Abstract
Sea cucumbers have been valued for many centuries as a tonic and functional food, dietary delicacies and important ingredients of traditional medicine in many Asian countries. An assortment of bioactive compounds has been described in sea cucumbers. The most important and abundant secondary metabolites from sea cucumbers are triterpene glycosides (saponins). Due to the wide range of their potential biological activities, these natural compounds have gained attention and this has led to their emergence as high value compounds with extended application in nutraceutical, cosmeceutical, medicinal and pharmaceutical products. They are characterized by bearing a wide spectrum of structures, such as sulfated, non-sulfated and acetylated glycosides. Over 700 triterpene glycosides have been reported from the Holothuroidea in which more than 145 are decorated with an acetoxy group having 38 different aglycones. The majority of sea cucumber triterpene glycosides are of the holostane type containing a C18 (20) lactone group and either Δ7(8) or Δ9(11) double bond in their genins. The acetoxy group is mainly connected to the C-16, C-22, C-23 and/or C-25 of their aglycone. Apparently, the presence of an acetoxy group, particularly at C-16 of the aglycone, plays a significant role in the bioactivity; including induction of caspase, apoptosis, cytotoxicity, anticancer, antifungal and antibacterial activities of these compounds. This manuscript highlights the structure of acetylated saponins, their biological activity, and their structure-activity relationships.
1. Introduction
Holothurians belong to the Animal kingdom, the phylum Echinodermata, and the class Holothuroidea (from the Greek holothurion, “sea polyps”). Holothuroidea are divided into three subclasses: Dendrochirotidae, Aspidochirotacea and Apodacea. The most studied holothurians belong to the families Holothuriidae, Cucumariidae, and Stichopodidae. Holothurians are sedentary marine invertebrates, commonly known as sea cucumbers, trepang, bêche-de-mer, khiar daryaei or gamat, which vary in size from an inch in length to up to three feet long. Sea cucumbers are commercially important and have been used in Asian traditional medicine since ancient times as tonics, and frequently reported as a treatment for certain diseases; and now are gaining popularity as a dietary supplement in western countries [1]. There is a dried sea cucumber market on the Internet (www.coastsidebio.com) and over-the-counter in encapsulated form as dietary health supplements and nutraceuticals for humans and companion animals in the United States and Canada [2]. In the Qin dynasty in China, sea cucumbers were considered as a good remedy equal to ginseng for “yin deficiency of kidney, ischemia, dysentery and ulcers” [3]. Many Asians consume sea cucumbers to cure disorders including asthma, hypertension, cancer and arthritis, as well as intestinal and urinary dysfunctions [4]. Further, they believe consumption of sea cucumbers enhances the immune system and possesses aphrodisiac properties. Thus, food supplements comprising sea cucumber extracts are currently utilized in the treatment of cancer patients in Korea, Japan, China and other countries [5].
Triterpene glycosides are well-known for their cytotoxic, antimicrobial, anticoagulant, hemolytic, antiviral, antiparasitic and antitumor properties. Some glycosides can prevent the growth [6], survival, invasion [7,8], and metastasis [9] of cancerous cells; others possess immunomodulatory activity [10], or inhibit the sodium–potassium ATPase [11], and even elicit apoptosis [12,13,14]. The wide spectrum of biological properties of the triterpene glycosides, particularly in high concentrations, is preferably associated with the interaction with Δ5(6) sterols of the cellular membrane resulting in a saponification that lyses the cell. The defense mechanism against predators could be due to membranotropic action of these compounds.
This study is the first comprehensive review on acetylated triterpene glycosides isolated from sea cucumbers, and describes the structure of all acetylated triterpene glycosides (145) reported to-date, and covers the effect of the acetoxy group upon the activity of glycosides and their structure-activity relationships. Therefore, the main aim of this review is to combine and summarize the literature about the structure, biological activities and distribution of acetylated glycosides from sea cucumbers.
2. Sea Cucumbers Triterpene Glycosides as Chemotaxonomic Markers
Sea cucumbers are a prolific source of bioactive secondary metabolites with the potential to cure or prevent several diseases. The bioactive compounds from sea cucumbers are well-known. This high chemical diversity is a potential source of nutraceutical, pharmaceutical and cosmetic agents, many of which have been of interest in pharmaceutical development. Echinoderms belonging to the class Holothuroidea generate a complex assortment of triterpene glycosides (holothurins), which are in charge of general toxicity and defense due to their membranotropic function.
The current knowledge of holothurian diversity is virtually unknown. However, over 1500 species have been reported. Biodiversity may provide chemical diversity which increases the chance of exploring novel therapeutic compounds. Chemical fingerprinting of triterpene glycosides in holothurians can give insight on the correct taxonomic position of a species. These congeners have the potential to be used as chemotaxonomic markers.
Many studies revealed that holothurians belonging to all the extant orders of the class Holothuroidea produce triterpene glycosides. Several triterpene glycosides are specific to different taxonomic groups of holothurians. These structural characteristics and features of triterpene glycosides have been applied to resolve taxonomic problems in the class Holothuroidea [15]. Sea cucumber triterpene glycosides have been used to reclassify and improve sea cucumber taxonomy as they are useful taxonomic markers [15,16]. Therefore, the occurrence of triterpene glycosides may be considered as a chemical marker and taxonomic character for this class [17]. Indeed, the combination of chemotaxonomic and morphological and conventional methods can become a strong tool to determine sea cucumber classification, phylogeny and evolution.
It has been claimed that S. chloronotus collected off the Great Barrier Reef produces stichoposides C, D and E and lack of their 25(26)-dehydro derivatives, while Kitagawa et al. (1981) have isolated these glycosides as well as their 25(26)-dehydro analogs from specimens of the same species collected from the Japanese coast [16]. Therefore, there is some evidence that the geographical location can have an effect on the saponin structure. However, this discrepancy might result from using different analytical instrumentation, as these compounds are major compounds that have been reported in many species of the genus Stichopus collected around the world.
3. The Structural Features of Holothurian Triterpene Glycosides
Triterpene glycosides are naturally highly polar compounds with low volatility, first discovered in higher plants. Saponins have also been reported in some marine invertebrates particularly echinoderms, octocorals and sponges. The presence of saponins in these classes is a unique characteristic among the animal kingdom, differentiating them from other echinoderms and from each other [18,19]. Saponins are complex compounds, heterosides, composed of a saccharide moiety (hydrophilic part, water-soluble), connected glycosidically to a hydrophobic aglycone (sapogenin), which has a triterpene or steroid backbone (lipo-soluble) [20,21].
These amphipathic compounds are generally perceived as highly active natural products and the sea cucumber saponins have been well characterized for their biological activities.
Indeed, the name ”saponin” originated from sapo (the Latin word for soap) since they possess surfactant properties and create stable, soap-like foams when shaken in aqueous solution [22,23]. They have been used as emulsification and foaming agents [22,24,25]. Saponins are constituents of many plant drugs and folk medicines, especially from the Orient. They are also consumed as preservatives, flavor modifiers, food additives, vaccine adjuvants and cholesterol-lowering agents.
The main characteristic feature of the holothurians is the presence of particular holostane type triterpene glycosides, and could be differentiated by several structural features. They include a number and position of double bonds in the core and lateral chain of the aglycone, number and position of sulfated groups in the sugar moieties, number, composition and sequence of saccharide residues in the saccharide chain, and the occurrence of hydroxy, epoxy, acetoxy and ketone groups in numerous positions of the aglycone.
Saponins are generally divided into three main groups in accordance with their aglycone (genin) structure: triterpenoidic, steroidal and steroid alkaloid glycosides [22]. Triterpenoid saponins have aglycones that consist of 30 carbons, whereas steroidal saponins possess aglycones with 27 carbons, which are rare in nature [22]. Sea cucumber saponins, commonly referred to as holothurins, are usually triterpene glycosides containing a holostane structure, derived from lanostane, which the majority belongs to rather than nonholostane [19,21,26]. The former is comprised of a lanostane-3β-ol type aglycone containing a γ-18(20)-lactone in the D-ring of tetracyclic triterpene (3β,20S-dihydroxy-5α-lanostano-18,20-lactone) structural characteristic [27,28] and can contain a shortened side (aliphatic) chain, and a carbohydrate moiety consisting of up to six monosaccharide units covalently connected to C-3 of the aglycone (cyclic system) [19,29,30,31,32]. The sugar moieties mainly consist of d-xylose (Xyl), d-quinovose (Qui), 3-O-methyl-d-glucose (MeGlc), 3-O-methyl-d-xylose (MeXyl) and d-glucose (Glc), and sometimes 3-O-methyl-d-quinovose (MeQui), 3-O-methyl-d-glucuronic acid (MeGlcA) and 6-O-acetyl-d-glucose (AcGlc) [32]. The monosaccharide unit which is linked to the C-3 of aglycone is always Xyl, whereas MeGlc and/or MeXyl are always the terminal sugars. The monosaccharaide residues bearing 3-O-methyl groups are always terminal ones. The great majority of saponins have Qui or Glc as the second unit in their carbohydrate chain. Apparently, 3-O-methlylation of the monosaccharide units is a termination signal, preventing the further elongation of a carbohydrate moiety in sea cucumber glycosides. The basic structure of monosaccharides (a) and the holostane type aglycone (b), which is a characteristic aglycone moiety for sea cucumber saponins, are shown in Figure 1.
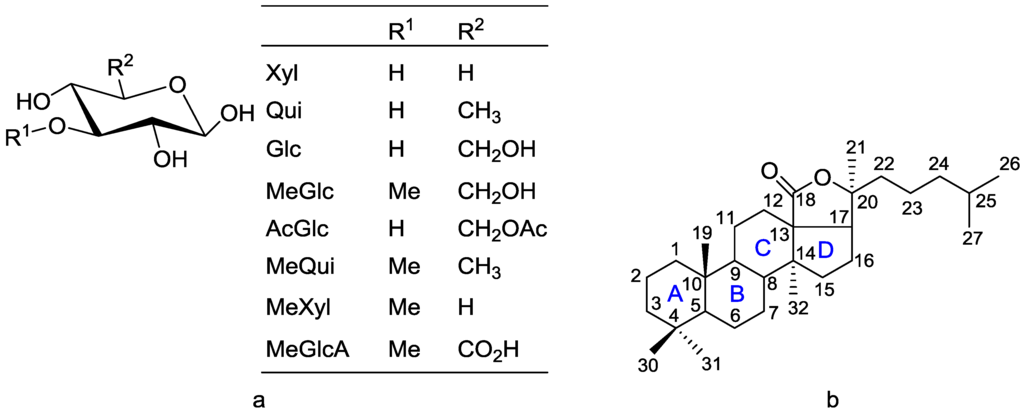
Figure 1.
Structure of monosaccharide (a) and the holostane aglycone (b), bearing an 18(20)-lactone, the characteristic aglycone moiety in sea cucumber glycosides.
Aglycones
The aglycone of sea cucumber triterpene glycosides are generally reported to contain either the 7(8) or 9(11)-double bond in their polycyclic core. Individual sea cucumber species contain one type of these aglycones in their triterpene glycosides. However, a few species of sea cucumbers have been reported to possess both types of aglycones in their triterpene glycosides. These are Cucumaria conicospermium [33], Pentacta australis [34], Neothyonidium magnum [35,36], Psolus fabricii [37,38], and Cucumaria frondosa [39]. The simultaneous existence of triterpene glycoside congeners having different types of unsaturation (either 7(8) or 9(11)) in the polycyclic core in some species of holothurians probably demonstrates the occurrence of common biosynthetic precursors for both type of glycosides (having 7(8)- or 9(11)-double bond). It also sheds light on the production of the polycyclic nucleus with a distinct and particular type of unsaturation at the initial stages of biosynthesis followed by oxidative transformations. Moreover, it has also paved the way that the biosynthesis of the aglycone moiety does not precede glycosylation, but is concurrent [40]. Generally aglycones with a Δ9(11) double bond are characteristic of sea cucumbers belonging to the family Holothuriidae of the order Aspidochirotida, whereas those with a Δ7(8) configuration are usually found in the order Dendrochirotida. Only Nobiliside A was described to contain a 7(8),9(11)-diene cyclic core [41]. However, an aglycone with such a structure (having both unsaturated bonds) is very unlikely.
The glycosides from an individual sea cucumber species may have both holostane aglycones, i.e., containing 18(20)-lactone characteristic for most of the identified sea cucumber glycosides, and nonholostane aglycones having no such lactone. They may also contain aglycones with saturated or unsaturated side chains.
The typical position of a ketone group in the sea cucumber triterpene glycosides is C-16 [15]. Thirty eight different structures have been described for the side chain of acetylated aglycones. Cucumarioside A1-2 is the only example of acetylated saponins with an acetyl moiety (6-OAc) linked to the terminal Glc of sugar residue [42] which is an unusual structure for a sea cucumber glycoside.
4. Distribution of Saponina
Over 700 triterpene glycosides have been reported in a wide range of sea cucumbers species collected from many areas including tropical Pacific, Indian, and Atlantic Oceans, the Mediterranean Sea, South America, the North Atlantic, North Pacific and Persian Gulf [43]. The existence of these compounds has been reported in all sea cucumber species studied. These glycosides are classified into four main structural categories based on their aglycone moieties: three holostane types containing a (1) 3β-hydroxyholost-9(11)-ene aglycone skeleton; (2) a 3β-hydroxyholost-7(8)-ene skeleton and (3) an aglycone moiety different to other two holostane type aglycones, and a nonholostane aglycone. The majority of saponins belong to the holostane type. Three of these possess a holostane skeleton, featuring a C-18/C-20 lactone. The presence of a 7(8) double bond alone is more characteristic of glycosides found in the family Stichopodidae. However, it is also reported in the family Holothuriidae which could represent a parallel and independent evolution or the mosaic type of biosynthesis of glycosides (independent sequence of reactions) in different taxa of sea cucumbers. It was stated that the biosynthesis of glycosides might occur through two pathways; the mosaic type of biosynthesis and the regulatory type of biosynthesis (a strictly determined sequence).
Having a nonlinear hexasaccharide chain is a characteristic feature of most of the major identified glycosides in the family Stichopodidae. Moraes et al. [16] categorized representatives of the family Stichopodidae into two groups on the basis of their glycoside compositions. The first group included species having Stichoposides and Thelenotosides and comprised S. hermanni, S. variegatus, S. chloronotus, Astichopus multifidus, T. ananas and T. anax. The second included species producing Holotoxins and contained P. californicus and Apostichopus japonicus. Nonetheless, our analysis could not confirm this observation as some common saponin congeners such as Holotoxin A1 were found in the viscera of S. hermanni [44]. Furthermore, the major triterpene glycosides (hexasaccharide) were common among the species belonging to these genera. In other words no distinction was seen between these two groups, and this species showed characteristics of both groups. Most of the identified glycosides had an oxidized group at C-23 of the lateral chain of the aglycone and an 18(20)-lactone structure, and contained non-sulfated sugar residues.
S. hermanni species mostly produced triterpenoic oligoglycosides lacking a sulfate group in their sugar units. In fact having a hexasaccharide chain increased the hydrophilic property of saponin congeners proposing they could be released in water surrounding sea cucumbers. Stichopus spp. and Thelonota spp. produce triterpene glycosides lacking a sulfate group in their sugar moieties.
Mass spectrometry (MS), in combination with the literature, is a rapid, sensitive, reliable and accurate technique to elucidate the structure of saponins [32,43,45]. In the positive MS analysis of saponins, isotopic distribution of the molecules can be detected around every cluster including the sodium and proton adducts, which are the most abundant species. However, the presence of acetate adducts clusters in the negative mode of ESI can be commonly observed. It is notable that this phenomenon is a typical characteristic future of using this type of ionization vehicle, and it should not be considered as acetylated compounds (ions) instantly. To ascertain whether they are acetylated compounds it is recommended to corroborate the data by conducting an MS analysis in the positive ion mode.
The notable abundance and diversity of sea cucumber saponins (more than 50 in one species) makes the purification of saponins from their natural sources a challenge. The high complexity of the saponin mixtures makes their structure elucidation and the evaluation of their potential biological activity difficult.
