Abstract
Six kinds of chitinous materials have been used as sole carbon/nitrogen (C/N) sources for producing α-glucosidase inhibitors (aGI) by Paenibacillus sp. TKU042. The aGI productivity was found to be highest in the culture supernatants using demineralized crab shell powder (deCSP) and demineralized shrimp shell powder (deSSP) as the C/N source. The half maximal inhibitory concentration (IC50) and maximum aGI activity of fermented deCSP (38 µg/mL, 98%), deSSP (108 µg/mL, 89%), squid pen powder (SPP) (422 µg/mL, 98%), and shrimp head powder (SHP) (455 µg/mL, 92%) were compared with those of fermented nutrient broth (FNB) (81 µg/mL, 93%) and acarbose (1095 µg/mL, 74%), a commercial antidiabetic drug. The result of the protein/chitin ratio on aGI production showed that the optimal ratio was 0.2/1. Fermented deCSP showed lower IC50 and higher maximum inhibitory activity than those of acarbose against rat intestinal α-glucosidase.
1. Introduction
Chitin and its derivatives hold great economic value due to their versatile activities and biotechnological applications. Among the natural chitin-containing resources, crab shells, shrimp shells, and squid pens have the highest chitin content. Conventionally, chitin is obtained from crab shells and shrimp shells by using an inorganic acid or a strong alkali for demineralization or deproteinization, respectively. However, these chemical treatments have several drawbacks, such as the creation of pollutant acid or alkali liquid. Furthermore, the utilization of the deproteinized liquid bioresources is reduced because of the presence of an alkali [1].
Among the fishery chitinous materials, squid pens contain the highest ratio of protein [2]. For the recycling squid pens in order to produce additional highly bioactive products other than chitin or chitosan, the utilization of squid pens has been estimated via microbial conversion [3,4,5,6,7,8,9,10,11,12,13,14,15]. Many microorganisms produce chitinolytic and/or proteolytic enzymes using squid pens as the sole carbon/nitrogen (C/N) source [3,4,13]. These findings provide promising results for the production of chitin or chitin oligomers from this chitinous material. In addition, investigations have also included the production of enzymes [3,4,5], exopolysaccharides [6,7,8,9], chito-oligomers [3], antioxidants [10], insecticidal materials [11,12], biofertilizers [13], and biosorbents [14,15]. These chitinous materials were utilized for another purpose in the present study, the synthesis of antidiabetic drugs via microbial conversion.
Over 90% of diabetes mellitus (DM) cases are type 2 (non-insulin-dependent DM). The use of α-glucosidase inhibitors (aGI), such as acarbose, miglitol, and voglibose has been reported in regard to the treatment of type 2 diabetes. These remedies entailed some problematic side effects, such as diarrhea, flatulence, and abdominal discomfort. Therefore, there is an interest in discovering new natural sources of aGI. Until now, aGI have been reported to be produced by microorganisms [2,16]. Among these aGI-producing microbes, only strains of Streptomyces [17,18], Bacillus [19,20,21,22,23], Stenotrophomonas maltrophilia [24], and Actinoplanes spp. SE-50 [25] have been studied extensively.
Many strains of Paenibacillus have been reported to use squid pens as the sole C/N source for producing exopolysaccharides [6,7,8,9]. Squid pens have been efficiently recycled via microbial fermentation to produce medicinal materials, and have also been used as the sole C/N source for the screening of aGI-producing bacterial strains. Our preceding studies revealed that Paenibacillus sp. TKU042, a bacterium isolated from Taiwanese soil, using squid pens as the sole C/N source, secreted acarbose-comparable aGI in the fermented nutrient broth. The optimization of culture conditions, pH and thermal stabilities, and the effect of fermented nutrient broth on mice were also explored [2].
In this study, six kinds of chitin-containing materials were used as the C/N source for aGI production by Paenibacillus sp. TKU042; other chitinolytic bacteria strains of Paenibacillus species were tested for aGI productivity. The effect of protein supplement and some cultivation parameters on the aGI productivity and the specific inhibition of Paenibacillus sp. TKU042 aGI were also investigated. Herein, the half maximal inhibitory concentration (IC50) and maximum activity were estimated and compared with those of fermented nutrient broth and acarbose.
