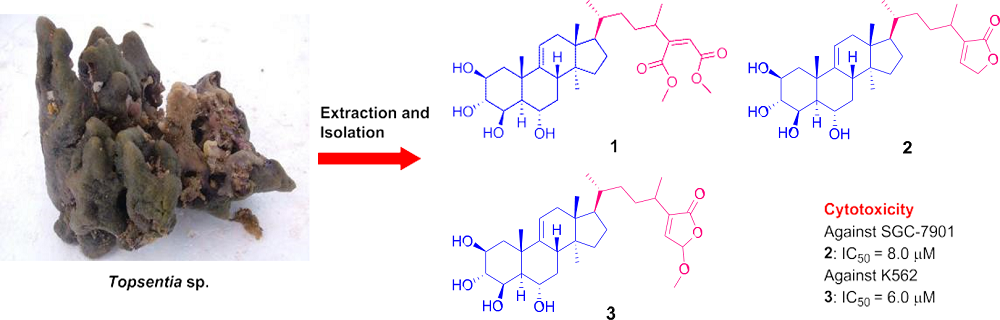
Topsensterols A–C, Cytotoxic Polyhydroxylated Sterol Derivatives from a Marine Sponge Topsentia sp.
Abstract
:1. Introduction
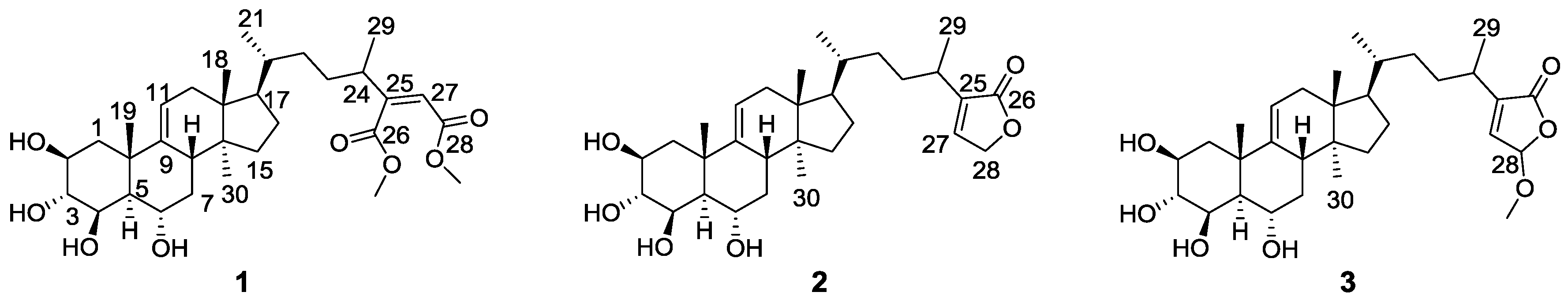
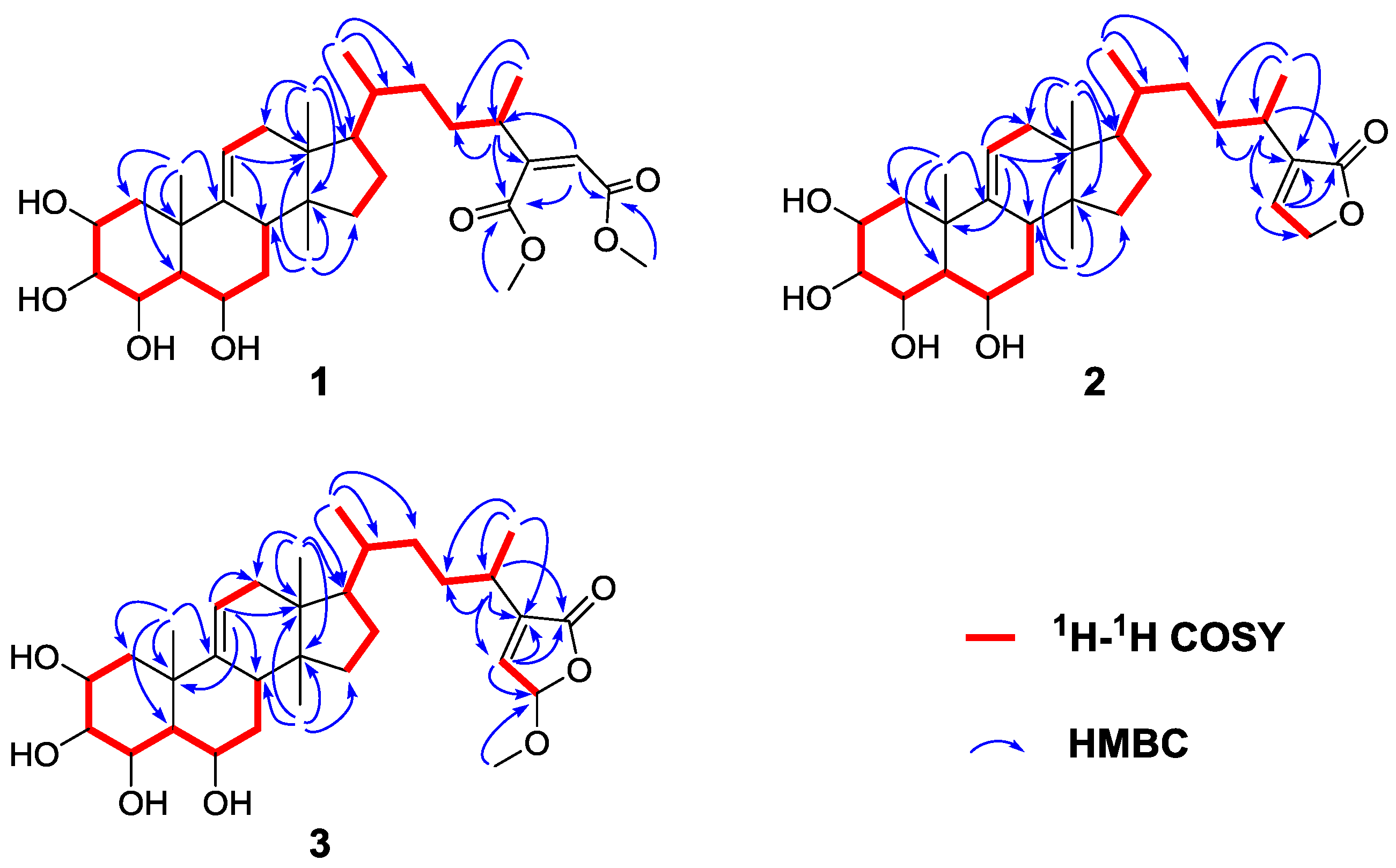
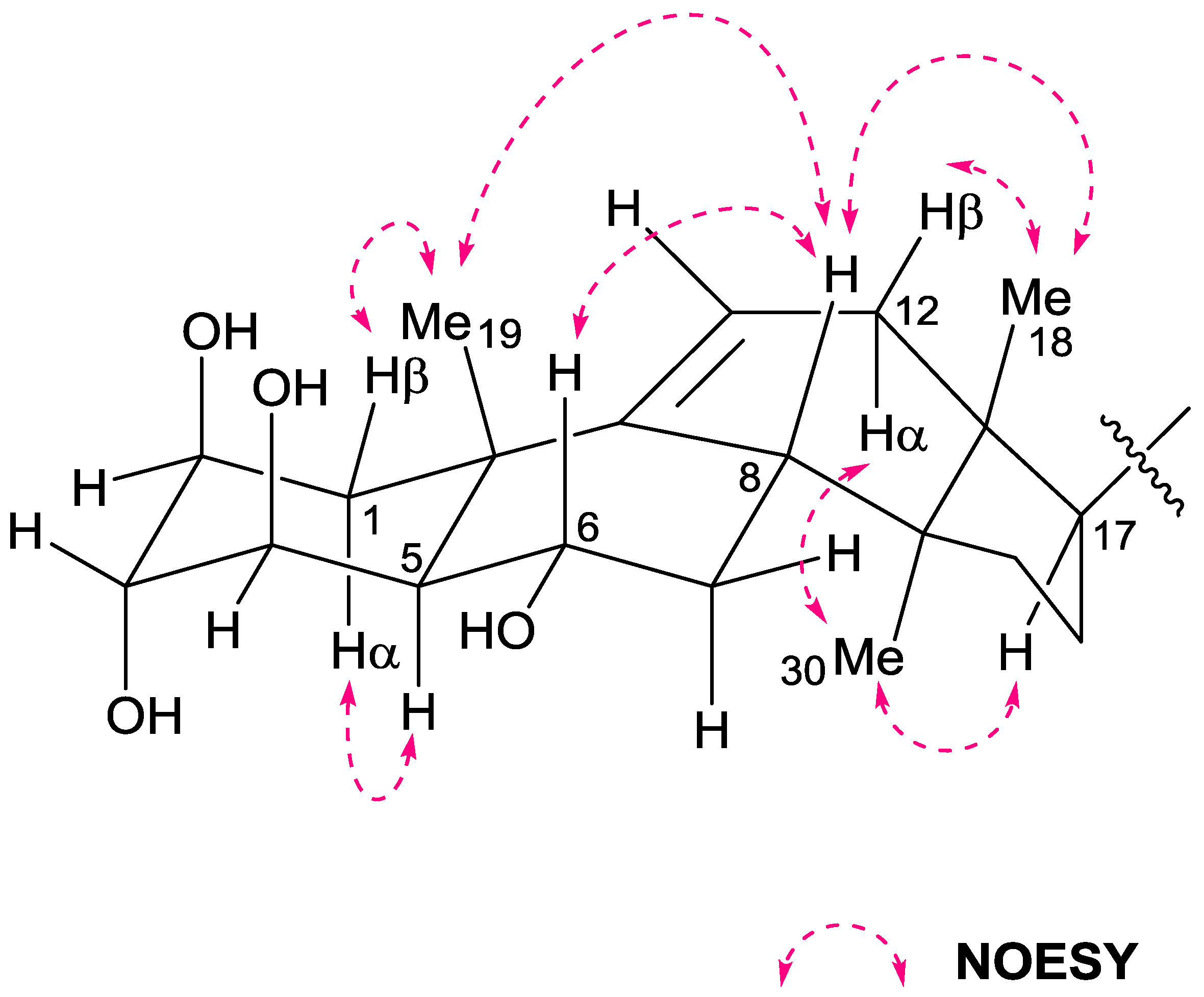
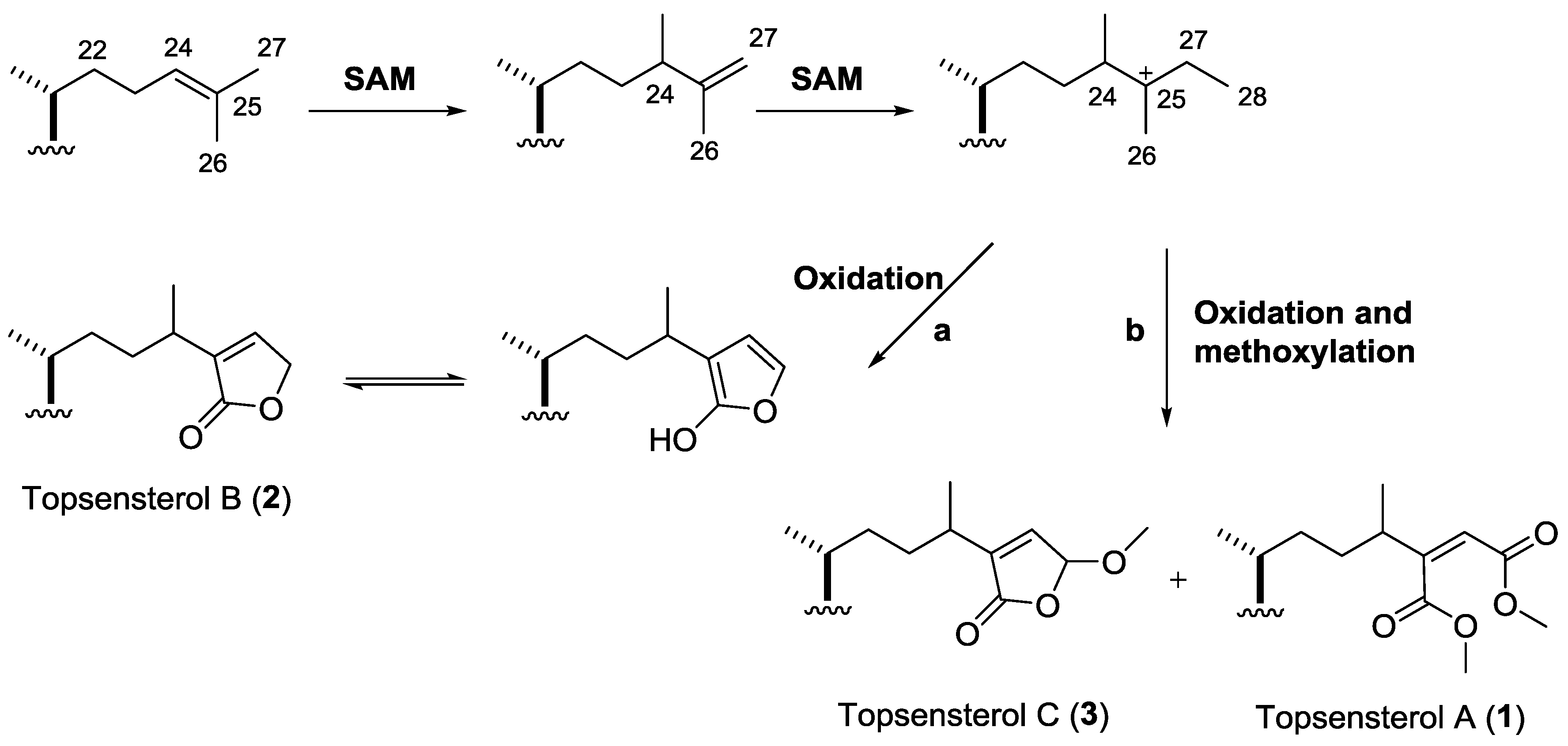
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, C.S.; Muller, W.E.; Schroder, H.C.; Guo, Y.W. Disulfide- and multisulfide-containing metabolites from marine organisms. Chem. Rev. 2012, 112, 2179–2207. [Google Scholar] [CrossRef] [PubMed]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Gomes, N.G.; Dasari, R.; Chandra, S.; Kiss, R.; Kornienko, A. Marine invertebrate metabolites with anticancer activities: Solutions to the “supply problem”. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Fattorusso, E.; Menna, M. Steroids from sponges: Recent reports. Steroids 1999, 64, 687–714. [Google Scholar] [CrossRef]
- Dai, J.; Sorribas, A.; Yoshida, W.Y.; Kelly, M.; Williams, P.G. Topsentinols, 24-isopropyl steroids from the marine sponge Topsentia sp. J. Nat. Prod. 2010, 73, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Whitson, E.L.; Bugni, T.S.; Chockalingam, P.S.; Concepcion, G.P.; Harper, M.K.; He, M.; Hooper, J.N.A.; Mangalindan, G.C.; Ritacco, F.; Ireland, C.M. Spheciosterol