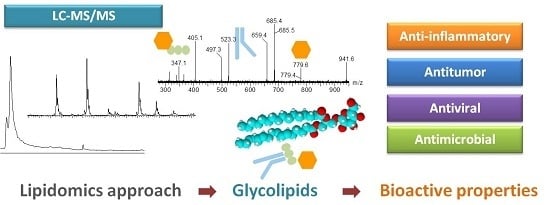
Lipidomic Approaches towards Deciphering Glycolipids from Microalgae as a Reservoir of Bioactive Lipids
Abstract
:1. Introduction
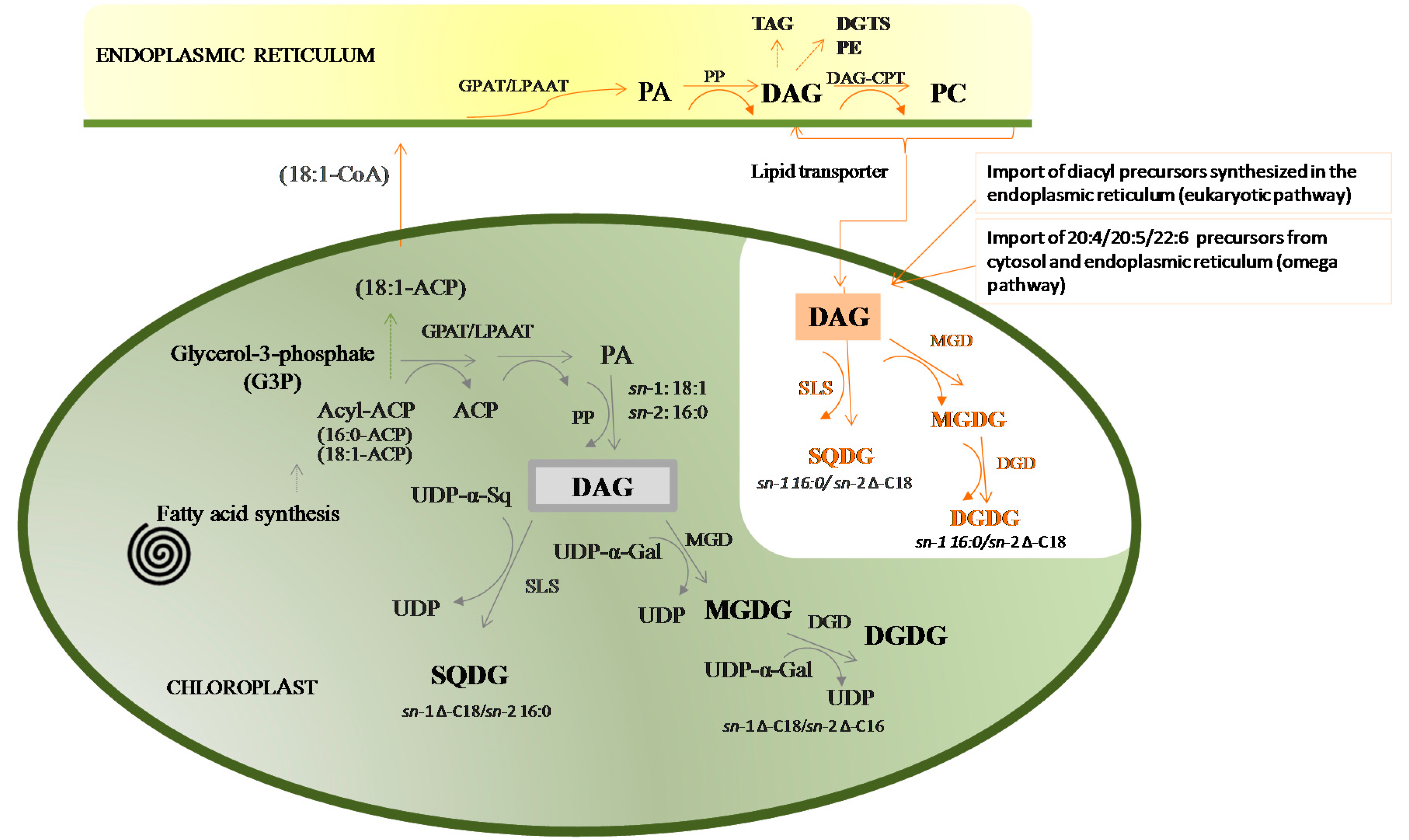
2. The Structure of Glycolipids
3. Biological Properties Associated with Glycolipids from Microalgae
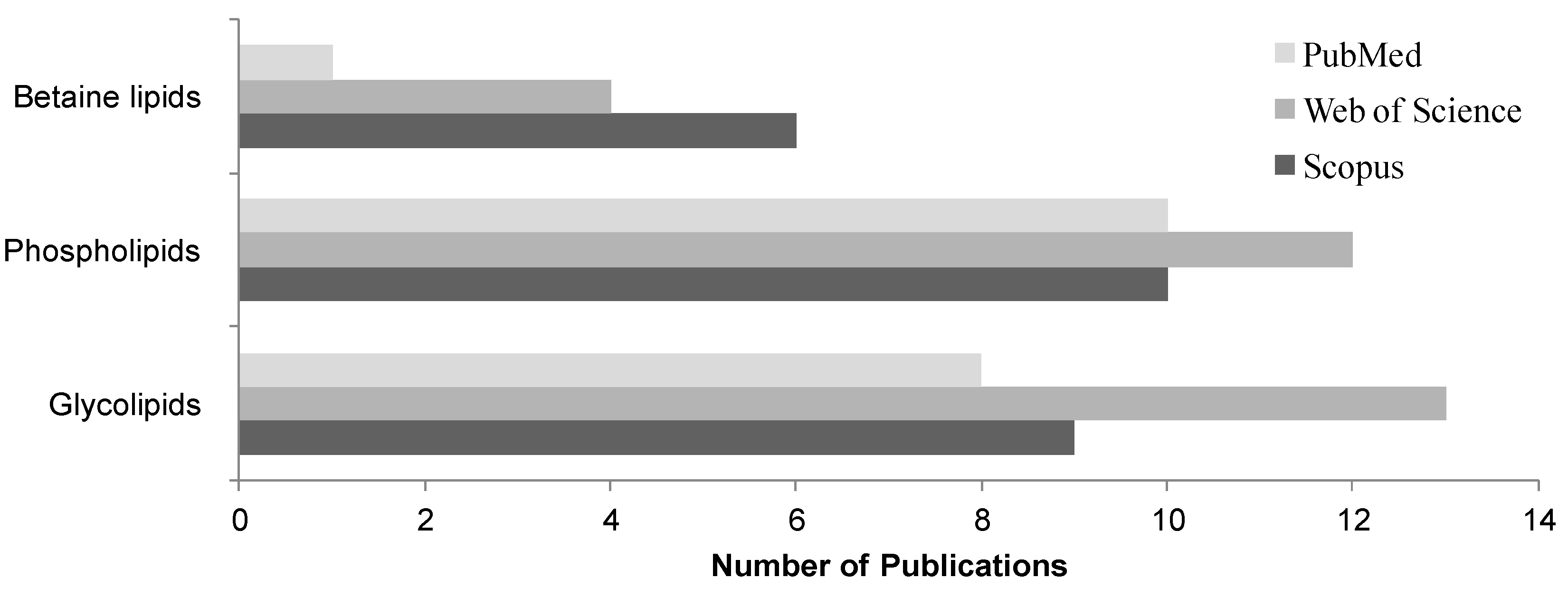
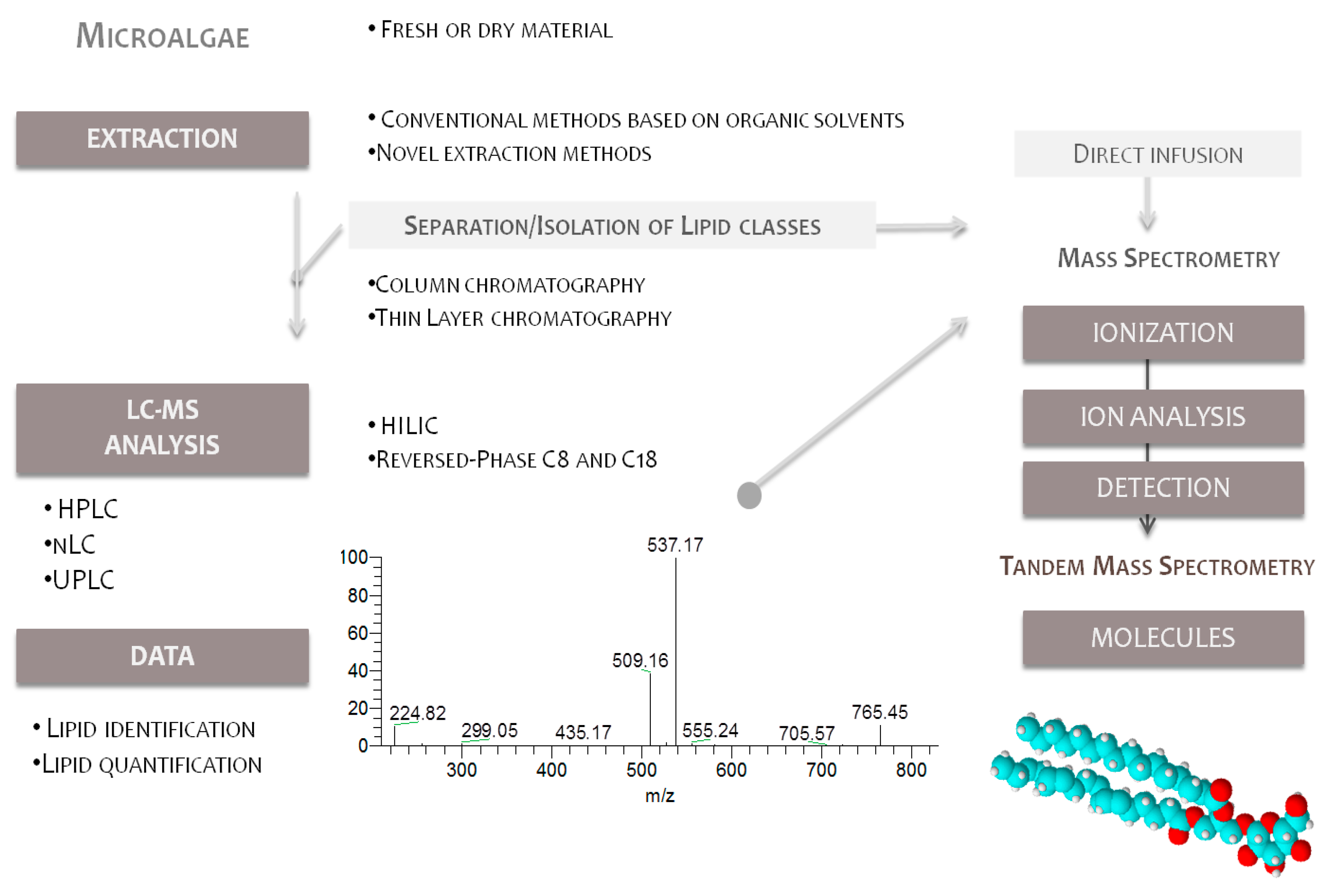
4. Lipidomic Approaches of Microalgae
4.1. Lipid Extraction and Isolation of Glycolipids
4.2. Mass Spectrometry Analysis
Analysis of Glycolipids by Mass Spectrometry
4.3. Studies Uncovering the Lipidome of Microalgae
4.3.1. Glycolipidomic Profiling on Cyanobacteria
4.3.2. Glycolipidomic Profiling on Chlorophyta
4.3.3. Glycolipidomic Profiling on Bacillariophyta
4.3.4. Glycolipidomic Profiling on Dinoflagellata
4.3.5. Glycolipidomic Profiling on Eustigmatophyta
4.3.6. Glycolipidomic Profiling on Prymnesiophyta
4.3.7. Glycolipidomic Profiling on Rhodophyta
5. Final Considerations and Perspectives
Acknowledgments
Conflicts of Interest
References
- Adl, S.M.; Simpson, A.G.; Lane, C.E.; Lukes, J.; Bass, D.; Bowser, S.S.; Brown, M.; Burki, F.; Dunthorn, M.V.; Heiss, A.; et al. The revised classification of eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–514. [Google Scholar] [CrossRef] [PubMed]
- Cavalier-Smith, T.; Chao, E.E. Phylogeny and megasystematics of phagotrophic heterokonts (kingdom Chromista). J. Mol. Evol. 2006, 62, 388–420. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication: National University of Ireland, Galway, Ireland. Available online: http://www.algaebase.org (accessed on 23 March 2016).
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef] [PubMed]
- Vigani, M.; Parisi, C.; Rodríguez-Cerezo, E.; Barbosa, M.J.; Sijtsma, L.; Ploeg, M.; Enzing, C. Food and feed products from micro-algae: Market opportunities and challenges for the EU. Trends Food Sci. Technol. 2015, 42, 81–92. [Google Scholar] [CrossRef]
- Chu, W. Biotechnological applications of microalgae. IeJSME 2012, 6, 24–37. [Google Scholar]
- Mostafa, S.S.M. Microalgal biotechnology: Prospects and applications. In Plant Science; InTech: Rijeka, Croatia, 2012; pp. 275–313. [Google Scholar]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, E.; Cifuentes, A. Benefits of using algae as natural sources of functional ingredients. J. Sci. Food Agric. 2013, 93, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L.; Guschina, I.A. The versatility of algae and their lipid metabolism. Biochimie 2009, 91, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, P.A.; Murata, N. Lipids in Photosynthesis: Structure, Function and Genetics; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 21–81. [Google Scholar]
- Hölzl, G.; Dörmann, P. Structure and function of glycoglycerolipids in plants and bacteria. Prog. Lipid Res. 2007, 46, 225–243. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Rodgers, R.P.; Marshall, A.G.; Hsu, C.S. Algae polar lipids characterized by online liquid chromatography coupled with hybrid linear quadrupole ion trap/fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2011, 25, 4770–4775. [Google Scholar] [CrossRef]
- Kim, S.-H.; Liu, K.-H.; Lee, S.-Y.; Hong, S.-J.; Cho, B.-K.; Lee, H.; Lee, C.-G.; Choi, H.-K. Effects of light intensity and nitrogen starvation on glycerolipid, glycerophospholipid, and carotenoid composition in Dunaliella tertiolecta culture. PLoS ONE 2013, 8, e72415. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; de Souza, L.M.; Sassaki, G.L.; Cavalcanti, J.F.; Villela Romanos, M.T.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Antiviral Sulfoquinovosyldiacylglycerols (SQDGs) from the Brazilian brown seaweed Sargassum vulgare. Mar. Drugs 2013, 11, 4628–4640. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Glycolipids from seaweeds and their potential biotechnological applications. Front. Cell. Infect. Microbiol. 2014, 4, 174. [Google Scholar] [CrossRef] [PubMed]
- Noda, H.; Amano, H.; Arashima, K.; Nisizawa, K. Antitumor activity of marine algae. Hydrobiologia 1990, 204, 577–584. [Google Scholar] [CrossRef]