2. Results and Discussion
2.1. Screening of Chitin-Containing Materials as C/N for α-Glucosidase Inhibitors Production
The effect of chitin-containing materials on aGI production by Paenibacillus sp. TKU042 was investigated with six kinds of chitinous materials: demineralized crab shell powder (deCSP), demineralized shrimp shell powder (deSSP), squid pen powder (SPP), shrimp head powder (SHP), fresh shrimp shell powder (frSSP), and cicada shell powder (CiSP) as the sole sources of C/N with the concentration of 1% (w/v). The protein–chitin–mineral salts compositions of shrimp shells and squid pen were 48%, 38%, and 14% and 61%, 38%, and 1%, respectively [26]. It is obvious that the mineral salts content in squid pen (1%, w/w) is much lower than in shrimp shell (14%, w/w). Therefore, only the crab shells and shrimp shells were decalcified and used as the C/N sources for aGI production.
The results of maximum aGI activity, aGI productivity, and cell growth were investigated, and are shown in Figure 1. After four days of fermentation, the maximum aGI activity (Figure 1A) of most C/N sources reached the highest level, 91–100% with the priority sequence of deCSP, deSSP, SHP, and SPP. The maximum aGI activities of deCSP, deSSP, and SHP remained thereafter; only SPP decreased dramatically to a level lower than 40% on the 6th day. The reason might be related to the higher content (around 60%) of protein in SPP. It is also possible that an additional inhibitory component was made and accumulated with increased time that affected the activity of the active compound. The other possibility could be the decreased stability of the active ingredient in SPP after the fourth day. A similar phenomenon was also found in Bacillus subtilis TKU007, which showed that SHP was a better C/N source than SSP for the production of chitosanase and protease [27] and nattokinase [28]. Although the protein content of SPP was higher than that of SSP, the resultant enzyme productivity was not raised [27]. This might be related to the ratio of chitin and protein in the C/N sources.
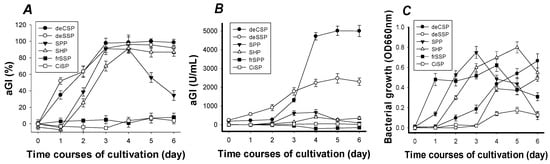
Figure 1.
Screening of chitinous materials as the Carbon/Nitrogen (C/N) source for α-glucosidase inhibitors (aGI) production by Paenibacillus sp. TKU042. (A) percentage, (B) enzyme units per mL and (C) bacterial growth per cultivation time (day). deCSP: Demineralized crab shell powder; deSSP: Demineralized shrimp shell powder; SPP: Squid pen powder; SHP: Shrimp head powder; fSSP: Fresh shrimp shell powder; CiSP: Cicada shell powder. OD: optical density.
The maximum aGI activity of frSSP and CiSP was lower than 20%, even though the culture time was lengthened to six days. The maximum aGI activity was speculated to be affected by the presence of high levels of mineral salts (in the case of frSSP and CiSP) or some additional factors that were eliminated during demineralization (in the case of deCSP and deSSP). As shown in Figure 1B, the aGI productivity reached its highest level after 4–5 days of fermentation. The highest was found in deCSP (5010 U/mL), deSSP (2476 U/mL), and then in the other four (SPP, SHP, frSSP, and CiSP) with aGI productivity lower than 660 U/mL. The same as the phenomenon of maximum activity found in Figure 1A, the aGI productivity of deCSP and deSSP remained steadily even after six days of fermentation.
To examine the relationship of aGI productivity and bacterial growth, the culture broth after removing the residual chitinous materials (C/N source) by centrifugation (Kubota 5922, Japan) at 150× g for 10 min was used for analyzing cell growth. As shown in Figure 1C, deCSP and deSSP were not the best C/N sources for cell growth. These results showed that the production of aGI may not have a direct relationship with cell growth.
The abovementioned comparisons of aGI production by Paenibacillus sp. TKU042 in different chitin-containing media are summarized in Table 1. The aGI activity of fermented nutrient broth (FNB) [2] and acarbose (an antidiabetic drug) were also compared. The aGI activities of the C/N sources all reached their highest level on the 4th day. The IC50 value was found in the priority sequence of deCSP (38 μg/mL), FNB (81 μg/mL), deSSP (108 μg/mL), SPP (422 μg/mL), SHP (455 μg/mL), and acarbose (1095 μg/mL). The maximum aGI activities were in the priority sequence of deCSP (98%), SPP (98%), FNB (93%), SHP (92%), SSP (89%), and then acarbose (74%).

Table 1.
Half maximal inhibitory concentration (IC50), maximum inhibition activity, and activity yield of the fermented chitinous materials (FCMs).
