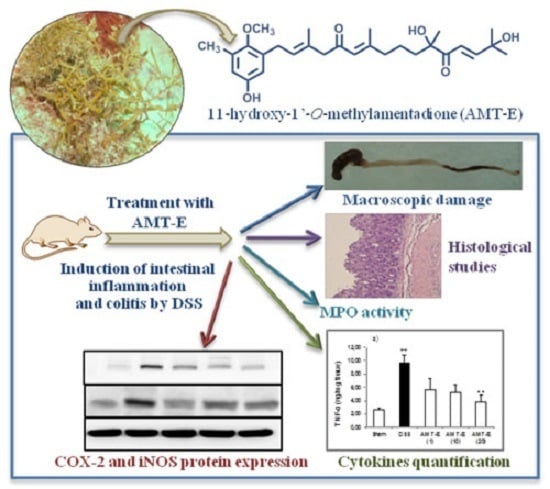
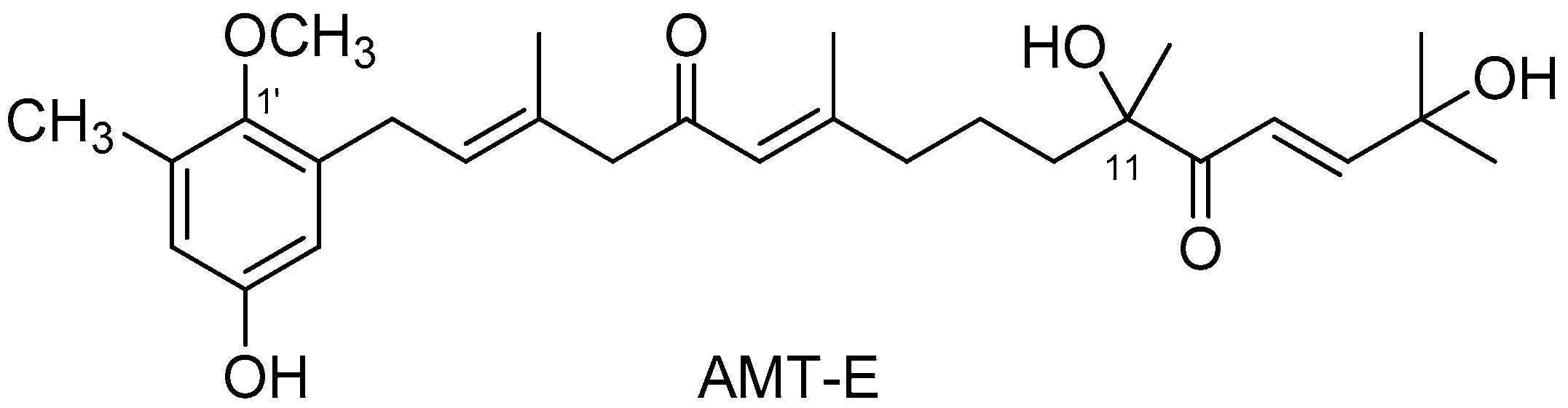
The Algal Meroterpene 11-Hydroxy-1′-O-Methylamentadione Ameloriates Dextran Sulfate Sodium-Induced Colitis in Mice
Abstract
:1. Introduction
2. Results
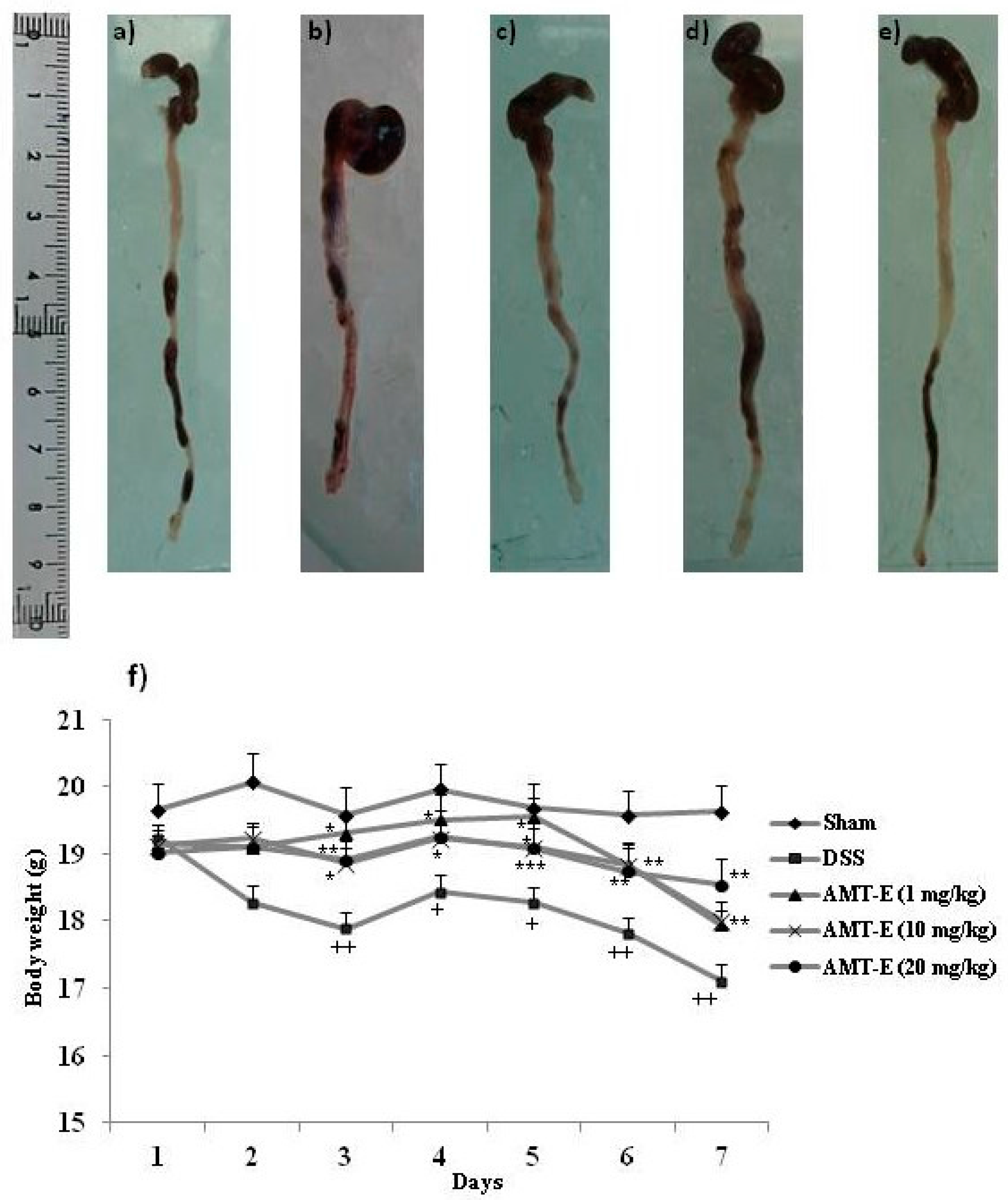
2.1. AMT-E Treatment Protects Mice against DSS-Induced Acute Colitis
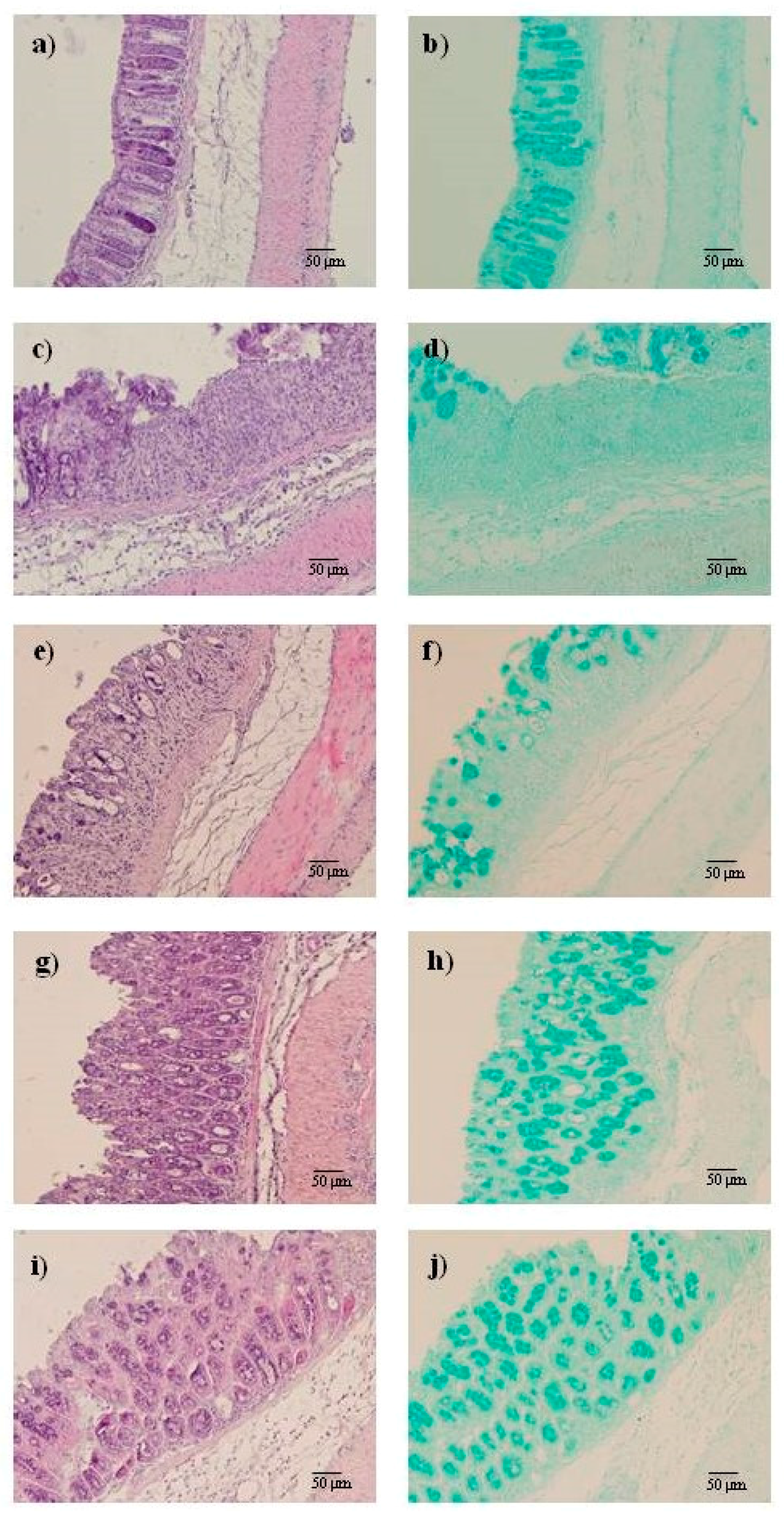
2.2. AMT-E Alleviates Microscopic Colon Damage and Increases Mucus Production
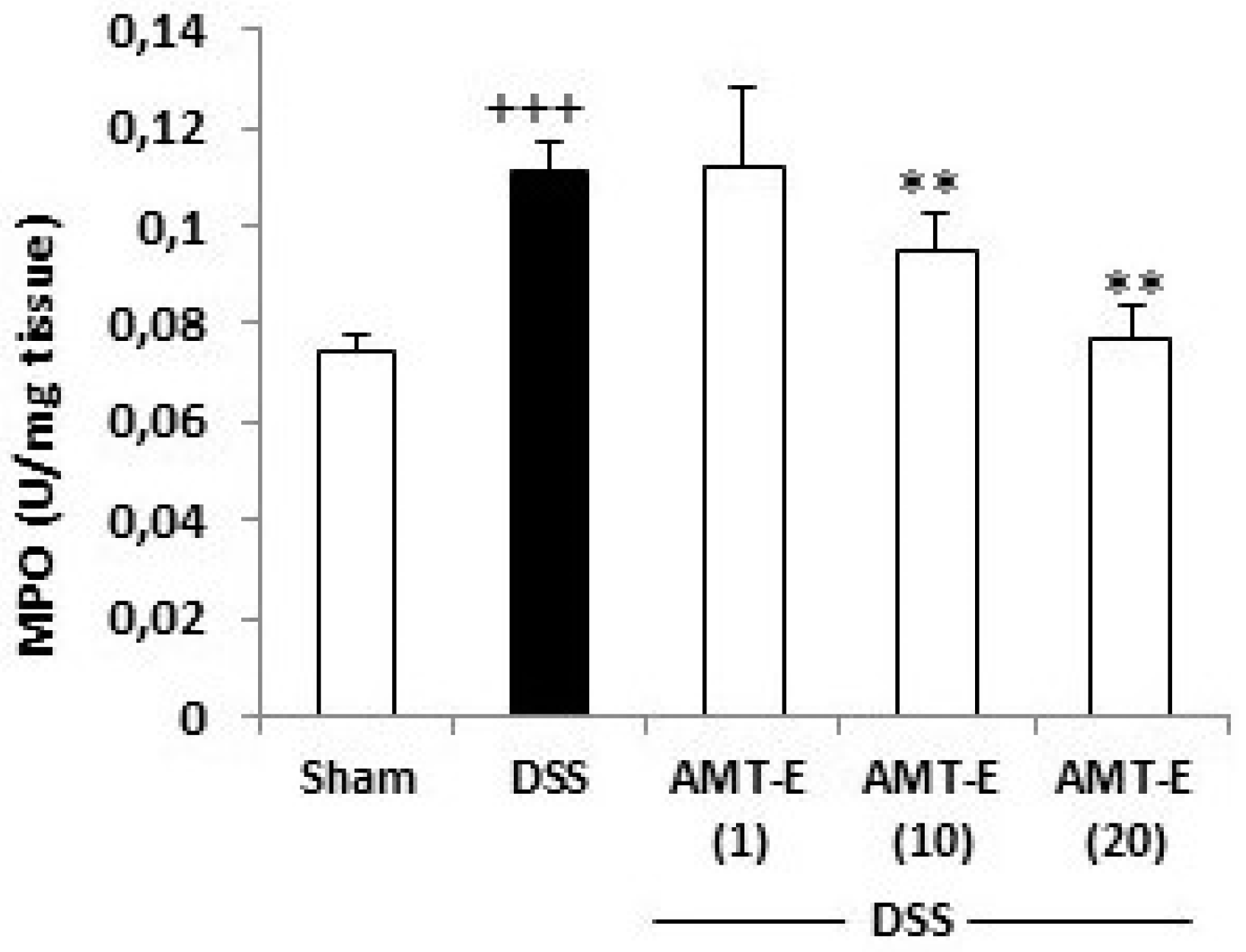
2.3. AMT-E Attenuates MPO Levels in DSS-Induced Colitis in Mice
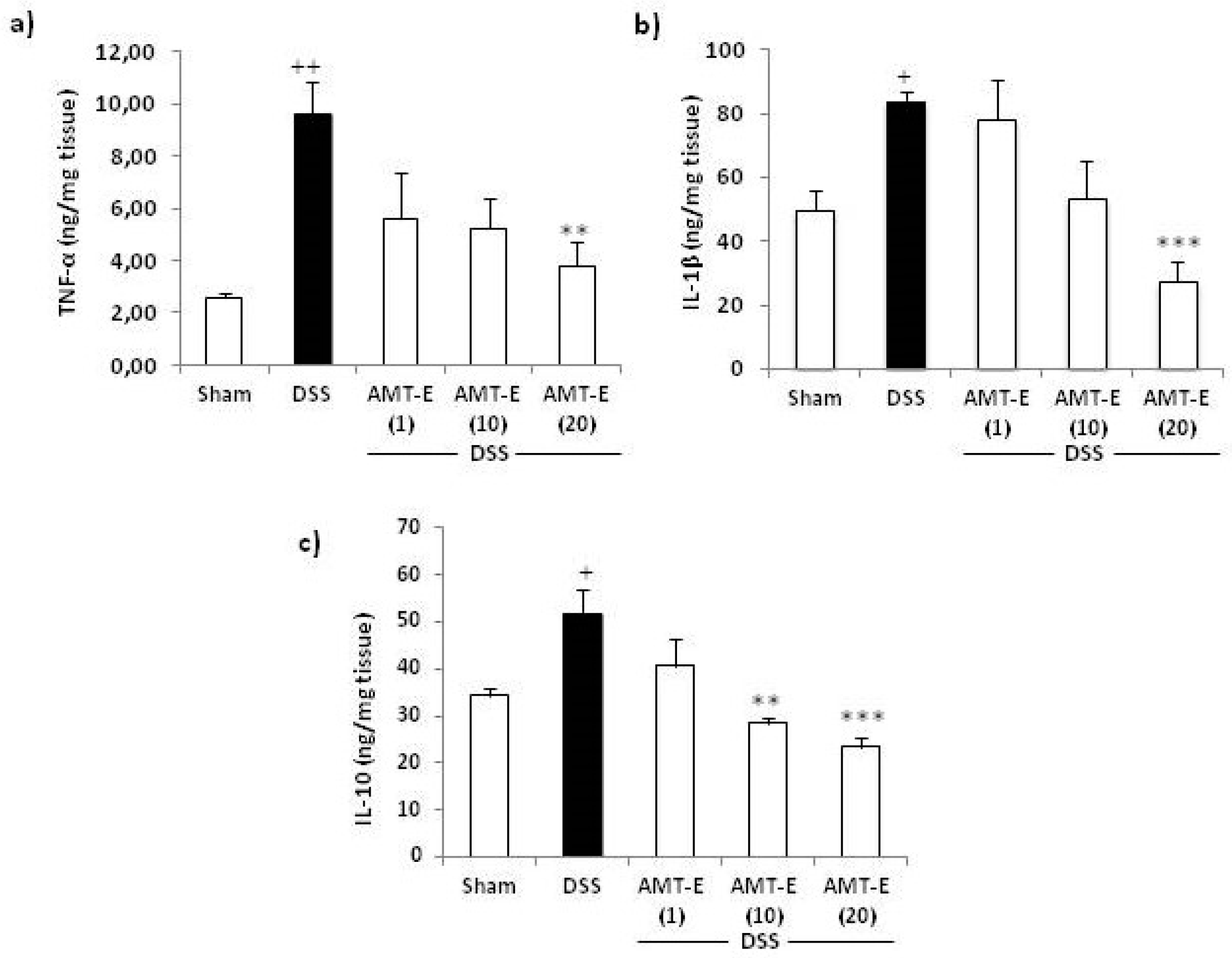
2.4. Effects of AMT-E on the Production of Cytokines in the Colon of DSS-Treated Mice
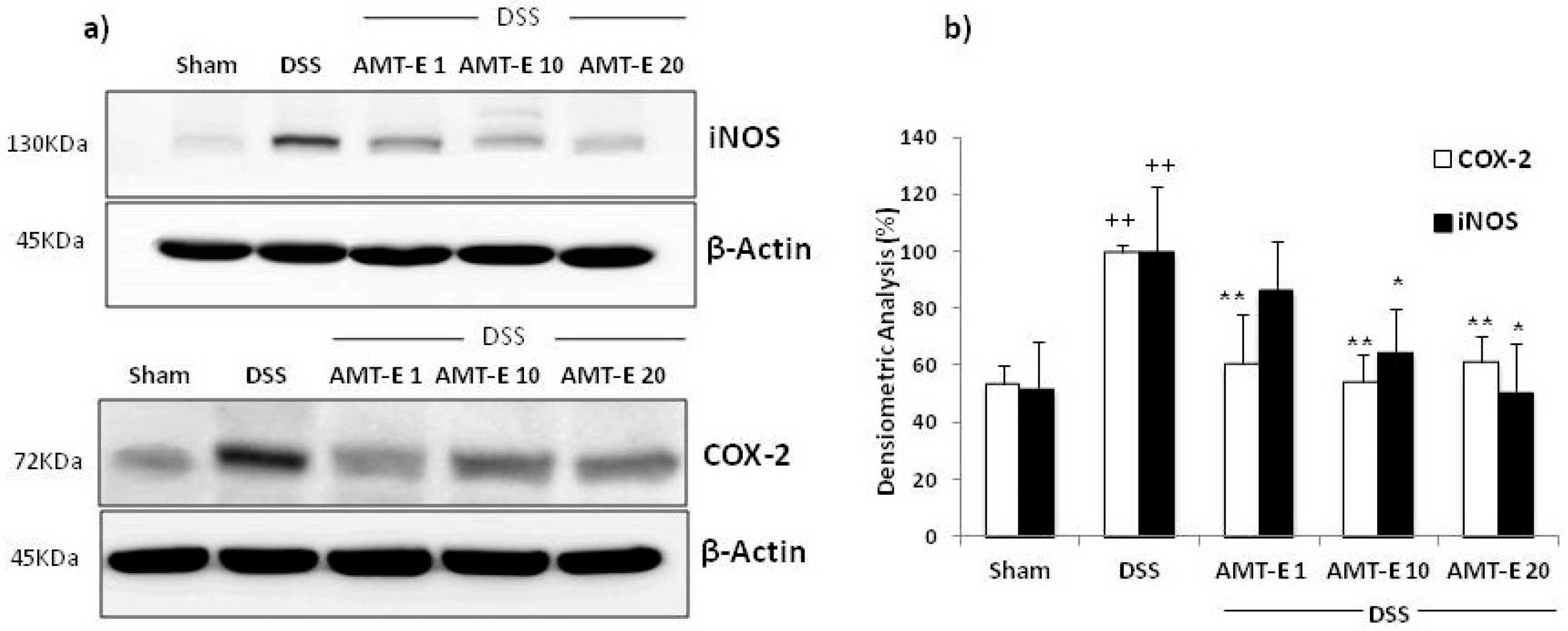
2.5. AMT-E Downregulates the Expression of COX-2 and iNOS in Colonic Mucosa
3. Discussion
4. Experimental Section
4.1. Experimental Animals
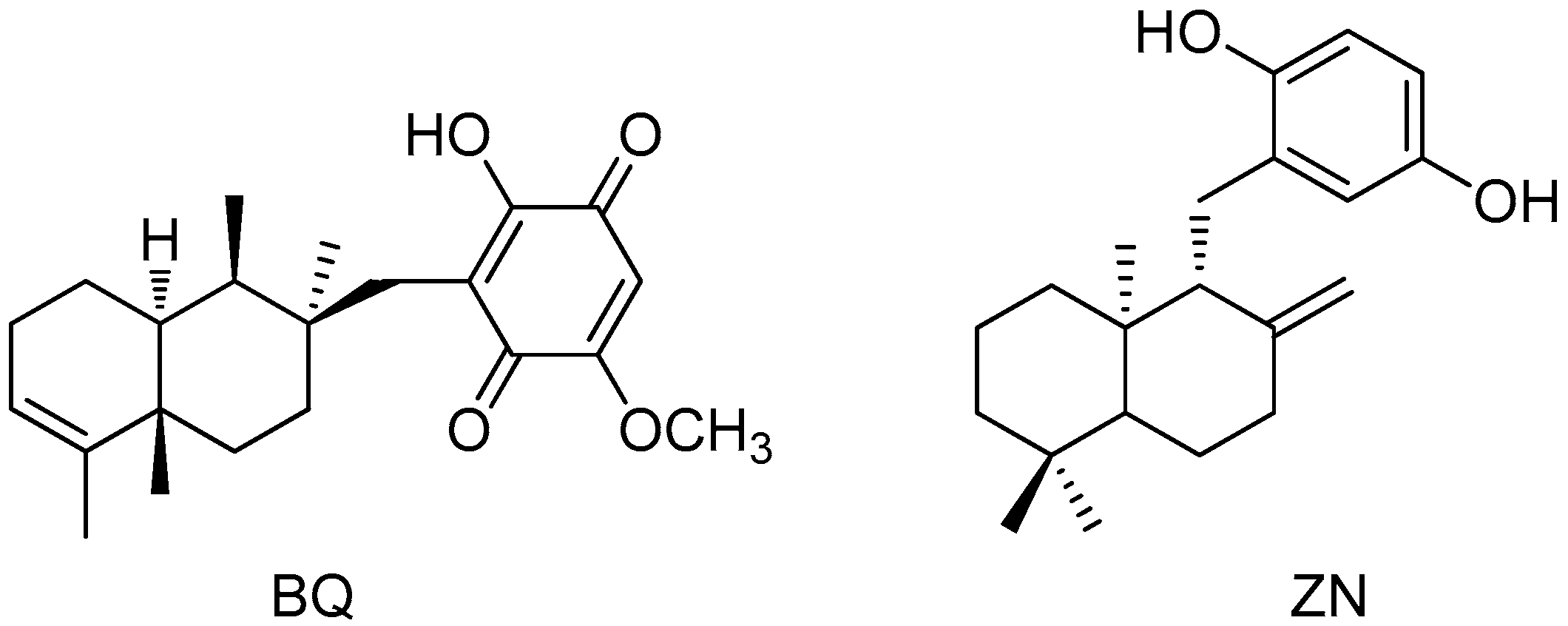
4.2. Isolation of 11-Hydroxy-1′-O-Methylamentadione (AMT-E)
4.3. Induction of DSS Colitis and Treatments
4.4. Histological Studies
4.5. Myeloperoxidase Activity Assay
4.6. Cytokines Assay
4.7. Extraction of Cytoplasmic Proteins and Western Blot Analysis
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mulder, D.J.; Noble, A.J.; Justinich, C.J.; Duffinc, J.M. A tale of two diseases: The history of inflammatory bowel disease. J. Crohns Colitis 2014, 8, 341–348. [Google Scholar] [PubMed]