- Naumann, I.; Darsow, K.H.; Walter, C.; Lange, H.A.; Buchholz, R. Identification of sulfoglycolipids from the alga Porphyridium purpureum by matrix-assisted laser desorption/ionisation quadrupole ion trap time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Reshef, V.; Mizrachi, E.; Maretzki, T.; Silberstein, C.; Loya, S.; Hizi, A.; Carmeli, S. New acylated sulfoglycolipids and digalactolipids and related known glycolipids from cyanobacteria with a potential to inhibit the reverse transcriptase of HIV-1. J. Nat. Prod. 1997, 60, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T. Anti-tumour-promoting glyceroglycolipids from the green alga, Chlorella vulgaris. Phytochemistry 1995, 40, 1433–1437. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Yu, G.; Guan, H. Total synthesis and structure-activity relationship of glycoglycerolipids from marine organisms. Mar. Drugs 2014, 12, 3634–3659. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chen, D.; Xu, J.; Zhou, C. Profiles of photosynthetic glycerolipids in three strains of Skeletonema determined by UPLC-Q-TOF-MS. J. Appl. Phycol. 2011, 23, 271–282. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Xu, J.; Zhou, C. Analysis of phospholipids in microalga Nitzschia closterium by UPLC-Q-TOF-MS. Chin. J. Oceanol. Limnol. 2010, 28, 106–112. [Google Scholar] [CrossRef]
- Vieler, A.; Wilhelm, C.; Goss, R.; Süß, R.; Schiller, J. The lipid composition of the unicellular green alga Chlamydomonas reinhardtii and the diatom Cyclotella meneghiniana investigated by MALDI-TOF MS and TLC. Chem. Phys. Lipids 2007, 150, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, D.; Yan, X.; Chen, J.; Zhou, C. Global characterization of the photosynthetic glycerolipids from a marine diatom Stephanodiscus sp. by ultra performance liquid chromatography coupled with electrospray ionization-quadrupole-time of flight mass spectrometry. Anal. Chim. Acta 2010, 663, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, X.; Xu, J.; Zhou, C. Precise identification of photosynthetic glycerolipids in microalga Tetraselmis chuii by UPLC-ESI-Q-TOF-MS. Sci. China Ser. C Life Sci. 2008, 51, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Petroutsos, D.; Amiar, S.; Abida, H.; Dolch, L.-J.; Bastien, O.; Rébeillé, F.; Jouhet, J.; Falconet, D.; Block, M.A.; McFadden, G.I.; et al. Evolution of galactoglycerolipid biosynthetic pathways—From cyanobacteria to primary plastids and from primary to secondary plastids. Prog. Lipid Res. 2014, 54, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Mühlroth, A.; Li, K.; Røkke, G.; Winge, P.; Olsen, Y.; Hohmann-Marriott, M.F.; Vadstein, O.; Bones, A.M. Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista. Mar. Drugs 2013, 11, 4662–4697. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. Membrane lipids: It’s only a phase. Curr. Biol. 2000, 10, 377–380. [Google Scholar] [CrossRef]
- Lu, N.; Wei, D.; Chen, F.; Yang, S.-T. Lipidomic profiling and discovery of lipid biomarkers in snow alga Chlamydomonas nivalis under salt stress. Eur. J. Lipid Sci. Technol. 2012, 114, 253–265. [Google Scholar] [CrossRef]
- Lu, N.; Wei, D.; Chen, F.; Yang, S.-T. Lipidomic profiling reveals lipid regulation in the snow alga Chlamydomonas nivalis in response to nitrate or phosphate deprivation. Process Biochem. 2013, 48, 605–613. [Google Scholar] [CrossRef]
- Yang, D.; Song, D.; Kind, T.; Ma, Y.; Hoefkens, J.; Fiehn, O. Lipidomic analysis of Chlamydomonas reinhardtii under nitrogen and sulfur deprivation. PLoS ONE 2015, 10, e0137948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Benning, C. Chloroplast lipid synthesis and lipid trafficking through ER-plastid membrane contact sites. Biochem. Soc. Trans. 2012, 40, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Boudière, L.; Michaud, M.; Petroutsos, D.; Rébeillé, F.; Falconet, D.; Bastien, O.; Roy, S.; Finazzi, G.; Rolland, N.; Jouhet, J.; et al. Glycerolipids in photosynthesis: Composition, synthesis and trafficking. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Shiraiwa, Y. Biosynthesis of lipids and hydrocarbons in algae. In Photosynthesis; Dubinsky, Z., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Benning, C. Biosynthesis and function of the sulfolipid sulfoquinovosyl diacylglicerol. Annu. Rev. Plant Physiol. 1998, 49, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.R.; Cardellina, J.H.; Fuller, R.W.; Weislow, O.S.; Kiser, R.F.; Snader, K.M.; Patterson, G.M.; Boyd, M.R. AIDS-antiviral sulfolipids from cyanobacteria (blue-green algae). J. Natl. Cancer Inst. 1989, 81, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Zianni, R.; Bianco, G.; Lelario, F.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. Fatty acid neutral losses observed in tandem mass spectrometry with collision-induced dissociation allows regiochemical assignment of sulfoquinovosyl-diacylglycerols. J. Mass Spectrom. 2013, 48, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Abrol, Y.P.; Ahmad, A. Sulphur in Plants; Kluwer Academic: Dordrecht, The Netherlands, 2003; pp. 189–219. [Google Scholar]