As for the productivity of aGI after fermentation under optimal conditions, deCSP showed the best yield of 26,316 kU/g, followed consecutively by FNB (12,346 kU/g), deSSP (9259 kU/g), SPP (2370 kU/g), and SHP (2198 kU/g). These tested chitinous materials were all potential C/N sources for aGI production by Paenibacillus sp. TKU042, due to their higher productivity than that of acarbose (913 kU/g).
The weight yields of the obtained culture supernatants were also compared. The weight yields of deCSP (2.03 g/L) and deSSP (1.60 g/L) were lower than that of FNB (6.5 g/L). These results showed that there were fewer contaminants and greater ease for further purification of the aGI from the culture supernatants of deCSP and deSSP than from that of FNB. These results showed that the deCSP and deSSP were the most remarkable C/N sources for aGI production by Paenibacillus sp. TKU042.
2.2. The Effect of Protein Supplement on α-Glucosidase Inhibitors Production
To analyze whether extra protein may enhance aGI production, the supplementation of 0.2% and 0.4% of protein (polypeptone/yeast extract = 4/6) in the deCSP (1%)-containing medium was studied. As shown in Figure 2, an inverse relationship between protein concentration and aGI productivity was found. The supplementation of free protein increased the bacterial growth (Figure 2A) but did not enhance the aGI productivity (Figure 2B). In the first two days, no significant difference in aGI productivity was found. The difference started to appear on the fourth day; the aGI productivity of these three C/N sources were in the order of 1% deCSP (4833 U/mL), 1% deCSP/0.2% protein (2512 U/mL), and then 1% deCSP/0.4% protein (1374 U/mL). According to the results, the ratio of protein/chitin appeared to play an important role in aGI production by Paenibacillus sp. TKU 042. The C/N sources (such as crab shell chitin or shrimp shell chitin) with chitin but only less than 1% of protein, were also found to be unsuitable for use as an inducer for the protease production by B. subtilis TKU007 [28] and Bacillus sp. TKU004 [29].
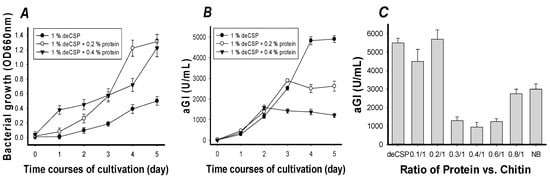
Figure 2.
Effects of protein supplementation on the cell growth (A) and aGI production (B,C) of Paenibacillus sp. TKU042.
To investigate whether the protein in deCSP was sufficient to play an important role in aGI production by Paenibacillus sp. TKU 042, the protein in the deCSP was removed by heat-alkali-deproteinization treatment [29]. The obtained deproteinized deCSP was then used as the chitin sample for investigating the effect of ratio of protein vs. chitin (0.1/1–0.8/1) on aGI production. As shown in Figure 2C, the best results were found in the ratio of protein vs. chitin of 0.2/1 (5700 U/mL) and 0.1/1 (4500 U/mL). The optimal ratio of protein vs. chitin (0.2/1) investigated in this study was approximately the same as the deCSP, which showed comparable aGI productivity of 5500 U/mL. Another source of chitin (chitin obtained from SPP) was fermented by Paenibacillus sp. TKU 042 at the same ratio of protein vs. chitin and cultivation conditions as above. Similarly, this bacterium strain showed the same manner of aGI production with aGI productivity of 5600 U/mL.
Nutrient broth (NB) containing proteins of polypeptone and yeast extract showed lower aGI productivity (3000 U/mL) than the ratio of protein vs. chitin (0.2/1), deCSP, and ratio of protein vs. chitin (0.1/1). These results showed that the supplementation of protein vs. chitin with a ratio higher than 0.2/1 decreased the aGI production. Therefore, deCSP was chosen for further optimization study.
2.3. Production of aGI from Demineralized Crab Shell Powder by Different Bacteria
To examine whether other bacteria strains, especially the strains of Paenibacillus species, would also produce aGI in the deCSP-containing medium, 16 stocked chitinolytic bacteria which were all isolated from Taiwanese soils were tested. As shown in Table 2, only the bacteria of Paenibacillus species produced aGI against the tested three α-glucosidases. The maximum aGI activity against α-glucosidases of Saccharomyces cerevisiae (S), Bacillus stearothermophilus (B), and rat (R) were all above 90%.

Table 2.
Comparison of the maximum inhibitory activity of α-glucosidase inhibitors produced by various bacteria using deCSP * as the C/N source.