- Loftus, E.V., Jr.; Sandborn, W.J. Epidemiology of inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2002, 31, 1–20. [Google Scholar] [CrossRef]
- Papadakis, K.A.; Targan, S.R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2000, 51, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Animal models of inflammatory bowel diseases: Illuminating the pathogenesis of colitis, ileitis and cancer. Dig. Dis. 2012, 30, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; Bolivar, S.; Ávila-Román, J.; Alcaide, A.; Fiorucci, S.; Motilva, V. Inhibition of chronic ulcerative colitis-associated adenocarcinoma development in mice by VSL#3. Inflamm. Bowel Dis. 2015, 21, 1027–1037. [Google Scholar] [PubMed]
- Yan, Y.; Kolachala, V.; Dalmasso, G.; Nguyen, H.; Laroui, H.; Sitaraman, S.V.; Merlin, D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS ONE 2009, 4, e6073. [Google Scholar] [CrossRef] [PubMed]
- Hussain, E.; Wang, L.-J.; Jiang, B.; Riaz, S.; Butt, G.Y.; Shi, D.-Y. A review of the components of brown seaweeds as potential candidates in cancer therapy. RSC Adv. 2016, 6, 12592–12610. [Google Scholar] [CrossRef]
- Ioannou, E.; Roussis, V. Natural Products from Seaweeds. In Plant-Derived Natural Products; Osburn, A.E., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 51–81. [Google Scholar]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Murphy, C.; Hotchkiss, S.; Worthington, J.; McKeown, S.R. The potential of seaweed as a source of drugs for use in cancer chemotherapy. J. Appl. Phycol. 2014, 26, 2211–2264. [Google Scholar] [CrossRef]