- Kim, Y.H.; Choi, J.; Yoo, J.S.; Park, Y.; Kim, M.S. Structural identification of glycerolipid molecular species isolated from Cyanobacterium Synechocystis sp. PCC 6803 using Fast Atom Bombardment Tandem Mass Spectrometry. Anal. Biochem. 1999, 267, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.G.; Lasiter, A.D.; Leblond, J.D. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. III. Four cold-adapted, peridinin-containing taxa and the presence of trigalactosyldiacylglycerol as an additional glycolipid. Eur. J. Phycol. 2009, 44, 441–447. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.; Arondel, V.; Bates, P.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.; et al. Acyl lipid metabolism. In The Arabidopsis Book; Keiko, T., Ed.; American Society of Plant Biologists: Rockville, MD, USA, 2013; Volume 8, pp. 1–65. [Google Scholar]
- Browse, J.; Somerville, C. Glycerolipid metabolism: Biochemistry and regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 467–506. [Google Scholar] [CrossRef]
- Browse, J.; Warwick, N.; Somerville, C.R.; Slack, C.R. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the “16:3” plant Arabidopsis thaliana. Biochem. J. 1986, 235, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Dormann, P.; Benning, C. Galactolipids rule in seed plants. Trends Plant Sci. 2002, 7, 112–118. [Google Scholar] [CrossRef]
- Okazaki, Y.; Kamide, Y.; Hirai, M.Y.; Saito, K. Plant lipidomics based on hydrophilic interaction chromatography coupled to ion trap time-of-flight mass spectrometry. Metabolomics 2013, 9, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Khozin-Goldberg, I.; Cohen, Z. Unraveling algal lipid metabolism: Recent advances in gene identification. Biochimie 2011, 93, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Khozin-Goldberg, I.; Didi-Cohen, S.; Shayakhmetova, I.; Cohen, Z. Biosynthesis of eicosapentaenoic acid (EPA) in the freshwater eustigmatophyte Monodus subterraneus (Eustigmatophyceae). J. Phycol. 2002, 38, 745–756. [Google Scholar] [CrossRef]
- Millar, A.A.; Smith, M.A.; Kunst, L. All fatty acids are not equal: Discrimination in plant membrane lipids. Trends Plant Sci. 2000, 5, 95–101. [Google Scholar] [CrossRef]
- Yao, L.; Gerde, J.A.; Lee, S.-L.; Wang, T.; Harrata, K.A. Microalgae lipid characterization. J. Agric. Food Chem. 2015, 63, 1773–1787. [Google Scholar] [CrossRef] [PubMed]
- Sandra, K.; Sandra, P. Lipidomics from an analytical perspective. Curr. Opin. Chem. Biol. 2013, 17, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Fusetani, N. Marine pharmacology in 2007–2008: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar] [PubMed]
- Amaro, H.; Guedes, A.; Malcata, F. Antimicrobial activities of microalgae: An invited review. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex: Badajoz, Spain, 2011; pp. 1272–1280. [Google Scholar]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Bergé, J.P.; Debiton, E.; Dumay, J.; Durand, P.; Barthomeuf, C. In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. J. Agric. Food Chem. 2002, 50, 6227–6232. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Gallant, P.; Stefanova, R.; Melanson, R.; O’Leary, S.J.B. Monogalactosyldiacylglycerols, potent nitric oxide inhibitors from the marine microalga Tetraselmis chui. Nat. Prod. Res. 2013, 27, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Stefanova, R.; Gallant, P.; McGinn, P.J. Mono- and digalactosyldiacylglycerols: Potent nitric oxide inhibitors from the marine microalga Nannochloropsis granulata. J. Appl. Phycol. 2013, 25, 349–357. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.S.; Hafting, J.T. Lipids isolated from the cultivated red alga Chondrus crispus inhibit nitric oxide production. J. Appl. Phycol. 2014, 26, 1565–1571. [Google Scholar] [CrossRef]
- Bruno, A.; Rossi, C.; Marcolongo, G.; di Lena, A.; Venzo, A.; Berrie, C.P.; Corda, D. Selective in vivo anti-inflammatory action of the galactolipid monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005, 524, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Chirasuwan, N.; Chaiklahan, R.; Kittakoop, P.; Chanasattru, W.; Ruengjitchatchawalya, M.; Tanticharoen, M.; Bunnag, B. Anti HSV-1 activity of sulphoquinovosyl diacylglycerol isolated from Spirulina platensis. Nat. Sci. 2007, 41, 311–318. [Google Scholar]