sulfates, PKCζ inhibitors from a Philippine sponge Spheciospongia sp. J. Nat. Prod. 2008, 71, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Guzii, A.G.; Makarieva, T.N.; Denisenko, V.A.; Dmitrenok, P.S.; Burtseva, Y.V.; Krasokhin, V.B.; Stonik, V.A. Topsentiasterol sulfates with novel iodinated and chlorinated side chains from the marine sponge Topsentia sp. Tetrahedron Lett. 2008, 49, 7191–7193. [Google Scholar] [CrossRef]
- Fusetani, N.; Takahashi, M.; Matsunaga, S. Topsentiasterol sulfates, antimicrobial sterol sulfates possessing novel side chains, from a marine sponge, Topsentia sp. Tetrahedron 1994, 50, 7765–7770. [Google Scholar] [CrossRef]
- Luo, X.; Li, F.; Shinde, P.B.; Hong, J.; Lee, C.O.; Im, K.S.; Jung, J.H. 26,27-Cyclosterols and other polyoxygenated sterols from a marine sponge Topsentia sp. J. Nat. Prod. 2006, 69, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.C.; Cardellinaii, J.H.; Tischler, M.; Snader, K.M.; Boyd, M.R. Ibisterol sulfate, a novel HIV-inhibitory sulfated sterol from the deep water sponge Topsentia sp. Tetrahedron Lett. 1993, 34, 389–392. [Google Scholar] [CrossRef]