- Dang, H.T.; Lee, H.J.; Yoo, E.S.; Shinde, P.B.; Lee, Y.M.; Hong, J.; Kim, D.K.; Jung, J.H. Anti-inflammatory constituents of the red alga Gracilaria verrucosa and their synthetic analogues. J. Nat. Prod. 2008, 71, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Na, H.J.; Moon, P.D.; Ko, S.G.; Lee, H.J.; Jung, H.A.; Hong, S.H.; Seo, Y.; Oh, J.M.; Lee, B.H.; Choi, B.W.; et al. Sargassum hemiphyllum inhibits atopic allergic reaction via the regulation of inflammatory mediators. J. Pharmacol. Sci. 2005, 97, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Sansom, C.E.; Larsen, L.; Perry, N.B.; Berridge, M.V.; Chia, E.W.; Harper, J.L.; Webb, V.L. An antiproliferative bis-prenylated quinone from the New Zealand brown alga Perithalia capillaris. J. Nat. Prod. 2007, 70, 2042–2044. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.A.; Abatis, D.; Paulus, K.; Bauer, R.; Vagias, C.; Roussis, V. Marine polyprenylated hydroquinones, quinones, and chromenols with inhibitory effects on leukotriene formation. Chem. Biodivers. 2005, 2, 901–909. [Google Scholar] [CrossRef] [PubMed]
- De los Reyes, C.; Zbakh, H.; Motilva, V.; Zubía, E. Antioxidant and anti-inflammatory meroterpenoids from the brown alga Cystoseira usneoides. J. Nat. Prod. 2013, 76, 621–629. [Google Scholar] [CrossRef] [PubMed]