- Hossain, Z.; Kurihara, H.; Hosokawa, M.; Takahashi, K. Growth inhibition and induction of differentiation and apoptosis mediated by sodium butyrate in Caco-2 cells with algal glycolipids. In Vitro Cell. Dev. Biol. 2005, 41, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Naumman, I. Sulfoquinovosyldiacylglyceride Antiviral Active Substanzen. Ph.D. Thesis, Fakultät der Universitt Erlangen-Nür, Erlangen, Germany, 2009. [Google Scholar]
- Mattos, B.B.; Romanos, M.T.V.; de Souza, L.M.; Sassaki, G.; Barreto-Bergter, E. Glycolipids from macroalgae: Potential biomolecules for marine biotechnology? Rev. Bras. Farmacogn. 2011, 21, 244–247. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Alejandro Cifuentes, A.; Ibáñez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.-M. Dietary Omega-3 fatty acids for women. Biomed. Pharmacother. 2007, 61, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bergé, J.P.; Barnathan, G. Fatty acids from lipids of marine organisms: Molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv. Biochem. Eng. Biotechnol. 2005, 96, 49–125. [Google Scholar] [PubMed]
- Simopoulos, A. The importance of the ratio of Omega-6/Omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Kagan, M.L.; Levy, A.; Leikin-Frenkel, A. Comparative study of tissue deposition of omega-3 fatty acids from polar-lipid rich oil of the microalgae Nannochloropsis oculata with krill oil in rats. Food Funct. 2015, 6, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, J.; Chen, J.J.; Chen, J.J.; Zhou, C.; Yan, X. The major lipid changes of some important diet microalgae during the entire growth phase. Aquaculture 2014, 428–429, 104–110. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Melanson, R.; Osborne, J.A.; O’Leary, S.J.B. Five new galactolipids from the freshwater microalga Porphyridium aerugineum and their nitric oxide inhibitory activity. J. Appl. Phycol. 2013, 25, 951–960. [Google Scholar] [CrossRef]
- Robertson, R.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.; Fitzgerald, G.; Ross, R.; Stanton, C. The anti-inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-stimulated human THP-1 Macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, P.; Milne, S.; Myers, D.; Brown, H. Lipidomics: A mass spectrometry based systems level analysis of cellular lipids. Curr. Opin. Chem. Biol. 2009, 13, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Bou Khalil, M.; Hou, W.; Zhou, H.; Elisma, F.; Swayne, L.A.; Blanchard, A.P.; Yao, Z.; Bennett, S.A.L.; Figeys, D. Lipidomics era: Accomplishments and challenges. Mass Spectrom. Rev. 2010, 29, 877–929. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, N.; Misaki, A. Glycolipids from Spirulina maxima: Structure and fatty acid composition. Agric. Biol. Chem. 1983, 47, 2349–2355. [Google Scholar] [CrossRef]
- Han, Y.; Wen, Q.; Chen, Z.; Li, P. Review of methods used for microalgal lipid-content analysis. Energy Proc. 2011, 12, 9444–9950. [Google Scholar] [CrossRef]
- Leblond, J.D.; Chapman, P.J. Lipid class distribution of highly unsaturated long chain fatty acids in marine dionflagellates. J. Phycol. 2000, 1108, 1103–1108. [Google Scholar] [CrossRef]
- Benning, C.; Huang, Z.H.; Gage, D.A. Accumulation of a novel glycolipid and a betaine lipid in cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch. Biochem. Biophys. 1995, 317, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Son, B.W.; Kim, J.C.; Lee, S.M.; Cho, J.C.; Choi, J.S.; Choi, H.D.; Song, J.C. New diacylgalactolipids from the marine Cyanophycean microalga Oscillatoria sp. Bull. Korean Chem. Soc. 2000, 21, 1138–1140. [Google Scholar]
- Son, B.W.; Cho, Y.J.; Cho, J.S.; Lee, W.K.; Kim, D.S.; Choi, H.D.; Choi, J.S.; Jung, J.H.; Im, K.S.; Choi, W.C. New galactolipids from the marine bacillariophycean microalga Nitzschia sp. Nat. Prod. Lett. 2001, 15, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Hu, P.; Liang, Q.-L.; Wang, Y.-M.; Luo, G.-A. Recent advances in lipidomics. Fenxi Huaxue/Chin. J. Anal. Chem. 2009, 37, 1390–1396. [Google Scholar] [CrossRef]