Many strains of Paenibacillus species have been reported to be potentially used in agricultural, industrial, health food, and medical utilizations, such as the biosynthesis of antifungal agents [30,31], biofertizlizers [32,33], enzymes [22,24,25,26]. Recently, Paenibacillus species have also been explored to use SPP as the sole C/N source for the production of exopolysaccharides [6,7,8,9], antimicrobial biosurfactants [7], antioxidants [6], and homogentisic acid [16,34]. Paenibacillus sp. TKU042 was then chosen as the aGI-producing strain for further investigation.
2.4. Optimization of Culture Conditions
The deCSP was confirmed as a potential C/N source and chosen for an optimization study of some parameters, including the cultivation temperature (25, 30, 34, and 37 °C), culture volume (50, 70, 100, 130, and 160 mL), the seed culture volume (0.5, 1, 2, and 4 mL), and the concentration of deCSP (0.5, 0.75, 1, 1.25, 1.6, and 2%) (the data are shown in Supplementary Figure S1). Overall, aGI were effectively produced by Paenibacillus sp. TKU042 within the 1.6% deCSP-containing medium (130 mL medium in 250 mL-Erlenmeyer flask) at 30 °C in a reciprocal shaker (Yi Der LM-570R, Jun Yang, New Taipei City, Taiwan) at 150 rpm for four days (Figure S1). The culture conditions before and after the optimization study are summarized in Table 3. After the optimization was studied, the aGI productivity was increased from 5000 to 12,400 U/mL (2.48-fold), and the IC50 value was reduced from 81 to 6.7 µg/mL (12.1-fold). The culture supernatant (fermented deCSP) obtained by culturing Paenibacillus sp. TKU 042 in the optimized condition was then used for further study.

Table 3.
Comparison of culture conditions before and after optimization.
2.5. Specific α-Glucosidase Inhibitors Activity of Fermented Demineralized Crab Shell Powder
To evaluate the potential of Paenibacillus sp. TKU 042 aGI to be developed as antidiabetic drugs, the inhibitory specificity of fermented deCSP was tested against six kinds of commercial enzymes, including α-glucosidases (S. cerevisiae, B. stearothermophilus, rice, rat intestine) and α-amylases (B. subtilis, porcine pancreas).
As shown in Table 4, fermented deCSP showed lower IC50 (15.9 µg/mL) and higher maximum inhibitory activity (97%) than those of acarbose (78 µg/mL, 91%) against rat intestinal α-glucosidase. The IC50 and maximum inhibitory activity against S. cerevisiae α-glucosidase of fermented deCSP (6.7 µg/mL, 99%) were also better than those of acarbose (1095 µg/mL, 74%). Acarbose showed better results of IC50 (0.042 µg/mL and 3.04 µg/mL) against α-glucosidases from B. stearothermophilus, and rice, respectively, compared to fermented deCSP (6.6 µg/mL, 6.7 µg/mL). No inhibitory activity was found for fermented deCSP against porcine α-amylase and B. subtilis α-amylase. For the evaluation of the potential inhibitors using as antidiabetic drugs, α-glucosidase from rat has been suggested as the most valuable resource among the tested enzymes, since it is a mammalian enzyme closer to that of human [2]. In this study, fermented deCSP showed stronger inhibition against rat α-glucosidase than that of acarbose. This result suggested that fermented deCSP may be a good candidate of antidiabetic drugs.

Table 4.
Specific inhibitory activity of fermented deCSP and acarbose against some enzymes.
2.6. Confirmation of α-Glucosidase Inhibitors Containing in Fermented Chitin-Containing Media
The same concentration (20 mg/mL) of the unfermented deCSP and 1–4 day-fermented deCSP solutions were analyzed by high-performance liquid chromatography (HPLC). The difference of the HPLC fingerprints of deCSP before and after fermentation can be clearly observed in Figure 3A. After fermentation, some new main peaks appeared at the retention time of 8–8.5, 23, and 27.5 min, or enhanced its area (the peak at the retention time of 27.5 min). Unfermented deCSP was tested for α-glucosidase inhibition, but no activity was observed. The differences in HPLC fingerprints and the inhibitory activity between unfermented and fermented deCSP led to the conclusion that the aGI were produced by fermentation and had not previously existed in the deCSP.