- De los Reyes, C.; Ortega, M.J.; Zbakh, H.; Motilva, V.; Zubía, E. Cystoseira usneoides: A brown alga rich in antioxidant and anti-inflammatory meroditerpenoids. J. Nat. Prod. 2016, 79, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Tseng, C.K.; Chang, C.F. Chinese seaweeds in herbal medicine. Hydrobiologia 1984, 116/117, 152–154. [Google Scholar]
- Ko, S.J.; Bu, Y.; Bae, J.; Bang, Y.M.; Kim, J.; Lee, H.; Beom-Joon, L.; Hyun, Y.H.; Park, J.W. Protective effect of Laminaria japonica with probiotics on murine colitis. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Ryu, B.; Park, J.W. Effects of Sargassum pallidum on 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice. J. Korean Orient. Intern. Med. 2010, 32, 224–241. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002, 20, 495–549. [Google Scholar] [CrossRef] [PubMed]
- Ferrándiz, M.L.; Sanz, M.J.; Bustos, G.; Payá, M.; Alcaraz, M.J.; De Rosa, S. Avarol and avarone, two new anti-inflammatory agents of marine origin. Eur. J. Pharmacol. 1994, 253, 75–82. [Google Scholar] [CrossRef]
- Gil, B.; Sanz, M.J.; Terencio, M.C.; De Giulio, A.; De Rosa, S.; Alacaraz, M.J.; Payá, M. Effects of marine 2-polyprenyl-1,4-hydroquinones on phospholipase A2 and some inflammatory responses. Eur. J. Pharmacol. 1995, 285, 281–288. [Google Scholar] [CrossRef]