- Watson, A.D. Thematic review series: Systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: A global approach to lipid analysis in biological systems. J. Lipid Res. 2006, 47, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Fiehn, O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends Anal. Chem. 2014, 61, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rappid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Kumar, R.R.; Rao, P.H.; Arumugam, M. Lipid extraction methods from microalgae: A comprehensive review. Front. Energy Res. 2015, 2. [Google Scholar] [CrossRef]
- Li, Y.; Naghdi, F.G.; Garg, S.; Adarme-Vega, T.C.; Thurecht, K.J.; Ghafor, W.A.; Tannock, S.; Schenk, P.M. A comparative study: The impact of different lipid extraction methods on current microalgal lipid research. Microb. Cell Fact. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; le Grandois, J.; Marchioni, E.; Zhao, M.; Ennahar, S.; Bindler, F. Improvement of total lipid and glycerophospholipid recoveries from various food matrices using pressurized liquid extraction. J. Agric. Food Chem. 2010, 58, 9912–9917. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-C.; Choi, W.-Y.; Oh, S.-H.; Lee, C.-G.; Seo, Y.-C.; Kim, J.-S.; Song, C.-H.; Kim, G.-V.; Lee, S.-Y.; Kang, D.-H.; et al. Enhancement of lipid extraction from marine microalga, Scenedesmus associated with high-pressure homogenization process. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Manning, S.; Montoya, M.; Keller, K.; Poenie, M. Extraction of algal lipids and their analysis by HPLC and Mass Spectrometry. J. Am. Oil Chem. Soc. 2012, 89, 1371–1381. [Google Scholar] [CrossRef]
- Herrero, M.; Vicente, M.J.; Cifuentes, A.; Ibáñez, E. Characterization by high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry of the lipid fraction of Spirulina platensis pressurized ethanol extract. Rapid Commun. Mass Spectrom. 2007, 21, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W.; Han, X. Lipid Analysis-Isolation, Separation, Identification and Lipidomic Analysis, 4th ed.; Oily Press: Bridgwater, UK; Woodhead Publishing Ltd.: Cambridge, UK, 2010. [Google Scholar]
- Martin, G.J.O.; Hill, D.R.A.; Olmstead, I.L.D.; Bergamin, A.; Shears, M.J.; Dias, D.A.; Kentish, S.E.; Scales, P.J.; Botté, C.Y.; Callahan, D.L. Lipid profile remodeling in response to nitrogen deprivation in the microalgae Chlorella sp. (Trebouxiophyceae) and Nannochloropsis sp. (Eustigmatophyceae). PLoS ONE 2014, 9, e103389. [Google Scholar] [CrossRef] [PubMed]
- Oradu, S.A.; Cooks, R.G. Multistep mass spectrometry methodology for direct characterization of polar lipids in green microalgae using paper spray ionization. Anal. Chem. 2012, 84, 10576–10585. [Google Scholar] [CrossRef] [PubMed]
- Guella, G.; Frassanito, R.; Mancini, I. A new solution for an old problem: The regiochemical distribution of the acyl chains in galactolipids can be established by electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Dodson, V.J.; Dahmen, J.L.; Mouget, J.-L.; Leblond, J.D. Mono- and digalactosyldiacylglycerol composition of the marennine-producing diatom, Haslea ostrearia: Comparison to a selection of pennate and centric diatoms. Phycol. Res. 2013, 61, 199–207. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Yan, X.; Zhao, P.; Chen, J.; Zhou, C.; Zhao, F.; Li, S. Lipidomic changes during different growth stages of Nitzschia closterium f. minutissima. Metabolomics 2013, 9, 300–310. [Google Scholar] [CrossRef]
- Gray, C.G.; Lasiter, A.D.; Leblond, J.D. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. I. Peridinin-containing taxa. Eur. J. Phycol. 2009, 44, 191–197. [Google Scholar] [CrossRef]
- Leblond, J.D.; Dahmen, J.L.; Evens, T.J. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. IV. Temperature-induced modulation of fatty acid regiochemistry as observed by electrospray ionization/mass spectrometry. Eur. J. Phycol. 2010, 45, 13–18. [Google Scholar] [CrossRef]
- Li, S.; Xu, J.; Jiang, Y.; Zhou, C.; Yu, X.; Zhong, Y.; Chen, J.; Yan, X. Lipidomic analysis can distinguish between two morphologically similar strains of Nannochloropsis oceanica. J. Phycol. 2015, 51, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Hummel, J.; Segu, S.; Li, Y.; Irgang, S.; Jueppner, J.; Giavalisco, P. Ultra performance liquid chromatography and high resolution mass spectrometry for the analysis of plant lipids. Front. Plant Sci. 2011, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wuhrer, M.; de Boer, A.R.; Deelder, A.M. Structural glycomics using hydrophilic interaction chromatography (HILIC) with mass spectrometry. Mass Spectrom. Rev. 2009, 28, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Okazaki, Y.; Saito, K.; Fiehn, O. LipidBlast templates as flexible tools for creating new in-silico tandem mass spectral libraries. Anal. Chem. 2014, 86, 11024–11027. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Liu, K.H.; Lee, Y.; DeFelice, B.; Meissen, J.K.; Fiehn, O. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods. 2013, 10, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Costello, C.E.; Vath, J.E. Tandem mass spectrometry of glycolipids. Methods Enzymol. 1990, 193, 738–768. [Google Scholar] [PubMed]