5. Acetylated Triterpene Glycosides
Decoration of saponins with acyl groups (acetoxy) contributes further structural diversity to the original saponins, which could be vital for the bioactivity of the compounds. Another structural feature that has been found only in this series of aglycones is the presence of an acetoxy group at C-16 and/or in the lateral chain of the aglycone (C-22 or C-23 and/or C-25). Based on a comprehensive literature research, acetylated saponins are mainly reported in the family Cucucmarridae; acetylated saponins are mainly identified in the orders Dendrochirotida and Aspidochirotida. Kolga hyaline is the only species of the order Elasipodida, reported to contain an acetylated triterpene glycoside [46]. However, the presence of acetylated saponins from the genus Holothuria is very rare, and only reported for Holothuria lessoni, H. forskalii, H. nobilis, H. hilla, H. fuscocinerea, H. (Microthele) axiloga and H. pervicax.
The position of acetoxy group at the aglycone creates a huge structural diversity of triterpene glycosides. Based on the position of the acetoxy group, we divided the acetylated saponins in three main groups. The first group contains an acetoxy group linked to the cyclic core of holostane type aglycone (C-16), the second group possesses an acetoxy moiety in their lateral chains of holostane type aglycone including C-22, C-23, C-24, C25, and the third group comprises of non-holostane aglycones. The majority of acetylated saponins (roughly two third) were in the first group bearing an acetoxy moiety at C-16 (Figure 2). Nobiliside C 66 is the only example of an acetylated triterpene glycoside containing a cyclic lateral moiety, while other glycosides possess a linear side chain.
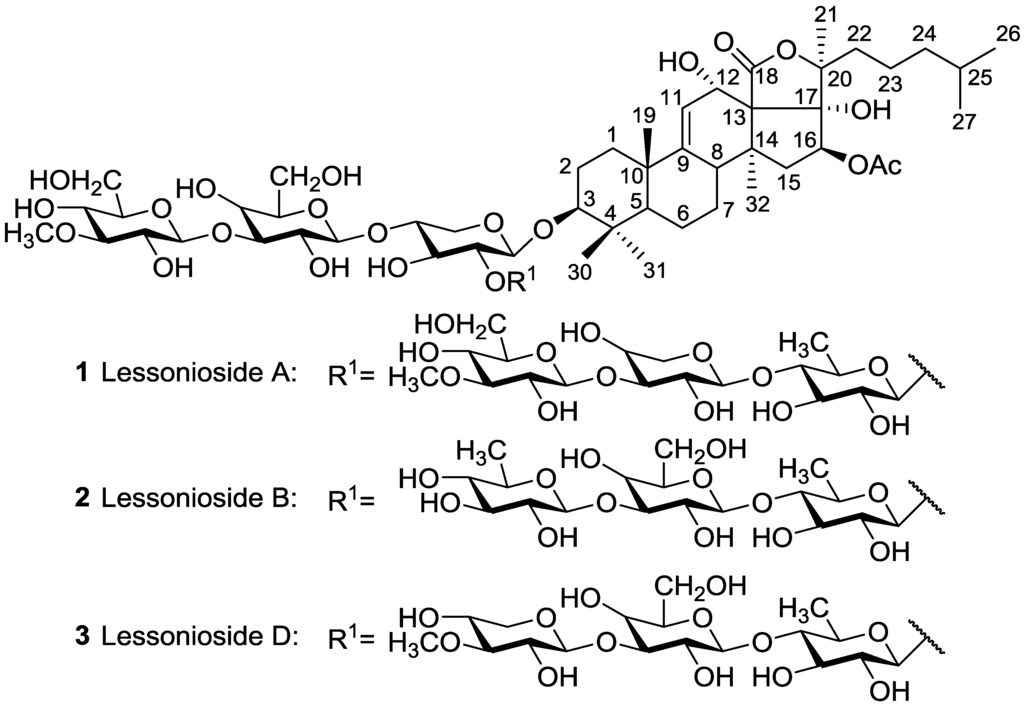
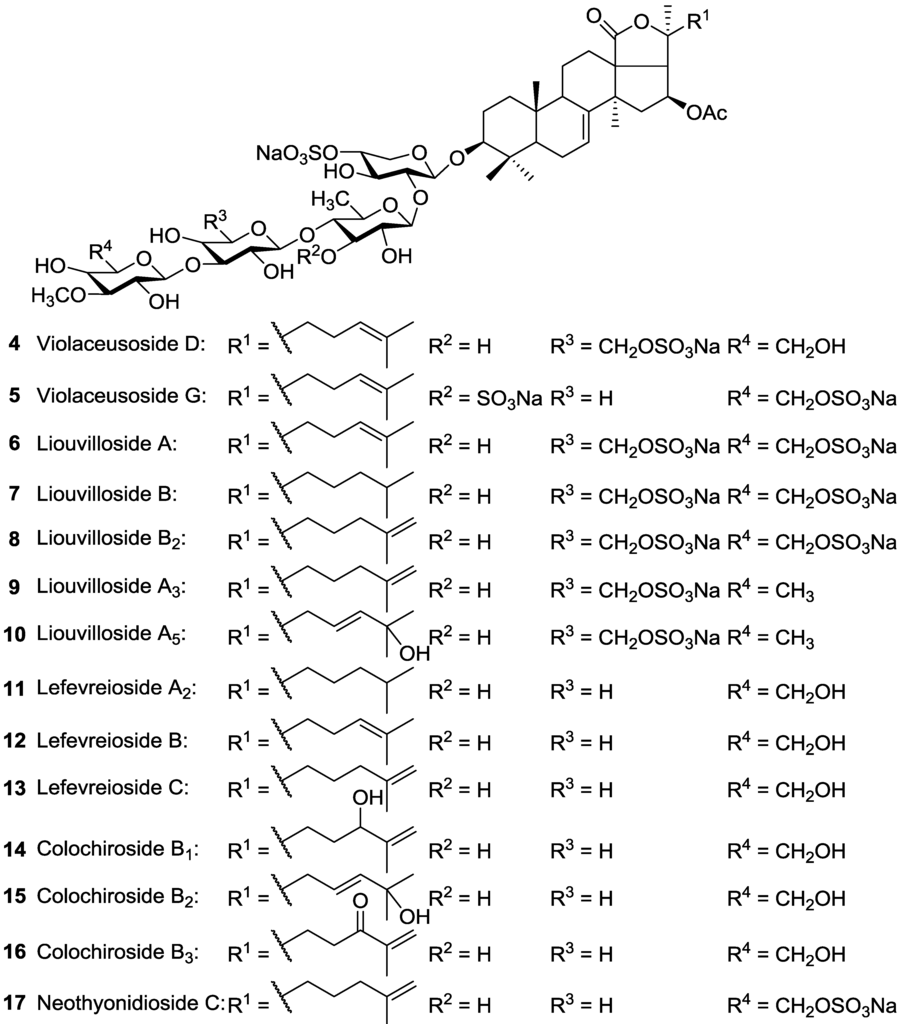
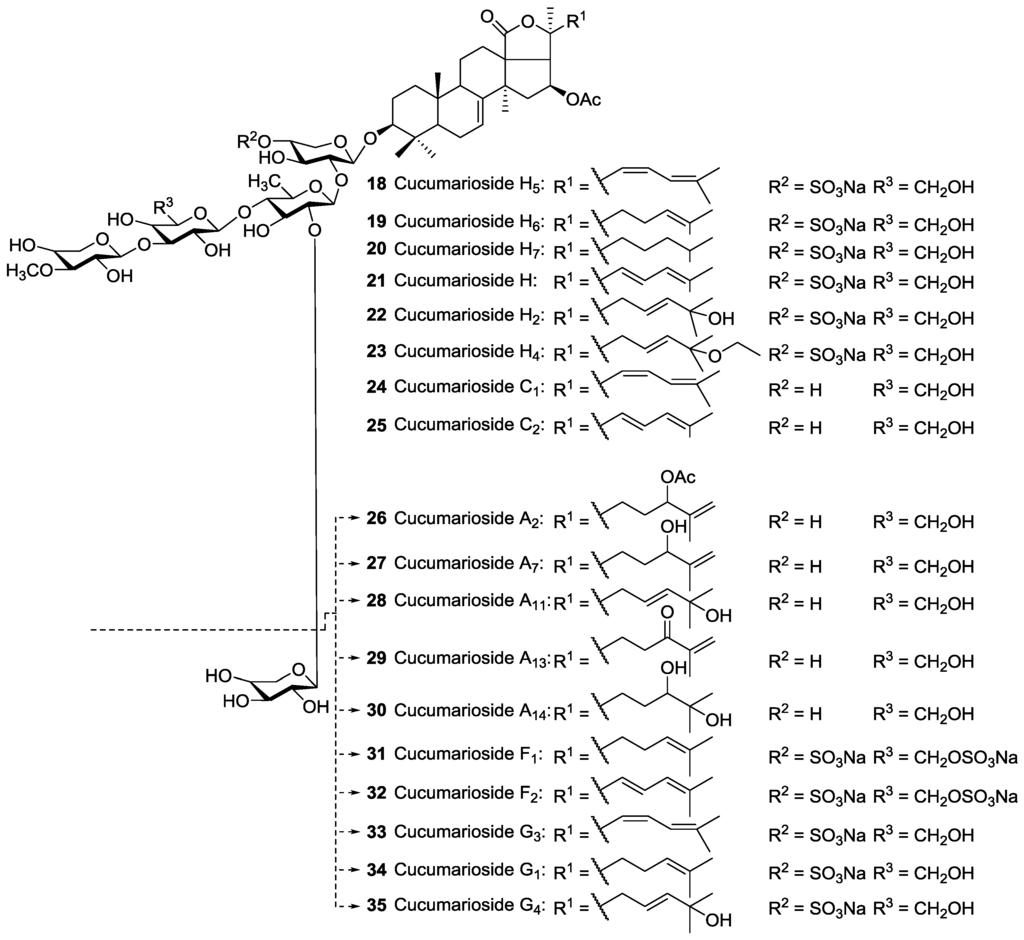
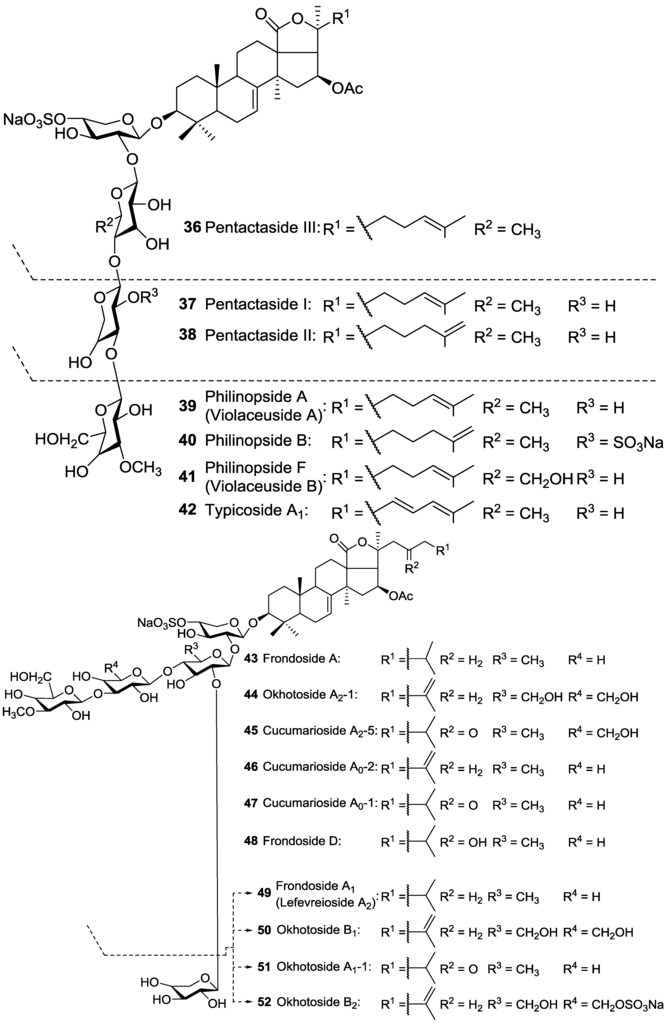
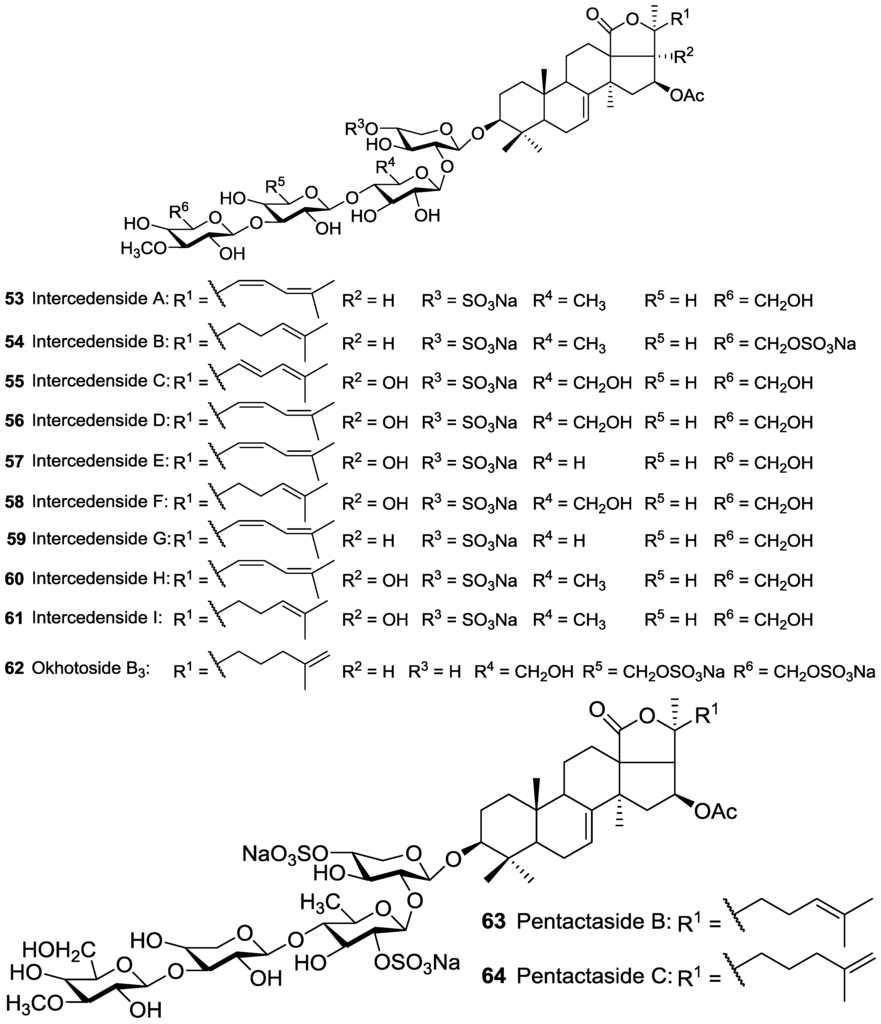
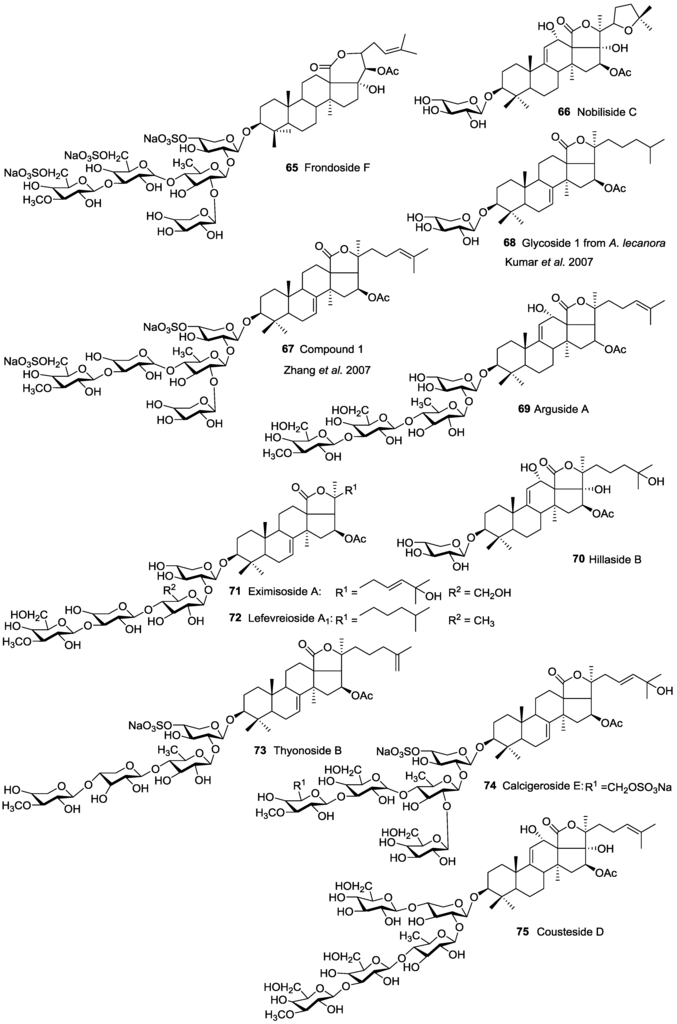
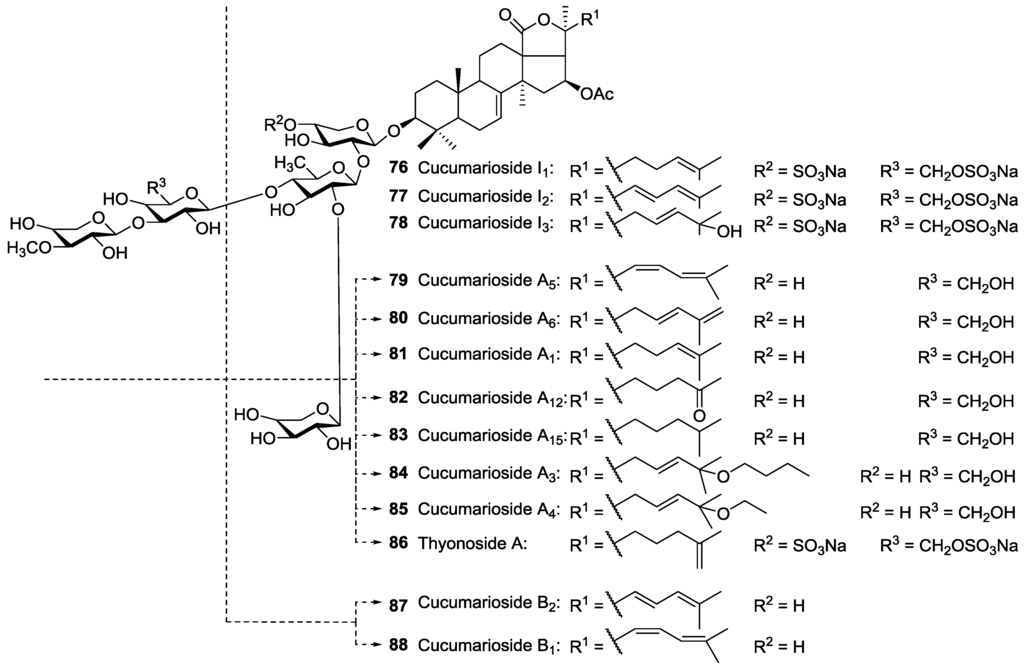
Figure 2.