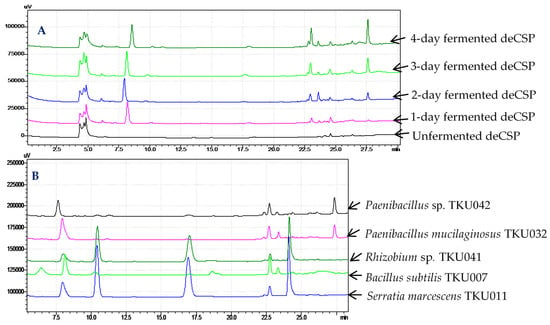
Figure 3.
High-performance liquid chromatography (HPLC) fingerprints of (A) crab shell powder (CSP) fermented by TKU042 with different time courses of cultivation and (B) CSP fermented by various bacteria in four days.
To further compare which peaks especially belong to Paenibacillus sp., the four day-fermented deCSP by Paenibacillus sp. TKU042, Paenibacillus mucilaginosus TKU032, Rhizobium sp. TKU041, B. subtilis TKU007, and Serratia marcescens TKU011 were analyzed. As shown in Figure 3B, only the peak at the retention time of 27.5 min was found in deCSP fermented by Paenibacillus sp. TKU042 and P. mucilaginosus TKU032. Combining these results with the data shown in Table 2, this peak may signify the active compounds of aGI. The isolation and identification of these aGI will be performed soon.
3. Materials and Methods
3.1. Materials
Crab shells, shrimp shells, and squid pens were acquired from Shin-Ma Frozen Food Co. (I-Lan County, Taiwan). Fresh shrimp shells were prepared by drying them by lyophilization. Shrimp head powder was obtained from Fwu-Sow Industry (Taichun, Taiwan). Cicada shells were collected from the campus of Tamkang University (New Taipei, Taiwan). Demineralized crab shells and decalcified shrimp shells were prepared via acid treatment [29]. Nutrient broth was purchased from Creative Life Science Co. (Taipei, Taiwan). Rat α-glucosidase (intestinal acetone powders from rat) was purchased from Sigma Aldrich (St. Louis, MO, USA). Acarbose, S. cerevisiae α-glucosidase, B. stearothermophilus α-glucosidase, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma Aldrich (Singapore). Rice α-glucosidase (type 4) and porcine pancreatic α-amylase (type VI-B) were purchased from Sigma Aldrich. Some reagents, solvents, and other common chemicals were available at the highest grade.
3.2. Biological Assays of Enzymatic Inhibition
Enzyme inhibitory activity was modified from the method of Kwon et al. [35]. Fifty microliters of the sample solution were mixed with the same volume of the α-glucosidase solution and 100 μL of potassium phosphate buffer, and kept at 37 °C for 20 min. The addition of 50 μL of the substrate p-nitrophenyl glucopyranoside (pNPG) started the reaction, and this step was maintained at 37 °C for 40 min. One hundred microliters of a 1 mol/L Na2CO3 solution was added to stop the reaction. The final mixture solution was measured at 410 nm. The inhibition was calculated by using the following formula:
where A is the absorbance at 410 nm of the reaction blank (no inhibitor/sample), and B is the absorbance at 410 nm of the reaction in the presence of the inhibitor/sample. The concentration of an inhibitor that can inhibit 50% of enzymatic activity under the assay conditions was defined as the IC50 value. The potassium phosphate buffer used was at a concentration of 0.1 mol/L, pH 7; this buffer was used for preparing enzymes, the sample, and the substrate solutions. S. cerevisiae, B. stearothermophilus, and rice α-glucosidases were used at concentrations of 0.25, 0.10, and 1.0 U/mL, respectively. The preparation of rat intestinal α-glucosidase solution was described in detail in a previous report [2]. The Statistical Analysis Software (SAS) version 9.4 (provided by SAS Institute Taiwan Ltd., Minsheng East Road, Section 2, Taipei, Taiwan 149-8) was employed to analyze the differences between the means of the calculated IC50 (µg/mL) and maximum inhibition (%) values at p < 0.01.