- Sun, H.H.; Gross, S.S.; Gunasekera, M.; Koehn, F.E. Weinbersterol disulfates A and B, antiviral steroid sulfates from the sponge Petrosia weinbergi. Tetrahedron 1991, 47, 1185–1190. [Google Scholar] [CrossRef]
- Fusetani, N.; Matsunaga, S.; Konosu, S. Bioactive marine metabolites II. Halistanol sulfate, an antimicrobial novel steroid sulfate from the marine sponge halichondria cf. moorei bergquist. Tetrahedron Lett. 1981, 22, 1985–1988. [Google Scholar] [CrossRef]
- Sarma, N.S.; Krishna, M.S.R.; Rao, S.R. Sterol ring system oxidation pattern in marine sponges. Mar. Drugs 2005, 3, 84–111. [Google Scholar] [CrossRef]
- Massey, I.J.; Djerassi, C. Mass spectrometry in structural and stereochemical problems. 252. Structural and stereochemical applications of mass spectrometry in the marine sterol field. Synthesis and electron impact induced mass spectral fragmentation of Δ24- and Δ24(28)-3β-hydroxy-Δ5-sterols. J. Org. Chem. 1979, 44, 2448–2456. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fromtling, R.A.; Galgiani, J.N.; Pfaller, M.A.; Espinel-Ingroff, A.; Bartizal, K.F.; Bartlett, M.S.; Body, B.A.; Frey, C.; Hall, G.; Roberts, G.D. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob. Agents Chemother. 1993, 37, 39–45. [Google Scholar] [CrossRef] [PubMed]
| H | 1 | 2 | 3 |
|---|---|---|---|