Acetylated triterpene glycosides bearing an acetoxy group at C-16 of their aglycones.
The second group contains an acetoxy group at the side chain of their aglycone (C-22, C-23, C-24 and C-25). For instance, Fuscocineroside A 90, Pervicoside A (Neothyoside A) 91, Marmoroside C 92, Neothyoside B 93, 25-acetoxy bivittoside D 94, Holothurinoside B 95, Pervicoside D 96 and Arguside D 97 possess an acetoxy group at C-25 of the lateral chain of aglycone (Figure 3), whereas many of other glycosides consist of an acetoxy group attached to C-23 (Figure 4).
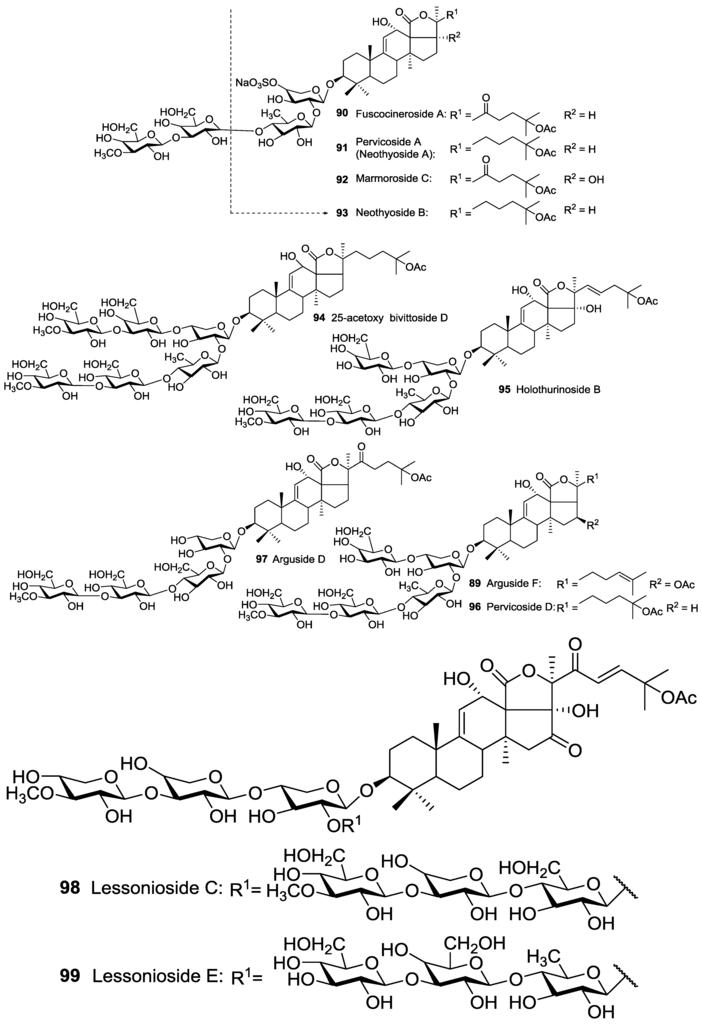
Figure 3.
Acetylated triterpene glycosides possessed an acetoxy group at C-25 of their aglycones.
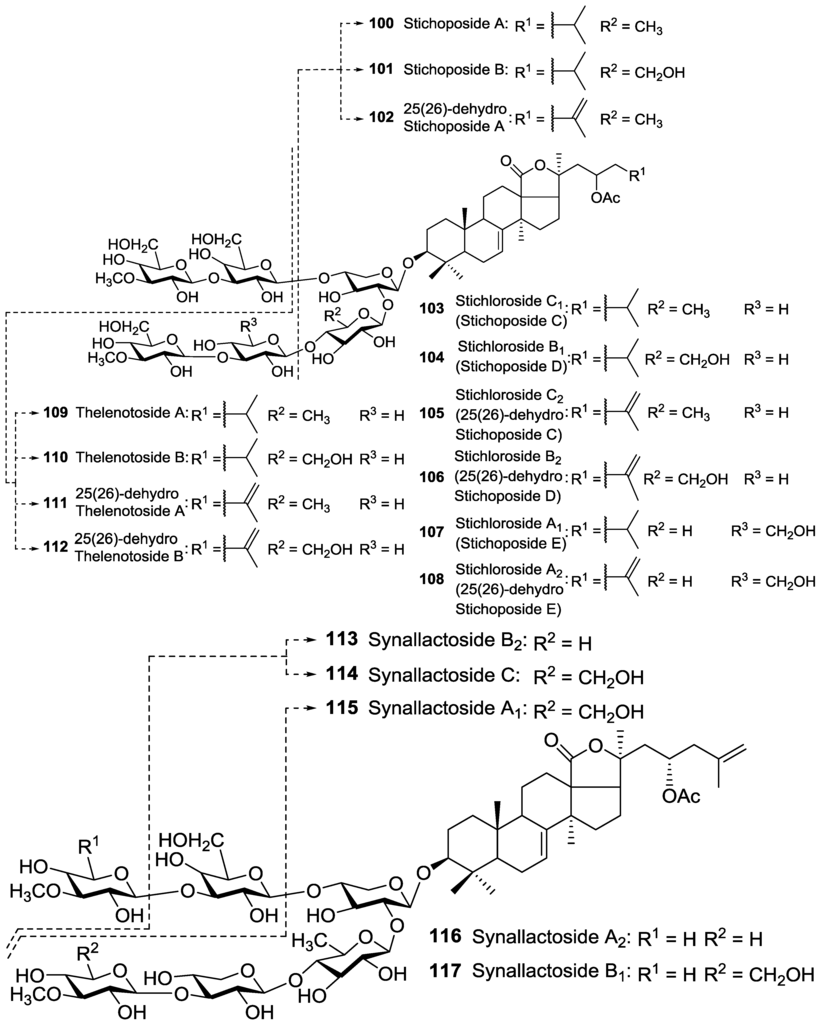
Figure 4.
Acetylated triterpene glycosides bearing an acetoxy moiety linked to C-23 of their aglycones.
A number of acetylated saponins have an acetoxy residue linked to C-22 of aglycone Figure 5 and Figure 6.
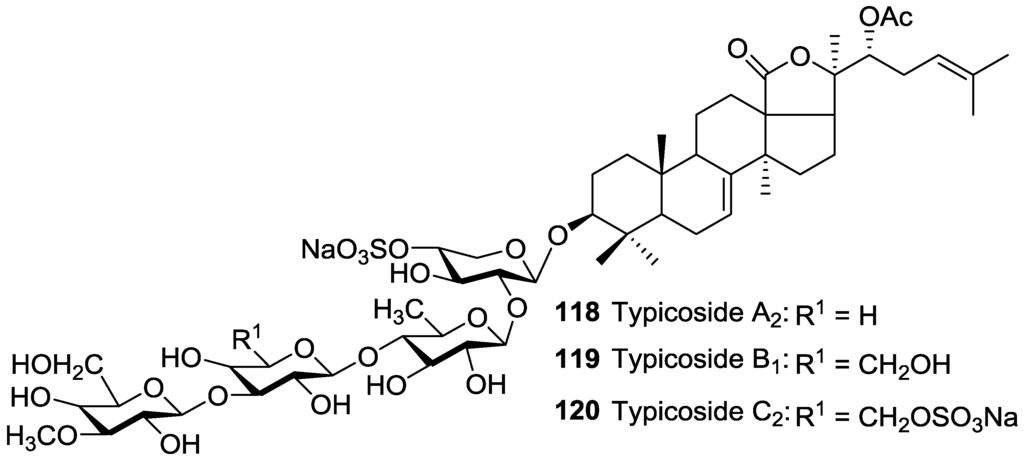
Figure 5.
Acetylated triterpene glycosides having an acetoxy moiety linked to C-22 of their aglycones.
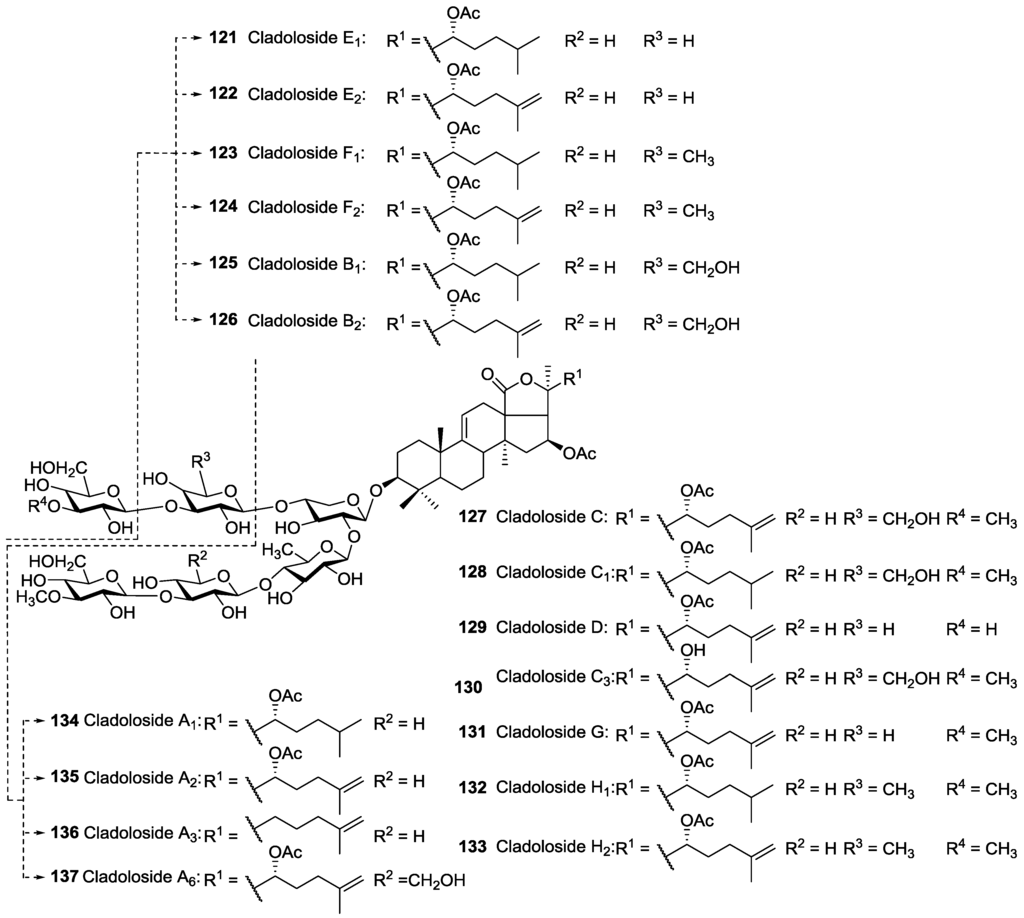
Figure 6.
Acetylated triterpene glycosides contained two acetoxy groups at C-16 and C-22 of their aglycones.
The majority of reported acetylated saponins possess only one acetoxy group in their structure, whereas saponins containing two O-acetic groups in their aglycone moieties have also been reported (Figure 6) [47].
Glycosides bearing two acetyl groups in their aglycone were reported from C. schmeltzii namely Cladolosides which possess 16,22-di-O-acetylated holostane aglycones with saturated or 25(26)-unsaturated lateral chains. In addition, Cucumarioside A2 26 was also reported to have two acetoxy moieties linked to C16, and C24 of aglycone, which is the only example of acetylated saponins bearing an acetoxy group at C-24 (Figure 2).
The comprehensive list of names of the acetylated saponin congeners from sea cucumbers, the site of collection, together with their taxonomic information is summarized in Table 1. This table also indicates a few saponin congeners bearing a non-holostane aglycone.

Table 1.
Distribution of acetylated triterpene glycosides in the sea cucumbers belonging to the class Holothuroidea.
6. Sulfated-Acetylated Compounds
Almost half the identified triterpenoid glycosides contain a sulfate group at the C-4 of the first xylose, and are called sulfated glycosides [123]. While the majority of sulfated glycosides contain a sulfate group at their Xyl residue, glycosides with sulfate groups bind to Glc, MeGlc, Qui and Me-Qui residues have also been reported. Some acetylated compounds also contained a sulfate group bonded to their sugar residues. For instance, Pervicoside A 91 from Holothuria pervicax was reported to contain a sulfate group attached to Xyl [59]. Although most of them are monosulfated glycosides, some of them are di- or trisulfated glycosides, mainly reported in the order Dendrochirotida. For instance, Liouvillosides A 6 and B 7, from Staurocucumis liouvillei are described as trisulfated compounds [103]. It was contended that sea cucumbers belonging to the family Cucumariidae often contain mono-, di- and trisulfated triterpene glycosides [33]. Honey-Escandón et al. [123] stated that the ability to expel (expellability), or the absence of Cuvierian tubules and the temporal or permanent concealing habits of the species in family Holothuriidae apparently could influence the occurrence of sulfated and non-sulfated compounds in these species, which is in agreement with the Kalinin groups’ finding [67,92]. It was also stated that all the holotoxins, thelenotosides and stichoposides reported from sea cucumbers belonging to the family Stichopodidae, lack a sulfate group [16].