Inhibition (%) = (A − B)/ A × 100
3.3. The Effects of Chitin-Containing Materials and the Protein Supplement on aGI Production Experiments
Various kinds of chitin-containing materials, such as deCSP, deSSP, SPP, SHP, frSSP, and CiSP (w/v) were used as the sole sources of C/N. The cultural medium conducted in this study contains 0.1% K2HPO4, 0.05% MgSO4·7H2O, and 1% chitin-containing materials. The deCSP, the material for aGI production with the most potential, was chosen for the following experiments (The effects of the protein supplement on aGI production). Amounts of 0.2% and 0.4% protein (polypeptone/yeast extract = 4/6) were supplemented to the medium containing 1% deCSP. The above fermentation processes were performed at 30 °C, 150 rpm (shaking speed), 100/250 mL (ratio of volume of medium/Erlenmeyer flask), and the seed culture inoculated was 1 mL (OD660nm = 0.25). The culture supernatants obtained by centrifugation at 500 rpm in 10 min to remove all the residues of chitinous materials were used to detect bacterial growth (measuring OD660nm), and the supernatants obtained by centrifugation at 12,000× g in 20 min to remove bacteria were conducted for Biological assays of enzymatic inhibition. The bacterial growth and inhibitory activity were detected daily; α-glucosidase from S. cerevisiae was used in the test, and the activity was expressed as % and U/mL. To investigate the optimal ratio of protein/chitin, the protein contained in the deCSP was removed by the method described in a previous study [29]; 0.1–0.8% free protein (polypeptone/yeast extract = 4/6) was then supplemented to the medium containing 1% chitin. The cultivation was performed as described above in four days; the supernatants obtained by centrifugation at 12,000 × g in 20 min were then used in the inhibition testing.
3.4. Optimization of Some Parameters for α-Glucosidase Inhibitors Productivity Production
Some major parameters, including the cultivation temperature (25, 30, 34, and 37 °C), culture volume (50, 70, 100, 130, and 160 mL), the concentration of deCSP (0.5%, 0.75%, 1%, 1.25%, 1.6%, and 2%), and the bacterial seed culture volume (0.5, 1, 2, and 4 mL) were considered for examination. The cultivation temperature experiments were conducted in a 250 mL Erlenmeyer flask with 100 mL of medium (initial pH 6.85) containing 0.05% MgSO4·7H2O, 0.1% K2HPO4, and 1% deCSP. Fermentation was performed in an incubator at the temperature range of 25–37 °C, with a shaking speed of 150 rpm for 4–5 days. The following experiments were designed based on the optimal conditions achieved from previous experiments. The S. cerevisiae α-glucosidase inhibition of the supernatants was tested and expressed as U/mL.
3.5. Conditions of High-Performance Liquid Chromatography Fingerprints Analysis
High-performance liquid chromatography fingerprints were analyzed under optimal conditions of mobile phase—0–5’ (0.1–1% methanol (MeOH)), 5–10’ (1–2% MeOH), 10–15’ (2–30% MeOH), 15–25’ (30–70% MeOH), and 25–35’ (70–100% MeOH)—and detected at 254 nm (ultraviolet detector), 0.6 mL/min (flow rate), and 25 °C (column temperature) using the C18 column.
4. Conclusions
Decalcified crab shells as the sole C/N source showed the best results for producing aGI compared to other tested chitin-containing materials by Paenibacillus sp. TKU042. The obtained culture supernatant showed higher aGI activity, lower IC50, and higher maximum inhibitory activity than those of acarbose. Among the tested 16 chitinolytic enzyme-producing bacteria, only the bacteria of Paenibacillus species produced aGI in the deCSP-containing medium. When the ratio of protein/chitin content in the liquid culture medium was adjusted to 0.2/1, the aGI activity obtained after fermentation was similar to that of decalcified crab shells. HPLC fingerprints showed the aGI produced by fermentation. All of these results suggest that chitin is a potential C/N source for producing aGI using Paenibacillus species. Furthermore, the culture supernatants might be useful candidates for treating type 2 diabetes. For evaluating the feasibility of commercialization of antidiabetic drugs using crab shells with a protein/chitin ratio of 0.2/1.0 and the bacteria of Paenibacillus species, the therapeutic effect ascertained in animal testing and the safety of human health when exposed to the aGI materials remains a challenge and requires a breakthrough.
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/15/11/350/s1. Figure S1: The effect of some parameters on aGIs synthesis.
Acknowledgments
This work was supported in part by a grant from the Ministry of Science and Technology, Taiwan (MOST 105-2313-B-032-001, MOST 106-2320-B-032-001-MY3).
Authors Contributions
S.-L.W. and V.B.N. conceived the study; S.-L.W. and V.B.N. designed and performed the study; S.-L.W. contributed reagents/materials/analysis tools; S.-L.W. and V.B.N. analyzed the data; V.B.N. and S.-L.W. wrote the paper.
Conflict of Interest
The authors declare no conflict of interest.