| 1α | 1.96 (dd, 13.0, 4.8) | 2.00 (m) | 1.98 (m) |
| 1β | 2.40 (m) | 2.46 (br d, 13.0) | 2.46 (br d, 14.0) |
| 2 | 4.25 (br s) | 4.28 (br s) | 4.28 (br s) |
| 3 | 3.91 (t, 2.4) | 3.98 (t, 2.8) | 3.97 (t, 3.2) |
| 4 | 3.98 (br d, 2.4) | 4.04 (br d, 2.8) | 4.03 (br d, 3.2) |
| 5 | 1.41 (dd, 10.8, 2.0) | 1.46 (dd, 10.8, 2.0) | 1.45 (dd, 10.8, 2.0) |
| 6 | 4.11 (td, 10.8, 4.4) | 4.16 (td, 10.8, 4.4) | 4.15 (td, 10.8, 4.4) |
| 7α | 1.51 (m) | 1.53 (m) | 1.53 (m) |
| 7β | 1.91 (m) | 1.91 (m) | 1.91 (m) |
| 8 | 1.95 (m) | 1.95 (m) | 1.95 (m) |
| 11 | 5.32 (d, 5.2) | 5.36 (d, 5.2) | 5.36 (br d, 5.2) |
| 12α | 2.12 (d, 17.5) | 2.15 (br d, 17.5) | 2.15 (br d, 17.5) |
| 12β | 1.93 (m) | 1.93 (m) | 1.93 (m) |
| 15a | 1.38 (m) | 1.38 (m) | 1.38 (m) |
| 15b | 1.44 (m) | 1.44 (m) | 1.44 (m) |
| 16a | 1.37 (m) | 1.37 (m) | 1.37 (m) |
| 16b | 1.92 (m) | 1.92 (m) | 1.92 (m) |
| 17 | 1.66 (q, 9.4) | 1.69 (q, 9.4) | 1.68 (q, 9.4) |
| 18 | 0.70 (s) | 0.73 (s) | 0.73 (s) |
| 19 | 1.34 (s) | 1.38 (s) | 1.38 (s) |
| 20 | 1.42 (m) | 1.42 (m) | 1.42 (m) |
| 21 | 0.91 (d, 6.4) | 0.95 (d, 6.4) | 0.95 (d, 6.4) |
| 22a | 1.06 (m) | 1.11 (m) | 1.11 (m) |
| 22b | 1.43 (m) | 1.48 (m) | 1.48 (m) |
| 23a | 1.42 (m) | 1.47 (m) | 1.47 (m) |
| 23b | 1.54 (m) | 1.55 (m) | 1.55 (m) |