7. Non-Holostane Acetylated Saponins
The non-holostane aglycones could serve as biosynthetic precursors, the so-called “hot metabolites”, of more oxidized holostane aglycones. Sea cucumber non-holostane (lanostane) glycosides are rare and, indeed, only 24 have been reported, of which eight are acetylated. They include Kurilosides A 139 and C 140, Frondosides A2-7 138, A2-8 142 and C 145, Psolusoside B 141 and Cucumariosides A8 143 and A9 144. They have been mainly found in sea cucumber species belonging to the order Dendrochirotida. Cucumariosides A8 143 and A9 144 contain MeXyl as terminal monosaccharide which are regarded as intermediates of glycosides biosynthesis in sea cucumbers. Psolusoside B 141 and Kuriloside C 140 were described as tetraglycoside saponins bearing uncommon non-linear carbohydrate structures. Another structural feature for Psolusoside B 141 is the presence of an unusual Glc to Glc (1→4) linkage. The presence of this type of saccharide structure is rare for sea cucumber glycosides, and their structures need to be reconfirmed.
Frondosides A2-7 138 and A2-8 142 reported to have isomeric structure and differed from each other only in the position of a double bond in their aglycone core, with some probability they are the same compound. The structures of non-holostane type acetylated triterpene glucosides are illustrated in Figure 7.
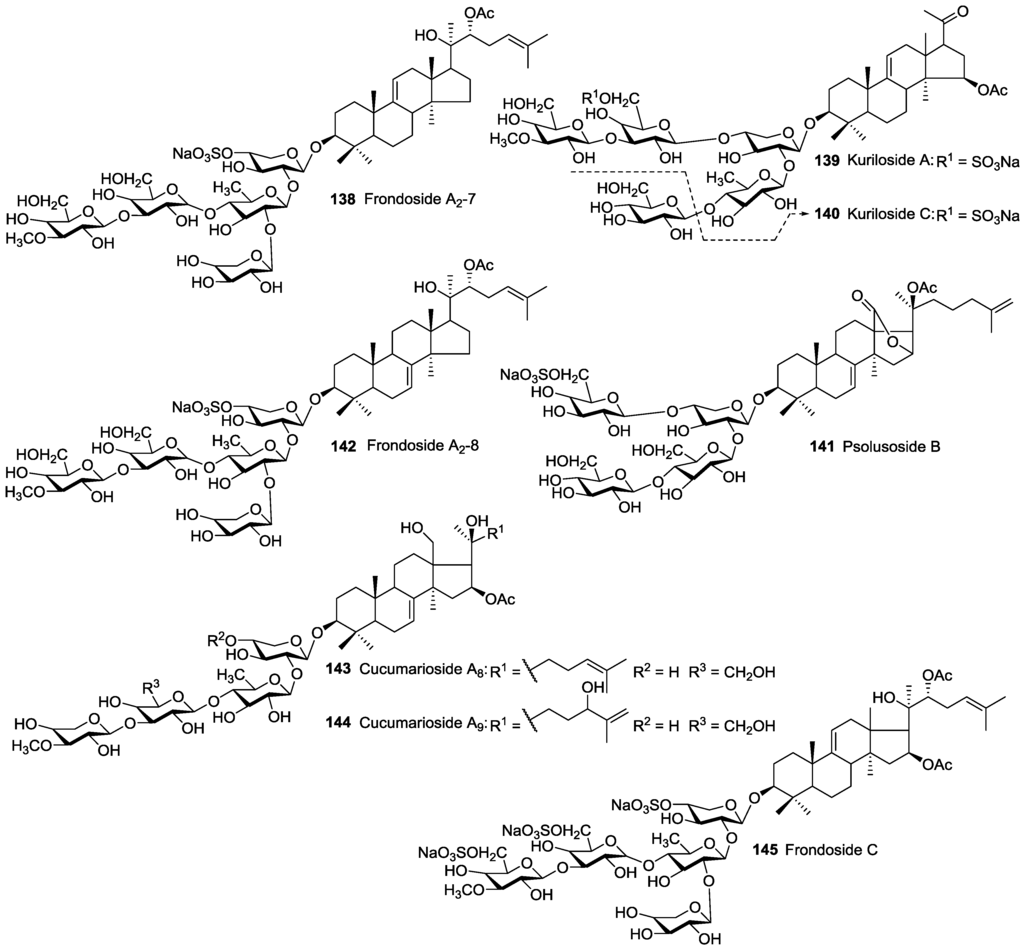
Figure 7.
Non-holostane acetylated triterpene glycosides from Holothuroidea.
8. Acetylated Saponins Having an Uncommon Structure
Our comprehensive review revealed several acetylated triterpene glycosides with unusual chemical structures. For instances, some acetylated glycosides possess trisulfated carbohydrate chains and some contain 3-MeQui as terminal monosaccharide, Figure 2.
The structures of Pentactasides I 37 and II 38 were described to contain a trisaccharide moiety which is an uncommon structural feature among holothurian glycosides and has been infrequently reported [93]. Philinopside B 40, a disulfated tetrasaccharide glycoside, was reported to possess C-2 sulfated Xyl as third saccharide residue which is a rare structural feature for sea cucumber triterpene glycosides. Liouvillosides A3 9 and A5 10 were also reported from Staurocucumis liouvillei as disulfated acetylated tetrasaccharide glycosides bearing very rare 3-O-MeQui as terminal monosaccharide by Antonov et al. [102,103]. Tetrasaccharide glycoside Violaceusoside G 5 was reported to possess C-3 sulfated Qui [99], while Pentactasides B 63 and C 64 stated to contain C-2 sulfated Qui as second sugar residue in their saccharide moieties [97].
The presence of 3-O-MeXyl in Synallactosides A2 116, B1 117 and B2 113 glycosides is a rare structural feature and not characteristic for glycosides from representatives of the family Stichopodidiae.
To date, two types of glycosides having a diene-system in the side chain of their aglycones have been reported; glycosides bearing the 22E,24-diene system from E. fraudatrix and E. pseudoquinquesemita and glycosides having the 22Z,24-diene system from E. fraudatrix and Mensamaria intercedens. However, Cucumarioside A6 80 was reported to contain a 23E,25-diene system which is an uncommon structure for sea cucumber glycosides.
A trisulfated pentaoside, Frondoside F 65, having an 18(22)-lactone instead of 18(20)-lactone and a saturated polycyclic nucleus in the aglycone was reported by Yayli [78]. These structural characteristics are uncommon and unprecedented for sea cucumber triterpene glycosides, and the published data raise many questions. The NMR and MS findings are not supporting the proposed structure. For instance, there is no correlation between protons at C-8 and C-9 in the 1H–1H-COSY spectrum, or no peak can find for quasi-molecular ion in the FAB mass spectrum data. Bearing an acetoxy group at C-20 and having a C-18(22)-lactone are unusual structures for triterpene glycosides of sea cucumbers in addition to the absence of a double bond in the nucleus of the aglycone moiety. In addition, the lateral chain of aglycone is connected to C-22 instead C-20 [78]. Therefore the structure suggested by Yayli [78] seems to be doubtful. A possible justification for these data could be that, in terms of ‘‘frondecaside’’, the author dealt with a very complicated cocktail of glycosides contacting aglycone moieties with both 7(8)- and 9(11)-double bonds whose signals were not accumulated in the NMR spectra. Furthermore, the author pointed out Frondosides A 43 and A1 49 as isomers, which is not correct [78], since they differ from each other in the numbers of monosaccharide units and are not isomers.
The structure of Thyonoside B 73 reported from Thyone aurea also appears to require additional confirmation [107] in order to be confirmed and accepted. In this glycoside, the terminal 3-O-MeXyl is linked to C-4 of the third monosaccharide unit (xylose) instead of C-3, which is described in all previously identified sea cucumber glycosides without exception.
9. Immunomodulatory Properties of Acetylated Saponins
It has been reported that triterpene glycosides isolated from sea cucumbers possess potent immunomodulatory activity. For instance, Frondoside A 43, reported as the major triterpene glycoside from sea cucumber Cucumaria frondosa, possesses immunomodulatory properties [124]. This group suggested that Frondoside A 43 induced prolonged lysosomal activity of mouse macrophages in vivo and in vitro with different concentrations, and stimulated the phagocytosis process of macrophages. Aminin et al. [124] demonstrated that Frondoside A 43 stimulated cell-based immunity namely phagocytosis with a very weak effect on the humoral immune response. In contrast to Frondoside A 43, Cumaside (a non-toxic complex of monosaulfated glycosides mainly Cucumarioside A2-2 from Cucumaria japonica) was stated to have a remarkable stimulatory effect on the humoral immune system [125]. Therefore, saponins can boost the immune system via their adjuvant properties. Monosulfated triterpene glycosides isolated from Cucumaria japonicus were described as the most effective immunostimulants, while di- and trisulfated saponins were reported as immunosuppressors [125].
Six monosulfated triterpene glycosides namely Frondoside A1 49, Okhotoside B1 50, Okhotoside A1-1 51, Frondoside A 43, Okhotoside A2-1 44, and Cucumarioside A2-5 45, isolated from C. okhotensis were reported to increase lysosomal activity of mouse macrophages and reactive oxygen species (ROS) formation in the macrophages in which the highest stimulatory activity of the lysosomal macrophage was induced by Frondoside A1 49, Frondoside A 43, and Cucumarioside A2-5 45 [85]. In addition, Silchenko et al. [117] investigated the immunomodulatory activity of the triterpene glycosides Cucumariosides I2 77, H 21, A5 79, A6 80, B2 87, and B1 88 isolated from Eupentacta fraudatrix on mouse peritoneal macrophages and found only Cucumariosides I2 77, A5 79, and B2 87 showing an increase in the lysosomal activity of macrophages. They reported that there is no direct correlation between lysosomal activity and cytotoxicity of the glycosides.
It might be concluded that some acetylated saponins possess a particular and selective stimulatory effect on the cellular immune system. Therefore, triterpene glycosides from sea cucumbers might introduce a new platform to cure and/or prevent diseases associated with the humoral and cellular-immune status, and could be useful as effective immunomodulatory agents.
10. Cytotoxicity and Anticancer Activity of Saponins
Saponins have been reported to be cytotoxic to mammalian cells primarily due to their membranolytic activity. However, this is dependent on concentration and other specific biological activities have been noted. There is a close relationship between the chemical structure of saponins and their biological activities. Observations from numerous studies confirm that the biological activity of saponins is influenced both by the aglycone and the carbohydrate moiety. The correlations between the structure and the cytotoxicity of triterpenoid glycosides have been described by several groups. It has been stated that having a linear carbohydrate chain is essential for the biological activity of saponins resulting in modifying the cellular membrane [92,126]. For instance, the presence of a linear tetrasaccharide fragment in carbohydrate chain increases the activity. In the case of triterpene saponins for example, acylation seems to increase their hemolytic potential [127]. The obtained results indicated that a free hydroxy at C-16 may be of importance in mediating cytotoxicity. However, the presence of a hydroxy at C-15 and acetyl group had a detrimental effect [127]. Generally, the presence of hydroxy group in the lateral chain of aglycone reduces the cytotoxicity of glycosides.
Triterpene glycosides retard tumor cell proliferation and stimulate apoptosis. The mode of actions could be selective inhibition by arrest of the cell cycle [128], specific cytotoxic activity affected by the saccharide moiety of the saponin structure [129], and non-specific cytotoxicity resulted from detergent function [130].
It has been reported that the presence of acetoxy groups usually enhances cytotoxic potency [130]. The effects of two monosulfated triterpene glycosides; Frondoside A 43 (acetylated) isolated from sea cucumber Cucumaria frondosa [76], and Cucumarioside A2-2 (non-acetylated) from C. japonica [131], on caspase activation and apoptosis of different human leukemia cells death-inducing capability were determined and compared [12]. Frondoside A 43 has an acetoxy group at C-16 of the aglycone and xylose at the third monosaccharide residue (as opposed to a 16-keto group and a Glc as the third sugar moiety in Cucumarioside A2-2). Therefore, the main structural differences between them are the functional group at C-16 and the third monosaccharide in their sugar chain. This study determined the acetyl group at C-16 as the structural feature responsible for increasing cytotoxicity of this compound, and showed that the presence of acetoxy group at C-16 could play a remarkable role in cytotoxicity and caspase activation of this compound [12]. Both compounds strongly induced apoptosis of leukemic cells, but the process was more rapid and potent in cells treated with Frondoside A 43 (acetylated), in comparison to Cucumarioside A2-2. They suggested that Frondoside A 43 results in caspase-independent cell death. Frondoside A 43 did not stimulate caspase activation before earlier apoptosis, whereas Cucumarioside A2-2 induced-apoptosis was caspase-dependent. Therefore, Frondoside A 43 may initiate apoptosis pathways in a caspase-independent manner. Jin et al. [12] stated that Frondoside A 43 is more toxic to the leukemia cells than Cucumariosides, and elicits apoptosis in HL-60 cells through the signal transduction perforin/granzyme pathway. However, Li et al. [13] reported that low concentrations of Frondoside A 43 stimulates apoptosis of pancreatic cancer cells through the mitochondrial and activation of caspase cascade. In addition, Frondoside A 43 was reported to hinder breast cancer cell invasion/migration by decreasing matrix metalloproteinase (MMP)-9 expression via blockage of nuclear translocation and transactivation of NF-κB and AP-1 [132]. Furthermore, Janakiram et al. [2] showed that Frondanol A5, (a glycolipid extract mixture of Frondoside A 43, lipid and chondroitin sulfate) inhibited proliferation and activated caspase-2 inducing apoptosis of colon cancer cells. On the other hand, additional acetylation of the hydroxyl group at C-4" of rhamnose was reported to significantly decrease the cytotoxic activity [127]. Kalinin and coworkers stated that the evolution of saponins from sea cucumber led from non-holostane glycosides with branched carbohydrate chains to holostane compounds containing linear carbohydrate moieties, and this transition increased the activity of saponin significantly [60].
It was also reported that Frondoside A 43 is a selective stimulant of cell-based immunity, but had no marked adjuvant property. The aglycone of Cucumarioside A2-2 isolated from C. japonica contains a 25(26)-terminal double bond and a C-16-keto group compared to Frondoside A [124]. Hence, the small differences in the structure of saponins may impact on the level of immunomodulatory properties.
The anti-metastatic property of Frondoside A 43 was also investigated against human breast cancer cell lines [133]. The authors stated that Frondoside A blocked the expression of TPA-induced MMP-9 likely through the downregulation of AP-1 and NF-κB signaling pathways in which impeded the activation of the PI3K/Akt, ERK1/2 and p38 MAPK signals, which ultimately results in downregulation of MMP-9 expression.
The cytotoxicity of Arguside A 69, bearing an acetoxy group at C-16, from Bohadschia argus was examined against four human tumor cell lines [49] which showed more activity towards HCT-116 cells than others. Furthermore, Intercedenside A 53, having an acetoxy group at C-16, was also pointed out to possess notable in vivo antineoplastic property against mouse Lewis lung cancer and mouse S180 sarcoma [89].
It has been documented that the existence of an 18(20)-lactone, and 9(11) double bond in lanostane of the aglycone moiety, and having a linear tetrasaccharide residue in the sugar chain are very crucial for the membranotropicity of these compounds [92,134]. Triterpene glycosides bearing a 9(11)-double bond, the presence of an 18(20)-lactone in the aglycone, with at least one oxygen group adjacent to this functional moiety is substantial for their biological activity [126,132].
In general, the structure of the carbohydrate chain is also a characteristic which defines the biological activity of these glycosides. For instance, it has been reported that Stichoposide A 100 (a disaccharide glycoside) and Stichoposide E 107 (a hexasaccharide glycoside having a Xyl as the second sugar moiety) showed lower membranotropic activity than other stichoposides [92]. The presence of a sulfate group can also influence the biological activity of triterpene glycosides considerably [27]. The presence of a sulfate group attached to the first Xyl of a linear tetrasaccharide residue of triterpene glycosides increases their activity. However, the activity of sulfated glycosides will influence with the type, sequence and order of the other monosaccharides in their sugar resides as well as the position of sulfate group. Stichoposide C (Stichloroside C1) 103 is a quinovose-containing hexaosides at its second position. It has been described that Stichoposide C stimulated apoptosis of human leukemia and colorectal cancer cells via the activation of both intrinsic and extrinsic pathways [14]. Apoptosis was shown to be induced by this compound in mouse CT-26 subcutaneous tumor and HL-60 leukemia cells in a dose-dependent manner and resulted in the activation of Fas (CD95) and caspase-8, cleavage of Bid, mitochondrial damage, and activation of caspase-3. Stichoposide C 103 was also reported to reduce tumor growth of HL-60 xenograft and CT-26 subcutaneous tumors and enhance ceramide generation in vivo through activation of acid and neutral types of sphingomyelinases (SMase) in response to apoptotic stimuli. It should be noted that in all types of cancer cells, anticancer substances enhance ceramide levels to variable degrees [135]. Apparently, Stichoposide C 103 targets SMase which leads to an increase in ceramide and apoptosis.