References
- Wang, S.L.; Liang, T. Microbial reclamation of squid pens and shrimp shells. Res. Chem. Intermed. 2017, 43, 3445–3462. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, A.D.; Kuo, Y.H.; Wang, S.L. Biosynthesis of α-glucosidase inhibitors by a newly isolated bacterium, Paenibacillus sp. TKU042 and its effect on reducing plasma glucose in mouse model. Int. J. Mol. Sci. 2017, 18, 700. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Chen, W.T.; Lin, Z.H.; Kuo, Y.H.; Nguyen, A.D.; Pan, P.S.; Wang, S.L. An amphiprotic novel chitosanase from Bacillus mycoides and its application in the production of chitooligomers with their antioxidant and anti-inflammatory evaluation. Int. J. Mol. Sci. 2016, 17, 1302. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D.; Huang, C.C.; Liang, T.W.; Nguyen, V.B.; Pan, P.S.; Wang, S.L. Production and purification of a fungal chitosanase and chitooligomers from Penicillium janthinellum D4 and discovery of the enzyme activators. Carbohydr. Polym. 2014, 108, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Liang, T.W.; Yen, Y.H. Bioconversion of chitin-containing wastes for the production of enzymes and bioactive materials. Carbohydr. Polym. 2011, 84, 732–742. [Google Scholar] [CrossRef]
- Wang, C.L.; Huang, T.H.; Liang, T.W.; Wang, S.L. Production and characterization of exopolysaccharides and antioxidant from Paenibacillus sp. TKU023. New Biotechnol. 2011, 28, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Wu, C.C.; Cheng, W.T.; Chen, Y.C.; Wang, C.L.; Wang, I.L.; Wang, S.L. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Appl. Biochem. Biotechnol. 2014, 172, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Wang, S.L. Recent advances in exopolysaccharides from Paenibacillus spp.: Production, isolation, structure, and bioactivities. Mar. Drugs 2015, 13, 1847–1863. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Tseng, S.C.; Wang, S.L. Production and characterization of antioxidant properties of exopolysaccharides from Paenibacillus mucilaginosus TKU032. Mar. Drugs 2016, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Liang, T.W.; Liu, K.C.; Hsu, Y.W.; Hsu, H.; Wang, S.L. Isolation and identification of a novel antioxidant with antitumor activity from Serratia ureilytica using squid pen as fermentation substrate. Mar. Biotechnol. 2011, 13, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Wang, C.Y.; Yen, Y.H.; Liang, T.W.; Chen, S.Y.; Chen, C.H. Enhanced production of insecticidal prodigiosin from Serratia marcescens TKU011 in media containing squid pen. Process Biochem. 2012, 47, 1684–1690. [Google Scholar] [CrossRef]
- Liang, T.W.; Chen, C.H.; Wang, S.L. Production of insecticidal materials from Pseudomonas tamsuii. Res. Chem. Intermed. 2015, 41, 7965–7971. [Google Scholar] [CrossRef]
- Wang, S.L.; Huang, T.Y.; Wang, C.Y.; Liang, T.W.; Yen, Y.H.; Sakata, Y. Bioconversion of squid pen by Lactobacillus paracasei subsp paracasei TKU010 for the production of proteases and lettuce enhancing biofertilizers. Biores. Technol. 2008, 99, 5436–5443. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Chen, S.Y.; Yen, Y.H.; Liang, T.W. Utilization of chitinous materials in pigment adsorption. Food Chem. 2012, 135, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Lo, B.C.; Wang, S.L. Chitinolytic bacteria-assisted conversion of squid pen and its effect on dyes and adsorption. Mar. Drugs 2015, 13, 4576–4593. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Utilization of fishery processing byproduct squid pens for Paenibacillus sp. fermentation on producing potent α-glucosidase inhibitors. Mar. Drugs 2017, 15, 274. [Google Scholar] [CrossRef] [PubMed]
- Kameda, Y.; Asano, N.; Yoshikawa, M.; Takeuchi, M.; Yamaguchi, T.; Matsui, K.; Horii, S.; Fukase, H. Valiolamine, a new α-glucosidase inhibiting amino-cyclitol produced by Streptomyces hygroscopicus. J. Antibiot. 1984, 37, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Ezure, Y.; Maruo, S.; Miyazaki, K.; Kawamata, M. Moranoline (1-deoxynojirimycin) fermentation and its improvement. Agric. Biol. Chem. Tokyo 1985, 49, 1119–1125. [Google Scholar]