- Terencio, M.C.; Ferrándiz, M.L.; Posadas, I.; Roig, E.; de Rosa, S.; De Giulio, A.; Payá, M.; Alcaraz, M.J. Suppression of leukotriene B4 and tumor necrosis factor α release in acute inflammatory responses by novel prenylated hydroquinone derivatives. Naunyn Schmiedebergs Arch. Pharmacol. 1998, 357, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Giannini, C.; D’Auria, M.V.; Payá, M. Modulatory effect of bolinaquinone, a marine sesquiterpenoid, on acute and chronic inflammatory processes. J. Pharmacol. Exp. Ther. 2003, 304, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Busserolles, J.; Payá, M.; D’Auria, M.V.; Gomez-Paloma, L.; Alcaraz, M.J. Protection against 2,4,6-trinitrobenzenesulphonic acid-induced colonic inflammation in mice by marine natural products bolinaquinone and petrosaspongiolide. Biochem. Pharmacol. 2005, 69, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Koyama, T.; Noguchi, H.; Ueda, Y.; Kitsuyama, R.; Shimizu, H.; Tanimoto, A.; Wang, K.Y.; Nawata, A.; Nakayama, T.; et al. Marine hydroquinone zonarol prevents inflammation and apoptosis in dextran sulfate sodium-induced mice ulcerative colitis. PLoS ONE 2014, 9, e113509. [Google Scholar] [CrossRef] [PubMed]
- Ermund, A.; Schutte, A.; Johansson, M.E.; Gustafsson, J.K.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Oehlers, S.H.; Flores, M.V.; Hall, C.J.; Crosier, K.E.; Crosier, F.S. Retinoic acid suppresses intestinal mucus production and exacerbates experimental enterocolitis. Dis. Model. Mech. 2012, 5, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Takagi, T.; Yoshikawa, T. Molecular fingerprints of neutrophil-dependent oxidative stress in inflammatory bowel disease. J. Gastroenterol. 