Stichoposide D 104 is a hexaosides which can stimulate apoptosis of human leukemia cells through both extrinsic and intrinsic pathways; however, the potency of this induction is two to five times lower than the induction caused by Stichoposide C 103 [132]. Stichoposide D 104 by activating Fas/ceramide synthase 6 (CerS6)/p38 kinase in lipid rafts contributed to its anti-leukemic activity [136]. This group contended that the difference in only one sugar between Stichoposide C 103 and Stichoposide D 104 may impact on both the potency and the molecular mechanisms for their activities. Therefore, the number, length and the type and linkage variation in sugar moieties significantly influence the bioactivity of saponins.
Cytotoxicity of Pentactasides B 63 and C 64 from Pentacta quadrangularis were tested against five human tumor cell lines [97,137]. Two disulfated acetylated (an acetyl group at position 16β) holostane glycosides reported to possess significant activity against all tumor cell lines with IC50 values between 0.09 and 2.30 µM. Both saponins differ only in the side chain of the triterpene aglycone.
Almarzouqi et al. [7] demonstrated that Frondoside A 43 markedly reduced the growth of human breast cancer cell line MDA-MB-231 tumor xenografts in athymic mice and inhibited the migration and invasion of these cells in a wound healing assay. In addition, it provoked the caspases 9 and 3/7 cell death pathways followed by the activation of p53. Further, Frondoside A 43 was shown to have potent antimetastatic activity in mice bearing mammary gland-implanted tumors to the lungs [8].
Activation of apoptosis is the most commonly described mechanism of the anticancer drugs. Frondoside A 43 was stated to increase the activities of caspases-3 and -7 in LNM35 lung cancer cells [9], and elicits apoptosis by increasing activities of caspases-9, -3, and -7, boosting bax (an apoptotic promotor), and declining bcl-2 (apoptotic inhibitor) and mcl-1 (promoting cell survival by preventing cell death) [13], while Philinopside A 39, an acetylated glycoside from Pentacta quadrangularis exhibited antitumor activity and stimulate apoptosis by inhibition of autophosphorylation of receptor tyrosine kinases [95]. In addition, they contended that Philinopside A 39 blocked all examined angiogenesis-related receptor tyrosine kinases (RTKs) widely, comprising fibroblast growth factor receptor-1 (FGFR1), vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor-β (PDGFβ), as well as epithelial growth factor receptor (EGFR). Besides, Philinopside A 39 was reported to block proliferation and the migration of human microvascular endothelial cells (HMECs) in a doses-dependent manner [95].
It has been demonstrated that the lateral chain of the aglycone could impact on both the cytotoxicity against tumor cells and the selective cytotoxicity in neoplastic versus normal cells of these compounds [138]. The cytotoxicity and hemolytic activities of Cucumariosides H5 18, H6 19, H7 20, H8, H 21, H2 22, A1 81, A3 84, A4 85, A5 79, A6 80, A12 82 and A15 83 isolated from Eupentacta fraudatrix were studied against mouse spleen lymphocytes and mouse erythrocytes [112,115,139]. Cucumariosides H5 18, H6 19, H7 20 and H 21 differ from each other in their lateral chain of aglycones. These glycosides were reported to exhibit different cytotoxicity activities. For instance, Cucumarioside H2 having a 25-hydroxy group in the lateral chain demonstrated low activity against mouse spleen lymphocytes and Ehrlich carcinoma cells. Therefore, the structure of the side chain of the aglycone influences noticeably the cytotoxic property of these glycosides.
The cytotoxic potency of Cucumariosides might influence by their amphiphilicity. It has been demonstrated that the presence of a 25-OH group in the lateral chain of aglycone moiety of triterpene glycosides (Cucumarioside H2 22) decline their cytotoxicity considerably, while the Cucumariosides bearing 25-ethoxy group (Cucumarioside H4 23) possess potent cytotoxic activity against lymphocytes and very high hemolytic activity [139].
Saponins were reported to possess anti-glioblastoma effects. Research and clinical findings showed that glioblastoma is one of the most common malignant tumors in the neurological system [140]. Tian et al. [141] described the anti-glioblastoma activity of saponins from five different species of sea cucumbers and found several saponins exhibiting significant anti-glioblastoma properties in vivo by in situ administration (interstitial chemotherapy). For instance, the authors contented that Fuscocineroside A 90 exhibited in vitro cytotoxicity against human cancer cell lines HL60 (Human myeloid leukemia cells). Furthermore, Fuscocineroside A 90 could remarkably prevent the proliferation, stimulate the apoptosis and suppress the surviving expression in U251 cells (human brain) in a doses and time-dependent manner, but shows slight cytotoxicity to normal human astrocytes [141]. They inferred that saponins influence with cancer cells through multiple mechanisms of action, such as interfering with cell cycle progression, stimulating apoptosis, inciting stabilization of microtubules, as well as several signal transduction pathways [141].
22-oxo-25-acetoxy-echinoside B from the sea cucumber Holothuria moebii was stated to reduce the proliferation of four different glioma cells [142]. It was reported this compound induced apoptosis in human glioblastoma U87-MG cells and decreased the production of a number of glioma metabolic enzymes of glycolysis and glutaminolysis.
Other researchers also pointed out that Philinopside A (Violaceuside A) 39 and Philinopside F (Violaceuside B) 41 possess cytotoxic activity towards HL60 and BEL-7402 cell lines by cell division inhibitions [100].
11. Antifungal Activity
The frequency, intensity and diversity of fungal infections affecting humans range from the superficial, like dermatophytosis, to the deeply invasive, such as candidiasis, has increased. Coupled with the development of resistance strains to the current antibiotics used in the clinics, there is a pressing need to develop new antifungal substances with diverse chemical structures and novel modes of action.
Antifungal activity of a number of triterpene glycosides isolated from sea cucumbers has been reported. Kitagawa et al. reported antifungal activity of six triterpene glycosides; Stichlorosides A1 107, A2 108, B1 104, B2 106, C1 103 and C2 105 from the body wall of Thelonota ananas and S. chloronotus, with Stichloroside B1 having the highest yield of 15% of the mixture [63,143]. These compounds were also reported in the body wall of S. hermanni by Kobayashi et al. [65]. Wang et al. [144] also reported a potent antifungal activity of the acetylated saponin, Stichloroside C1 103 isolated from S. japonicus.
In addition, Stichloroside A1 107, A2 108, B1 104, B2 106, C1 103 and C2 105 were reported by other workers to be antifungal as are Bivittoside C and D from Bohadschia argus (janome-namako); from Holothuria edulis (akamishikiri); Holothurin A and Echinoside A from Bohadschia graeffie (kurote-namako) [65].
We also investigated antifungal activity of isobutanol-enriched saponins and HPCPC fractions from the viscera of sea cucumber S. hermanni against Fusarium pseudograminearum, Pythium irregulare and Rhizoctonia solani using a modified disc diffusion agar assay [44]. Lessonioside A 1, previously reported in H. lessoni [43], isolated from this species shows strong antifungal activity toward the tested strain. Our limited results show that acetylated and mono-sulfated saponins usually exhibit higher antifungal activity compared to non-sulfated and non-acetylated compounds. Our results, though with a limited number of samples, indicate that saponins, bearing a double bond at the lateral chain of the aglycone, in particular Δ25, have a significant antifungal activity. This suggests that the Δ25 terminal double bond may increase the activity. This point of view correlates well with the data on the structure-activity relationship of triterpene glycosides reported by Wang et al. [3] who suggested that the 18(20) lactone moiety and Δ25 double bond may increase the activity.
The configuration and composition of the aglycone and glycone moieties appear to play a crucial role in the bioactivity. Wang’s group; however, contended that the position of double bond in the core of aglycone residue (Δ7, Δ8 or Δ9(11)) contributes little to the bioactivity [3,145], whereas Avilov et al. [146] claimed that the position of the double bond in the aglycone nucleus influence the activity. In addition, Yuan et al. [52] stated the presence of hydroxy or acetoxy groups at C-25 in the lateral chain of the aglycone reduces the antifungal activity of saponins. Further, it was contended triterpene glycosides bearing Qui as the second monosaccharide unit are the most active against fungi [137], which requires further research and evidence. Kitagawa et al. [147] stated that the presence of a linear tetrasaccharide moiety and a sulfate group are important for antifungal activity of saponins.
It has been reported that Thyonosides A 86 and B 73 isolated from the sea cucumber Thyone aurea showed interesting activity against murine tumor cell line, L1210, and herpes simplex virus type 1, HSV-1 [107].
Sea cucumbers could provide an impressive source of antifungal compounds due to saponins, which are worthy of further investigation. Therefore, more extensive research is required to understand the structure-activity relationship clearly. To conclude, the cytotoxic activity depends on both configuration of the aglycone nucleus and the sequence of saccharide moiety.
12. Molecular Effect of Saponins on Membranes
Many biological activities of holothurian saponins occur through their membranolytic function once a certain threshold concentration is reached. Triterpene glycosides have the ability to cause membrane perturbation; altering the membrane permeability, loss of barrier function, and the rupture of cell membrane. [60,132,148]. The interaction of glycosides (aglycone part) with the Δ5(6)-sterols of membranes (rafts), preferably with cholesterol (ergosterol in fungi), is the major factor, but not the only factor for the determination of the many biological activities of sea cucumber glycosides [92,149,150]. Glycosides bind to membrane sterols and create glycoside-sterol complexes in membranes, modifying its microviscosity, ion permeability and the activity of embedded membrane proteins. The formation of a complex is followed by association of these complexes into “two-dimensional micellar-type structures” within the membrane [150]. The hydrophilic sugar chains of the saponins, which are supposed to be centrally orientated in the micellar-like complex, result in the development of an aqueous pore and budding of the lipid a binary membrane due to the increased curvature stress. It is recognized that the holothuroid triterpene glycosides possess strong membranolytic function towards biological and model membranes containing Δ5-sterols due to formation of single-ion channels and larger pores (channels) which is the basis of hemolytic, and antifungal features of these substances, explaining the wide spectrum of their biological activities.
Triterpene glycosides influence the physicochemical properties of membranes such as stability, permeability and microviscosity of lipid bilayers and lipid-protein interaction and conformation of membrane proteins [151,152]; for instance, reduction or inhibition the activity of some membrane enzymes, in particular ATPases [60]. Such sterol/saponin interactions result in an efflux of some ions, substances of the nucleotide pool and peptides, disruption of ion homeostasis and osmolarity followed by lysis and cell death [92,149,153,154]. However, activities of sea cucumber glycosides in sub-cytotoxic doses are especially interesting. Low concentrations of triterpene glycosides from sea cucumbers and plants were reported to interfere with specific membrane transport proteins in cancer cells, parasites, bacteria, and fungi and alter their activities. For instance, Frondoside A 43 and CucumariosideA2-2 blocked the ATP-binding cassette (ABC) transporter (membrane transport P-glycoprotein; P-gp) and multidrug-resistance protein-1(MDR1) [155,156], in which they extrude or modify the lipophilic chemotherapeutical agents and drugs. Triterpene glycosides appear to act as competitive inhibitors for P-gp, multiple resistance-associated protein1 (MRP1), and Breast cancer resistance protein (BCRP) in cancer cells, or efflux pumps in bacteria (NorA) and fungi [156]. Because of their lipophilicity property, these terpenoids probably serve as substrates for P-gp and other ABC transporters.
Triterpene glycosides influence most of the normal functions of the membrane such as ion transport, and membrane permeability. Membrane transporters, which are modulated by triterpene glycosides, can be considered as potential therapeutic targets. Despite several studies that have investigated the membranotropic properties of sea cucumber glycosides during the past three decades; however, the molecular mechanisms of action of these compounds in biomembranes are not fully understood, so further investigation needs to be done.
Also, glycosides containing a 7(8)-double bond and lacking a C-16 ketone group possess more hemolytic activity than those with a 9(11)-double bond and a C-16 ketone group. In addition, Mal’tsev et al. [157] stated that glycosides containing quinovose as the second monosaccharide unit exhibited more hemolytic activity than other triterpene glycosides.
The ability of some triterpene glycosides to interact with glucocorticoid receptors is another intriguing mode of function for these secondary metabolites. Glucocorticoids are a class of important steroidal hormones which mediate the regulation of a manifold of physiological processes. In mammals, they play the regulatory roles in development, metabolism, neurobiology and apoptosis [158]. Due to the characteristic and similarity of structure of saponin aglycones and steroids, therefore, several pharmacological properties of saponins including anti-inflammatory, immunosuppressive and neuroprotective effects [159] as well as stimulation of adipogenesis [160] or apoptosis, are associated to interact with receptors of glucocorticoid hormones [150].
Several published data on the structure-activity relationships stressed that there is no correlation between the ability to cause hemolysis with other known activities of saponins such as their cytotoxic properties and anti-fungal or their applicability as adjuvants [150]. In short, the formation of a complex between glycoside and membranes sterols followed by the creation of single ion channels and more large pores are fundamental for hemolytic properties of sea cucumber glycosides. Table 2. summarizes the mode of action of acetylated triterpene glycosides from sea cucumbers on the membrane transporters.

Table 2.
Potential mechanisms of function of acetylated triterpene glycosides from sea cucumbers on cell membranes.
13. Chemical Nomenclature
This review revealed that ten acetylated triterpene glycoside compounds with identical chemical structures identified from different species of sea cucumbers have been given different names by different researchers. We have indicated the compounds having identifiable synonyms (the same structure, different names) in brackets in their chemical structures; Figure 2 to Figure 7 and Table 1. For instance, Pervicoside A 91 isolated from Holothuria pervicax (family Holothuriidae, order Aspidochirotida) [59] has an identical structure with Neothyoside A 91 reported in Neothyone gibbosa (family Sclerodactilidae, order Dendrochirotida) [122]. Another example, Violaceuside A 39 [100], had been described previously by another group from the sea cucumber Pentacta quadrangularis and called Philinopside A 39 [95]. These identifiable synonyms hampered the chemical identification and structure elucidation of compounds as well as distribution by genus.
In addition, Honey-Escandón et al. [123] reported thirteen non-acetylated triterpene glycosides having identifiable synonyms. A serious problem of homonyms (same name, different structures) was identified for the Nobiliside compounds [41,162]. The structures of two different compounds, Nobilisides B and C 66 sensu Zhang et al. [162] are identical to Nobilisides B and C 66 sensu Wu et al. [163]. Wu’s group also reported Nobilisides 1a and 2a, the latter consisting of the same structure as desulfated Holothurin A which is also highlighted by Caulier, et al. [164]. Later, Zhang et al. [165] ascribed the structure of two compounds, Nobilisides I (roman number one instead of capital i) and II, having the same formula as previously defined compounds, Holothurin A and Ananaside C, respectively [123].
Cucumariosides A4 85 and H4 23 were described to possess a very rare ethoxy moiety at C-25 of the lateral chain of their aglycone, and as the authors mentioned they are highly likely to be artefacts, formed during prolonged storage of the ethanolic extract or over extraction procedure [139], in addition to Cucumarioside H2 22 which is also artefact generated by ethanol/water extraction. The presence of butoxy and ethoxy groups in the side chain of Cucumariosides A3 84 and A4 85, respectively, is an unusual feature. These glycosides are very likely to be artificial products generated during the process of glycoside purification using EtOH for extraction and BuOH as a foam quencher during extract evaporation [115].
Wang et al. [144] reported the molecular formula for Stichloroside C1 103 from Apostichopus japonicus; however, the proposed structure of this compound is not compatible with the elemental composition and molecular formula and it hindered the identification of this compound.