- Cho, Y.S.; Park, Y.S.; Lee, J.Y.; Kang, K.D.; Hwang, K.Y.; Seong, S.I. Hypoglycemic effect of culture broth of Bacillus subtilis S10 producing 1-deoxynojirimycin. J. Korean Soc. Food Sci. Nutr. 2008, 37, 1401–1407. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Yamaki, K.; Yoshihashi, T.; Ohnishi, K.M.; Li, X.T.; Cheng, Y.Q.; Mori, Y.; Li, L.T. Purification and identification of 1-deoxynojirimycin (DNJ) in okara fermented by Bacillus subtilis B2 from Chinese traditional food (meitaoza). J. Agric. Food Chem. 2010, 58, 4097–4103. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.Y.; Hwang, K.Y.; Cho, Y.S.; Park, Y.S.; Kang, K.D.; Seong, S.I. Isolation and identification of a Bacillus sp. producing α-glucosidase inhibitor 1-deoxynojirimycin. Korean J. Microbiol. Biotechnol. 2011, 39, 49–55. [Google Scholar]
- Onose, S.; Ikeda, R.; Nakagawa, K.; Kimura, T.; Yamagishi, K.; Higuchi, O.; Miyazawa, T. Production of the α-glycosidase inhibitor 1-deoxynojirimycin from Bacillus species. Food Chem. 2013, 138, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Jung, H.; Karuppasamy, S.; Park, Y.S.; Cho, Y.S.; Lee, J.Y.; Seong, S.; Suh, J.G. Anti-diabetic effect of the soybean extract fermented by Bacillus subtilis MORI in db/db mice. Food Sci. Biotechnol. 2012, 21, 1669–1676. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Xue, Y.P.; Shen, Y.C. Production of valienamine by a newly isolated strain: Stenotrophomonas maltrophilia. Enzyme Microb. Technol. 2006, 39, 1060–1065. [Google Scholar] [CrossRef]
- Schmidt, D.D.; Frommer, W.; Junge, B.; Müller, L.; Wingender, W.; Truscheit, E.; Schäfer, D. α-Glucosidase inhibitors, new complex oligosaccharides of microbial origin. Naturwissenschaften 1977, 64, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Yeh, P.Y. Production of a surfactant- and solvent-stable alkaliphilic protease by bioconversion of shrimp shell wastes fermented by Bacillus subtilis TKU007. Process Biochem. 2006, 41, 1545–1552. [Google Scholar] [CrossRef]
- Wang, S.L.; Yeh, P.Y. Purification and characterization of a chitosanase from a nattokinase producing strain Bacillus subtilis TKU007 using shrimp shell powder as a medium. Process Biochem. 2008, 43, 132–138. [Google Scholar] [CrossRef]
- Wang, S.L.; Wu, Y.Y.; Liang, T.W. Purification and biochemical characterization of a nattokinase by bioconversion of shrimp shell with Bacillus subtilis TKU007. New Biotechnol. 2011, 28, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Kao, D.Y.; Wang, C.L.; Yen, Y.H.; Chern, M.K.; Chen, Y.H. A solvent stable metalloprotease produced by Bacillus sp. TKU004 and its application in the deproteinization of squid pen for beta-chitin preparation. Enzyme Microb. Technol. 2006, 39, 724–731. [Google Scholar] [CrossRef]
- Hong, C.E.; Kwon, S.Y.; Park, J.M. Biocontrol activity of Paenibacillus polymyxa AC-1 against Pseudomonas syringae and its interaction with Arabidopsis thaliana. Microbiol. Res. 2016, 185, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.J.; Lee, Y.S.; Kim, K.Y.; Jung, W.J. Antifungal activity of chitinase obtained from Paenibacillus ehimensis MA2012 against conidial of Collectotrichum gloeosporioides in vitro. Microb. Pathog. 2016, 96, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Tabacchioni, S. Ecology and biotechnological potential of Paenibacillus polymyxa: A minirevie. Indian J. Microbiol. 2009, 49, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Padda, K.P.; Chanway, C.P. Evidence of nitrogen fixation and growth promotion in canola (Brassica napus L.) by an endophytic diazotroph Paenibacillus polymyxa P2b-2R. Biol. Fertil. Soils 2016, 52, 119–125. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, H.T.; Zhang, L. J.; Lin, Z.H.; Kuo, Y.H. Conversion of squid pen to homogentisic acid via Paenibacillus sp. TKU036 and the antioxidant and anti-inflammatory activities of homogentisic acid. Mar. Drugs 2016, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Vattem, D.A.; Shetty, K. Evaluation of clonal herbs Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 107–118. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).