- Melo, T.; Alves, E.; Azevedo, V.; Martins, A.S.; Neves, B.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Lipidomics as a new approach for the bioprospecting of marine macroalgae: Unraveling the polar lipid and fatty acid composition of Chondrus crispus. Algal Res. 2015, 8, 181–191. [Google Scholar] [CrossRef]
- Da Costa, E.; Melo, T.; Alves, E.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Decoding bioactive polar lipid profile of the macroalgae Codium tomentosum from a sustainable IMTA system using a lipidomic approach. Algal Res. 2015, 12, 388–397. [Google Scholar] [CrossRef]
- Tatituri, R.V.V.; Brenner, M.B.; Turk, J.; Hsu, F.-F. Structural elucidation of diglycosyl diacylglycerol and monoglycosyl diacylglycerol from Streptococcus pneumoniae by Multiple-Stage Linear Ion-Trap Mass Spectrometry with Electrospray Ionization. J. Mass Spectrom. 2013, 47, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Navas-Iglesias, N.; Carrasco-Pancorbo, A.; Cuadros-Rodríguez, L. From lipids analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part II: Analytical lipidomics. TrAC Trends Anal. Chem. 2009, 28, 393–403. [Google Scholar] [CrossRef]
- Okazaki, Y.; Otsuki, H.; Narisawa, T.; Kobayashi, M.; Sawai, S.; Kamide, Y.; Kusano, M.; Aoki, T.; Hirai, M.Y.; Saito, K. A new class of plant lipid is essential for protection against phosphorus depletion. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Picchioni, G.A.; Watada, A.E.; Whitaker, B.D. Quantitative high-performance liquid chromatography analysis of plant phospholipids and glycolipids using light-scattering detection. Lipids 1996, 31, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Jamali, A.A.; Akbari, F.; Ghorakhlu, M.M.; de la Guardia, M.; Khosroushahi, A.Y. Applications of diatoms as potential microalgae in nanobiotechnology. BioImpacts 2012, 2, 83–89. [Google Scholar] [PubMed]

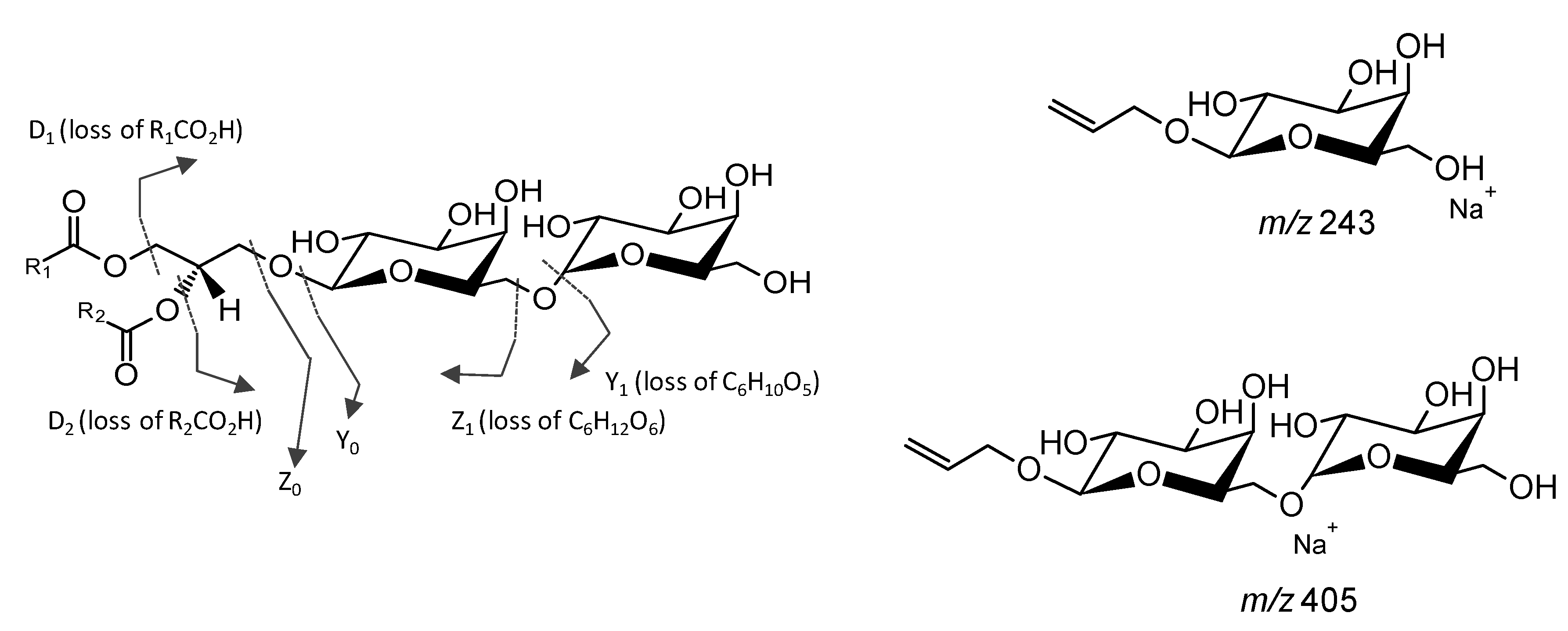
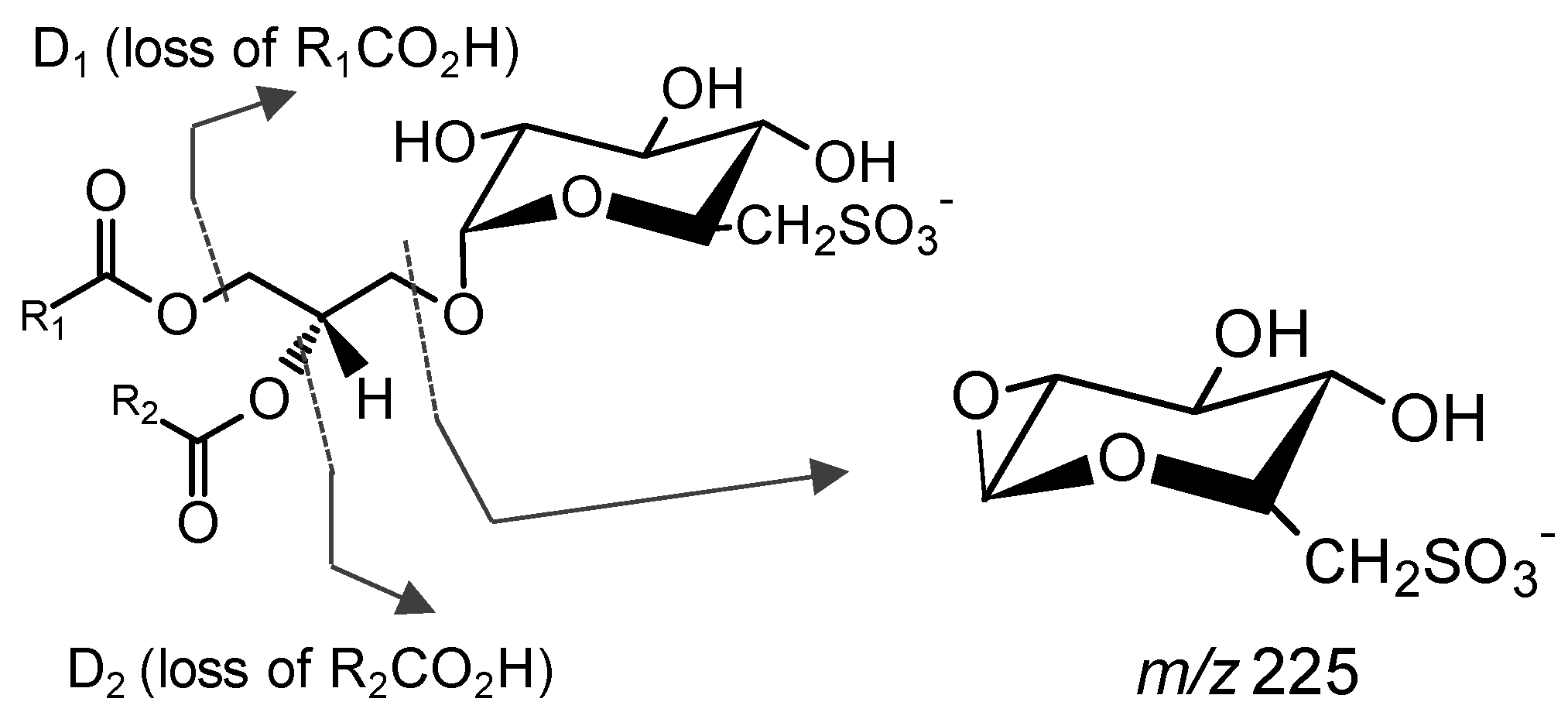
| Species Name | Glycolipid | Mechanism | Reference |
|---|---|---|---|
| Antiviral | |||
| Scytonema sp. Oscillatoria raoi Oscillatoria trichoides Oscillatoria limnetica Phormidium tenue | MGDG DGDG SQDG | Inhibit HIV-1 RT activity | [20] |
| Spirulina platensis | SQDG | [62] | |
| Antitumor | |||
| Chlorella vulgaris | MGDG DGDG | Antitumor properties expressed by the inhibitory effects of galactolipids on in vitro assay of TPA-induced EBV-EA activation on Raji cells | [21] |
| Anti-proliferative | |||
| Porphyridium cruentum | SQDG | Inhibition of mammalian r-DNA polymerase Inhibition of the growth cancer cell-lines on human colon (DLD-1), breast (MCF-7), prostate adenocarcinoma (PC-3) and malignant melanoma (M4 Beu) cancer cells | [57] |
| Antioxidant | |||
| Porphyridium cruentum | SQDG | Inhibition of superoxide generation by activated peritoneal mono nuclear cells (PMNs) | [57] |
| Anti-inflammatory | |||
| Porphyridium purpureum | SQDG | Glycolipids inhibited the NO production through the downregulation of iNOS expression | [64] |
| Tetraselmis chui Nannocloropsis granulata Porphyridium aerugineum | MGDG DGDG | Glycolipids inhibited the NO production through the downregulation of iNOS expression | [58,59,73] |
| Pavlova lutheri | SQDG MGDG DGDG | Downregulation of the production of cytokine IL-6, IL-8 | [74] |
| MGDG, DGDG, SQDG | 18:4/16:0 18:3/16:0 | Anti-inflammatory effect on croton oil-induced ear oedema and carrageenan-induced paw oedema | [61] |
| Supply n-3 PUFA | |||
| Nannocloropsis sp. | MGDG DGDG | Glycolipids in the algal oil may promote effective delivery of EPA to plasma and tissue | [71] |
| Species Name | MS Approach | Extraction | Abundant Molecular Species GL-R1/R2 (Total Number of Molecular Species) | GL% | Reference |
|---|---|---|---|---|---|
| Cyanobacterium | |||||
| Spirulina platensis | LC-MSn Q-TOF | Ethanol Pressurized liquid extraction (PLE) | MGMG 16:0, 16:2, 18:2, 18:3 SQDG 18:2/16:0, 18:3/16:0 (4 Lyso-MGDGs, 2 SQDGs) | [93] | |