14. Conclusions
The marine environment is a rich source of natural compounds with promising therapeutic applications. This review has focused on acetylated triterpene glycosides and attempted to summarize the current knowledge on acetylated triterpene glycosides from different species of sea cucumbers. Sea cucumbers are a rich source of drugs, functional food and traditional folk medicine, especially in some parts of Asia. Sea cucumbers have been used as a tonic and medicinal food for more than 1000 years in Southern Asian countries; as an ancient Chinese medical text highlighted unspecified elements in sea cucumber can improve/repair the human immune system, relieve chronic fatigue syndrome and relieve stress and mental exhaustion.
These days, the interest in natural products has increased for cosmetics or medicine and agriculture application due to their properties, which can boost the action of active pesticides or chemotherapeutics or even reverse multidrug resistance, at least partially, of adapted and resistant cells. The combined applications of these substances with a cytotoxic or antimicrobial agent may converse resistance in a synergistic manner.
A large number of sea cucumber triterpene glycosides demonstrate noticeable anticancer properties at their sub-cytotoxic doses which might indicate mechanisms independent of their cytotoxicity pathways. Preliminary findings revealed that saponins provide their anticancer activities through a number of mechanisms including arresting cell cycles, induction of apoptosis, blocking of migration/metastasis and invasion of tumor cells, and interfering with angiogenesis via receptor tyrosine kinases. However, the detailed mechanisms of the anticancer properties of these secondary metabolites still remain unclear and not understand fully.
Despite extensive studies on triterpene glycosides in plants, our knowledge about the modes of action of marine triterpene glycosides on membrane transporters and the structure-activity relationship is limited. Therefore, a comprehensive study about the mechanisms of action of these secondary metabolites should be carried out to evaluate their potential as novel remedies for treatment of different diseases. Several triterpene glycosides have shown to possess anticancer activity by stimulating apoptosis of cancerous cells, while the detailed mechanisms controlling the anti-tumor property of the compounds have not been fully described. Although some preliminary data indict differential structural configuration leads to different biological activities, understanding the structural features determining the biological properties of holothurian triterpene glycosides and their structure-activity relationship is important for emerging marine drugs.
Acknowledgments
We would like to express our sincerest thanks to the Australian SeaFood CRC for financially supporting this project and the Iranian Ministry of Health and Medical Education, and Kermanshah University of Medical Sciences for their scholarship to Y.B. The authors gratefully acknowledge the assistance provided by Elham Kakaei. No funds are available for covering the costs to publish in open access.
Author Contributions
Y.B. and C.M.M.F. contributed to writing of this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kiew, P.L.; Don, M.M. Jewel of the seabed: Sea cucumbers as nutritional and drug candidates. Int. J. Food Sci. Nutr. 2012, 63, 616–636. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Mohammed, A.; Zhang, Y.; Choi, C.-I.; Woodward, C.; Collin, P.; Steele, V.E.; Rao, C.V. Chemopreventive effects of Frondanol A5, a Cucumaria frondosa extract, against rat colon carcinogenesis and inhibition of human colon cancer cell growth. Cancer Prev. Res. Phila. 2010, 3, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Yuan, W.; Gong, W.; Tang, H.; Liu, B.; Krohn, K.; Li, L.; Yi, Y.; Zhang, W. Antifungal nortriterpene and triterpene glycosides from the sea cucumber Apostichopus japonicus Selenka. Food Chem. 2012, 132, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.W.; Lovatelli, A.; Vasconcellos, M.; Ye, Y. Managing Sea Cucumber Fisheries with an Ecosystem Approach; FAO Fisheries and Aquaculture Technical Paper No 520; FAO: Rome, Italy, 2010. [Google Scholar]
- Kim, C.G.; Kwak, J.-Y. Anti-cancer effects of triterpene glycosides, frondoside A and cucumarioside A2-2 isolated from sea cucumbers. In Handbook of Anticancer Drugs from Marine Origin; Kim, S.-K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 673–682. [Google Scholar]
- Zhao, Q.; Xue, Y.; Liu, Z.D.; Li, H.; Wang, J.F.; Li, Z.J.; Wang, Y.M.; Dong, P.; Xue, C.H. Differential effects of sulfated triterpene glycosides, holothurin A1, and 24-dehydroechinoside A, on antimetastasic activity via regulation of the MMP-9 signal pathway. J. Food Sci. 2010, 75, H280–H288. [Google Scholar] [CrossRef] [PubMed]
- Al Marzouqi, N.; Iratni, R.; Nemmar, A.; Arafat, K.; Ahmed Al Sultan, M.; Yasin, J.; Collin, P.; Mester, J.; Adrian, T.E.; Attoub, S. Frondoside A inhibits human breast cancer cell survival, migration, invasion and the growth of breast tumor xenografts. Eur. J. Pharmacol. 2011, 668, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kundu, N.; Collin, P.D.; Goloubeva, O.; Fulton, A.M. Frondoside A inhibits breast cancer metastasis and antagonizes prostaglandin E receptors EP4 and EP2. Breast Cancer Res. Treat. 2012, 132, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Attoub, S.; Arafat, K.; Gélaude, A.; Al Sultan, M.A.; Bracke, M.; Collin, P.; Takahashi, T.; Adrian, T.E.; de Wever, O. Frondoside A suppressive effects on lung cancer survival, tumor growth, angiogenesis, invasion, and metastasis. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Koy, C.; Dmitrenok, P.S.; Muller-Hilke, B.; Koczan, D.; Arbogast, B.; Silchenko, A.A.; Kalinin, V.I.; Avilov, S.A.; Stonik, V.A.; et al. Immunomodulatory effects of holothurian triterpene glycosides on mammalian splenocytes determined by mass spectrometric proteome analysis. J. Proteom. 2009, 72, 886–906. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, I.A.; Kalinin, V.I.; Gorshkov, B.A.; Stonik, V.A. Two different modes of inhibition of the rat brain Na+,K+-ATPase by triterpene glycosides, psolusosides A and B from the holothurian Psolus fabricii. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1999, 122, 101–108. [Google Scholar] [CrossRef]
- Jin, J.O.; Shastina, V.V.; Shin, S.W.; Xu, Q.; Park, J.I.; Rasskazov, V.A.; Avilov, S.A.; Fedorov, S.N.; Stonik, V.A.; Kwak, J.Y. Differential effects of triterpene glycosides, frondoside A and cucumarioside A2-2 isolated from sea cucumbers on caspase activation and apoptosis of human leukemia cells. FEBS Lett. 2009, 583, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Roginsky, A.B.; Ding, X.Z.; Woodward, C.; Collin, P.; Newman, R.A.; Bell, R.H., Jr.; Adrian, T.E. Review of the apoptosis pathways in pancreatic cancer and the anti-apoptotic effects of the novel sea cucumber compound, frondoside A. Ann. N. Y. Acad. Sci. 2008, 1138, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Park, E.S.; Shin, S.W.; Na, Y.W.; Han, J.Y.; Jeong, J.S.; Shastina, V.V.; Stonik, V.A.; Park, J.I.; Kwak, J.Y. Stichoposide C induces apoptosis through the generation of ceramide in leukemia and colorectal cancer cells and shows in vivo antitumor activity. Clin. Cancer Res. 2012, 18, 5934–5948. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Smirnov, A.V. Sea cucumbers triterpene glycosides, the recent progress in structural elucidation and chemotaxonomy. Phytochem. Rev. 2005, 4, 221–236. [Google Scholar] [CrossRef]
- Moraes, G.; Norhcote, P.C.; Kalinin, V.I.; Avilov, S.A.; Silchenko, A.S.; Dmitrenok, P.S.; Stonik, V.A.; Levin, V.S. Structure of the major triterpene glycoside from the sea cucumber Stichopus mollis and evidence to reclassify this species into the new genus Australostichopus. Biochem. Syst. Ecol. 2004, 32, 637–650. [Google Scholar] [CrossRef]
- Antonov, A.S.; Avilov, S.A.; Kalinovsky, A.I.; Anastyuk, S.D.; Dmitrenok, P.S.; Kalinin, V.I.; Taboada, S.; Bosh, A.; Avila, C.; Stonik, V.A. Triterpene glycosides from Antarctic sea cucumbers. 2. Structure of Achlioniceosides A1, A2, and A3 from the sea cucumber Achlionice violaecuspidata (=Rhipidothuria racowitzai). J. Nat. Prod. 2009, 72, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Buckingham, J.; Munro, M.H.G. Taxonomy and marine natural products research. In Handbook of Marine Natural Products; Fattorusso, E., Gerwick, W.H., Taglialatela-Scafati, O., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 3–54. [Google Scholar]
- Bahrami, Y.; Zhang, W.; Franco, C. Discovery of novel saponins from the viscera of the sea cucumber Holothuria lessoni. Mar. Drugs 2014, 12, 2633–2667. [Google Scholar] [CrossRef] [PubMed]
- Chapagain, B.P.; Wiesman, Z. Metabolite profiling of saponins in Balanites aegyptiaca plant tissues using LC (RI)-ESI/MS and MALDI-TOF/MS. Metabolomics 2008, 4, 357–366. [Google Scholar] [CrossRef]
- Kerr, R.G.; Chen, Z. In vivo and in vitro biosynthesis of saponins in sea cucumbers. J. Nat. Prod. 1995, 58, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Marston, A. Saponins; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Chaieb, I. Saponins as Insecticides: A Review. Tunis. J. Plant Prot. 2010, 5, 39–50. [Google Scholar]
- Kjellin, M.; Johansson, I. (Eds.) Surfactants from Renewable Resources; Wiley: Stockholm, Sweden, 2010.
- Güçlü-Üstünda, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.H.; Thanh, N.V.; Kiem, P.V.; Huong, L.M.; Minh, C.V.; Kim, Y.H. Two new triterpene glycosides from the Vietnamese sea cucumber Holothuria scabra. Arch. Pharm. Res. 2007, 30, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Himaya, S.W. Triterpene glycosides from sea cucumbers and their biological activities. Adv. Food Nutr. Res. 2012, 65, 297–319. [Google Scholar] [PubMed]
- Van Dyck, S.; Gerbaux, P.; Flammang, P. Qualitative and quantitative saponin contents in five sea cucumbers from the Indian Ocean. Mar. Drugs 2010, 8, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Chludil, H.D.; Muniain, C.C.; Seldes, A.M.; Maier, M.S. Cytotoxic and antifungal triterpene glycosides from the Patagonian sea cucumber Hemoiedema spectabilis. J. Nat. Prod. 2002, 65, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhang, W.; Yi, Y.H.; Liu, B.S.; Pan, M.X.; Wang, X.H. A novel sulfated holostane glycoside from sea cucumber Holothuria leucospilota. Chem. Biodivers. 2010, 7, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-L.; Li, L.; Yi, Y.-H.; Sun, P. Philinopsides E and F, two new sulfated triterpene glycosides from the sea cucumber Pentacta quadrangularis. Nat. Prod. Res. 2006, 20, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, Y.; Zhang, W.; Chataway, T.; Franco, C. Structural elucidation of novel saponins in the sea cucumber Holothuria lessoni. Mar. Drugs 2014, 12, 4439–4473. [Google Scholar] [CrossRef] [PubMed]
- Avilov, S.A.; Antonov, A.S.; Silchenko, A.S.; Kalinin, V.I.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A.; Riguera, R.; Jimenez, C. Triterpene glycosides from the far eastern sea cucumber Cucumaria conicospermium. J. Nat. Prod. 2003, 66, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Togawa, K.; Higuchi, R.; Komori, T.; Sasaki, T. Structures of four new triterpenoid oligoglycosides: DS-penaustrosides A, B, C, and D from the sea cucumber Pentacta australis. J. Nat. Prod. 1992, 55, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Avilov, S.A.; Kalinovskii, A.I.; Stonik, V.A. New triterpene glycoside from the holothurian Neothyonidium magnum. Chem. Nat. Compd. 1990, 26, 42–45. [Google Scholar] [CrossRef]
- Zurita, M.B.; Ahond, A.; Poupat, C.; Potier, P.; Menou, J.L. Invertébrés marins du lagon néo-calédonien, VII. Étude structurale d’un nouveau saponoside sulfaté extrait de l’holothurie, Neothyonidium magnum. J. Nat. Prod. 1986, 49, 809–813. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Kalinovsky, A.I.; Stonik, V.A. Structure of psolusoside A the major triterpene glycoside from holothurian Psolus fabricii. Chem. Nat. Compd. 1985, 21, 197–202. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Kalinovskii, A.I.; Stonik, V.A.; Dmitrenok, P.S.; Elkin, Y.N. Structure of psolusoside-B, triterpenoid glycoside from Psolus holothurians. Khim. Prir. Soedin. 1989, 3, 361–368. [Google Scholar]
- Silchenko, A.S.; Avilov, S.A.; Kalinovsky, A.I.; Dmitrenok, P.S.; Kalinin, V.I.; Morre, J.; Deinzer, M.L.; Woodward, C.; Collin, P.D. Glycosides from the North Atlantic sea cucumber Cucumaria frondosa V-Structures of five new minor trisulfated triterpene oligoglycosides, frondosides A7-1, A7-2, A7-3, A7-4, and isofrondoside C. Can. J. Chem. 2007, 85, 626–636. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjashchenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Stonik, V.A. 3β-O-Glycosylated 16β-acetoxy-9β-H-lanosta-7,24-diene-3β,18,20β-triol, an intermediate metabolite from the sea cucumber Eupentacta fraudatrix and its biosynthetic significance. Biochem. Syst. Ecol. 2012, 44, 53–60. [Google Scholar] [CrossRef]
- Wu, J.; Yi, Y.H.; Tang, H.F.; Wu, H.M.; Zou, Z.R.; Lin, H.W. Nobilisides A–C, three new triterpene glycosides from the sea cucumber Holothuria nobilis. Planta Med. 2006, 72, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, O.A.; Avilov, S.S.; Kalinovskii, A.I.; Stonik, V.A. A new acetylated glycoside from the holothurian Cucumaria japonica. Chem. Nat. Compd. 1992, 28, 518–519. [Google Scholar] [CrossRef]
- Bahrami, Y.; Franco, M.M.C. Structure elucidation of new acetylated saponins, Lessoniosides A, B, C, D, and E, and non-acetylated saponins, Lessoniosides F and G, from the viscera of the sea cucumber Holothuria lessoni. Mar. Drugs 2015, 13, 597–617. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, Y. Discovery of Novel Saponins as Potential Future Drugs from Sea Cucumber Viscera. Ph.D. Thesis, Flinders University, Adelaide, Australia, 2015. [Google Scholar]
- Demeyer, M.; De Winter, J.; Caulier, G.; Eeckhaut, I.; Flammang, P.; Gerbaux, P. Molecular diversity and body distribution of saponins in the sea star Asterias rubens by mass spectrometry. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 168, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjashchenko, P.V.; Fedorov, S.N.; Dmitrenok, P.S.; Yurchenko, E.A.; Kalinin, V.I.; Rogacheva, A.V.; Gebruk, A.V. Kolgaosides A and B, two new triterpene glycosides from the Arctic deep water sea cucumber Kolga hyalina (Elasipodida: Elpidiidae). Nat. Prod. Commun. 2014, 9, 1259–1264. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Dolmatov, I.Y.; Kalinin, V.I.; Stonik, V.A. Structure and biological action of Cladolosides B1, B2, C, C1, C2 and D, six new triterpene glycosides from the sea cucumber Cladolabes schmeltzii. Nat. Prod. Commun. 2013, 8, 1527–1534. [Google Scholar] [PubMed]