| 24 | 2.42 (m) | 2.52 (q, 6.4) | 2.53 (q, 6.4) |
| 27 | 5.86 (s) | 7.32 (br s) | 6.93 (br s) |
| 28 | 4.87 (br s) | 5.87 (br s) | |
| 29 | 1.12 (d, 6.8) | 1.21 (d, 6.8) | 1.20 (d, 6.8) |
| 30 | 0.82 (s) | 0.85 (s) | 0.85 (s) |
| 26-OCH3 | 3.70 (s) | ||
| 28-OCH3 | 3.66 (s) | 3.58 (s) |
| C | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 39.9, CH2 | 39.7, CH2 | 39.8, CH2 |
| 2 | 71.5, CH | 71.2, CH | 71.3, CH |
| 3 | 72.5, CH | 72.0, CH | 72.2, CH |
| 4 | 72.8, CH | 72.4, CH | 72.6, CH |
| 5 | 48.1, CH | 48.8, CH | 49.0, CH |
| 6 | 66.7, CH | 66.4, CH | 66.5, CH |
| 7 | 37.9, CH2 | 37.5, CH2 | 37.6, CH2 |
| 8 | 41.4, CH | 41.0, CH | 41.1, CH |
| 9 | 147.9, C | 147.4, C | 147.5, C |
| 10 | 39.8, C | 39.5, C | 39.5, C |
| 11 | 117.0, CH | 116.9, CH | 116.9, CH |
| 12 | 38.4, CH2 | 38.1, CH2 | 38.1, CH2 |
| 13 | 45.7, C | 45.4, C | 45.4, C |
| 14 | 48.1, C | 47.8, C | 47.9, C |
| 15 | 34.9, CH2 | 34.7, CH2 | 34.7, CH2 |
| 16 | 29.0, CH2 | 28.8, CH2 | 28.8, CH2 |
| 17 | 52.3, CH | 51.9, CH | 51.9, CH |
| 18 | 15.1, CH3 | 15.1, CH3 | 15.1, CH3 |
| 19 | 27.1, CH3 | 27.0, CH3 | 27.0, CH3 |
| 20 | 37.5, CH | 37.0, CH | 37.1, CH |
| 21 | 18.9, CH3 | 18.9, CH3 | 18.9, CH3 |
| 22 | 34.7, CH2 | 34.4, CH2 | 34.5, CH2 |
| 23 | 32.7, CH2 | 32.5, CH2 | 32.4, CH2 |