| Synechocystis sp. | Off-line TLC FAB-MSn | Bligh and Dyer | MGDG 18:1/16:0, 18:2/16:0, 18:3/16:0 MGMG 16:0 DGDG 18:1/16:0, 18:2/16:0 DGMG 16:0 SQDG 16:1/16:0, 18:1/16:0 SQMG 16:0 (4 MGDGs, 1 MGMG, 3 DGDGs, 1 DGMG, 5 SQDGs, 1 SQMG) | [42] | |
| Scytonema sp. | Off-line Silica column FAB-MS | Methanol/water Chloroform/methanol | SQTG 16:0/16:0/16:0, 16:1/16:0/16:0, 18:2/16:0/16:0, 18:3/16:0/16:0 (5 SQTGs) | [20] | |
| Oscillatoria raoi Oscillatoria trichoides | Off-line Silica column FAB-MS | Methanol/water Chloroform/methanol | MGDG 18:1/16:0, 18:2/16:0, 16:2/16:0 DGTG 16:1/16:0/16:0, 18:2/16:0/16:0, 18:3/16:0/16:0 DGDG 18:1/16:0, 18:2/16:0 SQDG 16:1/16:0, 16:2/16:0, 18:1/16:0 (4 MGTGs,1 DGDG, 7 MGDGs, 3 SQDGs) | [20] | |
| Chlorophyta | |||||
| Chlorella sp. | C8 LC-MSn ESI-Q-TOF | Bligh and Dyer | MGDG 18:3/16:3, 18:4/18:4 DGDG 20:5/14:0, 20:5/16:0 SQDG 16:1/16:0 | MDGD 32% DGDG 6.4% SQDG 36% | [72] |
| RP amide column LC-MSn ESI-QqQ | Bligh and Dyer | MGDG 18:2/16:3, 18:3/16:3 DGDG 18:2/16:3, 18:3/16:3 SQDG 16:1/16:0 (14 MGDGs, 16 DGDGs, 20 SQDG) | MGDG 42% DGDG 21% SQDG 7% | [95] | |
| LC-MSn | 2-Ethoxyethanol Hexane and Folch | DGDG 18:2/18:2 | [42] | ||
| Chlorella vulgaris | Off-line SPE-Si Off-line TLC LC-MSn ESI-QqQ | Isopropanol Chloroform/methanol | MGDG ∆-C18/∆-C16 DGDG ∆-C18/∆-C16 SQDG 16:0/16:0 (13 MGDGs; 12 DGDGs, 3 SQDGs) | [52] | |
| Kyo-Chlorella | Paper spray ionization-MS LTQ-Orbitrap | No extraction | MGDG 16:2/16:3, 18:3/16:3, 18:3/18:3 SQDG 18:0/16:0, 18:3/16:0, 18:3/18:3 (3 MGDGs, 3 SQDGs) | [96] | |
| Chlamydomonas reinhardtii | Off-line SPE-Si TLC LC-MSn ESI-QqQ | Isopropanol Chloroform/methanol | MGDG 20:5/14:0 DGDG 18:1/16:0 SQDG 16:0/16:0 (14 MGDGs; 16 DGDGs, 6 SQDGs) | [52] | |
| Nano ESI-MSn LTQ | Chloroform/methanol/water | MGDG 34:7, 36:3, 36:5 DGDG 34:7, 34:6, 34:1, 34:3, 34:2 SQDG 32:0, 34:3, 34.2, 34:1 (4 MGDGs; 5 DGDGs, 4 SQDGs) | [34] | ||
| Off line TLC MALDI-TOF | Folch et al. | MGDG 36:8 and 34:7 DGDG 34:6 and several 34:n, n = 1–7 SQDG 32:1 (5 MGDGs, 9 DGDGs and 2 SQDGs) | [26] | ||
| Clamydomonas nivalis | C18LC-MSn Q-TOF | Bligh and Dyer | MGDG 18:3/16:4, 18:3/16:3 DGDG 18:3/16:4, 18:1/16:3, 18:2/16:0 SQDG 16:0/16:0, 16:0/18:3 (2 MGDGs, 5 DGDGs, 2 SQDGs) MGDG 18:3/16:2, 18:3/16:3, 18:1/16:2 DGDG 18:3/16:0, 18:1/16:3, 18:2/16:0, 18:1/16:0 SQDG 16:0/16:0, 16:0/18:3, 16:0/18:2, 16:0/18:1 (3MGDGs, 4 DGDGs, 4 SQDGs) | MGDG 41% DGDG 13% SQDG 11% | [32] [33] |
| Dunaliella tertiolecta | ESI-MSn LTQ | Folch et al. | MGDG 18:3/16:3 DGDG 18:3/16:4, 18:3/16:3, 18:3/16:2, 18:4/16:0 SQDG 16:0/16:0, 18:3/16:0 2 MGDGs, 6 DGDGs, 4 SQDGs) | [15] | |
| Scenedesmus sp. | Off-line SPE-Si Off-line TLC LC-MSn ESI-QqQ | Isopropanol Chloroform/methanol | MGDG ∆-C18/∆-C16 DGDG ∆-C18/∆-C16 SQDG 16:0/16:0 (14 MGDGs; 16 DGDGs, 6 SQDGs) | [52] | |
| Tetraselmis chuii | C18 LC-MSn ESI-Q-TOF | Bligh and Dyer | MGDG 18:1/16:1, 18:1/16:0, 18:4/16:4, 18:3/16:4 DGDG 18:1/16:0, 18:2/16:0, 18:3/16:0 SQDG 18:1/16:0, 18:3/16:0 (11 MGDGs, 7 DGDGs, 16 SQDGs) | [28] | |
| Bacillariophyta | |||||
| Chaetoceros calcitrans | C8 LC-MSn ESI-Q-TOF | Bligh and Dyer | MGDG 18:3/16:3, 18:4/18:4 DGDG 20:5/14:0, 20:5/16:0 SQDG 16:1/16:0 | MGDG 8% DGDG 4% SQDG 26% | [72] [97] |
| Cyclotella meneghiniana | Off line TLC MALDI-TOF | Folch et al. | MGDG 36:6 and 32:6 DGDG 36:7 and 32:1 SQDG 32:1 (5 MGDGs, 9 DGDGs, 2 SQDGs) | [26] | |
| Haslea ostrearia | Off-line Silica Column ESI-LTQ-MSn | Bligh and Dyer | MGDG 18:3/16:3, 18:3/16:2, 18:2/16:3 DGDG 18:3/18:3, 18:3/16:0 (19 MGDGs, 5 DGDGs) | [98] | |
| Navicula perminuta | Off-line Silica Column ESI-LTQ-MSn | Bligh and Dyer | MGDG 18:2/16:0, 18:4/16:0, 20:5/16:3 DGDG 18:3/16:3 (3 MGDGs, 1 DGDGs) | [98] | |
| Phaeodactylum tricornutum | C8 LC-MSn ESI-Q-TOF | Bligh and Dyer | MGDG 18:3/16:3, 18:4/18:4, 16:1/16:0 DGDG 20:5/14:0, 20:5/16:0 SQDG 16:1/16:0 (21 MGDGs, 9 DGDGs) | MGDG 5.1% DGDG 3.7% SQDG 26% | [72] |
| Off-line Silica Column ESI-LTQ-MSn | Bligh and Dyer | MDGD 20:5/16:2, 20:5/16:3 DGDG 16:1/16:1, 16:1/16:0, 20:5/16:1 (20 MGDGs, 9 DGDGs) | [98] | ||
| Nitzschia closterium | C8 LC-MSn ESI-Q-TOF | Bligh and Dyer | MGDG 20:5/16:2, 20:5/16:3, 16:2/16:3 DGDG 20:5/16:2 SQDG 16:1/14:0 Lyso SQDG 16:0, 16:1 (4 MGDGs, 1 DGDG, 3 SQDGs, 3 Lyso-SQDGs) | [99] [25] | |
| Skeletonema sp. | C18 LC-MSn ESI-Q-TOF | Bligh and Dyer | MGDG 16:3/16:3, 20:5/16:1, 20:5/16:3 DGDG 20:5/16:1, 20:5/16:2, 16:1/16:1 SQDG 14:0/14:0, 14:0/16:0, 14:0/16:1, 14:0/16:3 (19 MGDGs, 9 DGDGs, 22 SQDGs) | MGDG 45-71% DGDG 11-49% SQDG 11-40% | [24] |