- Kumar, R.; Chaturvedi, A.K.; Shukla, P.K.; Lakshmi, V. Antifungal activity in triterpene glycosides from the sea cucumber Actinopyga lecanora. Bioorg. Med. Chem. Lett. 2007, 17, 4387–4391. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.S.; Yi, Y.H.; Li, L.; Zhang, S.L.; Han, H.; Weng, Y.Y.; Pan, M.X. Arguside A: A new cytotoxic triterpene glycoside from the sea cucumber Bohadschia argus Jaeger. Chem. Biodivers. 2007, 4, 2845–2851. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.S.; Yi, Y.H.; Li, L.; Sun, P.; Han, H.; Sun, G.Q.; Wang, X.H.; Wang, Z.L. Argusides D and E, two new cytotoxic triterpene glycosides from the sea cucumber Bohadschia argus Jaeger. Chem. Biodivers. 2008, 5, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Elbandy, M.; Rho, J.; Afifi, R. Analysis of saponins as bioactive zoochemicals from the marine functional food sea cucumber Bohadschia cousteaui. Eur. Food Res. Technol. 2014, 238, 937–955. [Google Scholar] [CrossRef]
- Yuan, W.H.; Yi, Y.H.; Tang, H.F.; Liu, B.S.; Wang, Z.L.; Sun, G.Q.; Zhang, W.; Li, L.; Sun, P. Antifungal triterpene glycosides from the sea cucumber Bohadschia marmorata. Planta Med. 2009, 75, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.H.; Yi, Y.H.; Li, L.; Liu, B.S.; Zhang, H.W.; Sun, P. Two triterpene glycosides from the sea cucumber Bohadschia marmorata Jaeger. Chin. Chem. Lett. 2008, 19, 457–460. [Google Scholar] [CrossRef]
- Yuan, W.H.; Yi, Y.H.; Tan, R.X.; Wang, Z.L.; Sun, G.Q.; Xue, M.; Zhang, H.W.; Tang, H.F. Antifungal triterpene glycosides from the sea cucumber Holothuria (Microthele) axiloga. Planta Med. 2009, 75, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Castro, R.; Riguera, R. Holothurinosides: New antitumour non sulphated triterpenoid glycosides from the sea cucumber Holothuria forskalii. Tetrahedron 1991, 47, 4753–4762. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Yi, Y.-H.; Tang, H.-F. Bioactive triterpene glycosides from the sea cucumber Holothuria fuscocinerea. J. Nat. Prod. 2006, 69, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yi, Y.H.; Tang, H.F.; Wu, H.M.; Zhou, Z.R. Hillasides A and B, two new cytotoxic triterpene glycosides from the sea cucumber Holothuria hilla Lesson. J. Asian Nat. Prod. Res. 2007, 9, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Chludil, H.D.; Murray, A.P.; Seldes, A.M.; Maier, M.S. Biologically active triterpene glycosides from sea cucumbers (Holothuroidea, Echinodermata). In Bioactive Natural Products; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 28, pp. 587–615. [Google Scholar]
- Kitagawa, I.; Kobayashi, M.; Son, B.W.; Suzuki, S.; Kyogoku, Y. Marine natural products. XIX: Pervicosides A, B, and C, lanostane-type triterpene-oligoglycoside sulfates from the sea cucumber Holothuria pervicax. Chem. Pharm. Bull. 1989, 37, 1230–1234. [Google Scholar] [CrossRef]
- Stonik, V.A.; Kalinin, V.I.; Avilov, S.A. Toxins from sea cucumbers (holothuroids): Chemical structures, properties, taxonomic distribution, biosynthesis and evolution. J. Nat. Toxins 1999, 8, 235–248. [Google Scholar] [PubMed]
- Elyakov, G.B.; Kuznetsova, T.A.; Stonik, V.A.; Levin, V.S.; Albores, R. Glycosides of marine invertebrates. IV. A comparative study of the glycosides from Cuban sublittoral holothurians. Comp. Biochem. Physiol. B Comp. Biochem. 1975, 52, 413–417. [Google Scholar] [CrossRef]
- Stonik, V.A.; Mal’tsev, I.I.; Kalinovskii, A.I.; Conde, C.; Elyakov, G.B. Glycosides of marine invertebrates. XI. Two new triterpene glycosides from holothurians of the family Stichopadidae. Chem. Nat. Compd. 1982, 18, 177–182. [Google Scholar] [CrossRef]
- Kitagawa, I.; Kobayashi, M.; Imamoto, T.; Yasuzawa, T.; Kyogoku, Y. The structures of six antifungal oligoglycosides, stichlorosides A1, A2, B1, B2, C1 and C2, from the sea cucumber Stichopus chloronotus Brandt. Chem. Pharm. Bull. 1981, 29, 2387–2391. [Google Scholar] [CrossRef]
- Maltsev, I.I.; Stonik, V.A.; Kalinovsky, A.I. Stichoposide-E—A new triterpene glycoside from holoturian stichopodeidae family. Chem. Nat. Compd. 1983, 19, 308–312. [Google Scholar]
- Kobayashi, M.; Hori, M.; Kan, K.; Yasuzawa, T.; Matsui, M.; Suzuki, S.; Kitagawa, I. Marine natural products. XXVII: Distribution of lanostane-type triterpene oligoglycosides in ten kinds of Okinawan Sea cucumbers. Chem. Pharm. Bull. 1991, 39, 2282–2287. [Google Scholar] [CrossRef]
- Stonik, V.A.; Mal’tsev, I.I.; Kalinovskii, A.I.; Elyakov, G.B. Glycosides of marine invertebrates. XII. Structure of a new triterpene oligoglycoside from holothurians of family Stichopodidae. Chem. Nat. Compd. 1982, 18, 182–186. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Anisimov, M.M.; Prokofieva, N.G.; Avilov, S.A.; Afiyatullov, S.S.; Stonik, V.A. Biological activities and biological role of triterpene glycosides from holothuroids (Echinodermata). Echinoderm Stud. 1996, 5, 139–181. [Google Scholar]
- Kelecom, A.; Daloze, D.; Tursch, B. Chemical studies of marine invertebrates—XX: The structures of the genuine aglycones of thelothurins A and B, defensive saponins of the Indo-pacific sea cucumber Thelonota ananas Jaeger (Echinodermata). Tetrahedron 1976, 32, 2313–2319. [Google Scholar] [CrossRef]
- Kelecom, A.; Daloze, D.; Tursch, B. Chemical studies of marine invertebrates—XXI: Six triterpene genins artifacts from thelothurins A and B, toxic saponins of the sea cucumber Thelonota ananas Jaeger (echinodermata). Biosynthesis of the thelothurins. Tetrahedron 1976, 32, 2353–2359. [Google Scholar] [CrossRef]
- Kelecom, A.; Tursch, B.; Vanhaelen, M. Chemical studies of marine invertebrates XIX. Glycosidic chain structure of thelothurins A and B, two new saponins from the Indo-pacific sea cucumber Thelonota ananas Jaeger (Echinodermata). Bull. Soc. Chim. Belg. 1976, 85, 277–292. [Google Scholar] [CrossRef]
- Stonik, V.A.; Mal’tsev, I.I.; Elyakov, G.B. The structure of thelenotosides A and B from the holothurian Thelenota ananas. Chem. Nat. Compd. 1982, 18, 590–593. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Avilov, S.A.; Antonov, A.A.; Kalinin, V.I.; Kalinovsky, A.I.; Smirnov, A.V.; Riguera, R.; Jimenez, C. Triterpene glycosides from the deep-water north-pacific sea cucumber Synallactes nozawai Mitsukuri. J. Nat. Prod. 2002, 65, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I.; Jayasandhya, P.; Rajan, G.C.; Padmakumar, K.P. Structures and biological activities of Typicosides A1, A2, B1, C1 and C2, triterpene glycosides from the sea cucumber Actinocucumis typica. Nat. Prod. Commun. 2013, 8, 301–310. [Google Scholar] [PubMed]
- Rodriguez, J.; Riguera, R. Lefevreiosides: Four new triterpene glycosides from the sea cucumber Cucumaria lefevrei. J. Chem. Res. 1989, 11, 342–343. [Google Scholar]
- Yayli, N.; Findlay, J.A. A triterpenoid saponin from Cucumaria frondosa. Phytochemistry 1999, 50, 135–138. [Google Scholar] [CrossRef]
- Girard, M.; Bélanger, J.; ApSimon, J.W.; Garneau, F.X.; Harvey, C.; Brisson, J.R. Frondoside A. A novel triterpene glycoside from the holothurian Cucumaria frondosa. Can. J. Chem. 1990, 68, 11–18. [Google Scholar] [CrossRef]
- Avilov, S.A.; Kalinin, V.I.; Drozdova, O.A.; Kalinovsky, A.I. Triterpene glycosides from the holothurian Cucumaria frondosa. Chem. Nat. Compd. 1993, 29, 216–218. [Google Scholar] [CrossRef]
- Yayli, N. Minor saponins from the sea cucumber Cucumaria frondosa. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 2001, 40, 399–404. [Google Scholar]
- Avilov, S.A.; Drozdova, O.A.; Kalinin, V.I.; Kalinovsky, A.I.; Stonik, V.A.; Gudimova, E.N.; Riguera, R.; Jimenez, C. Frondoside C, a new nonholostane triterpene glycoside from the sea cucumber Cucumaria frondosa: Structure and cytotoxicity of its desulfated derivative. Can. J. Chem. 1998, 76, 137–141. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Avilov, S.A.; Antonov, A.S.; Kalinovsky, A.I.; Dmitrenok, P.S.; Kalinin, V.I.; Woodward, C.; Collin, P.D. Glycosides from the sea cucumber Cucumaria frondosa. IV. Structure of frondosides A2-4, A2-7, and A2-8, three new minor monosulfated triterpene glycosides. Can. J. Chem. Rev. Can. Chim. 2005, 83, 2120–2126. [Google Scholar] [CrossRef]
- Avilov, S.A.; Kalinin, V.I.; Smirnov, A.V. Use of triterpene glycosides for resolving taxonomic problems in the sea cucumber genus Cucumaria (Holothurioidea, Echinodermata). Biochem. Syst. Ecol. 2004, 32, 715–733. [Google Scholar] [CrossRef]
- Drozdova, O.A.; Avilov, S.A.; Kalinovsky, A.I.; Stonik, V.A. A minor glycoside from the sea cucumber Cucumaria japonica. Khim. Prir. Soedin. 1992, 5, 593. [Google Scholar]
- Drozdova, O.A.; Avilov, S.A.; Kalinovskii, A.I.; Stonik, V.A.; Mil’grom, Y.M.; Rashkes, Y.V. New glycosides from the holothurian Cucumaria japonica. Chem. Nat. Compd. 1993, 29, 200–205. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I.; Kalinovsky, A.I.; Dmitrenok, P.S.; Fedorov, S.N.; Stepanov, V.G.; Dong, Z.; Stonik, V.A. Constituents of the sea cucumber Cucumaria okhotensis. Structures of okhotosides B1-B3 and cytotoxic activities of some glycosides from this species. J. Nat. Prod. 2008, 71, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Pislyagin, E.A.; Menchinskaya, E.S.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Immunomodulatory and anticancer activity of sea cucumber triterpene glycosides. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 41, pp. 75–94. [Google Scholar]
- Aminin, D.L.; Silchenko, A.S.; Avilov, S.A.; Stepanov, V.G.; Kalinin, V.I. Immunomodulatory action of monosulfated triterpene glycosides from the sea cucumber Cucumaria okhotensis: Stimulation of activity of mouse peritoneal macrophages. Nat. Prod. Commun. 2010, 5, 1877–1880. [Google Scholar] [PubMed]
- Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I.; Stonik, V.A.; Kalinovskii, A.I.; Dmitrenok, P.S.; Stepanov, V.G. Monosulfated triterpene glycosides from Cucumaria okhotensis Levin et Stepanov, a new species of sea cucumbers from sea of Okhotsk. Russ. J. Bioorgan. Chem. 2007, 33, 81–90. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Kalinovskii, A.I.; Afiyatullov, S.S. Triterpene glycosides of the holothurian Eupentacta pseudoquinquisemita. Chem. Nat. Compd. 1988, 24, 187–189. [Google Scholar] [CrossRef]
- Zou, Z.; Yi, Y.; Wu, H.; Wu, J.; Liaw, C.-C.; Lee, K.-H. Intercedensides A−C, three new cytotoxic triterpene glycosides from the sea cucumber Mensamaria intercedens Lampert. J. Nat. Prod. 2003, 66, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.S. Biological activities of sulfated glycosides from echinoderms. In Bioactive Natural Products; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 35, pp. 311–354. [Google Scholar]
- Zou, Z.; Yi, Y.; Wu, H.; Yao, X.; Du, L.; Jiuhong, W.; Liaw, C.-C.; Lee, K.-H. Intercedensides D−I, cytotoxic triterpene glycosides from the sea cucumber Mensamaria intercedens Lampert. J. Nat. Prod. 2005, 68, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides from sea cucucmbers (Holothuroidea, Echinodermata). Biological activities and functions. In Bioactive Natural Products; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 35, pp. 135–196. [Google Scholar]
- Han, H.; Xu, Q.Z.; Tang, H.F.; Yi, Y.H.; Gong, W. Cytotoxic holostane-type triterpene glycosides from the sea cucumber Pentacta quadrangularis. Planta Med. 2010, 76, 1900–1904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-L.; Li, L.; Yi, Y.-H.; Zou, Z.-R.; Sun, P. Philinopgenin A, B, and C, three new triterpenoid aglycones from the sea cucumber Pentacta quadrangularis. Mar. Drugs 2004, 2, 185–191. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, X.; Tian, F.; Yi, Y.; Xu, Q.; Li, L.; Tong, L.; Lin, L.; Ding, J. Philinopside A, a novel marine-derived compound possessing dual anti-angiogenic and anti-tumor effects. Int. J. Cancer 2005, 114, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.H.; Xu, Q.Z.; Li, L.; Zhang, S.L.; Wu, H.M.; Ding, J.; Tong, Y.G.; Tan, W.F.; Li, M.H.; Tian, F.; et al. Philinopsides A and B, two new sulfated triterpene glycosides from the sea cucumber Pentacta quadrangularis. Helv. Chim. Acta 2006, 89, 54–63. [Google Scholar] [CrossRef]
- Han, H.; Xu, Q.Z.; Yi, Y.H.; Gong, W.; Jiao, B.H. Two new cytotoxic disulfated holostane glycosides from the sea cucumber Pentacta quadrangularis. Chem. Biodivers. 2010, 7, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Tang, H.-F.; Yi, Y.-H. Cytotoxic triterpene glycosides from the sea cucumber Pseudocolochirus violaceus. Fitoterapia 2007, 78, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Yurchenko, E.A.; Dautov, S.S. Structures of Violaceusosides C, D, E and G, sulfated triterpene glycosides from the sea cucumber Pseudocolochirus violaceus (Cucumariidae, Dendrochirotida). Nat. Prod. Commun. 2014, 9, 391–399. [Google Scholar] [PubMed]
- Zhang, S.-Y.; Yi, Y.-H.; Tang, H.-F.; Li, L.; Sun, P.; Wu, J. Two new bioactive triterpene glycosides from the sea cucumber Pseudocolochirus violaceus. J. Asian Nat. Prod. Res. 2006, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.S.; Roccatagliata, A.J.; Kuriss, A.; Chludil, H.; Seldes, A.M.; Pujol, C.A.; Damonte, E.B. Two new cytotoxic and virucidal trisulfated triterpene glycosides from the Antarctic sea cucumber Staurocucumis liouvillei. J. Nat. Prod. 2001, 64, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Antonov, A.S.; Avilov, S.A.; Kalinovsky, A.I.; Dmitrenok, P.S.; Kalinin, V.I.; Taboada, S.; Ballesteros, M.; Avila, C. Triterpene glycosides from Antarctic sea cucumbers III. Structures of liouvillosides A4 and A5, two minor disulphated tetraosides containing 3-O-methylquinovose as terminal monosaccharide units from the sea cucumber Staurocucumis liouvillei (Vaney). Nat. Prod. Res. 2011, 25, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Antonov, A.S.; Avilov, S.A.; Kalinovsky, A.I.; Anastyuk, S.D.; Dmitrenok, P.S.; Evtushenko, E.V.; Kalinin, V.I.; Smirnov, A.V.; Taboada, S.; Ballesteros, M.; et al. Triterpene glycosides from antarctic sea cucumbers. 1. Structure of liouvillosides A1, A2, A3, B1, and B2 from the sea cucumber Staurocucumis liouvillei: New procedure for separation of highly polar glycoside fractions and taxonomic revision. J. Nat. Prod. 2008, 71, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Yurchenko, E.A.; Dolmatov, I.Y. Colochirosides B1, B2, B3 and C, novel sulfated triterpene glycosides from the sea cucumber Colochirus robustus (Cucumariidae, Dendrochirotida). Nat. Prod. Commun. 2015, 10, 1687–1694. [Google Scholar] [PubMed]