| 24 | 40.5, CH | 31.8, CH | 31.9, CH |
| 25 | 157.6, C | 139.6, C | 144.2, C |
| 26 | 170.7, C | 176.3, C | 172.8, C |
| 27 | 119.4, CH | 146.1, CH | 143.1, CH |
| 28 | 167.1, C | 71.7, CH2 | 104.0, CH |
| 29 | 19.3, CH3 | 18.8, CH3 | 18.8, CH3 |
| 30 | 19.0, CH3 | 18.9, CH3 | 18.9, CH3 |
| 26-OCH3 | 52.3, CH3 | ||
| 28-OCH3 | 52.7, CH3 | 57.0, CH3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Wu, X.-D.; Zhao, Q.; Wang, C.-Y. Topsensterols A–C, Cytotoxic Polyhydroxylated Sterol Derivatives from a Marine Sponge Topsentia sp. Mar. Drugs 2016, 14, 146. https://doi.org/10.3390/md14080146
Chen M, Wu X-D, Zhao Q, Wang C-Y. Topsensterols A–C, Cytotoxic Polyhydroxylated Sterol Derivatives from a Marine Sponge Topsentia sp. Marine Drugs. 2016; 14(8):146. https://doi.org/10.3390/md14080146
Chicago/Turabian StyleChen, Min, Xu-Dong Wu, Qing Zhao, and Chang-Yun Wang. 2016. "Topsensterols A–C, Cytotoxic Polyhydroxylated Sterol Derivatives from a Marine Sponge Topsentia sp." Marine Drugs 14, no. 8: 146. https://doi.org/10.3390/md14080146
APA StyleChen, M., Wu, X.-D., Zhao, Q., & Wang, C.-Y. (2016). Topsensterols A–C, Cytotoxic Polyhydroxylated Sterol Derivatives from a Marine Sponge Topsentia sp. Marine Drugs, 14(8), 146. https://doi.org/10.3390/md14080146