ± 0.03 e | 0.65 ± 0.05 d |
| 9 | 0.75 ± 0.03 b | 0.60 ± 0.11 c | 0.65 ± 0.08 d,e | 0.70 ± 0.04 d |
| 10 | 0.65 ± 0.05 d | 0.65 ± 0.09 c | 0.90 ± 0.22 a | 1.01 ± 0.06 a |
| 11 | 0.70 ± 0.14 c | 0.65 ± 0.04 c | -- | 0.70 ± 0.07 b,c |
| 12 | -- | -- | 0.70 ± 0.15 c | 0.60 ± 0.08 d |
| Control (+) | 1.10 ± 0.01 | 1.08 ± 0.05 | 1.23 ± 0.06 | 1.05 ± 0.01 |
| Control (−) | -- | -- | -- | -- |
| Folin–Ciocalteau | DPPH | FRAP | TEAC | ||
|---|---|---|---|---|---|
| Treatment | μg GAE/g | % Inhibition | μmol TE/g | μmol TE/g | μmol TE/g |
| 1 | 23.22 ± 0.27 g | 4.04 ± 0.16 e | 0.23 ± 0.01 e | 0.31 ± 0.00 e | 0.56 ± 0.01 i |
| 2 | 55.40 ± 0.54 b | 11.05 ± 1.03 a | 0.52 ± 0.02 a | 0.34 ± 0.01 c,d | 1.12 ± 0.02 d |
| 3 | 36.74 ± 0.45 e | 11.04 ± 0.93 a | 0.52 ± 0.01 a | 0.33 ± 0.01 c,d | 0.58 ± 0.00 h |
| 4 | 17.96 ± 0.27 i | 4.16 ± 0.08 e | 0.24 ± 0.01 e | 0.17 ± 0.02 f | 0.89 ± 0.01 e |
| 5 | 48.83 ± 0.76 d | 5.97 ± 1.97 d | 0.31 ± 0.00 d | 0.40 ± 0.01 a | 0.91 ± 0.01 e |
| 6 | 22.57 ± 0.17 h | 4.08 ± 0.50 e | 0.23 ± 0.01 e | 0.39 ± 0.00 a | 0.51 ± 0.01 j |
| 7 | 17.72 ± 0.32 i | 4.01 ± 0.04 e | 0.22 ± 0.01 e | 0.13 ± 0.03 g | 0.87 ± 0.01 f |
| 8 | 76.47 ± 0.71 a | 10.23 ± 0.46 b | 0.40 ± 0.01 b | 0.11 ± 0.01 g | 1.67 ± 0.01 a |
| 9 | 53.12 ± 0.61 c | 1.01 ± 0.16 f | 0.13 ± 0.00 f | 0.40 ± 0.01 a | 1.37 ± 0.02 c |
| 10 | 9.51 ± 0.29 j | 4.07 ± 1.04 e | 0.23 ± 0.01 e | 0.37 ± 0.00 b | 0.65 ± 0.01 g |
| 11 | 38.85 ± 1.01 e | 10.99 ± 0.64 a | 0.51 ± 0.02 a | 0.39 ± 0.01 a | 1.47 ± 0.02 b |
| 12 | 28.38 ± 0.56 f | 4.99 ± 1.04 c | 0.27 ± 0.01 c | 0.35 ± 0.01 c | 0.55 ± 0.01 i |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Cuéllar-Bermúdez, S.P.; García-Pérez, J.S.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; Ontiveros-Valencia, A.; Rostro-Alanis, M.; García-García, R.M.; Torres, J.A.; et al. Effect of Supercritical Carbon Dioxide Extraction Parameters on the Biological Activities and Metabolites Present in Extracts from Arthrospira platensis. Mar. Drugs 2017, 15, 174. https://doi.org/10.3390/md15060174
Esquivel-Hernández DA, Rodríguez-Rodríguez J, Cuéllar-Bermúdez SP, García-Pérez JS, Mancera-Andrade EI, Núñez-Echevarría JE, Ontiveros-Valencia A, Rostro-Alanis M, García-García RM, Torres JA, et al. Effect of Supercritical Carbon Dioxide Extraction Parameters on the Biological Activities and Metabolites Present in Extracts from Arthrospira platensis. Marine Drugs. 2017; 15(6):174. https://doi.org/10.3390/md15060174
Chicago/Turabian StyleEsquivel-Hernández, Diego A., José Rodríguez-Rodríguez, Sara P. Cuéllar-Bermúdez, J. Saúl García-Pérez, Elena I. Mancera-Andrade, Jade E. Núñez-Echevarría, Aura Ontiveros-Valencia, Magdalena Rostro-Alanis, Rebeca M. García-García, J. Antonio Torres, and et al. 2017. "Effect of Supercritical Carbon Dioxide Extraction Parameters on the Biological Activities and Metabolites Present in Extracts from Arthrospira platensis" Marine Drugs 15, no. 6: 174. https://doi.org/10.3390/md15060174
APA StyleEsquivel-Hernández, D. A., Rodríguez-Rodríguez, J., Cuéllar-Bermúdez, S. P., García-Pérez, J. S., Mancera-Andrade, E. I., Núñez-Echevarría, J. E., Ontiveros-Valencia, A., Rostro-Alanis, M., García-García, R. M., Torres, J. A., Chen, W. N., & Parra-Saldívar, R. (2017). Effect of Supercritical Carbon Dioxide Extraction Parameters on the Biological Activities and Metabolites Present in Extracts from Arthrospira platensis. Marine Drugs, 15(6), 174. https://doi.org/10.3390/md15060174