- Avilov, S.A.; Kalinovskii, A.I.; Stonik, V.A. Two new triterpene glycosides from the holothurian Duasmodactyla kurilensis. Chem. Nat. Compd. 1991, 27, 188–192. [Google Scholar] [CrossRef]
- Avilov, S.A.; Antonov, A.S.; Drozdova, O.A.; Kalinin, V.I.; Kalinovsky, A.I.; Riguera, R.; Lenis, L.A.; Jimenez, C. Triterpene glycosides from the far Eastern sea cucumber Pentamera calcigera II: Disulfated glycosides. J. Nat. Prod. 2000, 63, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, I.; Rinehart, K.L. Thyonosides A and B, two new saponins isolated from the holothurian Thyone aurea. Tetrahedron 2004, 60, 2987–2992. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Avilov, S.A.; Kalinina, E.Y.; Korolkova, O.G.; Kalinovsky, A.I.; Stonik, V.A.; Riguera, R.; Jimenez, C. Structure of eximisoside A, a novel triterpene glycoside from the Far-Eastern sea cucumber Psolus eximius. J. Nat. Prod. 1997, 60, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Stonik, V.A.; Kapustina, I.I.; Boguslavsky, V.M.; Dmitrenoik, A.S.; Kalinin, V.I.; Cordeiro, M.L.; Djerassi, C. Biosynthetic studies of marine lipids. 42. Biosynthesis of steroid and triterpenoid metabolites in the sea cucumber Eupentacta fraudatrix. Steroids 1993, 58, 508–517. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Kalinovsky, A.I.; Stonik, V.A. Structure of cucumariosides C1 and C2—Two new triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Chem. Nat. Compd. 1987, 23, 691–696. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Avilov, S.A.; Kalinovskii, A.I.; Stonik, V.A.; Mil’grom, Y.M.; Rashkes, Y.V. Cucumarioside G4—A new triterpenglycoside from the holothurian Eupentacta fraudatrix. Chem. Nat. Compd. 1992, 28, 600–603. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Kalinin, V.I. Structure of cucumariosides H5, H6, H7 and H8, triterpene glycosides from the sea cucumber Eupentacta fraudatrix and unprecedented aglycone with 16,22-epoxy-group. Nat. Prod. Commun. 2011, 6, 1075–1082. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological activity of Cucumariosides B1 and B2, two new minor non-sulfated unprecedented triosides. Nat. Prod. Commun. 2012, 7, 1157–1162. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of cucumariosides I1, I3, I4, three new minor disulfated pentaosides. Nat. Prod. Commun. 2013, 8, 1053–1058. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of Cucumariosides A1, A3, A4, A5, A6, A12 and A15, seven new minor non-sulfated tetraosides and unprecedented 25-keto, 27-norholostane aglycone. Nat. Prod. Commun. 2012, 7, 517–525. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and cytotoxic action of Cucumariosides A2, A7, A9, A10, A11, A13 and A14, seven new minor non-sulfated tetraosides and an aglycone with an uncommon 18-hydroxy group. Nat. Prod. Commun. 2012, 7, 845–852. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Menchinskaya, E.S.; Aminin, D.L.; Kalinin, V.I. Structure of cucumarioside I2 from the sea cucumber Eupentacta fraudatrix (Djakonov et Baranova) and cytotoxic and immunostimulatory activities of this saponin and relative compounds. Nat. Prod. Res. 2013, 27, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Popov, R.S.; Avilov, S.A.; Silchenko, A.S.; Kalinovsky, A.I.; Dmitrenok, P.S.; Grebnev, B.B.; Ivanchina, N.V.; Kalinin, V.I. Cucumariosides F1 and F2, two new triterpene glycosides from the sea cucumber Eupentacta fraudatrix and their LC-ESI MS/MS identification in the starfish Patiria pectinifera, a predator of the sea cucumber. Biochem. Syst. Ecol. 2014, 57, 191–197. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Dolmatov, I.Y.; Savchenko, A.M.; Kalinin, V.I. Triterpene glycosides from the sea cucumber cladolabes schmeltzii. II. Structure and biological action of cladolosides A1–A6. Nat. Prod. Commun. 2014, 9, 1421–1428. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Dolmatov, I.Y.; Kalinin, V.I. Structures and biological activities of cladolosides C3, E1, E2, F1, F2, G, H1 and H2, eight triterpene glycosides from the sea cucumber Cladolabes schmeltzii with one known and four new carbohydrate chains. Carbohydr. Res. 2015, 414, 22–31. [Google Scholar] [CrossRef]
- Encarnación, D.R.; Murillo, J.I.; Nielsen, J.; Christophersen, C. Neothyoside B, a triterpenoid diglycoside from the Pacific sea cucumber Neothyone gibbosa. Acta Chem. Scand. 1996, 50, 848–849. [Google Scholar] [CrossRef]
- Encarnacion, R.; Carrasco, G.; Espinoza, M.; Anthoni, U.; Nielsen, P.H.; Christophersen, C. Neothyoside A, proposed structure of a triterpenoid tetraglycoside from the Pacific sea-cucumber Neothyone gibbosa. J. Nat. Prod. 1989, 52, 248–251. [Google Scholar] [CrossRef]
- Honey-Escandón, M.; Arreguín-Espinosa, R.; Solís-Marín, F.A.; Samyn, Y. Biological and taxonomic perspective of triterpenoid glycosides of sea cucumbers of the family Holothuriidae (Echinodermata, Holothuroidea). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2015, 180, 16–39. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Agafonova, I.G.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Collin, P.D.; Woodward, C. Immunomodulatory properties of frondoside A, a major triterpene glycoside from the North Atlantic commercially harvested sea cucumber Cucumaria frondosa. J. Med. Food 2008, 11, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Agafonova, I.G.; Berdyshev, E.V.; Isachenko, E.G.; Avilov, S.A.; Stonik, V.A. Immunomodulatory properties of cucumariosides from the edible far-eastern holothurian Cucumaria japonica. J. Med. Food 2001, 4, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I. Bioactive marine natural products. Yakugaku Zasshi 1988, 108, 398–416. [Google Scholar] [PubMed]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef] [PubMed]
- Mujoo, K.; Haridas, V.; Hoffmann, J.J.; Wächter, G.A.; Hutter, L.K.; Lu, Y.; Blake, M.E.; Jayatilake, G.S.; Bailey, D.; Mills, G.B.; et al. Triterpenoid saponins from Acacia victoriae (Bentham) decrease tumor cell proliferation and induce Apoptosis. Cancer Res. 2001, 61, 5486–5490. [Google Scholar] [PubMed]
- Kuroda, M.; Mimaki, Y.; Hasegawa, F.; Yokosuka, A.; Sashida, Y.; Sakagami, H. Steroidal glycosides from the bulbs of Camassia leichtlinii and their cytotoxic activities. Chem. Pharm. Bull. 2001, 49, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Mimaki, Y.; Yokosuka, A.; Kuroda, M.; Sashida, Y. Cytotoxic activities and structure-cytotoxic relationships of steroidal saponins. Biol. Pharm. Bull. 2001, 24, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Avilov, S.A.; Tishchenko, L.Y.; Stonik, V.A. Structure of cucumarioside A2–2A triterpene glycoside from the holothurian Cucumaria japonica. Chem. Nat. Compd. 1984, 20, 759–760. [Google Scholar] [CrossRef]
- Park, J.-I.; Bae, H.-R.; Kim, C.G.; Stonik, V.A.; Kwak, J.-Y. Relationships between chemical structures and functions of triterpene glycosides isolated from sea cucumbers. Front. Chem. 2014, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.H.; Kim, Y.; Lee, S.J. Frondoside A has an anti-invasive effect by inhibiting TPA-induced MMP-9 activation via NF-κB and AP-1 signaling in human breast cancer cells. Int. J. Oncol. 2012, 41, 933–940. [Google Scholar] [PubMed]
- Miyamoto, T.; Togawa, K.; Higuchi, R.; Komori, T.; Sasaki, T. Constituents of holothuroidea, II. Six newly identified biologically active triterpenoid glycoside sulfates from the sea cucumber Cucumaria echinata. Liebigs Ann. Chem. 1990, 1990, 453–460. [Google Scholar] [CrossRef]
- Taha, T.A.; Mullen, T.D.; Obeid, L.M. A house divided: Ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim. Biophys. Acta 2006, 1758, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Park, E.S.; Shin, S.W.; Ju, M.H.; Han, J.Y.; Jeong, J.S.; Kim, S.H.; Stonik, V.A.; Kwak, J.Y.; Park, J.I. By activating Fas/ceramide synthase 6/p38 kinase in lipid rafts, Stichoposide D inhibits growth of leukemia xenografts. Oncotarget 2015, 6, 27596–27612. [Google Scholar] [CrossRef] [PubMed]
- Careaga, V.P.; Maier, M.S. Cytotoxic triterpene glycosides from sea cucumbers. In Handbook of Anticancer Drugs from Marine Origin; Kim, S.-K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 515–528. [Google Scholar]
- Wang, J.; Han, H.; Chen, X.; Yi, Y.; Sun, H. Cytotoxic and apoptosis-inducing activity of triterpene glycosides from Holothuria scabra and Cucumaria frondosa against HepG2 Cells. Mar. Drugs 2014, 12, 4274–4290. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Kalinin, V.I. Structures and cytotoxic properties of cucumariosides H2, H3 and H4 from the sea cucumber Eupentacta fraudatrix. Nat. Prod. Res. 2012, 26, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M.; et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 468, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.R.; Tang, H.F.; Lin, H.W.; Cheng, G.; Wang, S.W.; Zhang, X. Saponins: The potential chemotherapeutic agents in pursuing new anti-glioblastoma drugs. Mini Rev. Med. Chem. 2013, 13, 1709–1724. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ye, X.; Huang, H.; Peng, R.; Su, Z.; Lian, X.Y.; Zhang, Z. Bioactive sulfated saponins from sea cucumber Holothuria moebii. Planta Med. 2015, 81, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Kobayashi, M.; Inamoto, T.; Yasuzawa, T.; Kyogoku, Y.; Kido, M. Stichlorogenol and dehydrostichlorogenol, genuin aglycones of stichlorosides A1, A2, B1, B2, C1, and C2, from the sea cucumber Stichopus chloronotus (Brandt). Chem. Pharm. Bull. 1981, 29, 1189–1192. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, W.; Sun, G.; Tang, H.; Liu, B.; Li, L.; Yi, Y.; Zhang, W. New holostan-type triterpene glycosides from the sea cucumber Apostichopus japonicus. Nat. Prod. Commun. 2012, 7, 1431–1434. [Google Scholar] [PubMed]
- Wang, X.-H.; Zou, Z.-R.; Yi, Y.-H.; Han, H.; Li, L.; Pan, M.-X. Variegatusides: New non-sulphated triterpene glycosides from the sea cucumber Stichopus variegates Semper. Mar. Drugs 2014, 12, 2004–2018. [Google Scholar] [CrossRef] [PubMed]
- Avilov, S.A.; Silchenko, A.S.; Antonov, A.S.; Kalinin, V.I.; Kalinovsky, A.I.; Smirnov, A.V.; Dmitrenok, P.S.; Evtushenko, E.V.; Fedorov, S.N.; Savina, A.S.; et al. Synaptosides A and A1, triterpene glycosides from the sea cucumber Synapta maculata containing 3-O-methylglucuronic acid and their cytotoxic activity against tumor cells. J. Nat. Prod. 2008, 71, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Kobayashi, M.; Inamoto, T.; Fuchida, M.; Kyogoku, Y. Marine natural products. XIV. Structures of echinosides A and B, antifungal lanostane-oligosides from the sea cucumber Actinopyga echinites (Jaeger). Chem. Pharm. Bull. 1985, 33, 5214–5224. [Google Scholar] [CrossRef] [PubMed]
- Pislyagin, E.A.; Gladkikh, R.V.; Kapustina, I.I.; Kim, N.Y.; Shevchenko, V.P.; Nagaev, I.Y.; Avilov, S.A.; Aminin, D.L. Interaction of holothurian triterpene glycoside with biomembranes of mouse immune cells. Int. Immunopharmacol. 2012, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, M.M. Triterpene glycosides and the structural-functional properties of membranes. Nauchnye Doki. Vyss. Shkoly Biol. Nauki 1987, 10, 49–63. [Google Scholar] [PubMed]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, I.A.; Kalinovsky, A.I.; Ilyin, S.G.; Gorshkov, B.A.; Stonik, V.A. Physicochemical characteristics of interaction of toxic triterpene glycosides from holothurians with rat brain Na+-K+-ATPase. Toxicon 1989, 27, 937–945. [Google Scholar] [CrossRef]
- Popov, A.M.; Rovin Iu, G.; Anisimov, M.M.; Likhatskaia, G.N.; Strigina, L.I. Effect of triterpene glycosides on the stability of bilayer lipid membranes, containing different sterols. Biofizika 1982, 27, 827–831. [Google Scholar] [PubMed]
- Aminin, D.L.; Chaykina, E.L.; Agafonova, I.G.; Avilov, S.A.; Kalinin, V.I.; Stonik, V.A. Antitumor activity of the immunomodulatory lead Cumaside. Int. Immunopharmacol. 2010, 10, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.M. Comparative study of effects of various sterols and triterpenoids on permeability of model lipid membranes. J. Evol. Biochem. Physiol. 2003, 39, 314–320. [Google Scholar] [CrossRef]
- Menchinskaya, E.S.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S.; Andryjashchenko, P.V.; Kalinin, V.I.; Stonik, V.A. Inhibition of tumor cells multidrug resistance by cucumarioside A2-2, frondoside A and their complexes with cholesterol. Nat. Prod. Commun. 2013, 8, 1377–1380. [Google Scholar] [PubMed]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front. Microbiol. 2012, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mal’tsev, I.I.; Stekhova, S.I.; Shentsova, E.B.; Anisimov, M.M.; Stonik, V.A. Antimicrobial activity of glycosides from holothurians of the family Stichopodidae. Pharm. Chem. J. 1985, 19, 44–46. [Google Scholar] [CrossRef]
- Yudt, M.R.; Cidlowski, J.A. The glucocorticoid receptor: Coding a diversity of proteins and responses through a single gene. Mol. Endocrinol. 2002, 16, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-F.; Fan, X.-J.; Li, X.; Peng, L.-L.; Wang, G.-H.; Ke, K.-F.; Jiang, Z.-L. Ginsenoside Rg1 protects neurons from hypoxic–ischemic injury possibly by inhibiting Ca2+ influx through NMDA receptors and L-type voltage-dependent Ca2+ channels. Eur. J. Pharmacol. 2008, 586, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Yeh, C.-H.; Yeh, M.; Cheng, J.-T. Increase of adipogenesis by ginsenoside (Rh2) in 3T3-L1 cell via an activation of glucocorticoid receptor. Horm. Metab. Res. 2009, 41, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, I.A.; Gorshkov, B.A.; Stonik, V.A. Inhibition of rat brain Na+-K+-ATPase by triterpene glycosides from holothurians. Toxicon 1989, 27, 927–936. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dai, J.B.; Chen, L.L.; Ding, P.Y.; Wu, J. Screening of bioactive constituents from sea cucumber Holothuria nobilis using conidia of Pyricularia oryzae. Zhong Yao Cai 2008, 31, 1001–1003. [Google Scholar] [PubMed]
- Wu, J.; Yi, Y.; Zou, Z. Two new triterpene glycosides from sea cucumber Holothuria nobilis. Chin. Tradit. Herb. Drugs 2006, 37, 497. [Google Scholar]
- Caulier, G.; Van Dyck, S.; Gerbaux, P.; Eeckhaut, I.; Flammang, P. Review of Saponin Diversity in Sea Cucumbers Belonging to the Family Holothuriidae; SPC Beche-de-mer Information Bulletin #31; SPC: Noumea Cedex, New Caledonia, 2011; pp. 48–54. [Google Scholar]
- Zhang, J.J. Extraction, isolation, and structure elucidation of two new triterpene glycosides from sea cucumber Holothuria nobilis. Chin. Tradit. Herb. Drugs 2011, 42, 1467–1472. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).