2007, 42, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Treiner, E. Mucosal-associated invariant T cells in inflammatory bowel diseases: Bystanders, defenders, or offenders? Front. Immunol. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Correale, C.; Gasbarrini, A.; Danese, S. Mucosal biomarkers in inflammatory bowel disease: Key pathogenic players or disease predictors? World J. Gastroenterol. 2010, 16, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Chaparro, M. Use of a third anti-TNF after failure of two previous anti-TNFs in patients with inflammatory bowel disease: Is it worth it? Scand. J. Gastroenterol. 2015, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M.; Harrison, O.J.; Schiering, C.; Asquith, M.J.; Becher, B.; Powrie, F.; Maloy, K.J. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4+ Th17 cells. J. Exp. Med. 2012, 209, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Schmidt, C.; Stallmach, A. Depression and mucosal proinflammatory cytokines are associated in patients with ulcerative colitis and pouchitis—A pilot study. J. Crohns Colitis 2011, 5, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Kammermeier, J.; Elawad, M.; Glocker, E.O. Interleukin-10 and interleukin-10-receptor defects in inflammatory bowel disease. Curr. Allergy Asthma Rep. 2012, 12, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.; Lohler, J.; Rennick, D.; Rajewsky, K.; Muller, D. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Talero, E.; Alcaide, A.; Ávila-Román, J.; García-Mauriño, S.; Vendramini-Costa, D.; Motilva, V. Expression patterns of sirtuin 1-AMPK-autophagy pathway in chronic colitis and inflammation-associated colon neoplasia in IL-10-deficient mice. Int. Immunopharmacol. 2016, 35, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Barquero, L.; Villegas, I.; Sanchez-Calvo, J.M.; Talero, E.; Sanchez-Fidalgo, S.; Motilva, V.; Alarcón de la Lastra, C. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int. Immunopharmacol. 2007, 7, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; Sánchez-Fidalgo, S.; de la Lastra, C.A.; Illanes, M.; Calvo, J.R.; Motilva, V. Acute and chronic responses associated with adrenomedullin administration in experimental colitis. Peptides 2008, 29, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Grosso, M.; Petruzzelli, R.; Izzo, P.; Calì, G.; D’Armiento, F.P.; Rocco, A.; Nardone, G.; et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 2009, 21, 1209–e112. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.B.; Benoit, J.N.; Granger, D.N. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990, 186, 729–742. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
| Treatments (n = 12) | Sham a | DSS b | AMT-E c (1 mg/kg) | AMT-E c (10 mg/kg) | AMT-E c (20 mg/kg) |
|---|---|---|---|---|---|