| Off-line Silica Column ESI-LTQ-MSn | Bligh and Dyer | MGDG 16:1/16:3, 16:2/16:2 DGDG 20:5/16:1 (3 MGDGs,1 DGDGs) | [98] | ||
| Stephanodiscus sp. | C18 LC-MSn ESI-Q-TOF | Bligh and Dyer | MGDG 16:2/16:0, 16:0/16:1 DGDG 20:5/16:1, 16:1/16:0 SQDG 14:0/16:0, 16:0/16:1, 16:2/16:2, 20:5/18:4 (16 MGDGs, 9 DGDGs, 23 SQDGs) | MGDG 68% DGDG 6.3% SQDG 21% | [27] |
| Thalassiosira weissflogii | Off-line Silica Column ESI-LTQ-MSn | Bligh and Dyer | MDGD 14:0/16:1, 16:0/16:3 DGDG 20:5/16:2 (5 MGDGs, 1 DGDGs) | [98] | |
| Dinoflagellata | |||||
| Dinoflagellate spp. | Off-line Silica Column ESI-LTQ-MSn | Bligh and Dyer | MGDG 18:5/18:4, 18:5/18:5 DGDG 18:4/18:4, 18:5/18:4 (4 MGDG, 4 DGDG) MGDG 20:5/18:4, 20:5/18:5 DGDG 20:5/18:4, 20:5/18:5 (4 MGDGs, 4 DGDGs) | [100] | |
| Off-line Silica Column ESI-LTQ-MSn | Bligh and Dyer | MGDG 18:5/18:4, 18:4/18:4, 18:5/18:5 DGDG 18:4/18:4, 18:5/18:4, 18:5/18:5, 18:1/14:0 TGDG 18:1/14:0, 18:1/16:0, 18:1/18:1 (3 MGDGs, 4 DGDGs, 3 TGDGs) | [43] | ||
| Glenodinium sanguineum | C18 LC-MSn ESI-Q-IT | MGDG 20:5/∆-C18, 20:5/∆-C16, ∆-C18/∆-C16, ∆-C18/∆-C18 DGDG ∆-C18/∆-C16, ∆-C18/∆-C18 (10 MGDGs,11 DGDGs) | [97] | ||
| Pyrocystis sp. | Off-line Silica Column ESI-LTQ-MSn | Bligh and Dyer | MGDG 20:5/18:5, 20:5/18:4 DGDG 20:5/18:5, 20:5/18:4 TGDG 18:1/14:0, 22:6/16:0 (2MGDGs, 2DGDGs, 2 TGDGs) | [101] | |
| Eustigmatophyta | |||||
| Nannochloropsis oculata | C8 LC-MSn ESI-Q-TOF | Bligh and Dyer | MGDG 18:3/16:3, 18:4/18:4 DGDG 20:5/14:0, 20:5/16:0 SQDG 16:1/16:0 | MGDG 17% DGDG 12% SQDG 6.3% | [72] |
| nLC-MSn ESI FT-ICR | Chloroform/methanol | MGDG 20:5/20:5 DGDG 20:5/16:0, 20:5/16:1 SQDG 16:1/16:0 (13 MGDGs, 20 DGDGs, 33 SQDGs | [14] | ||
| Nannocloropsis oceanica | C8 LC-MS ESI-Q-TOF | Bligh and Dyer | MGDG 20:5/14:0, 20:5/16:0, ∆-C16/∆-C16, ∆-C18/∆-C16 DGDG 20:5/16:0, ∆-C18/∆-C16 SQDG ∆-C16/∆-C16, 20:5/16:0, (6 MGDGs, 4 DGDGs, 7 SQDGs) | [102] | |
| Nannochloropsis sp. | Off-line SPE-Si Off-line TLC LC-MSn ESI-QqQ | Isopropanol Chloroform/methanol | MGDG 20:5/14:0, DGDG 20:5/14:0, 20:5/16:0, 20:5/16:0 SQDG 16:1/16:0 (14 MGDGs; 16 DGDGs, 6 SQDGs) | [52] | |
| C8 LC-MSn ESI-Q-TOF | Bligh and Dyer | MGDG 18:3/16:3, 18:4/18:4 DGDG 20:5/14:0, 20:5/16:0 SQDG 16:1/16:0 | MGDG 13% DGDG 5.6% SQDG 18% | [72] | |
| LC-MSn ESI-QqQ | Bligh and Dyer | MGDG 20:5/14:0 DGDG 20:5/14:0 SQDG 18:3/16:0 (14 MGDGs, 16 DGDGs, 20 SQDGs) | MGDG 42% DGDG 14% SQDG 39% | [95] | |
| Paper spray ionization LTQ-Orbitrap-MSn | No need of extraction | MGDG 32:3, 32:5, 34:2, 34:6 SQDG 30:0, 32:1, 34:3, 36:6 (4 MGDGs, 4 SQDGs) | [96] | ||
| Labyrinthulomycetes | |||||
| Schizochytrium limacinum | Off-line SPE-Si Off-line TLC LC-MSn ESI-QqQ | Isopropanol Chloroform/methanol | MGDG 22:6/16:0 DGDG 18:1/18:0 SQDG 16:1/16:0 (14 MGDGs; 16 DGDGs, 6 SQDGs) | [52] | |
| Rhodophyta | |||||
| Porphyridium purpuream | Off Line TLC MALDI-QIT-TOF-MSn | Dichloromethane/methanol | SQDG 18:2/16:0, 20:4/16:0, 20:5/16:0 (3 SQDGs) | [19] | |
| Porphyridium aerugineum | Off-line Silica Column LC-Q-MSn | Methanol Ethyl acetate | MGDG 20:4/18:3, 20:4/16:0, 20:5/16:0, 20:5/18:3, 20:5/20:4 (5 MGDGs) | [73] | |
| Prymnesiophyta | |||||
| Isochrysis galbana | C8 LC-MSn ESI-Q-TOF | Bligh and Dyer | MGDG 18:3/16:3, 18:4/18:4, 22:6/16:0 DGDG 20:5/14:0, 20:5/16:0 SQDG 16:1/16:0 | MGDG 37% DGDG 11% SQDG 41% | [72] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Costa, E.; Silva, J.; Mendonça, S.H.; Abreu, M.H.; Domingues, M.R. Lipidomic Approaches towards Deciphering Glycolipids from Microalgae as a Reservoir of Bioactive Lipids. Mar. Drugs 2016, 14, 101. https://doi.org/10.3390/md14050101
Da Costa E, Silva J, Mendonça SH, Abreu MH, Domingues MR. Lipidomic Approaches towards Deciphering Glycolipids from Microalgae as a Reservoir of Bioactive Lipids. Marine Drugs. 2016; 14(5):101. https://doi.org/10.3390/md14050101
Chicago/Turabian StyleDa Costa, Elisabete, Joana Silva, Sofia Hoffman Mendonça, Maria Helena Abreu, and Maria Rosário Domingues. 2016. "Lipidomic Approaches towards Deciphering Glycolipids from Microalgae as a Reservoir of Bioactive Lipids" Marine Drugs 14, no. 5: 101. https://doi.org/10.3390/md14050101
APA StyleDa Costa, E., Silva, J., Mendonça, S. H., Abreu, M. H., & Domingues, M. R. (2016). Lipidomic Approaches towards Deciphering Glycolipids from Microalgae as a Reservoir of Bioactive Lipids. Marine Drugs, 14(5), 101. https://doi.org/10.3390/md14050101