| Colonic length (cm) | 7.93 ± 0.16 | 6.02 ± 0.12 +++ | 6.19 ± 0.10 | 7.00 ± 0.14 | 7.03 ± 0.16 ** |
| Treatments | Sham | DSS | AMT-E (1 mg/kg) | AMT-E (10 mg/kg) | AMT-E (20 mg/kg) |
|---|---|---|---|---|---|
| Colitis score | 0 | 3.93 ± 0.2 * | 2.34 ± 0.1 † | 2.12 ± 0.2 † | 1.82 ± 0.2 † |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zbakh, H.; Talero, E.; Avila, J.; Alcaide, A.; De los Reyes, C.; Zubía, E.; Motilva, V. The Algal Meroterpene 11-Hydroxy-1′-O-Methylamentadione Ameloriates Dextran Sulfate Sodium-Induced Colitis in Mice. Mar. Drugs 2016, 14, 149. https://doi.org/10.3390/md14080149
Zbakh H, Talero E, Avila J, Alcaide A, De los Reyes C, Zubía E, Motilva V. The Algal Meroterpene 11-Hydroxy-1′-O-Methylamentadione Ameloriates Dextran Sulfate Sodium-Induced Colitis in Mice. Marine Drugs. 2016; 14(8):149. https://doi.org/10.3390/md14080149
Chicago/Turabian StyleZbakh, Hanaa, Elena Talero, Javier Avila, Antonio Alcaide, Carolina De los Reyes, Eva Zubía, and Virginia Motilva. 2016. "The Algal Meroterpene 11-Hydroxy-1′-O-Methylamentadione Ameloriates Dextran Sulfate Sodium-Induced Colitis in Mice" Marine Drugs 14, no. 8: 149. https://doi.org/10.3390/md14080149
APA StyleZbakh, H., Talero, E., Avila, J., Alcaide, A., De los Reyes, C., Zubía, E., & Motilva, V. (2016). The Algal Meroterpene 11-Hydroxy-1′-O-Methylamentadione Ameloriates Dextran Sulfate Sodium-Induced Colitis in Mice. Marine Drugs, 14(8), 149. https://doi.org/10.3390/md14080149